Abstract
Purpose
The present article has investigated the added value of ultrasound (US) guidance on improving the efficacy of local triamcinolone injection via comparing two US-guided methods versus a conventional landmark-guided approach.
Methods
Eighty-one subjects with mild or moderate CTS were included and randomly assigned into three categories including landmark-guided, conventional US-guided midline approach and US-guided ulnar in-plane method. Primarily, participants in the three groups were relatively similar in terms of demographics and their clinical variables comprising visual analog scale (VAS) for pain, pain-free grip strength (PFGS), Boston CTS questionnaire (BCTQ), EDX parameters, and cross-sectional area (CSA) of median nerve measured by ultrasonography. Ten weeks after injection, the changes of clinical and para-clinical outcomes were reassessed for 76 patients who finished the study.
Results
Our findings showed that all three injection methods were associated with a significant and relatively similar improvement in clinical and electrodiagnostic parameters. The post-injection evaluation showed a statistically significant change in all variables except for symptom severity score (SSS) of BCTQ. The best effect-size values were observed for VAS [56%] and functional severity scale (FSS) of BCTQ [42%], both reported in the US-guided midline group. However, no significant difference was found between the groups regarding their improvement in any of the outcome variables (P value >0.05).
Conclusion
Based on the current data, all three injection methods were effective in improving electrodiagnostic findings and clinical symptoms of CTS. Although all approaches were relatively similar, US-guided midline approach was associated with slightly better outcomes.
Keywords: corticosteroid injection, electrodiagnosis, conservative treatment
Introduction
Carpal tunnel syndrome (CTS) or focal distal neuropathy of median nerve is the most prevalent nerve entrapment.1 Subjects often present with numbness, paresthesia or pain in radial-side fingers or palm; weakness and atrophy might occasionally occur in advanced cases.2,3 CTS is associated with the second-longest average time away from work and its costs are estimated to be $30,000 US per worker in the United States of America.2,4 EDX has traditionally been used as the gold standard test. There are multiple therapeutic options for this condition and surgical release of the retinaculum has been approved for moderate to severe grades.5
Non-operative choices could be applied in earlier grades.6 Some of these conservative options are oral medications like non-steroidal anti-inflammatory drugs (NSAIDs), resting wrist splint, physical agent modalities, and local injections including corticosteroid and platelet-rich plasma (PRP).7–11 Local corticosteroid injection has been approved as one of the most common conservative therapies which are still the best choice for rapid symptom relief.12–15 The injection could be performed through multiple approaches. Owing to the wide variety of injection protocols in different trials, one may argue over what the best approach is, yet ultrasonography (US) with real-time images could be a perfect method for musculoskeletal procedures. This is especially true for CTS injections in which US-guided methods have had promising results.15–21 This article has investigated the added value of ultrasound (US) guidance on improving the efficacy of local triamcinolone injection via comparing two US-guided methods versus a conventional landmark-guided approach in a longitudinal observational study.
Methods
Participants
Among the patients who presented or referred with CTS symptoms to our electrodiagnosis (EDX) clinic of Shohada-e-Tajrish hospital from April 2018 until September 2018, 132 subjects were enrolled out of 700 subjects. Firstly, the diagnosis, as well as the severity grade, was confirmed using EDX.22 Only patients between 18 and 65 years of age with mild or moderate grades of CTS who had symptoms lasting for at least 3 months were included. The exclusion criteria included pregnancy, severe CTS grade in EDX or thenar atrophy, history of diabetes mellitus, rheumatoid arthritis or thyroid disorders, prior relevant surgery or hand trauma, any local injection in the wrist during the last 6 months, and active cervical radiculopathy or other peripheral neuropathies in the upper extremity. Concerning the cases of bilateral hand involvement, we only included the worse side and the other side was excluded. The written informed consent was obtained from all subjects. Also, the ethics committee of Shahid Beheshti University of Medical Sciences approved the study protocol (No: IR.SBMU.MSP.REC.1396.534).
Interventions
In the next step, 81 eligible participants were randomly divided into three categories of equal size (27 people in each group) using a mobile application for random number generation. “In this study, we chose three popular injection methods based on the current literature. Every patient underwent a single injection of 40 mg triamcinolone through one of the following approaches: A) the conventional landmark-guided method of the midline, B) US-guided longitudinal midline in-plane approach, and C) US-guided ulnar in-plane method. The injections in groups B and C were performed via in-plane guidance of US [Figure 1A and B] using a 5–12 MHz linear array transducer of US machine (Philips® HD6 machine). In the blind conventional method, the wrist was placed on the table in supination and dorsiflexion. A 25-G needle was inserted just before the distal wrist crease between tendons of palmaris longus (PL) and flexor carpi radialis (FCR) at 45 degrees angle in the volar surface while subjects were lying in the supine position. After skin preparation under sterile conditions, 1.5 inches of the needle tip was placed just at the radial-side of PL tendon in groups A and B; and ulnar to PL in group C. A mixture of 1 mL of triamcinolone 40 mg plus to 1 mL of lidocaine 2% was injected into the carpal tunnel. In the midline in-plane US-guided approach, we placed the wrist on the same position and same needle features but the injection was done under the guidance of ultrasonography above the median nerve. Lastly, in the ulnar in-plane US-guided approach, the wrist was placed on the table in the same position as above mentioned and the injection was done at tunnel inlet from ulnar side lateral to ulnar artery, below the median nerve.
Figure 1.
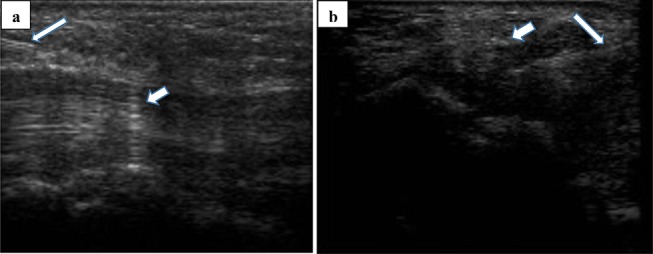
US-guided injections with demonstration of needle tip (long arrow) along with median nerve (short arrow) just before the carpal tunnel inlet. a) Ulnar in-plane approach: in this method, the wrist was placed on the table in slightly dorsiflexed and supinated position and the injection was done at tunnel inlet from ulnar side lateral to ulnar artery, below the median nerve. b) Midline in-plane approach: in this method, we placed the wrist on the same position as above mentioned and the same needle features but the injection was done above the median nerve.
All injections were performed by an expert physiatrist [SM.R] with 22 years of expertise in the musculoskeletal injection field. Subjects were asked to remain in the hospital for 30 mins to check any probable adverse event. They were instructed to wear a night-time prefabricated wrist splint for 10 weeks and also use only acetaminophen tablets as pain-killer, if necessary, within the first 48 hrs of injection. To remind patients and improve their adherence to protocol, a senior resident of physical medicine and rehabilitation [M.AD] was responsible for making a regular phone call every week after the injection. In this single-blinded randomized parallel trial, the assessor physicians were unaware of the groups. This investigation was registered in the Iranian Registry of Clinical Trials (IRCT) with the ID number of IRCT20130523013442N25.
Outcome Measures
Demographic and anthropometric characteristics of the participants such as age, gender, height, weight, body mass index (BMI), wrist circumference, and the severity grade were recorded. Our outcome measuring tools included clinical and para-clinical variables; clinical parameters were as follows: (1) visual analogue scale (VAS) for pain that was a 10-score scale in which “0” designated no pain, while “10” was an indicator of the worst pain ever experienced, (2) pain-free grip strength (PFGS) per kilogram assessed using a dynamometer, and (3) Boston CTS questionnaire (BQ) in two parts of symptom severity scale (SSS) and functional severity scale (FSS) with 11 and 8 questions of 5-choice response, respectively; higher scores had more severity. These three variables were our primary outcome measuring tools.
As the secondary outcomes, electrophysiologic and imaging features of disease were assessed in terms of US-measured nerve cross-sectional area (CSA) and some quantitative parameters of EDX. The latter included the amplitude (amp) and latency (lat) of sensory nerve action potential (SNAP), amp and latency of compound motor action potential (CMAP), and nerve conduction velocity (NCV). The EDX was done utilizing an electromyography machine (Medelec Synergy®, Manor Way, UK) by surface electrodes and a standard technique based on the American Association of Electrodiagnostic Medicine (AANEM) criteria established in 1999. A well-known neurophysiologic grading system was applied to determine mild and moderate CTS.22 The diagnostic US for measuring nerve CSA was conducted by another experienced physiatrist [SA.R] who was blinded to the subjects’ categories. US probe was placed at the level of pisiform just before the carpal tunnel inlet [Figure 2]. Nerve CSA was measured by placing electronic calipers around the inner margin of the nerve sheath just inside the hyper-echoic line. The measurements were repeated three times and the mean value was recorded as the final CSA. Ten weeks after injection, all clinical and para-clinical changes of patients who finished the study were reassessed. Finally, the data gathered were analyzed in SPSS Statistics V22 using Chi-square, one-way ANOVA, student’s and paired t-tests. The significant level was set at P < 0.05.
Figure 2.
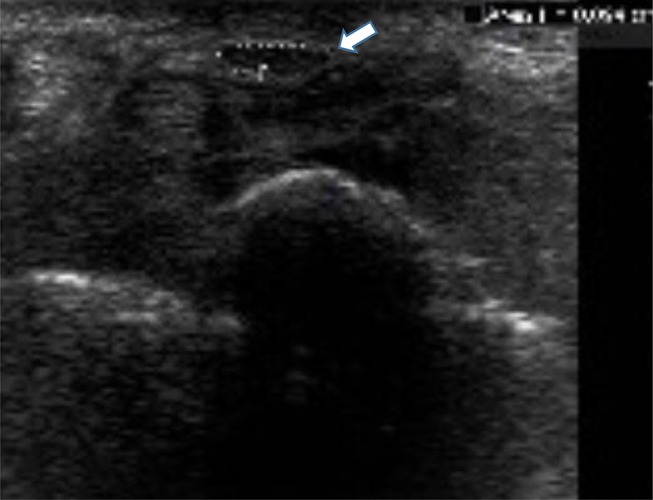
US-measuring of median nerve (white arrow) just before the carpal tunnel inlet.
Results
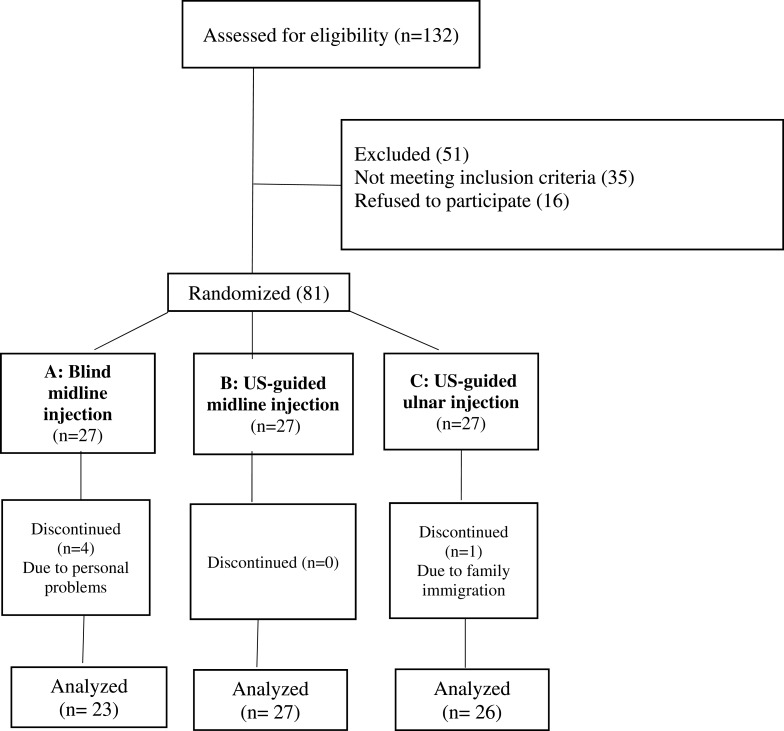
A total of 81 participants with the majority of moderate cases were included, among which five subjects did not continue the study due to personal reasons unrelated to our injections [Figure 3]. Eventually, 76 patients remained till the 10th-week visit [23, 27 and 26 patients in groups A, B, and C, respectively]. Primarily, all three groups were quite similar regarding their demographic and anthropometric parameters, as well as the severity grade; and no statistically significant difference was observed [Table 1].
Figure 3.
Flowchart of the study’s population.
Table 1.
Comparison Of Demographic Characteristics And Baseline Variables Among Patients Of Three Groups
| Variable | C: Ulnar In-Plane (n=26) | B: US-Midline In-Plane (n=27) | A: Landmark-Guided (n=23) | p value |
|---|---|---|---|---|
| Gender (M:F) | 7: 19 | 5: 22 | 5: 18 | 0.764 |
| Severity (Moderate: Mild) | 21: 5 | 18: 9 | 12: 11 | 0.107 |
| Age (mean ±SD) | 54.39 ± 9.3 | 54.56 ± 9/6 | 54.04 ± 10.3 | 0.974 |
| Dominant Hand (Rt: Lt) | 3: 23 | 3: 24 | 0: 23 | 0.247 |
| Study Hand (Rt: Lt) | 10: 16 | 14: 13 | 11: 12 | 0.589 |
| Weight (mean ± SD) | 73.73 ± 7.6 | 71.7 ± 8.18 | 72.83 ± 11.5 | 0.621 |
| Height (mean ± SD) | 167.58 ± 8.3 | 164.89 ± 7.6 | 165.13 ± 7.9 | 0.601 |
| Wrist Circumference (mean ± SD) | 17.25 ± 1.2 | 16.77 ± 1.8 | 17.35 ± 1.5 | 0.529 |
Regarding the pre- and post-treatment comparisons of our clinical parameters, a significant improvement was found within all three groups except for CSA measurement that did not reveal any dramatic change in two of the groups (A and C). In other words, the only significant change in nerve size was related to US-guided midline in-plane approach [from 0.177 to 0.128; MD=0.49 mm2; P=0.000]. Moreover, improvement in symptom severity score (SSS) of Boston questionnaire was not statistically significant in any group [Table 2]. Overall, among all clinical variables, significant improvement was observed for VAS, PFGS, and BQ-FSS within all three groups, as well as significant CSA decline in group B (Midline in-plane US-guided approach). The rest of pre- and post-injection comparisons for EDX variables have been shown in Table 3. As it could be found, all EDX variables were generally associated with significant improvement. SNAP “latency” and “amplitude” revealed significant changes with all three methods. Similarly, motor-fibers parameters including CMAP amp, latency and NCV showed a dramatic improvement in all groups.
Table 2.
Comparison Of Pre- And Post-Treatment Clinical Values In Each Group
| Variable | Ulnar In-Plane (C) | Midline In-Plane (B) | Landmark-Guided (A) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| VAS | 5.88 ± 1.98 | 2.41 ± 1.79 | 6.37 ± 2.55 | 2.8 ± 2.04 | 5.65 ± 2.67 | 3.04 ± 1.98 |
| P=0.009 | P=0.001 | P=0.023 | ||||
| PFGS [kg] |
23.23 ± 8.74 | 28.67 ± 10.41 | 20.61 ± 6.49 | 25.37 ± 9.12 | 23.03 ± 10.83 | 28.59 ± 12.7 |
| P=0.001 | P=0.000 | P=0.000 | ||||
| BQ-SSS | 31.92 ± 7.61 | 16.35 ± 6.21 | 33.0 ± 7.85 | 18.81 ± 7.99 | 33.26 ± 9.92 | 21.7 ± 10.47 |
| P=0.542 | P=0.089 | P=0.20 | ||||
| BQ-FSS | 20.54 ± 6.86 | 12.15 ± 5.28 | 21.67 ± 8.45 | 12.63 ± 4.03 | 22.52 ± 9.11 | 14.74 ± 7.58 |
| P=0.023 | P=0.019 | P=0.000 | ||||
| CSA [mm2] |
0.185 ± 0.08 | 0.163 ± 0.15 | 0.177 ± 0.07 | 0.128 ± 0.02 | 0.188 ± 0.2 | 0.131 ± 0.03 |
| P=0.777 | P=0.000 | P=0.374 | ||||
Note: Insignificant P values have been indicated in Bold format.
Abbreviations: mm2, millimeter square; kg, kilogram.
Table 3.
Comparison Of Pre- And Post-Treatment Electro-Diagnostic Values In Each Group
| Variable | Ulnar In-Plane (C) | Midline In-Plane (B) | Landmark-Guided (A) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| SNAP lat [ms] |
4.63±0.74 | 4.14±0.47 | 4.67±0.72 | 4.17±0.49 | 4.41±0.71 | 4.12±0.54 |
| P**=0.000 | P=0.000 | P=0.000 | ||||
| SNAP amp [uV] |
20.85±10.27 | 29.53±10.77 | 22.46±11.69 | 27.88±11.53 | 23.8±9.53 | 28.62±8.57 |
| P=0.000 | P=0.000 | P=0.000 | ||||
| CMAP lat [ms] |
4.77±0.8 | 4.35±0.57 | 4.86±0.86 | 4.35±0.56 | 4.6±0.76 | 4.32±0.66 |
| P=0.000 | P=0.000 | P=0.000 | ||||
| CMAP amp [mV] |
7.04±2.42 | 7.66±2.33 | 6.64±1.49 | 7.18±1.63 | 6.45±1.52 | 6.85±1.93 |
| P=0.000 | P=0.000 | P=0.000 | ||||
| NCV [m/s] |
50.76±4 | 53.6±4.51 | 52.07±5.27 | 54.74±5.74 | 53.82±4.39 | 56.47±6.05 |
| P=0.004 | P=0.000 | P=0.001 | ||||
Note: ** Paired t-test.
Abbreviations: ms, millisecond; m/s, meter per second; mV, millivolt; µV, microvolt.
Regarding between-groups comparisons, there was no significant difference among the three groups. The corresponding effect-size in terms of mean difference (MD) has been also demonstrated in Table 4. Except for two variables (CSA and SSS) which did not reveal significant changes, the best MD values with their percentage of changes have been indicated in each row. As displayed, maximum changes of almost all clinical variables including VAS [MD=3.57; 56%], and BQ-FSS [MD=9.04; 42%], as well as SNAP lat [MD=0.050], and CMAP lat [MD=0.051] belonged to midline in-plane US-guided approach. The highest amount of improvement in other EDX parameters including CMAP amp [MD=0.62; 9%], SNAP amp [MD=8.63; 41%], and NCV [MD=2.84; 6%] has been observed in ulnar in-plane approach. As could be found, the best efficacy among EDX parameters was associated with SNAP amp [41%], while the best effect-size of clinical variables was detected for VAS [56%]. Also, it should be pointed out that the blind approach only achieved the maximum improvement of PFGS [MD=5.56; 24%]. Finally, it should be kept in mind that during this observational study our approaches brought about no serious adverse events.
Table 4.
Comparison Of Improvement In Clinical And Electro-Diagnostic Variables Among Three Groups
| Post-Treatment Mean ± Sd [MD] |
C: Ulnar In-Plane (n=26) | B: Midline In-Plane (n=27) | A: Landmark-Guided (n=23) | P value (ANOVA) |
|---|---|---|---|---|
| VAS | 2.41 ± 1.79 [−3.47] |
2.8 ± 2.04 [−3.57] →56% |
3.04 ± 1.98 [−2.61] |
0.678 |
| PFGS (per kg) |
28.67 ± 10.41 [5.44] |
25.37 ± 9.12 [4.76] |
28.59 ± 12.7 [5.56] →24% |
0.913 |
| BQ-SSS | 16.35 ± 6.21 [−15.57] |
18.81 ± 7.99 [−14.19] |
21.7 ± 10.47 [−11.56] |
0.30** |
| BQ-FSS | 12.15 ± 5.28 [−8.39] |
12.63 ± 4.03 [−9.04] →42% |
14.74 ± 7.58 [−7.78] |
0.997 |
| CSA (per mm22) |
0.163 ± 0.15 [−0.022] |
0.128 ± 0.02 [−0.049] |
0.131 ± 0.03 [−0.057] |
0.472** |
| SNAP amp (per uV) |
29.53 ± 10.77 [8.63] →41% |
27.88 ± 11.53 [5.42] |
28.62 ± 8.57 [4.82] |
0.112 |
| SNAP lat (per ms) |
4.14 ± 0.47 [−0.49] |
4.17 ± 0.49 [−0.50] →1% |
4.12 ± 0.54 [−0.29] |
0.070 |
| CMAP amp (per mV) |
7.66 ± 2.33 [0.62] →9% |
7.18 ± 1.63 [0.54] |
6.85 ± 1.93 [0.40] |
0.704 |
| CMAP lat (per ms) |
4.35 ± 0.57 [−0.42] |
4.35 ± 0.56 [−0.51] →1% |
4.32 ± 0.66 [−0.28] |
0.637 |
| NCV (per m/s) |
53.6 ± 4.51 [2.84] →6% |
54.74 ± 5.74 [2.67] |
56.47 ± 6.05 [2.65] |
0.997 |
Notes: The best MD value in each row has been indicated in Bold format. **These two rows did not achieve any significant change within the groups; then we did not indicate the maximum amount of improvement among them.
Abbreviations: mm2, millimeter square; ms, millisecond; m/s, meter per second; mV, millivolt; µV, microvolt; kg, kilogram.
Discussion
In this study, we investigated three injection approaches for CTS treatment including landmark-guided method, US-guided ulnar and midline approaches. Developing these new approaches under guidance of high-resolution US machines could theoretically improve the accuracy and subsequently the efficacy of CTS injections. Our three groups were associated with significant improvement lasting for at least 10 weeks. Overall, the improvement of clinical variables such as FSS and VAS for pain was higher than that of US and EDX parameters. Moreover, no remarkable difference between the groups was observed. It might have various underlying causes which have prevented to detect any significant superiority. Here, we intend to discuss some of these important factors in common with other similar articles.
Among similar studies, Babaei-Ghazani et al, compared the clinical effectiveness of ultrasound-guided local triamcinolone injection “above” versus “below” the median nerve in CTS patients.23 They found that all outcome measures such as VAS, Boston questionnaire, EDX and US parameters improved significantly in both groups at 6 weeks, and these improvements were persevered up to 12-week follow-up. However, there was no significant difference in measured outcomes between the two groups in that study, exactly similar to our findings. One simple reason might be that the injections and the interventional procedures were performed only by experienced physicians, and not merely on the basis of US guidance. Therefore, in the future studies, the covariate analysis of “physicians’ expertise” on the added value of US guidance for injection precision should be evaluated.
In another study, Ustun et al, evaluated two parallel methods for local methylprednisolone injections in 46 CTS patients: a landmark-guided ulnar approach versus the US-guided ulnar out-plane method. Their follow-up moments were 6 and 12 weeks after injection. Although both methods were significantly effective in symptom relief and improved patients’ function, the investigators finally found an earlier onset and better improvement for the US-guided approach in comparison to the blind one.24 Another study performed by Karaahmet et al, investigated 40 CTS patients in two categories: 21 participants received steroid injection throughout the US-guided approach, while 19 subjects underwent the blind local injection. After 4 week follow-up, they concluded that US-guided injections might yield more effective results with earlier onset than the conventional landmark-guided method of injection. Unlike our findings, these two researches [Ustun24 and Karaahmet25] observed a definite superiority in favor of US-guided approach over the blind injection method. However, in contrast to our results, the latter study included only severe cases of CTS, then this conclusion could not be generalized to all grades of CTS.25
As the results showed (Table 4), VAS and the functional scale of Boston questionnaire had the highest amount of changes (56% and 42%, respectively), both of which belong to the midline in-plane group. Additionally, the only method which resulted in a significant decline of nerve size in US measurements was again the US-guided midline approach. In spite of all that, no dramatic superiority was found for a specific group regarding any parameter.
Besides, more accurate evaluations revealed that the VAS improved dramatically in all three groups, while symptom severity score (SSS) of Boston questionnaire did not significantly change. Considering the uniformity of questionnaire that makes the inter-rater bias negligible, it seems that there should be another explanation for this inconsistency. Since previously mentioned, the SSS has 11 questions which measure all related CTS symptoms including paresthesia, weakness, grip strength and pain, while pain is the only question in VAS assessment. Thus, we can state that the injections were associated with a significant change in terms of pain relief, much more than improvement in other symptoms. Eventually, Lee et al, performed a steroid injection trial via three approaches: ulnar in-plane, ulnar out-plane, and blind methods. They followed-up their patients at 4th and 12th-week intervals. These researchers concluded that the US-guided ulnar approach was associated with better outcomes than the other two methods.26 Lastly, Eslamian et al, studied 60 CTS subjects in two equal groups: blind injection of local methylprednisolone versus US-guided ulnar in-plane approach. They followed their patients for 3 months and proved that in spite of the significant changes in both groups, there was no significant difference between them,27 closely similar to our findings. However, they did not compare these results versus a more popular midline in-plane approach. As mentioned, a wise explanation could be the physicians’ lack of expertise or insufficiency of the utilized objective parameters. The use of more objective outcome measuring tools such as CSA is recommended for the future studies. Moreover, all the study procedures, especially injections and recording the results, should be done by junior residents who are still in their dynamic state of learning. Paying attention to these details can be helpful to clarify whether the application of US guidance may improve the accuracy and efficacy of steroid injections in CTS.
Conclusion
Based on the current data, all three methods of local triamcinolone injection in non-severe CTS patients were associated with dramatic changes in symptoms, particularly in terms of pain relief. Also, a significant change in electrodiagnostic and functional variables was observed via all three approaches. Although there was no dramatic superiority in comparison to these three methods, only the midline in-plane approach led to a significant reduction in US-measured nerve size after steroid injection.
Acknowledgments
This article has been extracted from the thesis written by Dr. Mohammad Ahmadi-Dastgerdi in School of Medicine, Shahid Beheshti University of Medical Sciences. Disclosure. It was also approved by the Physical Medicine and Rehabilitation Research Center of Shohada-e-Tajrish Educational Hospital.
Data Sharing Statement
The authors do not intend to share substantial data of this study. However, they are ready to share the de-identified file of data in excel format and all other study-related documents. It would be available for any period on the demand of editorial board via the corresponding author`s email.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim PT, Lee HJ, Kim TG, Jeon IH. Current approaches for carpal tunnel syndrome. Clin Orthop Surg. 2014;6(3):253–257. doi: 10.4055/cios.2014.6.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale AM, Harris-Adamson C, Rempel D, et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: pooled analysis of six prospective studies. Scand J Work Environ Health. 2013;39(5):495–505. doi: 10.5271/sjweh.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luckhaupt SE, Dahlhamer JM, Ward BW, Sweeney MH, Sestito JP, Calvert GM. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 National Health Interview Survey. Am J Ind Med. 2013;56(6):615–624. doi: 10.1002/ajim.22048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler JR, Gaughan JP, Ilyas AM. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: a meta-analysis. Clin Orthop Relat Res. 2011;469(4):1089–1094. doi: 10.1007/s11999-010-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrami MH, Shahraeeni S, Raeissadat SA. Comparison between the effects of progesterone versus corticosteroid local injections in mild and moderate carpal tunnel syndrome: a randomized clinical trial. BMC Musculoskelet Disord. 2015;16:322. doi: 10.1186/s12891-015-0752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008;77(1):6–17. [PMC free article] [PubMed] [Google Scholar]
- 7.Huisstede BM, Friden J, Coert JH, Hoogvliet P; European HANDGUIDE Group. Carpal tunnel syndrome: hand surgeons, hand therapists, and physical medicine and rehabilitation physicians agree on a multidisciplinary treatment guideline-results from the European HANDGUIDE study. Arch Phys Med Rehabil. 2014;95(12):2253–2263. doi: 10.1016/j.apmr.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 8.Rayegani SM, Bahrami MH, Eliaspour D, et al. The effects of low intensity laser on clinical and electrophysiological parameters of carpal tunnel syndrome. J Lasers Med Sci. 2013;4(4):182–189. [PMC free article] [PubMed] [Google Scholar]
- 9.Raeissadat SA, Reza-Soltani Z. Study of long term effects of laser therapy versus local corticosteroid injection in patients with carpal tunnel syndrome. J Lasers Med Sci. 2010;1:24–30. [Google Scholar]
- 10.Dinarvand V, Abdollahi I, Raeissadat SA, Mohseni Bandpei MA, Babaee M, Talimkhani A. The effect of scaphoid and hamate mobilization on treatment of patients with carpal tunnel syndrome. Anesthesiol Pain Med. 2017;7(5):e14621. doi: 10.5812/aapm.14621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raeissadat SA, Rayegani SM, Rezaei S, et al. The effect of polarized polychromatic noncoherent light (Bioptron) therapy on patients with carpal tunnel syndrome. J Lasers Med Sci. 2014;5(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Salman Roghani R, Holisaz MT, Tarkashvand M, et al. Different doses of steroid injection in elderly patients with carpal tunnel syndrome: a triple-blind, randomized, controlled trial. Clin Interv Aging. 2018;13:117–124. doi: 10.2147/CIA.S151290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dammers JW, Roos Y, Veering MM, Vermeulen M. Injection with methylprednisolone in patients with the carpal tunnel syndrome. A Randomised Double Blind Trial Testing Three Different Doses. J Neurol. 2006;253(5):574–577. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami MH, Raeissadat SA, Nezamabadi M, et al. Interesting effectiveness of ozone injection for carpal tunnel syndrome treatment: a randomized controlled trial. J Pain Res. 2019. in press article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P-C, Chuang C-H, Tu Y-K, Bai C-H, Chen C-F, Liaw M-Y. A Bayesian network meta-analysis: comparing the clinical effectiveness of local corticosteroid injections using different treatment strategies for carpal tunnel syndrome. BMC Musculoskelet Disord. 2015;16:363. doi: 10.1186/s12891-015-0815-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karimzadeh A, Bagheri S, Raeissadat SA, et al. The comparison of the effectiveness between different doses of local methylprednisolone injection versus triamcinolone in carpal tunnel syndrome: a double-blind clinical trial. J Pain Res. 2019;12:579–584. doi: 10.2147/JPR.S190652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayegani SM, Daneshtalab E, Bahrami MH, et al. Prevalence of accessory deep peroneal nerve in referred patients to an electrodiagnostic medicine clinic. J Brachial Plex Peripher Nerve Inj. 2011;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raeissadat SA, Rayegani SM, Langroudi TF, Khoiniha M. Comparing the accuracy and efficacy of ultrasound-guided versus blind injections of steroid in the glenohumeral joint in patients with shoulder adhesive capsulitis. Clin Rheumatol. 2017;36(4):933–940. doi: 10.1007/s10067-016-3393-8 [DOI] [PubMed] [Google Scholar]
- 19.DH JJ K, Park BK. Anatomical basis of ulnar approach in carpal tunnel injection. Pain Physician. 2013;16(3):E191–8. [PubMed] [Google Scholar]
- 20.Raeissadat SA, Shahraeeni S, Sedighipour L, Vahdatpour B. Randomized controlled trial of local progesterone vs corticosteroid injection for carpal tunnel syndrome. Acta Neurol Scand. 2017;136(4):365–371. doi: 10.1111/ane.12739 [DOI] [PubMed] [Google Scholar]
- 21.Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord. 2018;19:49. doi: 10.1186/s12891-018-1963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23(8):1280–1283. doi: [DOI] [PubMed] [Google Scholar]
- 23.Babaei-Ghazani A, Nikbakht N, Forogh B, et al. Comparison between effectiveness of ultrasound-guided corticosteroid injection above versus below the median nerve in mild to moderate carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2018;97(6):407–413. doi: 10.1097/PHM.0000000000000877 [DOI] [PubMed] [Google Scholar]
- 24.Ustun N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92(11):999–1004. doi: 10.1097/PHM.0b013e31829b4d72 [DOI] [PubMed] [Google Scholar]
- 25.Karaahmet Ö, Gürçay E, Kara M, Serçe A, Ünal ZK, Çakcı A. Comparing the effectiveness of ultrasound-guided versus blind steroid injection in the treatment of severe carpal tunnel syndrome. Turk J Med Sci. 2017;47(6):1785–1790. doi: 10.3906/sag-1704-97 [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: a prospective, randomized, single-blinded study. Medicine (Baltimore). 2014;93(29):e350. doi: 10.1097/MD.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eslamian F, Eftekharsadat B, Babaei-Ghazani A, Jahanjoo F, Zeinali M. A randomized prospective comparison of ultrasound-guided and landmark-guided steroid injections for carpal tunnel syndrome. J Clin Neurophysiol. 2017;34(2):107–113. doi: 10.1097/WNP.0000000000000342 [DOI] [PubMed] [Google Scholar]