Abstract
Epstein–Barr virus positivity (EBV(+)) and high‐microsatellite instability (MSI‐H) have been identified as molecular subgroups in gastric carcinoma. The aim of our study was to determine the prognostic and predictive relevance of these subgroups in the context of platinum/5‐fluorouracil (5‐FU) based preoperative chemotherapy (CTx). Additionally, we investigated the clinical relevance of the low‐MSI (MSI‐L) phenotype. We analysed 760 adenocarcinomas of the stomach or the gastro‐oesophageal junction encompassing 143 biopsies before CTx and 617 resected tumours (291 without and 326 after CTx). EBV was determined by PCR and in situ hybridisation for selected cases. MSI was analysed by PCR using five microsatellite markers and classified as MSI‐H and MSI‐L. Frequencies of EBV(+), MSI‐H and MSI‐L in the biopsies before CTx were 4.2, 10.5 and 4.9% respectively. EBV(+) or MSI‐H did not correlate with response, but MSI‐L was associated with better response (p = 0.011). In the resected tumours, frequencies of EBV(+), MSI‐H and MSI‐L were 3.9, 9.6 and 4.5% respectively. Overall survival (OS) was significantly different in the non‐CTx group (p = 0.014). Patients with EBV(+) tumours showed the best OS, followed by MSI‐H. MSI‐L was significantly associated with worse OS (hazard ratio [HR], 2.21; 95% confidence interval [CI], 1.21–4.04, p = 0.01). In the resected tumours after CTx, MSI‐H was also associated with increased OS (HR, 0.54; 95% CI, 0.26–1.09, p = 0.085). In multivariable analysis, molecular classification was an independent prognostic factor in the completely resected (R0) non‐CTx group (p = 0.035). In conclusion, MSI‐H and EBV(+) are not predictive of response to neoadjuvant platinum/5‐FU based CTx, but they are indicative of a good prognosis. In particular, MSI‐H indicates a favourable prognosis irrespective of treatment with CTx. MSI‐L predicts good response to CTx and its negative prognostic effect for patients treated with surgery alone suggests that MSI‐L might help to identify patients with potentially high‐benefit from preoperative CTx.
Keywords: microsatellite instability, Epstein–Barr virus, adenocarcinoma, gastric, gastro‐oesophageal junction, outcome, neoadjuvant chemotherapy, prognosis, molecular subtype
Introduction
Pre‐/peri‐operative chemotherapy (CTx) containing a platinum/5‐fluorouracil (5‐FU) combination is recommended for patients with advanced gastric carcinoma (GC) in western countries, but response rates are limited 1, 2, 3.
Recent studies suggest that molecular classification should be considered for optimal therapy planning. Molecular classification systems have been described by The Cancer Genome Atlas (TCGA) network and the Asian Cancer Research Group (ACRG). Both include tumours with microsatellite instability (MSI) as one subgroup 4, 5. MSI is characterised by accumulation of length alterations of microsatellite sequences and is commonly determined using five microsatellite markers 6. Depending on the number of unstable markers, MSI can be classified into high‐MSI (MSI‐H) (≥2/5 unstable markers) or low‐MSI (MSI‐L) (1/5 unstable marker). If there is no MSI, the tumour is considered as microsatellite stable (MSS). MSI‐H is related to DNA mismatch repair deficiency and is detected in about 7–24% of GC 4, 5, 7, 8, 9, 10, 11. MSI‐H has been related to good prognosis in GC in the majority of studies, but conflicting results of the prognostic significance for patients treated with CTx have been described 8, 9, 11, 12, 13, 14. Specifically a negative prognostic effect of MSI‐H for patients receiving neoadjuvant CTx has been reported based on the analysis of tumours resected after CTx, whereas another study reported the MSI‐H phenotype as a favourable prognostic marker also in this therapeutic setting 9, 14.
The MSI‐L phenotype has been described in various tumours, including GC, but the biological and clinical significance is largely unclear and MSI‐L and MSS tumours are frequently combined in one group 8, 11.
Another subgroup of the TCGA classification is formed by Epstein–Barr virus positive (EBV(+)) tumours, which represent about 4–10% of GC 4, 10, 15, 16.
EBV positivity was shown to be associated with better prognosis in GC patients though there are studies that find no clear correlation between EBV status and survival and the relevance of EBV positivity to predict response to neoadjuvant CTx is unclear 10, 15.
EBV positivity and MSI‐H were associated with good response in a clinical trial evaluating an immune check point inhibitor in metastatic GC 17.
In light of these new therapeutic options and against the background of still limited data available in the literature related to neoadjuvant CTx, further knowledge about the clinical relevance of MSI and EBV status in connection with classical treatment regimens is essential for the selection of the most appropriate treatment for each GC patient.
Thus the goal of our study was to determine the prognostic and predictive significance of EBV positivity and MSI for carcinomas of the stomach and gastro‐oesophageal junction in the context of preoperative platinum/5‐FU based CTx. As various clinical aspects are relevant in this therapeutic setting, we performed a comprehensive analysis of overall 760 tumours encompassing three different patient cohorts each with specific characteristics.
First, we analysed tumour biopsies before neoadjuvant CTx; this represents the most appropriate cohort to test for an association with therapy response as it allows inclusion of both non‐responding and completely or nearly completely responding patients. In addition, biopsies are the type of specimens that are available before the start of CTx in daily clinical practice. Second, we analysed the molecular subgroups in a relatively large cohort of resected tumours from patients treated with surgery alone and third, resected tumours from patients after neoadjuvant CTx, to determine if their prognostic role is comparable and might support the choice of subsequent treatment modalities. Finally, as the clinical significance of the MSI‐L phenotype in GC is poorly characterised, we aimed to fill this gap and analysed MSI in terms of MSI‐H and MSI‐L.
Material and methods
Patients
Resected tumours from 704 patients with gastric adenocarcinomas including tumours of the gastro‐oesophageal junction (AEG II and AEG III according to Siewert and Stein 18) that were treated between 2001 and 2013 at the Department of Surgery of the University of Heidelberg and between 2001 and 2012 at the Technical University of Munich were included in the study. Essentially the patient cohort was described previously 19. Tumours from 87 patients were excluded from this study and the final cohort of 617 tumours consisted of 291 tumours from patients treated with surgery alone and 326 tumours from patients after neoadjuvant CTx (Figure 1).
Figure 1.
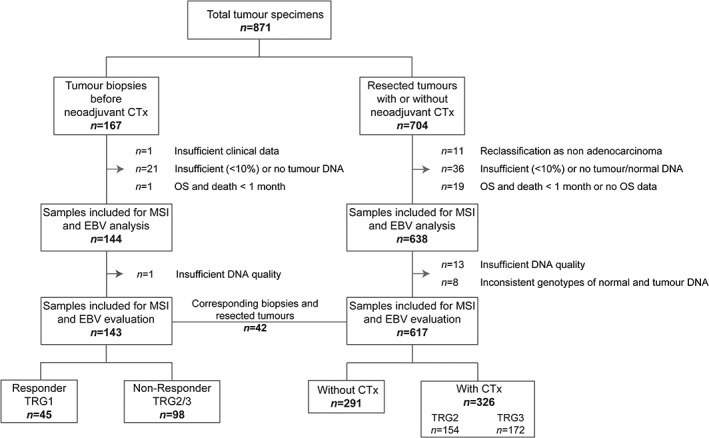
Flow chart diagram of patient and specimen inclusion.
Tumour biopsies before neoadjuvant CTx from 167 patients treated between 1993 and 2013 at the Department of Surgery of the Technical University of Munich were included. Limitation for inclusion was the availability of DNA or paraffin blocks with tumour and non‐tumorous tissues and 143 biopsies were finally analysed. Corresponding biopsies before and resected tumours after CTx from 42 patients were included (Figure 1). Previous studies had analysed biopsy specimens of 58 patients for MSI using a different panel of microsatellite markers 7, 20.
CTx and surgery
Patients were treated with platinum/5‐FU based chemotherapeutic regimens as detailed in supplementary material, Table S1. Comparison of overall survival (OS) of patients with resected tumours after CTx relating to treatment with platinum/5‐FU based regimens with and without taxanes or relating to regimens containing two or three drugs, revealed no statistically significant differences in either case (see supplementary material, Table S2).
All surgical approaches included an abdominal D2 lymphadenectomy and are described in detail in supplementary material, Supplementary materials and methods.
Response evaluation
Response to preoperative CTx was determined histopathologically and was classified into three tumour regression grades (TRG): TRG1, TRG2 and TRG3, which corresponded to <10, 10–50 and >50% residual tumour cells/tumour bed respectively. The prognostic relevance of this classification system has been demonstrated in previous studies 21, 22.
All three TRGs were present among the patients with tumour biopsies before CTx; patients with TRG1 were classified as responders, and those with TRG2 and TRG3 as non‐responders. Only tumours with TRG2 and TRG3 were present among the patients in the resected tumour cohort after CTx; these allow isolation of sufficient DNA from residual tumour cells.
Follow‐up and overall survival
Follow‐up was performed as described 19. OS was defined as the time between the date of operation and death by any cause.
Ethics statement
The study was in accordance with the Declaration of Helsinki and was approved by the local Institutional Review Boards at the Technical University of Munich (reference: 502/15s) and at the University of Heidelberg (reference: 301/2001).
DNA isolation
DNA from formalin fixed paraffin embedded (FFPE) tissues was isolated after manual microdissection from 8 μm thick sections after deparaffinisation and proteinase K digestions using the Maxwell extraction system according to the instructions of the manufacturer (Promega, Madison, WI, USA). Details are described in supplementary material, Supplementary materials and methods. Only samples with a tumour cell content of at least 10% were included for MSI analysis according to the described detection limit for MSI of 2–10% tumour alleles 23.
Analysis for MSI
MSI was determined by PCR analysing two mononucleotide repeats BAT25, BAT26 and three dinucleotide repeats D2S123, D5S346, D17S250 as recommended by the National Cancer Institute 6. Details are described in supplementary material, Supplementary materials and methods. Tumours with additional alleles at specific microsatellite markers compared to the corresponding normal tissue were classified as MSI. According to a standardised definition, MSI‐H was defined if at least two of the five markers showed MSI and as MSI‐L if one of the five markers showed MSI 6. Tumours without MSI were classified as MSS. MSI‐L cases were confirmed by a second independent PCR.
Detection of EBV
Screening for EBV was performed by a PCR based assay using primers for amplification of EBV specific DNA in the BamHI‐W and BamHI‐K regions of the virus as described 24. Tumours with positive signals in the PCR assay were further analysed by chromogenic in situ hybridisation using the EBV early RNA Probe and the iViEW Blue detection kit (Ventana, Roche, Tucson, AZ, USA) on an automated system (Ventana Medical System, Roche) according to the instructions of the manufacturer. EBV positivity was defined when positive staining after in situ hybridisation was present in the nuclei of the tumour cells.
Statistical analysis
Chi‐squared tests or Fisher's exact tests were used for hypothesis testing of differences between the relative frequencies. Kaplan–Meier estimates of survival rates were compared by log rank tests. Relative risks were estimated by hazard ratios (HRs) from univariable Cox proportional hazard models or from Firth's corrected Cox‐regression. A multivariable Cox proportional hazards model was built by stepwise forward variable selection using likelihood‐ratio tests of pre‐therapeutically and post‐therapeutically available clinical factors.
The pre‐therapeutically available factors were: sex, age (continuous variable), histological type according to Laurén (intestinal versus non‐intestinal), tumour localisation (proximal, middle, distal, total) and clinically determined tumour stage (cT2 versus cT3/cT4). The post‐therapeutic factors were: sex, age, histological type according to Laurén, tumour localisation, depth of tumour invasion (pT2 versus pT3/pT4), lymph node involvement (pN0 versus pN+), R‐category (R0 versus R+) and status of metastasis (M0 versus M+). Statistical analyses were performed using SPSS, Version 25 (IBM Corp., Armonk, NY, USA). Exploratory 5% significance levels (two‐tailed) were used for hypothesis testing.
Results
Study enrolment and patient characteristics
Our study population consisted of different GC cohorts. The biopsy cohort encompassed patients with pre‐therapeutic tumour biopsies before CTx with inclusion of responding (TRG1) and non‐responding (TRG2/3) patients to accurately determine the predictive and prognostic value of the molecular subgroups for CTx treatment. Of 167 pre‐therapeutic biopsies, which were initially evaluated for the study, 24 were excluded. Among the 143 analysed biopsies, 45 of the patients showed TRG1, 34 showed TRG2 and 64 showed TRG3 in the resected specimens. The OS of patients in relation to TRG was significantly different (log rank p < 0.01) and is shown in supplementary material, Figure S1. Only some difference in OS was observed between patients with TRG2 and TRG3, therefore both groups were classified as non‐responders and patients with TRG1 as responders.
The resected tumour cohort encompassed initially 704 patients and 87 were excluded. Of the remaining 617 resected tumours, 291 were from patients treated with surgery alone and 326 were from patients after treatment with CTx among them 154 with TRG2 and 172 with TRG3. An overview of the enrolment of patients with the respective exclusion criteria is shown in Figure 1. Clinical characteristics of the patients included for analysis are summarised in Table 1.
Table 1.
Patient characteristics
| Resected specimens | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumour biopsies before neoadjuvant CTx | All | Without neoadjuvant CTx | After neoadjuvant CTx | ||||||
| Category | Value | n | % | n | % | n | % | n | % |
| Cases | Total | 143 | 100 | 617 | 100 | 291 | 100 | 326 | 100 |
| Age (years) | Median | 61.1 | 64.6 | 68.1 | 61.3 | ||||
| Range | 23.1–78.0 | 28.3–90.9 | 32.1–90.9 | 28.3–81.2 | |||||
| Follow‐up period (month) | Median | 69.6 | 57.9 | 58.8 | 56.7 | ||||
| 95% CI | 61.6–77.6 | 53.1–62.7 | 50.7–66.9 | 47.4–66.0 | |||||
| Overall survival (month) | Median | 48.1* | 44.6 | 85.0 | 32.4 | ||||
| 95% CI | 26.2–70.0 | 30.2–59.0 | 51.7–118.3 | 23.0–41.8 | |||||
| Sex | Male | 109 | 76.2 | 453 | 73.4 | 193 | 66.3 | 260 | 79.8 |
| Female | 34 | 23.8 | 164 | 26.6 | 98 | 33.7 | 66 | 20.2 | |
| Localisation | Proximal | 100 | 69.9 | 301 | 48.8 | 97 | 33.3 | 204 | 62.6 |
| Middle | 23 | 16.1 | 153 | 24.8 | 84 | 28.9 | 69 | 21.2 | |
| Distal | 14 | 9.8 | 131 | 21.2 | 92 | 31.6 | 39 | 12.0 | |
| Total/linitis | 6 | 4.2 | 28 | 4.5 | 14 | 4.8 | 14 | 4.3 | |
| N/A | 0 | 0 | 4 | <1 | 4 | 1.4 | 0 | 0 | |
| Laurén histological subtype | Intestinal | 72 | 50.3 | 347 | 56.2 | 155 | 53.3 | 192 | 58.9 |
| Non‐intestinal | 71 | 49.7 | 270 | 43.8 | 136 | 46.7 | 134 | 41.1 | |
| Tumour grade | G1/2 | 33 | 23.1 | 125 | 20.3 | 80 | 27.5 | 45 | 13.8 |
| G3/4 | 110 | 76.9 | 400 | 64.8 | 210 | 72.5 | 190 | 58.3 | |
| N/A | 0 | 0 | 92 | 14.9 | 1 | 0 | 91 | 27.9 | |
| cT | cT2 | 8 | 5.6 | 144 | 23.3 | 129 | 44.3 | 15 | 4.6 |
| cT3/cT4 | 131 | 91.6 | 471 | 76.3 | 161 | 55.3 | 310 | 95.1 | |
| N/A | 4 | 2.8 | 2 | <1 | 1 | <1 | 1 | <1 | |
| (y) pT† | (y) pT0 | 9 | 6.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| (y) pT1 | 12 | 8.4 | 56 | 9.1 | 42 | 14.4 | 14 | 4.3 | |
| (y) pT2 | 20 | 14.0 | 79 | 12.8 | 47 | 16.2 | 32 | 9.8 | |
| (y) pT3 | 81 | 56.6 | 328 | 53.2 | 139 | 47.8 | 189 | 58.0 | |
| (y) pT4 | 19 | 13.3 | 154 | 25.0 | 63 | 21.6 | 91 | 27.9 | |
| N/A | 2 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (y) pN | Negative | 61 | 42.7 | 189 | 30.6 | 104 | 35.7 | 85 | 26.1 |
| Positive | 80 | 55.9 | 428 | 69.4 | 187 | 64.3 | 241 | 73.9 | |
| N/A | 2 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metastasis status | No | 97 | 67.8 | 534 | 86.5 | 272 | 93.5 | 262 | 80.4 |
| Yes | 44 | 30.8 | 83 | 13.5 | 19 | 6.50 | 64 | 19.6 | |
| N/A | 2 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Resection status | R0 | 117 | 81.8 | 469 | 76.0 | 235 | 80.8 | 234 | 71.8 |
| R1 | 24 | 16.8 | 148 | 24.0 | 56 | 19.2 | 92 | 28.2 | |
| N/A | 2 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour regression status | TRG1 | 45 | 31.4 | 0 | |||||
| TRG2 | 34 | 23.8 | 154 | 47.2 | |||||
| TRG3‡ | 64 | 44.8 | 172 | 52.8 | |||||
| Response | Responder | 45 | 31.5 | 0 | 0 | ||||
| (TRG1) | |||||||||
| Non‐Responder | 98 | 68.5 | 326 | 100 | |||||
| (TRG2/3)‡ | |||||||||
| EBV status | Positive | 6§ | 4.2 | 24 | 3.9 | 8§ | 2.7 | 16 | 4.9 |
| Negative | 137 | 95.8 | 593 | 96.1 | 283 | 97.3 | 310 | 95.1 | |
| MSI status | MSS | 121 | 84.6 | 530 | 85.9 | 241 | 82.8 | 289 | 88.7 |
| MSI‐L | 7§ | 4.9 | 28 | 4.5 | 15§ | 5.2 | 13 | 4.0 | |
| MSI‐H | 15 | 10.5 | 59 | 9.6 | 35 | 12.0 | 24 | 7.4 | |
N/A, not available.
OS was defined as time between the date of operation and death by any cause. For two patients who were not operated; the date of start of CTx was used.
Classification according to 7th Edition UICC 2007.
Two patients with tumour progression during CTx were not operated on; they were classified as TRG3 and a Non‐responder respectively.
One tumour biopsy and one resected tumour without neoadjuvant CTx were positive for both MSI‐L and EBV.
Frequency of EBV and MSI in the biopsy and the resected tumour cohorts
EBV(+) was detected in 6 (4.2%) of the 143 tumour biopsies and MSI‐H and MSI‐L were found in 15 (10.5%) and 7 (4.9%) of the samples respectively (Table 1).
In the resected tumour cohorts, 24 (3.9%) of the 617 tumours were EBV positive, and 59 (9.6%) and 28 (4.5%) showed MSI‐H and MSI‐L, respectively (Table 1). Considering the type of unstable markers among the MSI‐L tumours, 33 (94%) of the 35 MSI‐L tumours showed instability at one of the three dinucleotide repeats that are included in the marker panel used for the determination of MSI.
The MSI status of the 42 paired biopsies before CTx and resected tumours after CTx were the same in all cases. None of these pairs was EBV(+).
All MSI‐H tumours were negative for EBV. One biopsy and one resected tumour were positive for both MSI‐L and EBV. These two patients were excluded from further analyses and clinical parameters were compared for the four molecular subgroups, EBV(+), MSI‐H, MSI‐L and MSS/EBV(−), taking the latter as reference.
EBV, MSI and association with patient characteristics
Association with clinical characteristics was analysed for the 616 patients with resected tumours. EBV(+) was associated with male sex (p = 0.015), tumour localisation in the middle of the stomach (p = 0.033) and poor differentiation (p = 0.01). MSI‐H was associated with older age (p < 0.001), distal tumour localisation (p = 0.05) and absence of metastasis (p = 0.038). MSI‐L was more frequent in intestinal GC (p = 0.04) (see supplementary material, Table S3).
EBV, MSI and response to neoadjuvant CTx
EBV(+) and MSI‐H were not associated with response to CTx in the pre‐therapeutic biopsies before CTx (p = 0.626 and p = 1.00 respectively). In contrast, MSI‐L demonstrated a significant association with better response (p = 0.011). Five (83%) of six MSI‐L biopsies were of responding patients with TRG1 in the resected specimens after CTx compared to 33 (28%) of 116 MSS/EBV(−) tumours (Figure 2 and Table 2).
Figure 2.

EBV and MSI status of pre‐therapeutic biopsies and response to neoadjuvant CTx. Comparison of the four molecular subgroups with response to neoadjuvant CTx is shown. *P value of Chi‐square test each compared with MSS/EBV(−). Significant value in bold. †One tumour biopsy from a responding patient (TRG1) was positive for both MSI‐L and EBV, and was excluded from analysis.
Table 2.
EBV and MSI status of tumour biopsies before neoadjuvant CTx and resected tumours after neoadjuvant CTx and their association with response and tumour regression
| MSS/EBV(−) (n) | EBV(+) (n) | P value* | MSI‐L (n) | P value* | MSI‐H (n) | P value* | |
|---|---|---|---|---|---|---|---|
| Tumour biopsies before neoadjuvant CTx (n = 142) | |||||||
| Response | |||||||
| Responder (TRG1) | 33 | 2† | 0.626 | 5† | 0.011 | 4 | 1.00 |
| Non‐responder (TRG2/3) | 83 | 3 | 1 | 11 | |||
| Resected tumours after neoadjuvant CTx (n = 326) | |||||||
| Tumour regression grade‡ | |||||||
| TRG2 | 136 | 8 | 0.989 | 6 | 0.796 | 4 | 0.002 |
| TRG3 | 137 | 8 | 7 | 20 | |||
P value of Chi‐square test or Fisher's exact test compared to MSS/EBV(−).
One tumour biopsy from a responding patient (TRG1) was positive for both; MSI‐L and EBV, and was excluded from analysis.
TRG1 tumours were not included in the group of resected tumours after neoadjuvant CTx due to no or only extremely small amounts of residual tumour cells.
Significant p values are shown in bold.
Other groups reported differential responses of MSI‐H tumours to perioperative CTx 9 and we therefore additionally compared the prevalence of MSI‐H between the TRG2 and TRG3 group in our resected cohort after CTx. A significant difference was observed as in the TRG3 group 20 (12%) of 172 were MSI‐H compared to 4 (3%) of 154 in the TRG2 group (p = 0.002) (Table 2).
EBV, MSI and survival in the biopsy cohort
Comparison of OS of patients with biopsies before CTx regarding the four subgroups showed no statistically significant difference (overall log rank p = 0.565) (Figure 3A). Essentially in line with an association of MSI‐L with better response to CTx, MSI‐L tumours showed the best OS (MSI‐L: HR, 0.47; 95% confidence interval [CI], 0.12–1.93, p = 0.297; MSI‐H: HR, 0.66, 95% CI, 0.29–1.53, p = 0.333; EBV(+): HR, 0.83, 95% CI, 0.26–2.64, p = 0.754). All survival data including the 1, 3 and 5 year OS rates are summarised in Table 3.
Figure 3.
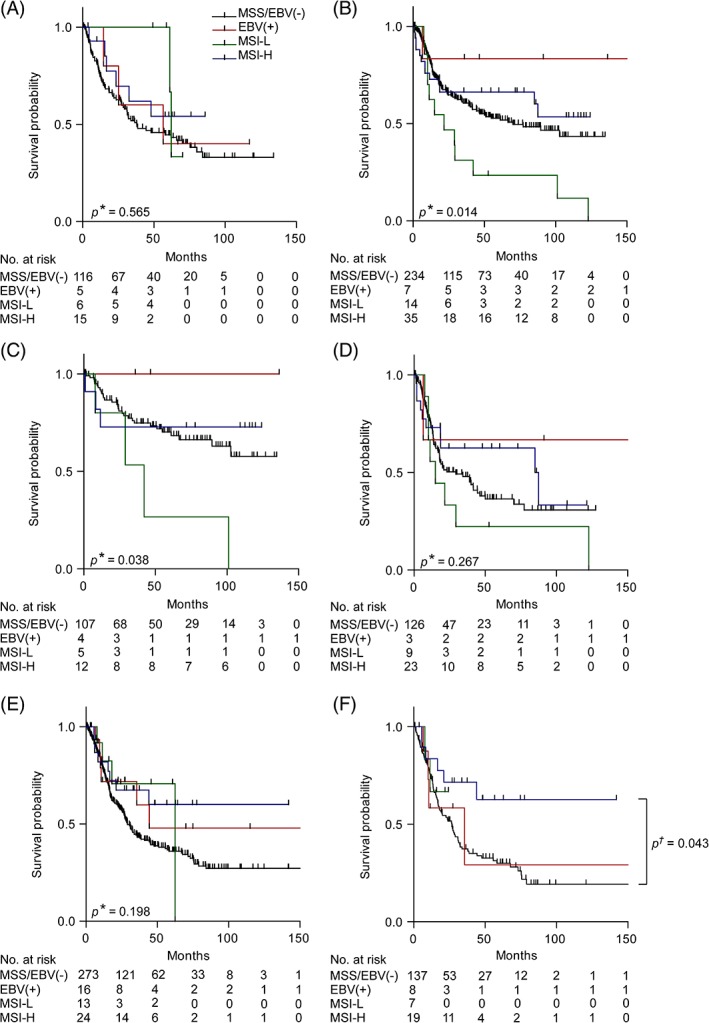
Discrimination of patient survival by EBV and MSI status. Kaplan–Meier curves of the EBV(+), MSI‐L, MSI‐H and MSS/EBV(−) patients are shown. Tumour biopsies before neoadjuvant CTx (A). Resected tumours from patients treated without neoadjuvant CTx: all patients (B), subgroups with clinical tumour stage cT2 (C) and cT3/cT4 (D). Resected tumours from patients after neoadjuvant CTx: all patients (E) and subgroup with TRG3 (F). *P value of log rank test (overall); † P value of Cox's regression.
Table 3.
Survival data of the patient cohorts and subgroups in association with EBV and MSI status
| EBV and MSI status | No. | Events | Survival probability (%) | Median survival (month) | HR | P value* | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | (95% CI) | (95% CI) | |||||
| Tumour biopsies before neoadjuvant CTx | MSS/EBV(−) | 116 | 66 | 74.6 | 52.0 | 44.6 | 37.9 (17.4–58.4) | 1 ref. | |
| EBV(+) | 5 | 3 | 100 | 60.0 | 40 | 56.5 (0.0–123.7) | 0.83 (0.26–2.64) | 0.754 | |
| MSI‐L | 6 | 2 | 100 | 100 | 100 | 62.2 (60.6–63.9) | 0.47 (0.12–1.93) | 0.297 | |
| MSI‐H | 15 | 6 | 92.2 | 61.9 | 54.2 | nr | 0.66 (0.29–1.53) | 0.333 | |
| Total | 142 | 77 | 78.3 | 55.1 | 47.3 | 48.1 (26.2–70.0) | |||
| Resected tumours without neoadjuvant CTx (total) | MSS/EBV(−) | 234 | 96 | 81.6 | 60.6 | 52.8 | 70.0 (32.7–107.3) | 1 ref. | |
| EBV(+) | 7 | 1 | 83.3 | 83.3 | 83.3 | nr | 0.26 (0.04–1.87) | 0.181 | |
| MSI‐L | 14 | 12 | 62.3 | 31.2 | 23.4 | 21.7 (1.0–42.4) | 2.21 (1.21–4.04) | 0.01 | |
| MSI‐H | 35 | 13 | 72.9 | 66.2 | 66.2 | nr | 0.81 (0.45–1.44) | 0.465 | |
| Total | 290 | 122 | 79.7 | 60.4 | 53.8 | 85.0 (52.1–117.9) | |||
| Resected tumours without neoadjuvant CTx (cT2) | MSS/EBV(−) | 107 | 30 | 91.8 | 74.7 | 70.2 | nr | 1 ref. | |
| EBV(+) | 4 | 0 | 100 | 100 | 100 | nr | 0.43† (0–3.06) | 0.495 | |
| MSI‐L | 5 | 4 | 80 | 53.3 | 26.7 | 42.2 (13.7–70.7) | 3.88† (1.24–9.51) | 0.023 | |
| MSI‐H | 12 | 3 | 72.7 | 72.7 | 72.7 | nr | 0.81† (0.22–2.20) | 0.713 | |
| Total | 128 | 37 | 89.7 | 74.5 | 69.7 | 42.2(13.7–70.7) | |||
| Resected tumours without neoadjuvant CTx (cT3/cT4) | MSS/EBV(−) | 126 | 65 | 73.4 | 48.3 | 36.7 | 29.3 (11.4–47.2) | 1 ref. | |
| EBV(+) | 3 | 1 | 66.7 | 66.7 | 66.7 | nr | 0.35 (0.05–2.56) | 0.300 | |
| MSI‐L | 9 | 8 | 55.6 | 22.2 | 22.2 | 15 (4.2–25.8) | 1.52 (0.72–3.20) | 0.272 | |
| MSI‐H | 23 | 10 | 72.9 | 62.5 | 62.5 | 85 (20.1–149.9) | 0.70 (0.36–1.36) | 0.289 | |
| Total | 161 | 84 | 72.1 | 49.2 | 40.7 | 34.3 (17.6–51) | |||
| Resected tumours after neoadjuvant CTx (total) | MSS/EBV(−) | 273 | 152 | 77.4 | 43.3 | 36 | 29.1 (24.6–33.6) | 1 ref. | |
| EBV(+) | 16 | 6 | 71.8 | 59.8 | 47.9 | 44.4 | 0.64 (0.29–1.46) | 0.290 | |
| MSI‐L | 13 | 4 | 82.5 | 70.7 | 70.7 | 62.4 | 0.64 (0.24–1.74) | 0.385 | |
| MSI‐H | 24 | 8 | 81.9 | 67.5 | 60 | nr | 0.54 (0.26–1.09) | 0.085 | |
| Total | 326 | 170 | 77.7 | 46.5 | 39 | 32.4 (23.0–41.8) | |||
| Resected tumours after neoadjuvant CTx (TRG3) | MSS/EBV(−) | 137 | 83 | 73.3 | 37.3 | 30 | 26.7 (19.2–34.2) | 1 ref. | |
| EBV(+) | 8 | 4 | 58.3 | 29.2 | 29.2 | 35.6 (0–73.6) | 0.91 (0.33–2.47) | 0.847 | |
| MSI‐L | 7 | 2 | 66.7 | 0 | 0 | nr | 0.79 (0.19–3.21) | 0.736 | |
| MSI‐H | 19 | 6 | 82.5 | 69.8 | 59.9 | nr | 0.43 (0.19–0.98) | 0.043 | |
| Total | 171 | 95 | 73.2 | 41.0 | 33.4 | 27.4 (20.8–34.0) | |||
ref., reference; nr, not reached.
P value of Cox's regression.
HRs were calculated according to Firth's correction.
EBV, MSI and survival in the resected non‐CTx cohort
Analysis of OS of patients in the resected cohort was separately performed in the groups stratified according to CTx (yes/no). In the non‐CTx group a statistically significant difference of OS regarding the four molecular groups was observed (overall log rank p = 0.014) (Figure 3B). Patients with EBV(+) tumours showed the best OS followed by MSI‐H tumours (EBV(+): HR, 0.26; 95% CI, 0.04–1.87, p = 0.181; MSI‐H: HR, 0.81; 95% CI, 0.45–1.44, p = 0.465). Patients with MSI‐L tumours showed a statistically significantly worse OS (HR, 2.21; 95% CI, 1.21–4.04, p = 0.01) (Table 3).
Subgroup analysis within the resected non‐CTx cohort stratified according to clinical tumour stage demonstrated a pronounced difference – especially regarding MSI‐L – in the cT2 group (overall log rank p = 0.038) (Figure 3C,D). All patients with EBV(+) tumours were alive and patients with MSI‐L tumours showed a significantly worse OS compared to the MSS/EBV(−) negative tumours (EBV(+): HR, 0.43; 95% CI, 0–3.06, p = 0.495; MSI‐H: HR, 0.81, 95% CI, 0.22–2.20, p = 0.713; MSI‐L: HR, 3.88; 95% CI, 1.24–9.51, p = 0.023) (Table 3).
Multivariable analysis was performed for the total resected non‐CTx cohort. Analysing the molecular subgroups and the pre‐therapeutically available clinical factors (age, sex, cT, histological type according to Laurén, tumour localisation) revealed cT (p < 0.001), age (p = 0.001) and the molecular classification (overall p = 0.027) as significant prognostic factors. Interestingly, considering the molecular subgroups separately, MSI‐H emerged as an independent prognostic factor (Table 4).
Table 4.
Multivariable analysis of survival including pre‐ and post‐therapeutically available clinical factors in the resected non‐CTx cohort
| HR | 95% CI | P value* | |
|---|---|---|---|
| Pre‐therapeutic factors† | |||
| Clinical tumour stage | |||
| cT2 | 1 | – | <0.001 |
| cT3/4 | 2.74 | 1.84–4.07 | |
| Age | 1.03 | 1.01–1.05 | 0.001 |
| Molecular classification | 0.027 | ||
| MSS/EBV(−) | 1 | – | – |
| EBV(+) | 0.20 | 0.03–1.46 | 0.113 |
| MSI‐L | 1.53 | 0.83–2.84 | 0.175 |
| MSI‐H | 0.55 | 0.30–1.00 | 0.049 |
| Post‐therapeutic factors‡ | |||
| pN | |||
| pN0 | 1 | – | <0.001 |
| pN1 | 3.15 | 1.95–5.10 | |
| Age | 1.03 | 1.01–1.04 | 0.004 |
| Resection status | |||
| R0 | 1 | – | 0.020 |
| R1 | 1.68 | 1.08–2.60 | |
| Localisation | 0.026 | ||
| Proximal | 1 | – | – |
| Middle | 0.67 | 0.42–1.05 | 0.079 |
| Distal | 0.52 | 0.33–0.83 | 0.006 |
| Total | 1.09 | 0.51–2.31 | 0.830 |
| Post‐therapeutic factors (R0 resected, non‐CTx cohort) | |||
| pN | |||
| pN0 | 1 | – | <0.001 |
| pN1 | 2.67 | 1.59–4.48 | |
| Age | 1.03 | 1.01–1.05 | 0.004 |
| Molecular classification | 0.035 | ||
| MSS/EBV(−) | 1 | – | – |
| EBV(+) | 0.23 | 0.03–1.66 | 0.144 |
| MSI‐L | 1.80 | 0.88–3.71 | 0.110 |
| MSI‐H | 0.55 | 0.28–1.10 | 0.090 |
| pT§ | |||
| pT1/2 | 1 | – | 0.023 |
| pT3/4 | 1.36 | 1.04–1.78 | |
P value of forward likelihood ratio Cox's regression model.
Pre‐therapeutic factors included: age, sex, localisation, Laurén subtypes, clinical tumour stage, molecular classification.
Post‐therapeutic factors included: age, sex, localisation, Laurén subtypes, pT, pN, M‐status, R‐status, molecular classification.
Classification according to 7th Edition UICC 2007.
Including the post‐therapeutically available factors revealed only the clinical parameters pN (p < 0.001), age (p = 0.004) and R‐category (p = 0.020) as independent prognostic factors (Table 4). Analysis of the subgroup of only completely resected patients (R0 group) revealed pN (p < 0.001), age (p = 0.004), the molecular classification (p = 0.035) and pT (p = 0.023) as independent prognostic factors (Table 4).
EBV, MSI and survival in the resected cohort after neoadjuvant CTx
In the CTx group, differences in OS were not statistically significant (overall log rank p = 0.198) (Figure 3E). However, an obviously better OS was observed for patients with MSI‐H tumours (HR, 0.54; 95% CI, 0.26–1.09, p = 0.085). These results are included in Table 3.
Subgroup analysis in the TRG2 and TRG3 groups separately revealed a significantly better OS for patients with MSI‐H tumours in the TRG3 group (HR, 0.43; 95% CI, 0.19–0.98, p = 0.043) (Figure 3F and Table 3).
Discussion
Molecular subtypes in GC have been identified, but knowledge about their clinical relevance in particular in the context of preoperative CTx is limited 4, 5, 9, 14, 17, 25, 26, 27. In this study, we addressed this issue and analysed the prognostic and predictive significance of four molecular subgroups namely EBV(+), MSI‐H, MSI‐L and MSS/EBV(−) in pre‐therapeutic tumour biopsies of GC patients before platinum/5‐FU based neoadjuvant CTx and in resected tumours of patients with or without neoadjuvant CTx.
One of the most interesting finding of our study was a better response to neoadjuvant CTx of MSI‐L tumours in the pre‐therapeutic biopsy cohort. The patients also showed an increased OS, although the difference was statistically not significant, likely due to low‐sample size. Interestingly, MSI‐L seems to have a differential prognostic role depending on the specific treatment of the patients as in our resected cohort treated with surgery alone MSI‐L demonstrated a negative prognostic effect. Usually, pre‐/perioperative CTx is recommended for patients with advanced tumour stages (cT3/cT4), but some experts endorse that patients with cT2 tumours can also be treated 2, 28. As clinical staging is relatively imprecise and the negative prognostic effect of MSI‐L was particularly prominent for patients with clinically staged cT2 tumours in our study, the determination of MSI‐L may contribute to improved management of GC patients in this context. The analysis of MSI‐L is based on a simple, cost efficient multiplex PCR assay. Thus, assuming confirmation by other studies, MSI‐L could represent an attractive marker for routine clinical application, even considering the relatively low number of 4–5% of patients demonstrating the MSI‐L phenotype in their tumours. MSI‐L has been detected in various tumour entities including GC over a range of 4–20% 7, 8, 29. However, it has to be emphasised that the detection rate of MSI‐L is dependent on the number and on the type of the microsatellite markers tested 30. In our study, instability in MSI‐L tumours was mainly restricted to alterations at dinucleotide repeat markers. An association of MSI‐L with MSI preferentially at dinucleotide repeats and with worse prognosis has been demonstrated in colorectal cancer, which is compatible with our results 29.
In contrast to the MSI‐H phenotype with a well‐known molecular background related to defects in one of the four DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2, the origin and biological significance of the MSI‐L phenotype is largely unclear and controversially discussed. MSI‐L has been related to elevated mutation rates, to defects in specific DNA repair genes and/or induction by DNA damaging agents 30, 31. Based on our results, it is tempting to speculate that MSI‐L may reflect a particular type of impaired DNA repair and numerous proteins involved in these complex mechanisms represent possible candidates in that scenario. Comparing the frequencies of MSI‐L among the pre‐therapeutic biopsies and the resected tumours after CTx in our study, one could expect a decrease of MSI‐L in resected tumours after CTx given the association of MSI‐L with tumours of responding patients, which are not present in the resected tumour group. However, we found only a slight difference (4.90% in the biopsies, 3.98% in the resected tumours). Although highly speculative, induction of MSI‐L by a DNA damaging agent or tumour heterogeneity may counteract this assumed decrease.
Regarding MSI‐H, the majority of studies has demonstrated an association of this type of MSI with good prognosis in GC, which is essentially in line with our findings for patients in the resected cohorts 13, 27, 32, 33. In the context of CTx, however, different results have been reported 8, 9, 11, 12, 14. An attenuated or negative prognostic effect of MSI‐H has recently been reported for patients treated with adjuvant CTx 11, 34, 35. In addition, a negative prognostic effect of MSI based on the analysis of the resected tumours after CTx was proposed for patients who underwent preoperative CTx in the MAGIC trial 9. Our findings of no negative prognostic significance of MSI‐H when pre‐therapeutic tumour biopsies before CTx were analysed and the good prognostic effect of MSI‐H in the groups of resected patients both with and without CTx, do not support these results. Our data from tumour biopsies before neoadjuvant CTx may allow for a more comprehensive conclusion about the relevance of MSI‐H for response in terms of tumour regression and OS in the setting of neoadjuvant CTx than an analysis of resected specimens after CTx, in which tumours from patients with near to complete and complete response are naturally missing. Comparing the frequency of MSI‐H between the tumours with TRG2 and TRG3 after CTx in our study, we found an enrichment of MSI‐H in the TRG3 group. This is somewhat in line with the data from others mentioned above 9 and, indeed, argues for an association of MSI‐H with compromised response. However, patients with MSI‐H tumours in the TRG3 group still showed a significantly better OS than MSS/EBV(−) patients, thus underlining the positive prognostic effect of MSI‐H, even for patients with no or only minor response to CTx. This is essentially in line with a recent study analysing gastric and gastro‐oesophageal junction cancer patients undergoing neoadjuvant CTx 14. The frequency and the significant associations of MSI‐H which we found with patient age, tumour location or status of metastasis confirms results reported by others 13, 34.
EBV was detected in 4–5% of our tumours, which is similar to a recent report 10, 16. We did not observe an association of EBV(+) with response to CTx. However, in the non‐CTx resected cohort a better OS was observed for patients with EBV(+) tumours. In addition, an association of EBV(+) with tumour location and male sex was found, which is in line with results reported by others 10, 25.
Regarding multivariable analysis for survival performed in the non‐CTx cohort, our results confirm the well‐known prognostic impact of lymph node involvement and completeness of tumour resection as independent prognostic factors 36. The prognostic relevance of our molecular classification was underlined as it emerged as an independent prognostic factor in the multivariable analysis of only the completely resected patients and when considering only pre‐therapeutically available factors.
Despite the comprehensive analysis of a very large cohort of patients comprising 760 tumour samples overall, our study has limitations which are mainly related to its retrospective nature. Regarding the analysis of the pre‐therapeutic biopsies, the availability of DNA or suitable tumour tissues presented the main limiting factor for inclusion of patients. Further limitations are that our analysis was not performed in the context of a randomised clinical trial testing different treatment regimens but refers to a sample series from daily clinical practice of a local hospital with some variations regarding surgical approaches and treatment protocols. Thus, our study has to be considered an explorative analysis. Further prospective studies are needed to confirm our results and a comprehensive molecular analysis of MSI‐L tumours should be performed to clarify the biological background of this particular type of MSI.
To conclude, in our study MSI‐H and EBV were not predictive of response to neoadjuvant platinum/5‐FU based CTx, but they were indicative of a good prognosis. In particular, considering MSI‐H, this was evident in principal regardless of the therapeutic approach chosen. MSI‐L, however, was predictive of good response to CTx. Furthermore, the negative prognostic effect of MSI‐L observed for patients treated with surgery alone, even in the group with clinically determined earlier tumour stages, indicates that MSI‐L might help to delineate patients with potentially high‐benefit from preoperative platinum/5‐FU based treatment. Clearly, additional studies are mandatory to confirm our results and the MSI‐L phenotype warrants further investigation to elucidate its role in chemosensitivity and tumour development.
Author contributions statement
MKo, BG, WW and GK planned and conducted the study. MKo, BG, MKr, SB, AN, MR, TS, LI, DM, PM, MMG and LB collected the data. MKo, BG, MKr, JSH, MJ, AN, AH, KO, WW, GK interpreted the data. MKo, BG and GK drafted the manuscript. MJ, AN, AH, MMG, KO and WW reviewed the manuscript. All authors have approved the final drafts submitted.
Supporting information
Supplementary Material and Methods
Figure S1. Discrimination of patient survival by tumour regression grade (TRG)
Table S1. Chemotherapy regimens of the preoperatively treated patients
Table S2. Drug regimens and survival of the preoperatively treated patients
Table S3. EBV and MSI status of resected tumours without and after neoadjuvant CTx and association with patient`s characteristics
Acknowledgements
This work was supported by the ‘Deutsche Krebshilfe’, German Cancer Aid (grant no. 70112177 to GK, AN and WW, Technical University of Munich, Germany; grant no. 70112380 to TS and MG, University of Heidelberg, Germany).
Conflict of interest statement: WW has a consulting role for AZ, Takeda, Lilly, Novartis, Pfizer, Merck, BMS, MSD, Roche, Boehringer Ingelheim, Amgen and has research funding from Roche, BMS, MSD, Bruker. All remaining authors have declared no conflicts of interest.
References
*Cited only in supplementary material.
- 1. Cunningham D, Allum WH, Stenning SP, et al Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 2. Smyth EC, Verheij M, Allum W, et al Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016; 27: v38–v49. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Batran SE, Homann N, Schmalenberg H. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4‐AIO): a multicenter, randomized phase 3 trial. J Clin Oncol 2017; 35: abstr 4004. [Google Scholar]
- 4. The Cancer Genome Atlas Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cristescu R, Lee J, Nebozhyn M, et al Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015; 21: 449–456. [DOI] [PubMed] [Google Scholar]
- 6. Boland CR, Thibodeau SN, Hamilton SR, et al A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248–5257. [PubMed] [Google Scholar]
- 7. Napieralski R, Ott K, Kremer M, et al Methylation of tumor‐related genes in neoadjuvant‐treated gastric cancer: relation to therapy response and clinicopathologic and molecular features. Clin Cancer Res 2007; 13: 5095–5102. [DOI] [PubMed] [Google Scholar]
- 8. An JY, Kim H, Cheong JH, et al Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5‐FU based chemotherapy after R0 resection. Int J Cancer 2012; 131: 505–511. [DOI] [PubMed] [Google Scholar]
- 9. Smyth EC, Wotherspoon A, Peckitt C, et al Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the Medical Research Council adjuvant gastric Infusional chemotherapy (MAGIC) Trial. JAMA Oncol 2017; 3: 1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hewitt LC, Inam IZ, Saito Y, et al Epstein–Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi‐Centre study. Eur J Cancer 2018; 94: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SY, Choi YY, An JY, et al The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer 2015; 137: 819–825. [DOI] [PubMed] [Google Scholar]
- 12. Choi YY, Bae JM, An JY, et al Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta‐analysis. J Surg Oncol 2014; 110: 129–135. [DOI] [PubMed] [Google Scholar]
- 13. Polom K, Marano L, Marrelli D, et al Meta‐analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018; 105: 159–167. [DOI] [PubMed] [Google Scholar]
- 14. Haag GM, Czink E, Ahadova A, et al Prognostic significance of microsatellite‐instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer 2019; 144: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 15. Camargo MC, Kim WH, Chiaravalli AM, et al Improved survival of gastric cancer with tumour Epstein–Barr virus positivity: an international pooled analysis. Gut 2014; 63: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Genitsch V, Novotny A, Seiler CA, et al Epstein–Barr virus in gastro‐esophageal adenocarcinomas – single center experiences in the context of current literature. Front Oncol 2015; 5: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim ST, Cristescu R, Bass AJ, et al Comprehensive molecular characterization of clinical responses to PD‐1 inhibition in metastatic gastric cancer. Nat Med 2018; 24: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 18. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85: 1457–1459. [DOI] [PubMed] [Google Scholar]
- 19. Bauer L, Hapfelmeier A, Blank S, et al A novel pretherapeutic gene expression‐based risk score for treatment guidance in gastric cancer. Ann Oncol 2018; 29: 127–132. [DOI] [PubMed] [Google Scholar]
- 20. Ott K, Vogelsang H, Mueller J, et al Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin‐based chemotherapy in gastric carcinoma. Clin Cancer Res 2003; 9: 2307–2315. [PubMed] [Google Scholar]
- 21. Becker K, Langer R, Reim D, et al Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011; 253: 934–939. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt T, Sicic L, Blank S, et al Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 2014; 110: 1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berg KD, Glaser CL, Thompson RE, et al Detection of microsatellite instability by fluorescence multiplex polymerase chain reaction. J Mol Diagn 2000; 2: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huber M, Pavlova B, Muhlberger H, et al Detection of the Epstein–Barr virus in primary adenocarcinoma of the lung with signet‐ring cells. Virchows Arch 2002; 441: 25–30. [DOI] [PubMed] [Google Scholar]
- 25. The Cancer Genome Atlas Network . Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sohn BH, Hwang JE, Jang HJ, et al Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin Cancer Res 2017; 23: 4441–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang SC, Ng KF, Yeh TS, et al Subtraction of Epstein–Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer 2019. 10.1002/ijc.32215. [DOI] [PubMed] [Google Scholar]
- 28. Moehler M, Baltin CT, Ebert M, et al International comparison of the German evidence‐based S3‐guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer 2015; 18: 550–563. [DOI] [PubMed] [Google Scholar]
- 29. Lee SY, Kim DW, Lee HS, et al Low‐level microsatellite instability as a potential prognostic factor in sporadic colorectal cancer. Medicine 2015; 94: e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hile SE, Shabashev S, Eckert KA. Tumor‐specific microsatellite instability: do distinct mechanisms underlie the MSI‐L and EMAST phenotypes? Mutat Res 2013; 743‐744: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koi M, Tseng‐Rogenski SS, Carethers JM. Inflammation‐associated microsatellite alterations: mechanisms and significance in the prognosis of patients with colorectal cancer. World J Gastrointest Oncol 2018; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pereira MA, Ramos M, Faraj SF, et al Clinicopathological and prognostic features of Epstein–Barr virus infection, microsatellite instability, and PD‐L1 expression in gastric cancer. J Surg Oncol 2018; 117: 829–839. [DOI] [PubMed] [Google Scholar]
- 33. Marrelli D, Polom K, Pascale V, et al Strong prognostic value of microsatellite instability in intestinal type non‐cardia gastric cancer. Ann Surg Oncol 2016; 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 34. Choi YY, Kim H, Shin SJ, et al Microsatellite instability and programmed cell death‐ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg 2019; 270: 309–316. [DOI] [PubMed] [Google Scholar]
- 35. Janjigian YY, Sanchez‐Vega F, Jonsson P, et al Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018; 8: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reim D, Loos M, Vogl F, et al Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single‐center European institution. J Clin Oncol 2013; 31: 263–271. [DOI] [PubMed] [Google Scholar]
- 37. Ott K, Bader FG, Lordick F, et al Surgical factors influence the outcome after Ivor–Lewis esophagectomy with intrathoracic anastomosis for adenocarcinoma of the esophagogastric junction: a consecutive series of 240 patients at an experienced center. Ann Surg Oncol 2009; 16: 1017–1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material and Methods
Figure S1. Discrimination of patient survival by tumour regression grade (TRG)
Table S1. Chemotherapy regimens of the preoperatively treated patients
Table S2. Drug regimens and survival of the preoperatively treated patients
Table S3. EBV and MSI status of resected tumours without and after neoadjuvant CTx and association with patient`s characteristics


