Abstract
Objective
Evaluate safety and effectiveness of VYC-12 (Juvéderm Volite; an injectable crosslinked hyaluronic acid gel designed to improve skin quality attributes such as surface smoothness and hydration) for facial intradermal injection.
Materials and methods
In a prospective, single-arm study, subjects with moderate/severe cheek skin roughness per Allergan Skin Roughness Scale (ASRS) received VYC-12 in the cheeks and forehead, and/or neck, with touch-up treatment to correct asymmetry 30 days later and optional repeat treatment 9 months after last treatment. The primary effectiveness measure was ASRS responder rate (percentage of cheeks with ≥1-point improvement from baseline) at month 1. Skin hydration was instrument-assessed.
Results
Of 131 subjects treated, 31 (23.7%) received touch-up treatment. ASRS responder rate was 96.2% at month 1, 76.3% at month 4, 34.9% at month 6, and 87.1% after repeat treatment. Responder rate in cheeks with severe baseline roughness was 93.8%, 83.1%, and 52.3% at months 1, 4, and 6, respectively. Skin hydration improved significantly (P<0.01) from baseline at all timepoints through month 9. Injection site responses were as expected. All treatment-related adverse events were mild/moderate.
Conclusion
VYC-12 safely and effectively improved skin smoothness up to 6 months and hydration lasting 9 months.
Keywords: hyaluronic acid, injectable dermal filler, Juvéderm Volite, skin aging
Introduction
Various attributes contribute to overall skin quality, such as texture or roughness, fine lines, hydration, and elasticity.1,2 Good skin quality is an important component of attractiveness and implies well-being, good health, and youthfulness.3–6 The desire for a healthy and youthful appearance has increased the demand for minimally invasive cosmetic procedures that address skin quality concerns. For example, chemical peels and laser skin resurfacing have increased in popularity over the last 2 decades, exceeding 1.9 million procedures in the United States in 2017.7 While these procedures may improve the appearance of the skin, there is limited information on how they may improve attributes of skin quality, such as hydration and elasticity. Intrinsic factors, such as age, and extrinsic factors, such as exposure to sun and cigarette smoke, contribute to structural changes in the skin associated with poor skin topography, resulting in the development of wrinkles, fine lines, and uneven complexion.1,8–10 These structural changes include increased disorganization of elastic fibers as well as the reduction of collagen and hyaluronic acid (HA) in the dermis.11–13 Intradermal injections of HA fillers have been reported to improve skin quality, hypothetically by stabilizing the extracellular matrix that supports intradermal fibroblasts and by increasing hydration in the dermis.14–16
VYC-12 (Juvéderm Volite; Allergan plc, Dublin, Ireland) is an injectable, crosslinked HA gel that was designed to treat superficial cutaneous depressions such as fine lines, and for additional improvement of skin quality attributes such as hydration.17 VYC-12 belongs to a family of HA gels based on the Vycross technology platform (Allergan plc). This study evaluated the safety and effectiveness of VYC-12 injectable gel for the treatment of facial roughness and fine lines and other skin quality attributes, including instrument-measured hydration, smoothness, and deformation.
Methods
Study Design
This prospective, single-center, single-arm study (ClinicalTrials.gov Identifier: NCT02877069) was conducted in France from September 2015 through October 2016. The treatment period, up to 11 months, consisted of 1 initial treatment, 1 touch-up treatment 30 days later to correct asymmetry, 9 months of follow-up after the last treatment (initial or touch-up), an optional repeat treatment at month 9, and 1 month of follow-up after the optional retreatment. Six European injecting physicians (from Italy, Belgium, Germany, Switzerland, Spain, and the United Kingdom) were brought to France to administer the study treatments.
The study was conducted in compliance with the principles of Good Clinical Practice, current standards of International Organization for Standardization guideline 14155, all applicable laws and regulations, and ethical principles for clinical research originating from the Declaration of Helsinki. An ethics committee (Comité de Protection de Personnes SUD-EST-IV, Lyon, France) approved the protocol before any subjects were enrolled. All subjects provided written informed consent before participation.
Subjects
Eligible subjects were adults (aged ≥18 years) with moderate or severe cheek skin roughness based on a live, investigator-assessed severity score of 2 or 3 for both cheeks on the validated, 5-point photonumeric Allergan Skin Roughness Scale (ASRS).18 Subjects were excluded for dermal fillers or other tissue-augmenting cosmetic procedures in the face or neck in the last 12 months; botulinum toxin injections in the face or neck in the last 6 months; semipermanent fillers or permanent facial implants anywhere in the face or neck; oral or topical antiwrinkle product in the face or neck in the last 30 days (permitted if initiated more than 30 days earlier and maintained throughout study); dental procedure within 6 weeks; current cutaneous inflammatory or infectious processes (eg, acne, herpes), abscess, unhealed wound, or cancerous or precancerous lesion on the face or neck; and tendency toward hypertrophic scarring. Drugs known to increase coagulation time were withdrawn for 10 days before and 3 days after study treatment.
Treatment
Treating physicians were instructed to inject VYC-12 (without lidocaine) intradermally in the cheeks and forehead on day 0, with an option to treat the neck, using 32-gauge (1/2 inch or 3/16 inch at the injecting physician’s discretion) ultra-thin wall needles in multiple microdepot injections over the treatment area. The spacing and depth of the intradermal injections were determined by the injecting physician, with spacing of injections restricted to no greater than 1 centimeter apart. Each injection was approximately 0.01 mL; volume accuracy could be assessed by checking for a decrease of 0.1 mL of the product in the syringe after 10 injections. Injectors were instructed not to draw blood or raise a wheal and to adjust injection volume should this occur. Select subjects received touch-up treatment 30 days after initial treatment of the cheeks, if deemed appropriate by the investigator to correct asymmetry. Variability in appearance of the cheeks after initial treatment was possible given the small injection amounts and potential for nonuniformity of spacing. Injection volume inconsistencies were also possible because of the small injections required. Optional repeat treatment was at the subject’s discretion 9 months after the last treatment (initial or touch-up). The maximum allowable total injection volume was 7 mL (4 mL in the face and 3 mL in the neck) for the initial treatment; 5 mL (3 mL in the face and 2 mL in the neck) could be administered for the touch-up treatment. At repeat treatment, subjects could once again receive up to 7 mL.
Assessments
Non-injecting, independent investigators at the study site rated skin roughness for all treated cheeks using the ASRS18 at screening, 30 days after initial treatment (before touch-up), months 1, 4, 6, and 9 after the last treatment (initial or touch-up), and month 1 after repeat treatment (month 1R). The anatomic areas rated were from oral commissure to preauricular cheek and from zygoma to mandible. At the same time points, investigators assessed cheek fine lines using the validated 5-point Allergan Fine Lines Scale (AFLS).19 The anatomic areas rated were 1 cm in from the nasolabial fold to the left preauricular cheek and from the inferior orbital rim to above the mandible. The descriptors of each grade of the ASRS and AFLS are listed in Table 1.
Table 1.
Allergan Skin Roughness Scale And Allergan Fine Lines Scale Descriptors
| Term (Grade) | Allergan Skin Roughness Scale | Allergan Fine Lines Scale |
|---|---|---|
| None (0) | Smooth visual skin texture | No fine lines |
| Minimal (1) | Slightly coarse and uneven visual skin texture | 1–2 superficial lines |
| Moderate (2) | Moderately coarse and uneven visual skin texture; may have early elastosis | 3–5 superficial lines |
| Severe (3) | Severely coarse visual skin texture, crosshatched fine lines; may have some elastosis | Greater than 5 superficial lines; no crosshatching |
| Extreme (4) | Extremely coarse visual skin texture, crosshatched deep creases; extreme elastosis | Diffuse superficial lines; crosshatching |
Instrument measures of skin hydration, smoothness, and skin deformation parameters were performed on the cheek, forehead, and neck of one side of the face on day 0 and at 30 days after initial treatment (before touch-up), months 1, 4, 6, and 9 after the last treatment, and month 1R. Target anatomic zones were selected for measurements in each area (cheek: on the zygoma at the upper cheek, at the point where a vertical line from the lateral canthus intersects with the most prominent portion of the zygoma; forehead: on the horizontal midline of the forehead, halfway between the eyebrows and hairline, just lateral to the vertical midline of the face; neck: on the sternocleidomastoid muscle midway between the jaw and sternum). Instrument software and anatomic markings were used to reposition subjects so that the same zone was measured at each time point. Skin hydration was measured using the MoistureMeterD instrument (Delfin Technologies Ltd., Kuopio, Finland) with the XS 5 and S 15 probes (depth of effective measurement: 0.5 and 1.5 mm, respectively). Skin smoothness was measured from images captured using a fringe projection system (DermaTOP, EOTECH SA, Marcoussis, France). The deformability of the skin was measured using the Cutometer Multi Probe Adapter 580 (Courage+Khazaka electronic GmbH, Cologne, Germany). Evaluations were performed by Dermscan (Lyon, France) technicians.
Subjects assessed their pain immediately after completion of the initial treatment using an 11-point scale (0=no pain; 10=worst pain imaginable) and completed a 30-day diary after each treatment reporting the presence and severity (mild, moderate, or severe) of injection site responses (ISRs) commonly associated with HA-based fillers (ie, redness, pain after injection, tenderness to touch, firmness, swelling, lumps/bumps, bruising, itching, and discoloration). Investigators monitored and recorded adverse events (AEs) throughout the study. AEs were summarized by system organ class and preferred term and further tabulated by severity.
Statistics
All effectiveness and safety analyses were performed on the safety population, which included all treated subjects. The primary effectiveness end point was the proportion of treated cheeks with at least a 1-point improvement from baseline (ie, ASRS responder rate) in investigator-rated ASRS score at month 1 after last injection (initial or touch-up), analyzed descriptively, with the corresponding 95% confidence interval (CI) summarized. The secondary effectiveness measures were instrument-assessed cheek skin smoothness, hydration, and skin deformation. The paired t-test was used to analyze mean changes from baseline in these parameters, with descriptive statistics, including 95% CI for the mean change. Instrument-assessed forehead and neck skin smoothness, hydration, and skin deformation were additional effectiveness measures, analyzed using descriptive statistics. Three measurements were taken for each measure and the mean was reported. Analyses of instrument measurements of neck and forehead skin included only subjects who had received treatment in those areas. The AFLS responder rate (ie, the proportion of cheeks with at least a 1-point improvement from baseline), another additional effectiveness measure, was determined only for cheeks that had AFLS scores of 2 (moderate) or 3 (severe) at baseline.
Results
Subjects
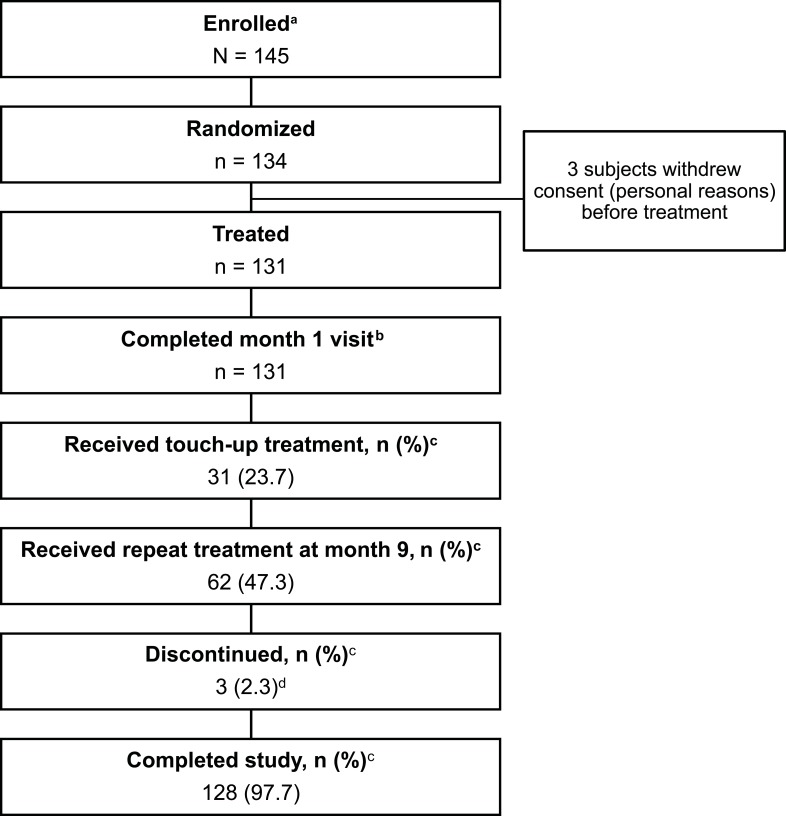
Subject disposition is shown in Figure 1. Of 131 treated subjects, 128 (97.7%) completed the study. Treated subjects were primarily female (88.5%) and had a median age of 54 years (range: 32–72 years; Table 2). Baseline cheek ASRS score was 2 (moderate) in 75.1% of subjects and 3 (severe) in 24.9%. Fitzpatrick skin types II and III were predominant.
Figure 1.
Subject disposition. aSubjects who signed the informed consent form were considered enrolled. bIncluded in the primary effectiveness analysis. cThe denominator is the number of treated subjects (n=131). d3 subjects were lost to follow-up.
Table 2.
Baseline Characteristics
| Characteristic | Subjects (N=131) |
|---|---|
| Age, median (range), years | 54 (32–72) |
| Female, n (%) | 116 (88.5) |
| Fitzpatrick skin type, n (%) | |
| II | 46 (35.1) |
| III | 70 (53.4) |
| IV | 14 (10.7) |
| V | 1 (0.8) |
| Body mass index, median (range), kg/m2 | 23.7 (17.0–32.5) |
| Cheek skin ASRS score, n (%)a | n=261 |
| 2 (Moderate) | 196 (75.1) |
| 3 (Severe) | 65 (24.9) |
| Cheek skin AFLS score, n (%)a | n=261 |
| 1 (Minimal) | 65 (24.9) |
| 2 (Moderate) | 135 (51.7) |
| 3 (Severe) | 54 (20.7) |
| 4 (Diffuse superficial lines; crosshatching) | 7 (2.7) |
| Exposure to sunlight, median (range), hours per day | 1.5 (0–8.0) |
| Smoking history, n (%) | |
| Never smoked/used tobacco | 81 (61.8) |
| Currently smokes/uses tobacco | 33 (25.2) |
| Formerly smoked/used tobacco | 17 (13.0) |
Note: aNumbers and percentages of treated cheeks.
Abbreviations: ASRS, Allergan Skin Roughness Scale; AFLS, Allergan Fine Lines Scale.
Treatment Characteristics
Of the 131 subjects who received initial treatment, only about one fourth received the touch-up treatment (Figure 1), and only in the cheeks (Table 3). Less than half of subjects (n=62) opted for repeat treatment 9 months after the last treatment (Table 3); 47.5% of cheeks, 45.0% of foreheads, and 31.3% of necks received repeat treatment. The median total volume injected in the cheeks, forehead, and neck was 3.9 mL for initial and touch-up treatments combined, and 2.6 mL for repeat treatment. Topical anesthetic was applied before all initial treatments and at the investigator’s discretion for touch-up (48.4%; 12.9% for repeat).
Table 3.
Injection Volume
| Median (Range), mL | ||||
|---|---|---|---|---|
| Initial (n=131) | Touch-Up (n=31) | Initial + Touch-Up (n=131) | Repeat (n=62) | |
| Cheek | n=261a; 1.3 (0.4–2.5)b | n=46a; 1.0 (0.03–2.0)b | n=261a; 1.3 (0.4–3.3)b | n=124a; 1.0 (0.5–2.0)b |
| Forehead | n=120; 0.2 (0.05–1.5) | n=0 | n=120; 0.2 (0.05–1.5) | n=54; 0.2 (0.1–0.5) |
| Neck | n=96; 0.9 (0.4–3.0) | n=0 | n=96; 0.9 (0.4–3.0) | n=30; 0.5 (0.1–1.7) |
| Total | 3.6 (1.1–7.0) | 1.0 (0.05–3.0) | 3.9 (1.1–8.0) | 2.6 (1.2–5.0) |
Notes: aNumber of treated cheeks. bVolume per individual cheek.
For the initial and touch-up treatments, needle length selection was evenly distributed between the 1/2-inch and the 3/16-inch needle for each treatment area (Supplemental Table 1). For repeat treatment, the 1/2-inch needle was selected more often. Injection spacing was less than 1 cm apart in the majority of cheeks for the initial, touch-up, and repeat treatments. In the forehead and neck areas, the spacing of injections was fairly evenly distributed between less than 1 cm and 1 cm apart (Supplemental Table 1).
Primary Effectiveness Measure: ASRS Responder Rate
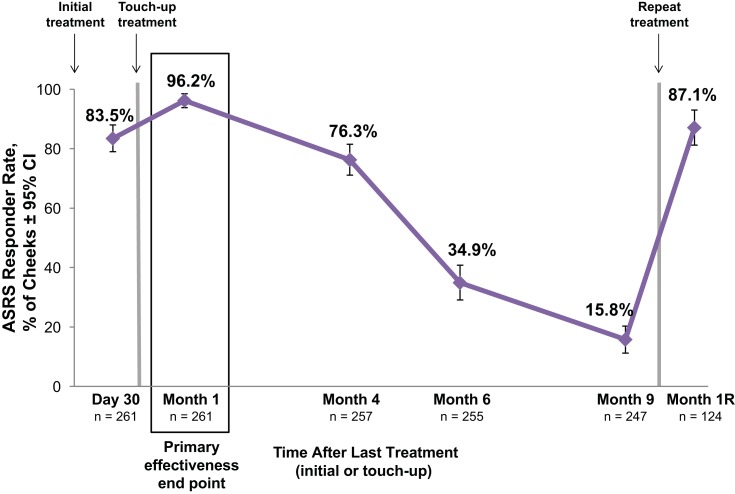
More than 95% of cheeks were ASRS responders at month 1 after the last treatment, and more than 75% of cheeks maintained a response at month 4 (Figure 2). At month 1R, the ASRS responder rate was 87%, which was comparable to the 84% rate at day 30 after initial treatment. For cheeks that had severe roughness at baseline (ASRS score=3; n=65 cheeks), the ASRS responder rate was 93.8% at month 1, 83.1% at month 4, 52.3% at month 6, 25.8% at month 9, and 100% at month 1R (repeat treatment: n=35 cheeks).
Figure 2.
Allergan Skin Roughness Scale (ASRS) responder rates after treatment with VYC-12. The responder rate is the percentage of cheeks with ≥1-point improvement from baseline in cheek ASRS score based on the investigator’s assessment. Each cheek of the 131 treated subjects (261 treated cheeks) was rated separately.
Abbreviations: CI, confidence interval; Month 1R, 1 month after repeat treatment.
Improvement In Fine Lines: AFLS Responder Rate
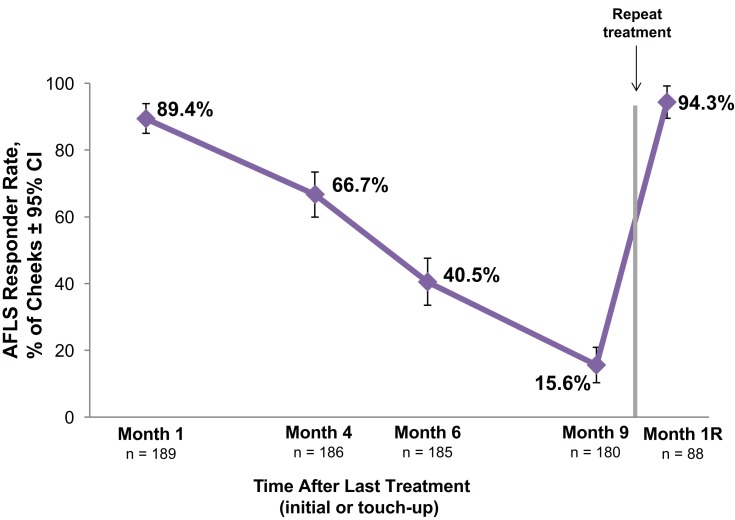
For cheeks that had moderate or severe fine lines (AFLS score 2 or 3) at baseline (189 cheeks), the AFLS responder rate was nearly 90% at month 1, 67% at month 4, and exceeded 90% after repeat treatment (Figure 3).
Figure 3.
Allergan Fine Lines Scale (AFLS) responder rate for VYC-12 treated cheeks with AFLS scores of 2 (moderate) or 3 (severe) at baseline. The responder rate is the percentage of cheeks with ≥1-point improvement from baseline in cheek AFLS score based on the investigator’s assessment.
Abbreviations: CI, confidence interval; Month 1R, 1 month after repeat treatment.
Instrument Measures Of Skin Hydration, Smoothness, And Skin Deformation
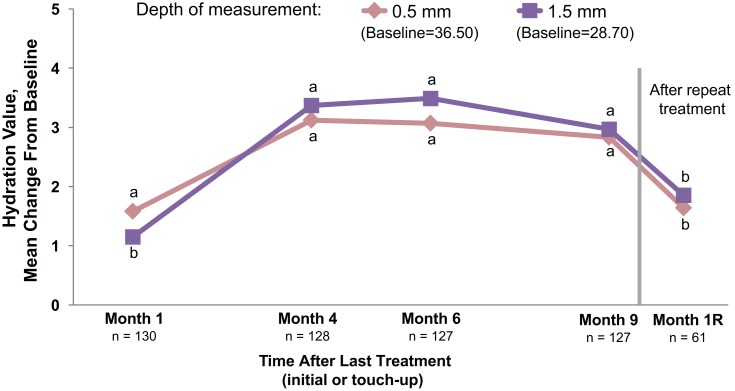
Cheek skin hydration measured at depths of 0.5 and 1.5 mm showed statistically significant improvements from baseline at months 1, 4, 6, and 9 after initial/touch-up treatment and after repeat treatment (month 1R; P≤0.012 for all; Figure 4). All instrument measures of cheek skin smoothness (average roughness, average relief, and amplitude) numerically decreased from baseline at month 1, indicating a smoothing effect (Supplemental Table 2). The change in average relief was statistically significant at month 1 (P=0.018) but not at later time points. Small changes from baseline in cheek skin deformation parameters occurred, with statistically significant changes observed in 6 parameters at month 1 but lacking consistency over time (Supplemental Table 2). In treated foreheads and necks, changes in hydration, smoothness, and skin deformation demonstrated a pattern similar to that in cheeks.
Figure 4.
Cheek skin hydration measured using the MoistureMeter D instrument. Increases indicate improved skin hydration. Month 1R=1 month after repeat treatment. aP<0.001. bP≤0.012. Paired t-test was used to test for mean changes from baseline.
Safety And Tolerability
The mean subject-reported pain score after initial treatment was 4.3 (0=no pain; 10=worst pain imaginable). Most subjects reported ISRs after initial and touch-up treatment (Table 4). The ISRs were as expected for dermal filler treatment and were typically mild or moderate. After the initial and touch-up treatments, severe ISRs reported by more than 5% of subjects included redness (6.1% [8/131]) and bruising (6.1% [8/131]). ISRs in all categories were lower after repeat treatment than after initial/touch-up treatment (Table 4). No severe ISRs occurred in more than 5% of subjects after repeat treatment. Most ISRs resolved within 1 week of each treatment.
Table 4.
Incidence Of Injection Site Responses In Any Treatment Area
| n (%)a | ||||
|---|---|---|---|---|
| Initial (n=131) | Touch-Up (n=31) | Initial + Touch-Up (n=131) | Repeat (n=62) | |
| Any ISR | 130 (99.2) | 28 (90.3) | 130 (99.2) | 54 (87.1) |
| ISR category | ||||
| Redness | 127 (96.9) | 18 (58.1) | 127 (96.9) | 53 (85.5) |
| Swelling | 121 (92.4) | 15 (48.4) | 122 (93.1) | 44 (71.0) |
| Tenderness to touch | 118 (90.1) | 16 (51.6) | 118 (90.1) | 45 (72.6) |
| Bruising | 114 (87.0) | 11 (35.5) | 114 (87.0) | 46 (74.2) |
| Firmness | 114 (87.0) | 13 (41.9) | 115 (87.8) | 43 (69.4) |
| Lumps/bumps | 112 (85.8) | 11 (35.5) | 112 (85.5) | 42 (67.7) |
| Pain after injection | 107 (81.7) | 7 (22.6) | 107 (81.7) | 42 (67.7) |
| Itching | 39 (29.8) | 4 (12.9) | 39 (29.8) | 13 (21.0) |
| Discoloration | 38 (29.0) | 2 (6.5) | 40 (30.5) | 16 (25.8) |
Note: aDenominator is the number of subjects who recorded in diaries after the treatment.
Abbreviation: ISR, injection site response.
There were 20 (15.3% [20/131]) subjects who experienced treatment-related AEs (Table 5). All treatment-related AEs were mild to moderate in severity. The only treatment-related AE that occurred in more than 5% of subjects was injection site mass (9.2% [12/131]). The masses were intradermal ovoid masses on the neck, generally 2 to 3 mm wide (range: 1 to 5 mm), decreased in size over time, and were not associated with inflammation in any subject. The majority of treatment-related AEs (95% [19/20]) began within 2 days of treatment. One subject reported 2 treatment-related AEs on the neck 122 days after initial treatment. These 2 events included injection site nodules and injection site erythema of moderate severity. Both events resolved after treatment with oral methylprednisolone.
Table 5.
Treatment-Related Adverse Events
| Treatment-Related Adverse Events | |
|---|---|
| Subjects with treatment-related adverse events, n/N (%) | 20/131 (15.3) |
| Event, n (%) | |
| Injection site mass | 12 (9.2) |
| Injection site bleeding | 4 (3.1) |
| Injection site hematoma | 3 (2.3) |
| Injection site erythema | 1 (0.8) |
| Injection site nodule | 1 (0.8) |
For nearly all subjects with AEs (95% [19/20]), the events resolved within 30 days (35% [7/20]) or prior to study completion (60% [12/20]). One subject had an ongoing moderate injection site mass on the neck at study end. No deaths or serious AEs related to treatment occurred.
Discussion
This is the first large prospective study to evaluate the long-term safety and effectiveness of an injectable HA gel for the treatment of skin roughness and fine lines and other skin quality attributes. Results from 131 subjects showed that VYC-12 results in improvements in skin topography lasting up to 6 months. At 1 and 4 months after treatment, respectively, more than 90% and 75% of cheeks had a reduction in skin roughness. Of note, response rates were generally higher and more durable in cheeks with severe roughness at baseline, for which ASRS response rates exceeded 80% at month 4 and 50% at month 6. Additionally, more than 90% of cheeks with moderate to severe fine lines at baseline showed improvement in the severity of fine lines at 1 month after initial treatment. Instrument measures showed significant improvements in cheek skin smoothness and hydration after treatment, with hydration showing improvement through 9 months. Although significantly different from baseline, the improvement in hydration at the month 1R visit was less than that measured at the month 9 visit. The interval between the month 9 retreatment and the month 1R visit may have been too short to see the effects of VYC-12 on hydration. This is supported by the lag between baseline hydration and maximum hydration values observed at 4 months after initial treatment. Treated necks and foreheads also showed improvements in these measures after initial treatment. Repeat treatment administered 9 months after the initial or touch-up treatment resulted in improvements in cheek skin roughness, fine lines, and hydration similar to those seen 1 month after treatment using a lower injection volume.
The safety and effectiveness of other HA fillers for the treatment of various skin quality attributes was evaluated in several small studies enrolling from 6 to 30 subjects.15,16,20–22 In these studies, enrolled subjects received injections at 3 separate visits at 2- to 4-week intervals, with an average total injected volume of HA filler between 3 mL and 6 mL in the face.15,16,20,22 One study demonstrated significantly improved instrument measures of cheek skin deformation and roughness for up to 4 months after the last of the 3 treatment sessions.15 In our study, improvements in attributes of skin quality were maintained for up to 6 months after initial/touch-up treatment with VYC-12. Further, skin hydration improvements lasted up to 9 months after the initial/touch-up treatment.
VYC-12 injectable gel was safe and well tolerated in all treated areas (cheek, forehead, and neck). ISRs were mainly mild or moderate in severity and typically lasted 1 week or less, as expected for dermal filler treatment. Adverse events were consistent with those observed in other clinical trials of HA-based fillers.23,24 No treatment-related AEs occurred after the 6-month database lock, and none were reported after repeat treatment. There was a single report of delayed-onset nodules (incidence: 0.8%), which is consistent with the approximate 0.5% incidence previously reported for HA fillers.25 Injection-site masses in the neck may have been due to the inherent difficulty of ensuring consistent injection volumes in this area, particularly with the small injection amounts required. The neck skin is thin compared with the cheek and forehead skin26 so greater volumes in some areas of the neck may lead to visible, palpable masses at those sites. Even with the use of a topical anesthetic, subjects reported a mean pain score of 4.3 following the initial treatment, which could have influenced the decision to request retreatment at month 9. The commercially available product has been formulated with lidocaine to make the injection process more comfortable for the patient and reduce the need for conventional anesthetics.27
One limitation of the study was that the detection of minor changes in skin topography may have been beyond the discrimination capacity of the human eye.28,29 Thus, assessments made using the photonumeric scales may have underestimated the extent of changes due to treatment with VYC-12.
Conclusions
VYC-12 injectable gel is safe and effective for improvements in skin roughness, reduction of fine lines, and improvements in hydration in the face and neck, with improvements in roughness lasting up to 6 months and improvements in hydration lasting 9 months. Subjects receiving repeat treatment at 9 months experienced improvements in skin roughness, fine lines, and hydration at 1 month after retreatment that were similar to those after initial treatment, but with a lower volume of VYC-12 injected.
Acknowledgments
This study was funded by Allergan plc. Medical writing and editorial support for this article were provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, New Jersey, and was funded by Allergan plc.
Abbreviations
AEs, adverse events; AFLS, Allergan Fine Lines Scale; ASRS, Allergan Skin Roughness Scale; CI, confidence interval; HA, hyaluronic acid; ISRs, injection site responses.
Data Sharing Statement
Data reported in this manuscript are available within the article [and/or] its supplementary tables. Allergan will share de-identified patient-level data and/or study-level data, including protocols and clinical study reports, for phase 2-4 trials completed after 2008 that are registered on ClinicalTrials.gov or EudraCT. The indication studied in the trial must have regulatory approval in the United States and/or the European Union and the primary manuscript from the trial must be published prior to data sharing. To request access to the data, the researcher must sign a data use agreement. All shared data are to be used for non-commercial purposes only. More information can be found on http://www.allerganclinicaltrials.com/.
Disclosure
F. Niforos, C. Leys, M. Cavallini, P. Ogilvie, M. Safa, and J. Chantrey are investigators for Allergan plc, Marlow, UK. J. Chantrey reports personal fee from Allergan as a teaching consultant, during the conduct of the study and outside the submitted work. A. Marx and S. Abrams are employees of Allergan plc, Irvine, CA. R. Hopfinger was an employee of Allergan plc, Irvine, CA at the time of this study. The authors report no other conflicts of interest in this work.
References
- 1.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672. doi: 10.1001/archderm.144.5.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi JW, Kwon SH, Huh CH, Park KC, Youn SW. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19(1):e349–e355. doi: 10.1111/j.1600-0846.2012.00650.x [DOI] [PubMed] [Google Scholar]
- 3.Fink B, Grammer K, Thornhill R. Human (Homo sapiens) facial attractiveness in relation to skin texture and color. J Comp Psychol. 2001;115(1):92–99. [DOI] [PubMed] [Google Scholar]
- 4.Jones AL, Kramer RS, Ward R. Signals of personality and health: the contributions of facial shape, skin texture, and viewing angle. J Exp Psychol Hum Percept Perform. 2012;38(6):1353–1361. doi: 10.1037/a0027078 [DOI] [PubMed] [Google Scholar]
- 5.Lai M, Oruc I, Barton JJ. The role of skin texture and facial shape in representations of age and identity. Cortex. 2013;49(1):252–265. doi: 10.1016/j.cortex.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Tsankova E, Kappas A. Facial skin smoothness as an indicator of perceived trustworthiness and related traits. Perception. 2016;45(4):400–408. doi: 10.1177/0301006615616748 [DOI] [PubMed] [Google Scholar]
- 7.American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. 2018. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf Accessed: July18, 2019.
- 8.Trojahn C, Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int. 2015;2015:318586. doi: 10.1155/2015/318586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003 [DOI] [PubMed] [Google Scholar]
- 10.Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: clinical perspectives and clinical methods in the evaluation of ageing skin. Int J Cosmet Sci. 2008;30(5):323–332. doi: 10.1111/j.1468-2494.2008.00455.x [DOI] [PubMed] [Google Scholar]
- 11.Nkengne A, Bertin C. Aging and facial changes–documenting clinical signs, part 1: clinical changes of the aging face. Skinmed. 2013;11(5):281–286. [PubMed] [Google Scholar]
- 12.Lee DH, Oh JH, Chung JH. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83(3):174–181. doi: 10.1016/j.jdermsci.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 13.Lapiere CM. The ageing dermis: the main cause for the appearance of ‘old’ skin. Br J Dermatol. 1990;122(Suppl 35):5–11. doi: 10.1111/j.1365-2133.1990.tb16119.x [DOI] [PubMed] [Google Scholar]
- 14.Landau M, Fagien S. Science of hyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;136(5 Suppl):188s–195s. doi: 10.1097/PRS.0000000000001823 [DOI] [PubMed] [Google Scholar]
- 15.Kerscher M, Bayrhammer J, Reuther T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol Surg. 2008;34(5):720–726. doi: 10.1111/j.1524-4725.2008.34176.x [DOI] [PubMed] [Google Scholar]
- 16.Streker M, Reuther T, Krueger N, Kerscher M. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face, hand, and decolletage. J Drugs Dermatol. 2013;12(9):990–994. [PubMed] [Google Scholar]
- 17.JUVÉDERM® Ultra Volite B [package insert]. Pringy, France: Allergan; 2017. [Google Scholar]
- 18.Donofrio L, Carruthers A, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial skin texture. Dermatol Surg. 2016;42(suppl 1):S219–S226. doi: 10.1097/DSS.0000000000000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carruthers J, Donofrio L, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial fine lines. Dermatol Surg. 2016;42(suppl 1):S227–S234. doi: 10.1097/DSS.0000000000000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BM, Han DG, Choi WS. Rejuvenating effects of facial hydrofilling using Restylane Vital. Arch Plast Surg. 2015;42(3):282–287. doi: 10.5999/aps.2015.42.3.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh NK, Kim MJ, Lee YW, Choe YB, Ahn KJ. A split-face study of the effects of a stabilized hyaluronic acid-based gel of nonanimal origin for facial skin rejuvenation using a stamp-type multineedle injector: a randomized clinical trial. Plast Reconstr Surg. 2016;137(3):809–816. doi: 10.1097/01.prs.0000480686.68275.60 [DOI] [PubMed] [Google Scholar]
- 22.Seok J, Hong JY, Choi SY, Park KY, Kim BJ. A potential relationship between skin hydration and stamp-type microneedle intradermal hyaluronic acid injection in middle-aged male face. J Cosmet Dermatol. 2016;15(4):578–582. doi: 10.1111/jocd.2016.15.issue-4 [DOI] [PubMed] [Google Scholar]
- 23.Raspaldo H, Chantrey J, Belhaouari L, Saleh R, Murphy DK. Juvéderm Volbella with Lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial. Plast Reconstr Surg Glob Open. 2015;3(3):e321. doi: 10.1097/GOX.0000000000000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SC, Downie JB, Shamban A, et al. Lip and perioral enhancement with hyaluronic acid dermal fillers in individuals with skin of color. Dermatol Surg. 2019;45(7):959–967. doi: 10.1097/DSS.0000000000001842 [DOI] [PubMed] [Google Scholar]
- 25.Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929–939. doi: 10.1097/DSS.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 26.Chopra K, Calva D, Sosin M, et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthet Surg J. 2015;35(8):1007–1013. doi: 10.1093/asj/sjv079 [DOI] [PubMed] [Google Scholar]
- 27.Levy PM, De BK, Raspaldo H. A split-face comparison of a new hyaluronic acid facial filler containing pre-incorporated lidocaine versus a standard hyaluronic acid facial filler in the treatment of naso-labial folds. J Cosmet Laser Ther. 2009;11(3):169–173. doi: 10.1080/14764170902833142 [DOI] [PubMed] [Google Scholar]
- 28.Regan D. Orientation discrimination for bars defined by orientation texture. Perception. 1995;24(10):1131–1138. doi: 10.1068/p241131 [DOI] [PubMed] [Google Scholar]
- 29.Gray R, Regan D. Spatial frequency discrimination and detection characteristics for gratings defined by orientation texture. Vision Res. 1998;38(17):2601–2617. doi: 10.1016/s0042-6989(97)00461-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. 2018. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf Accessed: July18, 2019.