Abstract
Parkinson’s disease (PD) patients frequently develop cognitive impairment. There is a need for brief clinical assessments identifying PD patients at high risk of progressing to dementia. In this study, we look into predicting dementia in PD and underlying structural and functional correlates to cognitive decline in PD. We included 175 patients with PD, 30 with PD dementia, 51 neurologically healthy controls and 121 patients with Alzheimer’s disease (AD) from Skane University Hospital, BIOFINDER cohorts. All underwent cognitive tests, including MMSE, 10-word list delayed recall (ADAS-cog), A Quick Test of cognitive speed (AQT), Letter S fluency, Clock Drawing Test (CDT) and pentagon copying. In non-demented patients with PD, abnormal AQT and CDT results predicted an increased risk of subsequent development of dementia (hazard ratio 2.2 for both). When comparing the cognitive profile between PD and AD, decreased performance on AQT, which measures attention and processing speed, was more typical in PD. Lastly, we investigated the underlying structural and functional correlates for the PD-specific test AQT with magnetic resonance imaging. In PD patients, decreased performance on AQT was associated with i) cortical thinning in temporoparietal regions, ii) changes in diffusion MRI, especially in the cingulum tract, and iii) decreased functional connectivity in posterior brain networks.
Subject terms: Neuroscience, Brain
Introduction
Parkinson´s disease (PD) is the second most common neurodegenerative disease1 and a common cause of motor symptoms, cognitive decline and dementia. The overall prevalence of PD is approximately 1% of the population >60 years of age. It has been estimated that 75% of patients with PD that survive more than 10 years develop dementia with a point prevalence of close to 30%2, although some studies suggest a lower cumulative prevalence of 46%3. The development of dementia is a serious problem that severely worsen the quality of life of patients and their relatives4 and increases the costs for society5.
Accurately predicting and diagnosing PD with dementia (PDD) is not only important for allocating necessary resources and care-giver support6, but also for treating the patients correctly. Randomized clinical trials (RCT) have shown positive cognitive effects of acetylcholinesterase inhibitors7 and memantine8 in PDD. Prediction of PDD may in the future also be valuable for choosing and evaluating neuromodulatory treatments.
Previously, memory and executive impairment has been shown to predict dementia in PD9 as well as impaired pentagon copying10. However, perhaps due to underlying heterogeneous pathologies, predicting dementia in PD remains a challenge, and the underlying pathology needs to be more elucidated.
The three aims of this study were to (1) evaluate the performance of various brief cognitive tests for predicting subsequent development of PD dementia, (2) identify tests specific to the cognitive decline in PD, and (3) examine the underlying brain correlates of the cognitive deficits in PD. First, we studied which cognitive tests could predict development of dementia in patients with PD (n = 175) during a mean of 5.5 (SD 2.3) years of follow-up. Next, patients with PDD at baseline (n = 30) were compared to an independent cohort of AD patients (n = 121) to evaluate which tests were specific for PDD.
Finally, in an attempt to shed light on specific patterns of pathology in PD dementia. we evaluated the underlying functional and structural correlates of declining processing speed in PD with magnetic resonance imaging (MRI) modalities, evaluating cortical thickness, white matter tracts and functional connectivity.
Methods
Participants
All participants were recruited at Skane University Hospital in southern Sweden and included patients with PD, PDD, AD as well as neurologically healthy controls. The cases are part of the prospective and longitudinal Swedish BioFINDER study (Biomarkers For Identifying Neurodegenerative Disorders Early and Reliably) described in previous publications from our group11 and at http://biofinder.se. This study started in 2008 and is still ongoing. The PD patients met the National Institute of Neurological Disorders and Stroke Diagnostics Criteria for PD12. The patients with PDD met the clinical diagnostic criteria for dementia associated with PD according to Emre et al.13. The healthy controls (HC) served as a control group and were neurologically and cognitively healthy. The AD patients met the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) criteria for dementia and the criteria for probable AD defined by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer´s and Related Disorders Society14.
Clinical evaluation
The study participants underwent cognitive evaluation according to the proposed algorithm and instruments for establishing the diagnosis of PDD at Level I by Dubois et al.15 and the DSM-V criteria16. The evaluation was performed at baseline and 2nd year for up to 10 years (follow-up range: 1.5–10 years). Conversion to dementia was evaluated at every time point (2, 4, 6, 8 or 10 year), or by chart review in the case of dropouts. A physician with experience in movement disorders performed a detailed history and neurological exam at every visit, as well as a review of the patient’s charts. A research nurse performed the cognitive testing. In this study, MMSE was chosen as the measure of global cognitive function. To measure episodic memory, the 10-word list delayed recall from the Alzheimer´s Disease Assessment Scale – cognition (ADAS-cog) was used17. Phonemic fluency was measured with Letter S fluency18 and visuospatial function with the Clock Drawing Test (Shulman’s scoring method19) and pentagon copying20, scored using the scoring method suggested by Caffara et al.21. Attention and processing speed was measured with AQT22–24. Briefly, AQT is a time trial of naming forms, colours and lastly the combination of coloured geometrical figures. The test aims to measure processing speed and executive function. It is a time trial where the figures are described by form and colour first separately, then in combination. The measured variable is the time it takes to name of 40 colors/forms. Motor function was assessed using UPDRS-III25. All patients were examined in the ON-state. For the longitudinal analysis of clinical progression in the PD cohort, all PD patients (n = 175) that were non-demented at baseline were followed clinically for up to ten years, (mean 5.3, SD 2.3, range 1.5–10). A physician with experience in neurodegenerative disorders evaluated the patients for presence of PDD at baseline or conversion to PDD at every follow up visit, based on clinical test scores and global functioning at home and in society. For dropouts, patients’ medical records were also reviewed for information about conversion to PDD during the follow up period. In the longitudinal analysis, a predefined AQT Color Form cutoff of >80 seconds was used (+2 SD above mean in a reference population26) and a predefined cutoff of <4 points was used for the Clock Drawing Test19.
MR imaging acquisition and processing
For investigation of regional brain changes associated with cognitive impairment, we performed MR imaging in 108 cases with PD and 17 cases with PDD. The clinical information closest to the MRI scan was analysed. All MR imaging was performed on 3 T Siemens Skyra MR equipment using the standard 20 channel headcoil. Coronal MPRAGE comprised 180 slices, voxel size 1 × 1 × 1 mm3. The resting state-fMRI protocol comprised 256 T2*-weighted echo planar imaging volumes sensitized to the BOLD effect (echo time 30 ms; repetition time 1850 ms; 33 axial slices; matrix 64 × 64; voxel size 3 × 3 × 3.75 mm3). Subjects were instructed to lie still with their eyes closed, without thinking of anything in particular and without falling asleep. DKI data comprised 99 volumes with 52 continuous slices, three volumes with b = 0 s/mm2 and 96 volumes with b-values of 250, 500, 1000, and 2750 s/mm2, distributed over 6, 6, 20, and 64 directions27. The voxel size was 2.3 × 2.3 × 2.3 mm3 and FOV 294 × 294 × 120 mm3. Motion and eddy current distortions were corrected using an extrapolation-based method for improved high b-value performance. Image volumes were smoothed using an isotropic 3D Gaussian kernel with a full-width at half maximum of 2.3 mm28–30. DKI parameter maps of fractional anisotropy (FA), mean kurtosis (MK), and mean diffusivity (MD)31 were obtained using in-house developed software which fitted the diffusion and kurtosis tensors by nonlinear optimization.
Freesurfer analysis
Using the free image analysing tool Freesurfer, available at http://surfer.nmr.mgh.harvard.edu, we analysed T1MPRAGE MR images.
Freesurfer software package is a validated tool for measuring cortical thickness32. Non-brain tissue is removed and grey and white matter is separated. The post processing results were quality controlled with visual inspection prior to analysis. Three patients were excluded due to corrupted data. Using the QDEC application in the free Freesurfer software package, we correlated the AQT scores, as well as the results from the other cognitive tests (MMSE, CDT, Pentagon copying, Letter S fluency and Delayed Word recall), to cortical thickness as the dependent variable in a whole brain analysis, using Monte Carlo simulation of 1.3. The model was balanced for age and years of education.
DTI/tractography
Tractography using deterministic tracking based on constrained spherical deconvolution (CSD) was performed to generate the left and right dorsal cingulum (CG), the hippocampal CG, the corticospinal tract (CST) the fornix (FX), the uncinate fasciculus (UF), the superior longitudinal fasciculus (SLF), the inferior longitudinal fasciculus (ILF), and the inferior fronto-occipital fasciculus (IFO)33. One seed-ROI covering the full extent of the specific tract and logical AND- and NOT-ROIs were defined in Montreal Neurological Institute (MNI) 152 standard-space and warped back to native-space utilizing the warp-fields generated by FLIRT and FNIRT34. All tracts were visually inspected and ROIs were adjusted in order to secure location of the tract in the intended anatomical region with the number of streamlines below 100. The average parameter estimate for each tract was extracted and used in the subsequent analyses. Since the differences we aimed to elucidate could be relatively subtle, we abstained from a whole brain approach WBA, because of the risk of it being to insensitive considering the multiple comparison issues with such voxel-based approach.
Functional network analysis
fMRI scans were preprocessed using the Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm). In brief, the following steps were applied: removal of first six volumes, correction for time offsets between slices, realignment, normalization to the MNI template, temporal high-pass filtering (0.01–0.08 Hz), regression of six head motion parameters, and white matter and cerebrospinal fluid signals. To remove the influence of volumes affected by motion, we applied a scrubbing procedure using root-mean-square intensity differences between volumes N to volumes N + 1 with DVARS35. In this study, 1 PD and 2 PDD patients showed head motion > =3 mm translation and rotation, and for this reason they were excluded from the analyses.
Functional brain networks were constructed for each subject using a set of nodes representing brain regions. These nodes were connected by edges representing the statistical interdependence in blood oxygen level-dependent (BOLD) signals. The nodes were defined using the 200 cortical and subcortical regions provided by the Craddock atlas36. The edges were calculated as the Pearson correlation coefficients between the regional time series of all possible pairs of regions, resulting in a 200 × 200 correlation matrix for each subject. To assess whether functional connectivity between the previous brain regions was associated with AQT performance, we used the network-based statistic (NBS) software package37.
Statistical analysis
SPSS 25 was used for all statistical analysis except for the Freesurfer data where the built in application Query Estimated Design Contrast, QDEC, was used38. For comparisons between the different diagnostic groups at baseline (PD, PDD, and HC), a univariate linear model corrected for age, sex, years of education and disease duration was applied with corrections for multiple comparisons using the Bonferroni method. The PD and AD patients were compared with a similar model, corrected for MMSE and age as a measure of global cognitive function and disease burden. For correlation analysis between cognitive tests and tractography, partial Pearson correlations were performed, corrected for age, sex, years of education, disease duration and brain volume. Since these analyses were exploratory in nature, we did not correct for multiple comparisons. For evaluation of the predictive value of cognitive tests on subsequent development of dementia, we performed cox regression analysis corrected for age, sex, disease duration and years of education.
In Freesurfer, the built-in application QDEC was used for analysis. The analysis was adjusted for age, sex, years of education and disease duration. A Monte Carlo simulation was used and p < 0.05 was considered significant. For the functional network analysis, a regression analysis was carried out to test whether AQT scores correlated with functional connectivity in the whole patient group, while controlling for age, sex, education and disease duration. Correction for multiple comparisons was performed using a false discovery rate (FDR) of 0.001 (1000 permutations).
Ethics
All individuals gave their written informed consent. The study procedure was approved by the local ethics committee at Lund University, Sweden, and conducted according to the Helsinki Declaration.
Results
Baseline characteristics
The baseline demographics of the participants are presented in Table 1. Group comparisons revealed differences in letter S fluency and pentagon drawing between HC and PD, but the differences were relatively small. The PDD patients performed worse on all cognitive scores except CDT than the PD and HC patients.
Table 1.
Baseline demographics.
| HC (n = 51) | PD (baseline) (n = 175) | PDD (n = 30) | |
|---|---|---|---|
| Baseline age, years | 65 (8.5) | 65 (10) | 72.5 (6.6) |
| Gender (1 = female) | 0.56 | 0.37 | 0.3 |
| Years of education | 13.3 (3.2) | 13 (4.0) | 11 (4.5) |
| Disease Duration Years | 5.1 (4.9) | 14 (6.8) | |
| Levodopa equivalents | 455 (455) | 881 (549) | |
| UPDRS-III | 1.7 (2.6) | 16 (10)c | 35 (13)f |
| MMSE (0–30 points) | 28.3 (1.5) | 28.5 (1.3) | 22 (5.0)f i |
| Wordlist delayed recall | 2 (1.7) | 3 (2.1) | 6 (3.0)f i |
| (0–10 errors) | |||
| AQT Color Form (sec) | 63 (11) | 71 (29) | 181 (104)f i |
| AQT Color (sec) | 24 (4.5) | 27 (7.0) | 52 (30)f i |
| AQT Form (sec) | 35 (7.5) | 37 (9.3) | 75 (37)f i |
| Letter S fluency | 17.5 (5.9) | 14.7 (5.7)a | 7.9 (4.1)f i |
| Clock Drawing Test (0–5p) | 4.1 (0.7) | 4.2 (0.9) | 4.6 (0.6) |
| Pentagon Drawing (0–13p) | 12 (0.9) | 11.3 (1.3)b | 7.5 (4.0)f i |
Mean presented with standard deviations within parenthesis.
To adjust for multiple comparisons with Bonferroni method, all p-values were multiplied by 3. All comparisons were corrected for age, gender, years of education and disease duration.
aSignificant difference (p < 0.05), comparison between HC and PD.
bSignificant difference (p < 0.01), comparison between HC and PD.
cSignificant difference (p < 0.001), comparison between HC and PD.
dSignificant difference (p < 0.05), comparison between HC and PDD.
eSignificant difference (p < 0.01), comparison between HC and PDD.
fSignificant difference (p < 0.001), comparison between HC and PDD.
gSignificant difference (p < 0.05), comparison between PD and PDD.
hSignificant difference (p < 0.01), comparison between PD and PDD.
iSignificant difference (p < 0.001), comparison between PD and PDD.
Prediction of PDD conversion
Next, we studied which cognitive tests could predict subsequent development of dementia in non-demented PD patients. The patients were followed between 1.5 and 10 years with an average of 5.5 (SD 2.3) years. In total 41 (23%) PD patients converted to dementia during follow-up. We applied a Cox regression model corrected for age, sex, disease duration and years of education. Z-scores of the results on the cognitive tests were used for comparability and the results are presented in Table 2. In brief, AQT Form, AQT Color Form and the CDT at baseline indicated an increase hazard ratio (HR) of developing dementia during follow up. Further, the significant predictors (AQT and CDT) were dichotomised into normal and abnormal scores using the predefined cutoffs CDT < 4 points and AQT Color Form >80 seconds. Using these cutoffs, AQT Color Form predicted dementia development with an hazard ratio (HR) of 2.2 (95% CI 1.08–4.5) and the CDT had a similar HR of 2.2 (95% CI 1.08–4.6).
Table 2.
Single test risk stratification of PD dementia conversion.
| Cognitive test | HR | p-value | 95% CI |
|---|---|---|---|
| MMSE | 1.2 | 0.39 | 0.80–1.8 |
| AQT Color Form | 1.6 | 0.04 | 1.01–2.7 |
| AQT Color | 1.2 | 0.2 | 0.9–1-0.6 |
| AQT Form | 1.6 | 0.001 | 1.2–2.0 |
| 10-world list delayed recall | 1.3 | 0.12 | 0.9–1.9 |
| Letter S fluency | 0.7 | 0.13 | 0.48–1.1 |
| Months backwards | 1.1 | 0.5 | 0.79–1.6 |
| Pentagon drawing | 0.56 | 0.55 | 0.314–1.01 |
| Clock Drawing Test | 1.4 | 0.032 | 1.03–2.0 |
COX regression evaluating the z-scores of the cognitive tests as predictors for conversion to dementia in non-demented PD patients. Each cognitive test model was adjusted for age, sex, years of education and disease duration. HRs are shown based on test z-scores for comparison between tests.
Comparison of AQT and the clock drawing Test in PDD and AD
Next, we compared the cognitive profile of our PDD patients to that of an independent AD cohort to evaluate if any of the two predictive tests with very similar predictive power (AQT and Clock Drawing Test) were more specific to PD. The model was corrected for age and MMSE as proxy of disease severity. We found that the PD patients exhibited slower processing speed on all AQT tasks whereas there was no difference on the Clock Drawing Test (Table 3). As expected, the AD patients performed worse on the memory task (word list delayed recall).
Table 3.
Comparison of cognitive profile between AD and PDD patients.
| AD (n = 121) | PDD (n = 30) | P-value | |
|---|---|---|---|
| Baseline age, years | 77 (5.5) | 72 (6.0) | <0.001 |
| Years of education | 10 (3.2) | 11 (4.5) | 0.143 |
| MMSE | 22 (4.0) | 22 (5.0) | 0.868 |
| Wordlist delayed recall (0–10 errors) | 8.5 (1.6) | 6.0 (2.9) | <0.001 |
| AQT Color Form (sec) | 108 (35) | 175 (98) | <0.001 |
| AQT Color (sec) | 38 (14) | 52 (20) | <0.001 |
| AQT Form (sec) | 60 (25) | 74 (37) | 0.003 |
| Clock Drawing Test (0–5p) | 3.1 (1.3) | 3.1 (10.9) | 0.61 |
Data are shown as mean values with standard deviations within parenthesis.
Cognitive test comparisons were adjusted for age, years of education and global cognitive function (MMSE).
Associations between AQT and MR imaging
Since our results suggested that AQT not only predicted conversion to PDD in PD but also seems more typical to PD than AD, we investigated the underlying structural and functional correlates to this seemingly PD specific test in an attempt to elucidate underlying pathological patterns. This was done by analysing associations between processing speed (measured with AQT Color Form) and cortical thickness, white matter tracts and functional networks in patients with PD or PDD who underwent MRI (n = 125). Clinical data for this subset of the cohort is presented in a supplement (Supplementary Table 1).
Analysis of all available cortical thickness measures adjusted for age and years of education, revealed that reduced performance on AQT was significantly correlated with cortical thinning in the entorhinal, inferior parietal and inferior temporal cortex in the left hemisphere and the lateroorbitofrontal cortex in the right hemisphere (Table 4).
Table 4.
Correlations between AQT and cortical thickness.
| Regions left side | p-value | Regions right side | p-value |
|---|---|---|---|
| Enthorihnal | <0.05 | Lateroorbitofrontal | <0.05 |
| Inferiotemporal | <0.05 | ||
| Inferiopearietal | <0.05 |
QDEC analysis adjusted for age, years of education and disease duration.
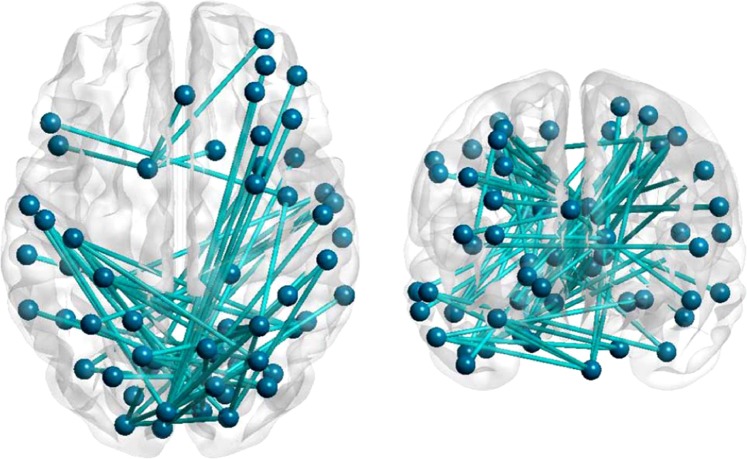
Next, white matter tracts were evaluated for correlations with declining processing speed measured with AQT, in an exploratory manner (Table 5). We mainly found correlations between declining processing speed and discrepancies in the cingulum tract using the mean diffusivity, MD, parameter, see Table 4. Anomalies could also be found in the inferior fronto-occipital fasciculus, ILF and inferior longitudinal fasciculus, SLF, whereas the corticospinal tract, included as a reference tract, fornix and uncinate fasciculus were not affected. Finally, we studied the associations between AQT Color Form and connectivity using resting-state functional MRI. We identified a network of 60 regions and 72 connections showing a negative correlation with AQT performance in patients (p < 0.001) (Fig. 1). This network mainly consisted of posterior cortical regions such as the precuneus, superior parietal gyri, occipital gyri, cuneus and lingual gyri. In addition, the brainstem, cerebellum and some frontal and temporal regions also correlated negatively with AQT scores, while controlling for age, sex, education and disease duration.
Table 5.
Partial correlations between AQT and white-matter tracts corrected for age, sex, disease duration, brain volume and years of education.
| Tract | Correlation dx (right) | p-value | Correlation sin (left) | p-value |
|---|---|---|---|---|
| CD MD | 0.274 | 0.007 | 0.222 | 0.03 |
| CV MD | 0.274 | 0.007 | 0.162 | 0.133 |
| CST MD | −0.065 | 0.525 | −0.043 | 0.676 |
| UF MD | 0.192 | 0.059 | 0.139 | 0.175 |
| SLF MD | 0.093 | 0.366 | 0.17 | 0.096 |
| ILF MD | 0.214 | 0.035 | 0.2 | 0.05 |
| IFO MD | 0.22 | 0.024 | 0.148 | 0.148 |
| FX MD | 0.036 | 0.728 |
CD, Dorsal cingulum; CV, Ventral cingulum; CST, Cortico spinal tract, IFO, Inferior fronto-occipital fasciculus, ILF, inferior longitudinal fasciculus, SLF, Superior longitudinal fasciculus, UF, Uncinate fasciculus, FX fornix, represented in column dx but is not side specific.
Figure 1.
Functional connection network analysis adjusted for age, sex, years of education and disease duration.
Discussion
In this study, we found that both AQT and the Clock Drawing Test predicted increased risk of developing dementia in PD (HR 2.2). When comparing cognitive profiles between PDD and AD, AQT was more specific to PDD, and we therefore further examined the underlying structural correlates of AQT performance (i.e. declining processing speed) in PD and PDD. We found that poor performance on AQT was associated with cortical atrophy in temporoparietal areas (mainly in the left hemisphere), diffusion changes in certain white matter tracts, mainly the cingulum tract, and decreased connectivity in posterior networks.
A few cognitive tests have previously been shown to predict conversion to dementia in PD. For example, Barker et al. found evidence that both verbal fluency, with a relative risk (RR) of 9.4 (95% CI 1.9–47.5) for a global decline, and inaccurate pentagon copying, with a RR of 5.2 (95% CI 1.9–14.1), predicted dementia in PD39. This could not be replicated in our cohort (Table 2). In the present study, we instead found that AQT and the Clock Drawing Test predicted dementia in PD, but not MMSE, pentagon copying, verbal fluency or word list delayed recall. The prediction of dementia using AQT is in agreement with previous findings showing that conversion to dementia can be predicted with tests of attention and executive function9.
Even though both AQT and Clock Drawing Test predicted subsequent development of dementia, AQT was more specific to the cognitive decline in PDD. When comparing the cognitive profile between PDD and AD, AQT scores were worse in the PDD group, but no difference was seen for Clock Drawing Test scores. As expected, the AD patients performed worse on delayed recall, but delayed recall did not seem to increase the risk of developing dementia in PD, suggesting that decline in episodic memory is not a typical or early feature in PDD. This is in accordance with previous studies showing that the cognitive profile in PD is initially more marked by dysexecutive symptoms and decreasing processing speed40,41, whereas AD patients decline more in episodic memory42,43, even though multiple cognitive domains can be affected in both disorders40,43.
The neuropathology of PDD is complex. Likely, a combination of α-synuclein pathology and AD-like pathology (amyloid-β and tau aggregates) is responsible for the cognitive symptoms44. Which pathology is more important is not fully elucidated45, but some authors suggest that α-synuclein pathology predominates46. It has been suggested that the cognitive symptoms are not solely a result of dopaminergic loss but also cholinergic insufficiency (which is thought to cause attention deficits)46,47, as well as degeneration of other neuronal cell types in the cortex. Previous studies of cortical thickness in different stages of PD have shown correlations between disease stage and cortical thinning48. For example, Zarei et al. found correlations between decreased MMSE and cortical thinning in several cortical areas in PD, including the posterior cingulate and precuneus. In our study, worse AQT scores correlated to decreased thickness in the posterior cingulate cortex as well as in the inferior parietal and temporal areas.
When it comes to structural connectivity, Kamagata et al. found decreased FA values in the cingulum tract in PD and PDD49. In our study we also found weak correlations between decreased processing speed and increased MD values in the cingulum as well as in the ILF and IFO tracts connecting the occipital lobe to the temporal lobe and frontal lobe, respectively50. While other studies have found evidence of frontostriatal involvement in dysexecutive syndromes in PD51, we also found associations between AQT and decreased connectivity in posterior networks and tracts with occipital connections. This could represent deficits in rudimentary visual processing and set-shifting, which is in agreement with the brain regions that are involved during the AQT Color Form task as visualized using fMRI and regional cerebral blood flow measures (rCBF)52.
A limitation of the study is that a larger battery might have identified other predictive tests for dementia conversion in PD, but, nonetheless, our cognitive battery included all tests recommended by the Movement Disorder Society Task Force15 and even some additional ones. The structural MRI findings were considered exploratory in nature, and therefore not corrected for multiple comparisons. Further studies are therefore warranted to confirm these findings. Strengths of the study are the relatively large and well described cohort, the large proportion of the original cohort that underwent MRI, and the long and structured follow-up.
We conclude that the dementia in PD is predominantly characterized by declining processing speed and executive dysfunction, which can be evaluated with the readily available test AQT. AQT can also predict subsequent development of dementia. Using the predefined cutoff of >80 seconds for AQT Color Form, clinicians can identify PD patients who have increased risk of developing dementia. Declining processing speed seems to have structural correlates in the cingulum tract and posterior functional networks. Further studies are needed to develop more precise methods and markers to predict cognitive decline in PD, and to shed more light on the underlying structural basis of cognitive decline in PD.
Supplementary information
Acknowledgements
Work at the authors’ research center was supported by the European Research Council, the Swedish Research Council, the Knut and Alice Wallenberg foundation, the Marianne and Marcus Wallenberg foundation, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Brain Foundation, The Parkinson foundation of Sweden, The Parkinson Research Foundation, the Skåne University Hospital Foundation, and the Swedish federal government under the ALF agreement. OH has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, Fujirebio, and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, Roche, and Fujirebio.
Author contributions
Mattis D Jalakas (1) A B C, (2) A B C, (3) A. Sebastian Palmqvist: (1) A B C, (2) A B C, (3) B. Danielle van Westen: (1) A B C, (2), (3) B. Sara Hall: (1) A, (2), (3) B. Olof Lindberg: (1) C, (2) B, (3) B. Daniel Svärd: (1) C, (2), (3) B. Joanna Pereira: (1) C, (2), (3) B. Oskar Hansson: (1) A B C, (2) A B C, (3) B. (1) Research Project: A. Conception, B. Organization, C. Execution. (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique. (3) Manuscript: A. Writing of the first draft, B. Review and Critique
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mattis Jalakas, Email: Mattis.Jalakas@med.lu.se.
Sebastian Palmqvist, Email: sebastian.palmqvist@med.lu.se.
Oskar Hansson, Email: Oskar.Hansson@med.lu.se.
Supplementary information
is available for this paper at 10.1038/s41598-019-51505-1.
References
- 1.Lebouvier T, et al. The second brain and Parkinson’s disease. The European journal of neuroscience. 2009;30:735–741. doi: 10.1111/j.1460-9568.2009.06873.x. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84:1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 4.Lawson RA, et al. Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Parkinsonism Relat Disord. 2016;27:47–53. doi: 10.1016/j.parkreldis.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association A. s. 2017 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2017;13:325–273. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 6.Meireles J, Massano J. Cognitive impairment and dementia in Parkinson’s disease: clinical features, diagnosis, and management. Front Neurol. 2012;3:88. doi: 10.3389/fneur.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Laar T, De Deyn PP, Aarsland D, Barone P, Galvin JE. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: a review of clinical data. CNS Neurosci Ther. 2011;17:428–441. doi: 10.1111/j.1755-5949.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarsland D, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. The Lancet. Neurology. 2009;8:613–618. doi: 10.1016/s1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 9.Levy G, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 10.Kaul S, Elble RJ. Impaired pentagon drawing is an early predictor of cognitive decline in Parkinson disease. Movement disorders: official journal of the Movement Disorder Society. 2014;29:427–428. doi: 10.1002/mds.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall S, et al. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84:57–63. doi: 10.1212/WNL.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Emre M, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois B, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 16.Association., A. P. Diagnostic and statistical manual of mental disorders (5th ed) (2013).
- 17.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. The American journal of psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 18.Gomez RG, White DA. Using verbal fluency to detect very mild dementia of the Alzheimer type. Archives of Clinical Neuropsychology. 2006;21:771–775. doi: 10.1016/j.acn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::AID-GPS242>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Helmes E. Cognitive screening of older adults: the utility of pentagon drawing. International psychogeriatrics. 2013;25:413–419. doi: 10.1017/s1041610212001998. [DOI] [PubMed] [Google Scholar]
- 21.Caffarra P, et al. The Qualitative Scoring MMSE Pentagon Test (QSPT): A New Method for Differentiating Dementia with Lewy Body from Alzheimer’s Disease. Behavioural Neurology. 2013;27:213–220. doi: 10.3233/BEN-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvitting AS, Wimo A, Johansson MM, Marcusson J. A Quick Test of Cognitive Speed (AQT)- Usefulness in dementia evaluations in primary care. Scand J Prim Health Care. 2013;31:13–19. doi: 10.3109/02813432.2012.751699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subirana-Mirete J, Bruna O, Virgili C, Signo S, Palma C. Processing speed in the aging process: screening criteria for the Spanish Quick Test of Cognitive Speed. Percept Mot Skills. 2014;119:417–429. doi: 10.2466/22.PMS.119c22z2. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi F, Awata S, Sakuma N, Inagaki H, Ijuin M. Reliability and validity of A Quick Test of Cognitive Speed for detecting early-stage dementia in elderly Japanese. Psychogeriatrics. 2012;12:75–82. doi: 10.1111/j.1479-8301.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 25.Metman LV, et al. Test-retest reliability of UPDRS-III, dyskinesia scales, and timed motor tests in patients with advanced Parkinson’s disease: an argument against multiple baseline assessments. Mov Disord. 2004;19:1079–1084. doi: 10.1002/mds.20101. [DOI] [PubMed] [Google Scholar]
- 26.Petrazzuoli Ferdinando, Palmqvist Sebastian, Thulesius Hans, Buono Nicola, Pirrotta Enzo, Cuffari Alfredo, Cambielli Marco, D’Urso Maurizio, Farinaro Carmine, Chiumeo Francesco, Marsala Valerio, Wiig Elisabeth H. A Quick Test of Cognitive Speed: norm-referenced criteria for 121 Italian adults aged 45 to 90 years. International Psychogeriatrics. 2014;26(9):1493–1500. doi: 10.1017/S1041610214000787. [DOI] [PubMed] [Google Scholar]
- 27.Poot DH, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE transactions on medical imaging. 2010;29:819–829. doi: 10.1109/tmi.2009.2037915. [DOI] [PubMed] [Google Scholar]
- 28.Veraart Jelle, Fieremans Els, Jelescu Ileana O., Knoll Florian, Novikov Dmitry S. Gibbs ringing in diffusion MRI. Magnetic Resonance in Medicine. 2015;76(1):301–314. doi: 10.1002/mrm.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrone D, et al. The effect of Gibbs ringing artifacts on measures derived from diffusion MRI. Neuroimage. 2015;120:441–455. doi: 10.1016/j.neuroimage.2015.06.068. [DOI] [PubMed] [Google Scholar]
- 30.Kellner Elias, Dhital Bibek, Kiselev Valerij G., Reisert Marco. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine. 2015;76(5):1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- 31.Latt J, et al. Regional values of diffusional kurtosis estimates in the healthy brain. J Magn Reson Imaging. 2013;37:610–618. doi: 10.1002/jmri.23857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardinale F, et al. Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 2014;12:535–542. doi: 10.1007/s12021-014-9229-2. [DOI] [PubMed] [Google Scholar]
- 33.Tournier JD, et al. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42:617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 38.Worker A, et al. Cortical Thickness, Surface Area and Volume Measures in Parkinson’s Disease, Multiple System Atrophy and Progressive Supranuclear Palsy. PLoS ONE. 2014;9:e114167. doi: 10.1371/journal.pone.0114167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 40.Aarsland D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceravolo R, Pagni C, Tognoni G, Bonuccelli U. The Epidemiology and Clinical Manifestations of Dysexecutive Syndrome in Parkinson’s Disease. Front Neurol. 2012;3:159. doi: 10.3389/fneur.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noe E, et al. Comparison of dementia with Lewy bodies to Alzheimer’s disease and Parkinson’s disease with dementia. Mov Disord. 2004;19:60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 43.Weintraub S, Wicklund AH, Salmon DP. The Neuropsychological Profile of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Compta Y, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hattori T, et al. Cognitive Status Correlates with White Matter Alteration in Parkinson’s Disease. Hum Brain Mapp. 2012;33:727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gratwicke J, Jahanshahi M, Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138:1454–1476. doi: 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrou M, Kotagal V, Bohnen NI. An update on brain imaging in parkinsonian dementia. Imaging in medicine. 2012;4:201–213. doi: 10.2217/iim.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarei M, et al. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84:875–882. doi: 10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamagata K, et al. White matter alteration of the cingulum in Parkinson disease with and without dementia: evaluation by diffusion tensor tract-specific analysis. AJNR. American journal of neuroradiology. 2012;33:890–895. doi: 10.3174/ajnr.A2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ASHTARI MANZAR. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Developmental Medicine & Child Neurology. 2011;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- 51.Rae CL, et al. White matter pathology in Parkinson’s disease: The effect of imaging protocol differences and relevance to executive function. NeuroImage. 2012;62:1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiig EH, et al. Parietal lobe activation in rapid, automatized naming by adults. Percept Mot Skills. 2002;94:1230–1244. doi: 10.2466/pms.2002.94.3c.1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.