Abstract
Background
The anticancer effects of cordyceps on various tumors have been reported. However, little is known about the role of selenium (Se)-enriched Cordyceps militaris in non-small cell lung cancer (NSCLC). In this study, the effects of Se-enriched Cordyceps militaris on cell proliferation, cell apoptosis and cell cycle in NSCLC cell line NCI-H292 and A549 were investigated.
Methods
CCK-8 assay was used to determine the appropriate concentrations of Se-enriched Cordyceps militaris in NSCLC (namely NCI-H292 and A549) cells. Colony formation assay, flow cytometric and Hoechst 33342 staining assays, and flow cytometric analysis were separately employed to assess the effect of increased Se-enriched Cordyceps militaris on NSCLC cell viability, cell apoptosis and cell-cycle distribution. Finally, the qPCR and Western blot assays were, respectively, applied to evaluate the effects of Se-enriched Cordyceps militaris on the expression of pro-apoptotic member BAX and the anti-apoptotic member BCL-2, as well as of G2/M cell cycle regulatory proteins CDK1 and cyclin B1.
Results
The concentration of Se-enriched Cordyceps militaris was 0, 4, 8, 12 mg/mL for NCI-H292 cells, and 0, 12.5, 25, 50 mg/mL for A549 cells. NSCLC cells treated with increased Se-enriched Cordyceps militaris showed the inhibited cell viability. Se-enriched Cordyceps militaris induced NSCLC cell apoptosis in concentration-dependent manner. Consistently, Se-enriched Cordyceps militaris diminished the ratio of anti-apoptotic member BCL-2 and pro-apoptotic member BAX at mRNA and protein levels in NSCLC cells. The percentage in G2/M phase was increased in NSCLC cells treated with increased Se-enriched Cordyceps militaris. Downregulation of G2/M cell cycle regulatory proteins CDK1 and cyclin B1 at mRNA and protein levels in NSCLC cells further confirmed the effects of Se-enriched Cordyceps militaris on cell cycle.
Conclusion
This study demonstrated the inhibitory role of Se-enriched Cordyceps militaris in cell proliferation and its facilitating role in cell apoptosis and cell cycle in NSCLC cells, suggesting an alternative therapeutic strategy for NSCLC treatment.
Keywords: Se-enriched Cordyceps militaris, NSCLC, proliferation, apoptosis, cell cycle
Introduction
Lung cancer, one of the leading causes of cancer-related deaths around the world, is classified into two major types, including small cell lung cancer (SCLC) and non-SCLC (NSCLC).1 NSCLC, accounting for nearly 85% of lung cancer, is the most common type of lung cancer and consists of squamous cell carcinoma and adenocarcinoma.2 Traditional Chinese medicines are considered to be useful medicines for survival, and have been used in Asia for thousands of years.3 Despite the great progresses in the diagnosis and treatments, the overall 5-year survival rate of patients with NSCLC is still less than 20%.4,5 Thus, developing the novel possible treatments and drugs for improving the outcome of NSCLC is an urgent assignment.
Cordyceps militaris and Cordyceps sinensis are representative species of Cordyceps mushrooms.6 Cordyceps militaris has been used as a traditional medicine in Asian countries for a long time.7 Different types of Cordyceps militaris extract were reported to have various pharmacological activities, including anti-tumor, anti-inflammatory and anti-oxidative activities.8,9 Lee et al have revealed that the anti-cancer effect of nucleosides-enriched ethanol extract of Cordyceps militaris is highly associated with cell cycle arrest and mitochondrial apoptosis in human colorectal carcinoma RKO cells.6 Park et al have reported that Cordyceps militaris induces the apoptosis of A549 cells through a signaling cascade of death receptor-mediated extrinsic and mitochondria-mediated intrinsic caspase pathways.10 Reis et al have documented that the methanolic extract of Cordyceps militaris inhibits the proliferation of various human carcinoma cell lines.11 Especially in NSCLC, it has been reported that the methanolic extract of Cordyceps militaris affects the cell viability of NCI-H460 cells through involving DNA damage and p53 activation.12 However, Cordyceps militaris is composed of various components, and the components related to its anti-cancer effects remain to be further elucidated.
Selenium (Se) is a key trace element with multiple functions and its chemical forms are divided into inorganic Se compounds and organic Se-containing compounds.13 Cordyceps militaris can absorb inorganic Se compounds from the substrate and convert it to organic Se compounds in fruiting bodies.14 Se has been reported as an essential role in physiological functions, such as anti-oxidation,15 anti-cancer,16 immunity stimulation,17 inhibiting HIV.18 Hu et al have demonstrated the effective antioxidant activities of Se-biofortified Cordyceps militaris.14 However, the biological roles of Se-enriched Cordyceps militaris in NSCLC have not been precisely elucidated.
This study aimed to investigate the functional role of Se-enriched Cordyceps militaris in the human NSCLC cell line NCI-H292 and A549. Functional assays and qPCR as well as Western blot analyses were performed to elucidate that the water extract of Se-enriched Cordyceps militaris inhibited cell proliferation, induced cell apoptosis and cell cycle arrest at G2/M phase. This study may give a new insight into the clinical treatments for NSCLC.
Materials And Methods
Cell Culture And Transfection
Human lung cancer cell lines NCI-H292 and A549 were obtained from Conservation Genetics of the Chinese Academy of Sciences Cell Bank (Shanghai, People’s Republic of China). Cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified incubator with 5% CO2 at 37°C for 24 hrs.
Isolation Of Se-Enriched Cordyceps militaris
The water extract used in this study was obtained from cultivated fruiting bodies of Se-enriched Cordyceps militaris (Shengfeng Pharmaceutic CO., LID, Enshi, People’s Republic of China). The isolation of Se-enriched Cordyceps militaris was similar to the previous report described.19 In brief, 20 g of Se-enriched Cordyceps militaris was added to 400 mL of double distilled water and boiled for 30 mins in a microwave oven on medium heat. Next, 12,000 g of the obtained solution was centrifuged for 15 mins. Finally, the supernatant was dried for 24 hrs using a vacuum freezing drying oven and 8.58 g of Se-enriched Cordyceps militaris (42.9%) was obtained.
Cell Counting Kit-8 (CCK-8) Assay
To evaluate the effect of Se-enriched Cordyceps militaris on cell viability of NSCLC cells, CCK-8 assay was performed according to the manufacture’s protocol. In brief, NCI-H292 and A549 cells were seeded into each well of 96-well plates at a density of 5000 cells/well. After incubation for 24 hrs, NCI-H292 and A549 cells were treated with varying concentrations of Se-enriched Cordyceps militaris (5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 mg/mL) for 24 hrs. Next, 10 μL of CCK-8 reagent was added to each well, and cells were cultured for 24 hrs. The optical density (OD) value of each well was measured using the Multiskan FC (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 450 nm wavelength.
Colony Formation Assay
NCI-H292 and A549 cells were planted into 24-well plates at a density of 2×105 cells/well and incubated for 24 hrs. A549 cells were treated with 0, 12.5, 25, and 50 mg/mL of Se-enriched Cordyceps militaris, while NCI-H292 cells were treated with 0, 4, 8, and 12 mg/mL of Se-enriched Cordyceps militaris. After incubation for 24 hrs, NCI-H292 and A549 cells were digested, and then plated into 6-well plates at a density of 500 cells/well. The suspension was extracted at day 10, fixed with 4% paraformaldehyde (Servicebio, Wuhan, People’s Republic of China) for 15 mins and stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 30 mins. The number of cell colonies with over 50 cells was counted using a microscope (Leica, Wetzlar, Germany).
Flow Cytometric Assay
To detect the effect of Se-enriched Cordyceps militaris on cell apoptosis of NSCLC cells, flow cytometry assay was conducted using Annexin V-FITC/PI apoptosis detection kit (Beyotime Biotechnology, Shanghai, People’s Republic of China) according to the manufacturer’s instructions. NCI-H292 and A549 cells were seeded into 6-well plates and treated with varying concentrations of Se-enriched Cordyceps militaris (0, 12.5, 25, and 50 mg/mL for A549 cells, and 0, 4, 8, and 12 mg/mL for NCI-H292 cells). The centrifuged NCI-H292 and A549 cells were collected for apoptosis analysis and washed with ice-cold phosphate-buffered saline, and then stained with (FITC)-Annexin V and propidium iodide (PI). The apoptosis rate of NCI-H292 and A549 cells was measured and analyzed using Annexin V-FITC/PI apoptosis detection kit (Beyotime Biotechnology, Shanghai, People’s Republic of China).
Cell Cycle Analysis
NCI-H292 and A549 cells were seeded into 6-well plates and treated with varying concentrations of Se-enriched Cordyceps militaris (0, 12.5, 25, and 50 mg/mL for A549 cells, and 0, 4, 8, and 12 mg/mL for NCI-H292 cells). NCI-H292 and A549 cells were fixed with 70% ethanol. Then cells were incubated with RNA enzyme A (0.5 mg/mL) and PI (0.1 mg/mL), and measured by a flow cytometry (BD Biosciences, San Jose, CA, USA). The percentage of cells in the G0/G1, S, and G2/M phases were evaluated using Cell Quest acquisition software (BD Biosciences, USA).
Hoechst 33342 Staining Analysis
NCI-H292 and A549 cells were planted into 6-well plates at a density of 1×106 cells/well, and then treated with different concentrations of Se-enriched Cordyceps militaris water extract (0, 12.5, 25, and 50 mg/mL for A549 cells, and 0, 4, 8, and 12 mg/mL for NCI-H292 cells). After incubation for 24 hrs, the clear supernatant extract was supplemented with 1mg/mL of Hoechst 33342 and incubated in the dark for 30 mins. Nuclei were taken using a fluorescence microscope.
RNA Isolation And qPCR Analysis
Total RNA was extracted from NCI-H292 and A549 cells treated with varying concentrations of Se-enriched Cordyceps militaris (0, 12.5, 25, and 50 mg/mL for A549 cells, and 0, 4, 8, and 12 mg/mL for NCI-H292 cells) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacture’s instruction. 1 μL of total RNA was used to synthesize complementary DNA (cDNA) using the RevertAidTM H Minus First Strand cDNA synthesis Kit (Takara, Otsu, Japan). qPCR analysis was conducted using SYBR PrimerScript RT-PCR kit (Takara, Biotechnology, Inc., Dalian, People’s Republic of China) on a CFX Connect™ Real-Time PCR Detection System (BIO-RAD Laboratories, Inc., California, USA). GAPDH was used as the internal control. Fold changes of genes were calculated by the relative quantification (2−ΔΔCt) method. The sequences of the primers were listed as follows: cyclin B1, 5ʹ-CCCCTGCAGAAGAAGACCTG-3ʹ (forward) and 5ʹ-TGACTGCTTGCTCTTCCTCA-3ʹ (reverse); CDK1, 5ʹ-CCCTTTAGCGCGGATCTACC-3ʹ (forward) and 5ʹ-CATGGCTACCACTTGACCTGT-3ʹ (reverse); BCL-2, 5ʹ-CCGCGACTCCTGA TTCATTG-3ʹ (forward) and 5ʹ-AGTCTACTTCCTCTGTGATGTTGTA-3ʹ (reverse); BAX, 5ʹ-AAACTGGTGCTCAAGGCCC-3ʹ (forward) and 5ʹ-AAAGTAGGAGAGGAGGCCGT-3ʹ (reverse); GAPDH, 5ʹ-ACGGATTTGGTCGTATTGGGCG-3ʹ (forward) and 5ʹ-GCTCCTGGAAGATGGTGATGGG-3ʹ (reverse).
Western Blot Analysis
After treated with varying concentrations of Se-enriched Cordyceps militaris (0, 12.5, 25, and 50 mg/mL for A549 cells, and 0, 4, 8, and 12 mg/mL for NCI-H292 cells), NCI-H292 and A549 cells were lysed with lysis buffer (Beyotime Biotechnology, Shanghai, People’s Republic of China) in the presence of the protease inhibitors (Roche, Basel, Switzerland). Equal amounts of protein from each sample were electrophoresed in 12% SDS-PAGE gel, and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Subsequently, PVDF membranes were blocked in 1 × tris-buffered saline containing 0.1% Tween-20 (TBST) with 5% skim milk for 1 h. PVDF membranes were incubated with different primary antibodies (cyclin B1, CDK1, BCL-2, BAX, 1:1000, ABclonal) at 4ºC overnight, followed by the corresponding secondary antibody (goat anti-mouse/anti-rabbit, 1:10,000, ABclonal) labeled with horseradish peroxidase (HRP) at 37°C for 1 h. GAPDH was used as the internal control. Signals were visualized by an enhanced ECL kit (Pierce, Rockford, IL, USA).
Statistical Analysis
Each experiment was independently conducted in triplicate and all data were presented as mean ± standard deviation (SD). The differences among multiply groups (>2) were analyzed by one-way ANOVA. P value less than 0.05 was considered statistical significance.
Results
Identification Of Inhibitory Concentration (IC50) Of Se-Enriched Cordyceps militaris In NSCLC Cells
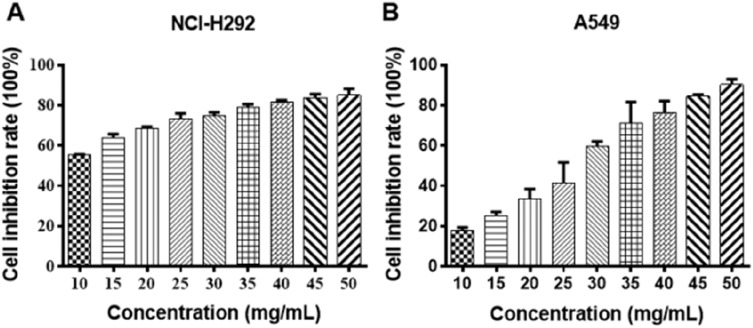
NCI-H292 and A549 cells were treated with different concentrations of Se-enriched Cordyceps militaris (5, 10, 15, 20, 25, 30, 35, 40, 45, 50 mg/mL). As displayed in Figure 1A and B, the cell inhibition rate of both NCI-H292 and A549 cells were increased caused by the increment of concentrations of Se-enriched Cordyceps militaris. The IC50 value (the concentration at which 50% inhibition of cell growth was achieved) of Se-enriched Cordyceps militaris in NCI-H292 and A549 cells were 8.409 mg/mL and 25.02 mg/mL, respectively. Based on the above results, we determined the concentration of Se-enriched Cordyceps militaris used in the following experiments. Concretely, for NCI-H292 cells, the concentration of Se-enriched Cordyceps militaris was 0, 4, 8, 12 mg/mL; for A549 cells, the concentration of Se-enriched Cordyceps militaris was 0, 12.5, 25, 50 mg/mL.
Figure 1.
The effects of Se-enriched Cordyceps militaris on cell inhibition rate of NCI-H292 and A549 cells.
Notes: (A) With the increased concentrations of Se-enriched Cordyceps militaris, both NCI-H292 and (B) A549 cells showed the gradual enhancement in the cell inhibition rate.
Abbreviation: Se, selenium.
Se-Enriched Cordyceps militaris Represses Cell Proliferation In NSCLC Cells
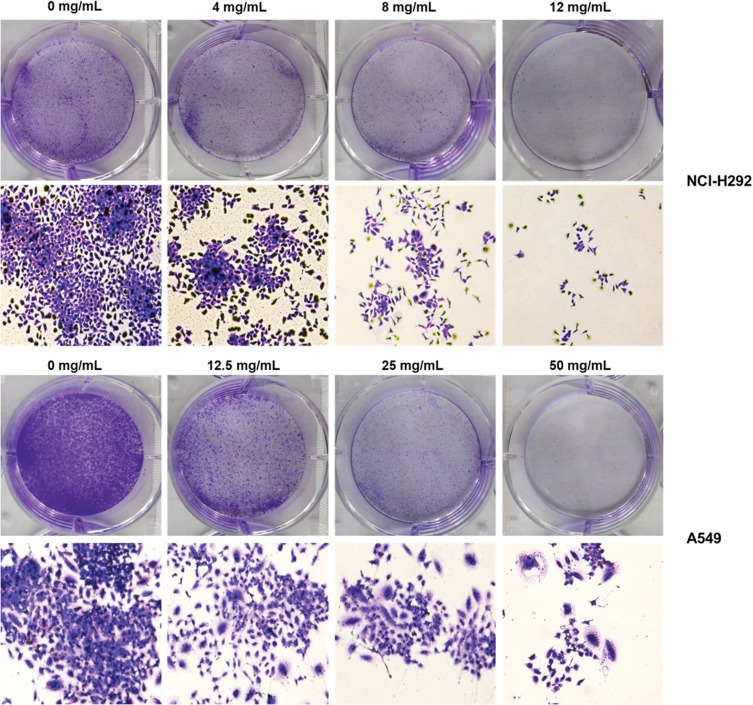
To evaluate the cell proliferation of NCI-H292 and A549 cells treated by different concentrations of Se-enriched Cordyceps militaris, colony formation assay was conducted. The results obtained from colony formation assay exhibited the reduction of the colony formation number in both NCI-H292 and A549 cells after treated with the increased concentrations of Se-enriched Cordyceps militaris (Figure 2), suggesting the inhibitory role of Se-enriched Cordyceps militaris in cell proliferation in NSCLC cells.
Figure 2.
The effects of Se-enriched Cordyceps militaris on cell proliferation of NCI-H292 and A549 cells. The colony formation number were reduced in both NCI-H292 and A549 cells caused by the increased concentrations of Se-enriched Cordyceps militaris.
Abbreviation: Se, selenium.
Se-Enriched Cordyceps militaris Induces Cell Apoptosis In NSCLC Cells
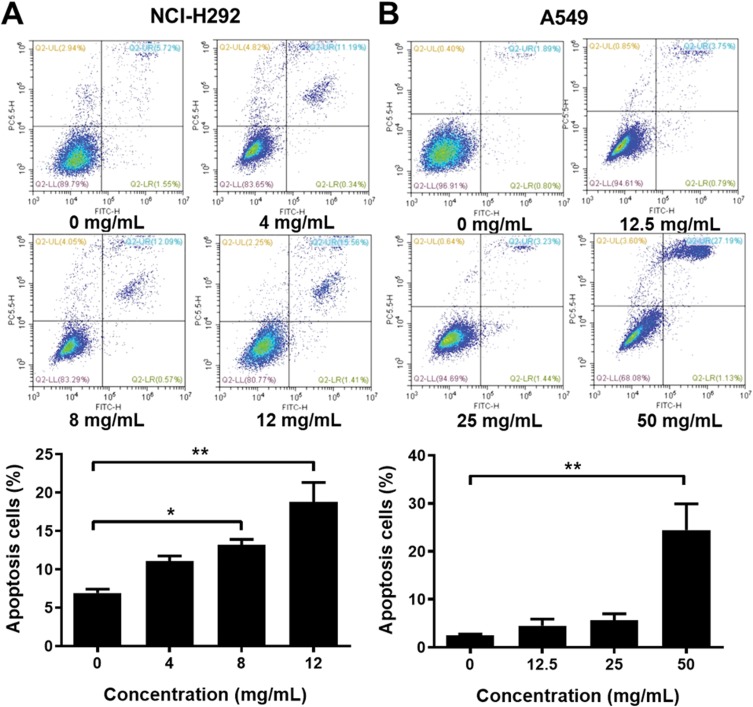
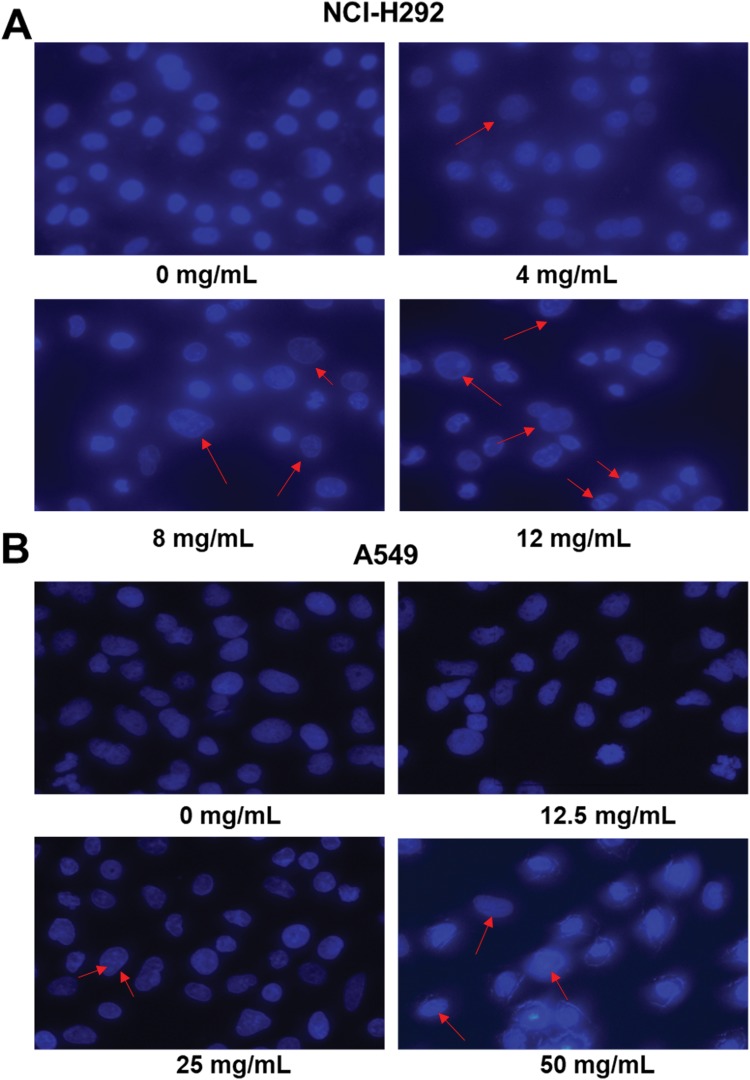
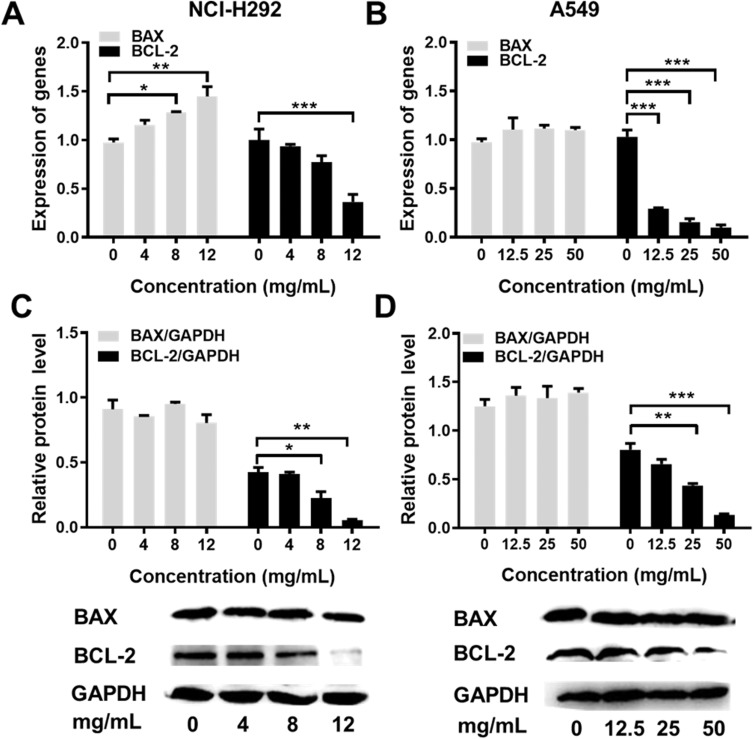
To analyze the cell apoptosis rate of NCI-H292 and A549 cells treated by different Se-enriched Cordyceps militaris, flow cytometry analysis was firstly performed. As shown in Figure 3A and B, the apoptosis rate of both NCI-H292 and A549 cells was gradually increased with the increase of concentration of Se-enriched Cordyceps militaris. For NCI-H292 cells, the apoptosis rate was 7.27% at 0 mg/mL, 11.53% at 4 mg/mL, 12.66% at 8 mg/mL, 16.97% at 12 mg/mL. Specially, the apoptosis rate was evidently raised when NCI-H292 cells were treated by Se-enriched Cordyceps militaris at a concentration of 8 or 12 mg/mL (Figure 3A, P<0.05, P<0.01). For A549 cells, the apoptosis rate was 2.69% at 0 mg/mL, 4.54% at 12.5 mg/mL, 4.67% at 25 mg/mL, 28.32% at 50 mg/mL. Specially, the apoptosis rate showed a significant increase when A549 cells were treated by Se-enriched Cordyceps militaris at a concentration of 12 mg/mL (Figure 3B, P<0.01). To further confirm the effects of Se-enriched Cordyceps militaris on cell apoptosis of NCI-H292 and A549 cells, Hoechst 33342 staining analysis was conducted. Likewise, the gradually increased cell apoptosis was observed in both NCI-H292 and A549 cells treated with varying concentrations of Se-enriched Cordyceps militaris (Figure 4). In addition, the effects of Se-enriched Cordyceps militaris on the expression of pro-apoptotic member BAX and the anti-apoptotic member BCL-2 were also analyzed by qPCR and Western blot assays. Generally, the ratio of expression levels of BCL-2 and BAX indicates the cell apoptosis. As shown in Figure 5A and B, the expression of BCL-2 at mRNA level was gradually reduced as the concentration of Se-enriched Cordyceps militaris increased in both NCI-H292 and A549 cells. The expression of BAX at mRNA level was increased mildly as the concentration of Se-enriched Cordyceps militaris increased in both NCI-H292 and A549 cells (Figure 5A and B). As for their protein level, BCL-2 expression presented an obvious decrease when cells were treated with 8 or 12 mg/L of Se-enriched Cordyceps militaris while BAX expression changed little at the same condition (Figure 5C and D). It followed that the ratio of BCL-2/BAX at both mRNA and protein levels was reduced as the concentration of Se-enriched Cordyceps militaris increased in both NCI-H292 and A549 cells, suggesting the promoted cell apoptosis. Taken together, these results revealed that Se-enriched Cordyceps militaris could induce cell apoptosis in NSCLC cells.
Figure 3.
The effects of Se-enriched Cordyceps militaris on NSCLC cell apoptosis analyzed by the flow cytometry analysis.
Notes: (A) The photos (upper panel) and quantitative analyses (lower panel) of the effects of Se-enriched Cordyceps militaris on cell apoptosis of NCI-H292 and (B) A549. *P<0.05; **P<0.01.
Abbreviations: Se, selenium; NSCLC, non-small cell lung cancer.
Figure 4.
The effects of Se-enriched Cordyceps militaris on NSCLC cell apoptosis analyzed by the Hoechst 33342 staining assay.
Notes: (A) The gradually increased cell apoptosis was observed in both NCI-H292 and (B) A549 cells caused by the increment of concentrations of Se-enriched Cordyceps militaris. The red arrows indicate nuclear condensation or apoptosis.
Abbreviations: Se, selenium; NSCLC, non-small cell lung cancer.
Figure 5.
The effects of Se-enriched Cordyceps militaris on the expressions of BAX and BCL-2.
Notes: (A) The mRNA expression level of BAX and BCL-2 were detected by qPCR assay in NCI-H292 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 4, 8, and 12 mg/mL) (B) and in A549 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 12.5, 25, and 50 mg/mL). (C) The protein expression level of BAX and BCL-2 were detected by Western blot assay in NCI-H292 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 4, 8, and 12 mg/mL) (D) and in A549 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 12.5, 25, and 50 mg/mL). *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: Se, selenium; qPCR, quantitative polymerase chain reaction.
Se-Enriched Cordyceps militaris Causes Cell-Cycle Arrest At G2/M Phase NSCLC Cells
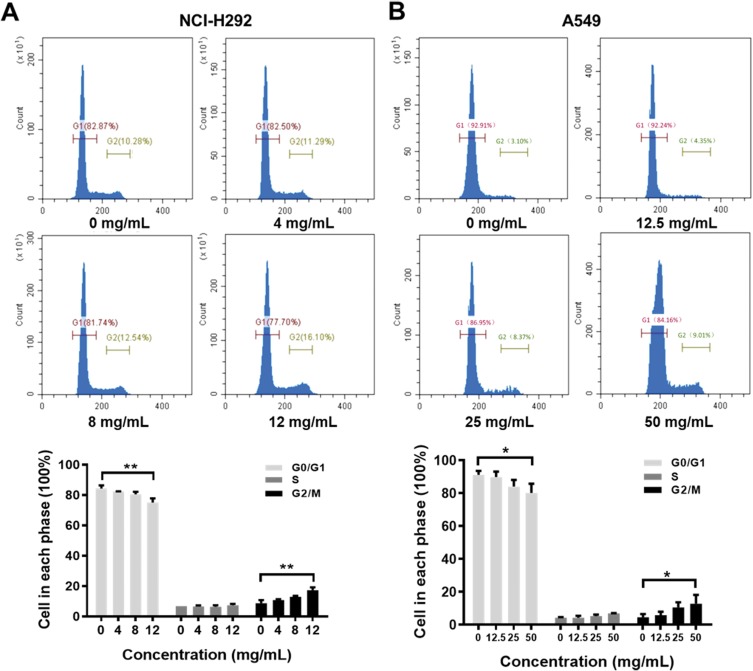
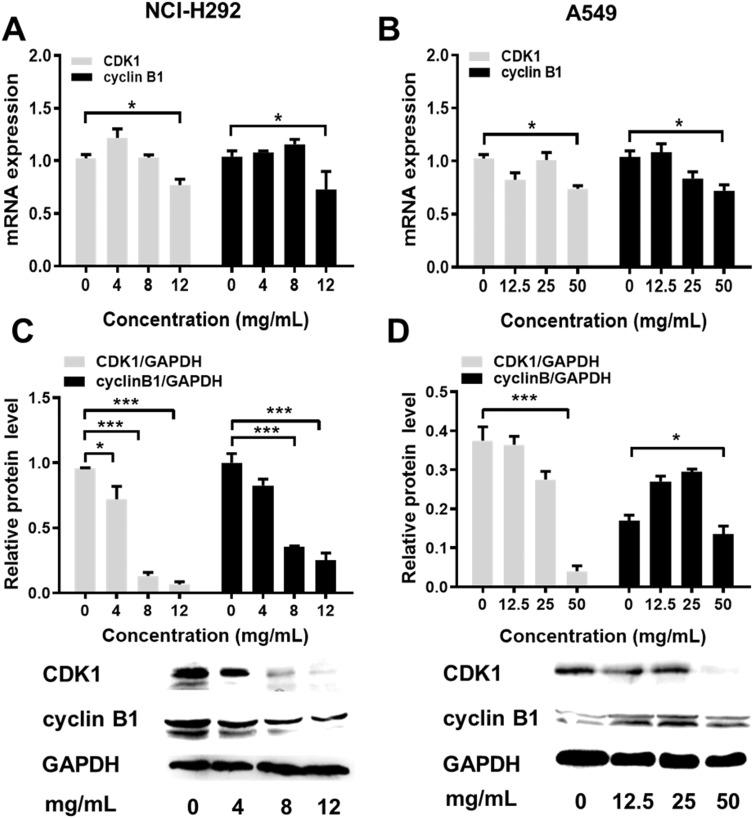
Flow cytometric analysis was performed to assess the effects of Se-enriched Cordyceps militaris on cell-cycle distribution in NSCLC cells. As shown in Figure 6A and B, the proportion of G0/G1 phase were reduced in NCI-H292 and A549 cells, ranging from 82.87% to 77.10% and from 92.91% to 84.16%, respectively. However, the proportion of G2/M phase was increased in NCI-H292 and A549 cells, ranging from 10.28% to 16.10% and from 3.10% to 9.01%, respectively (Figure 6A and B). Next, the effects of Se-enriched Cordyceps militaris on the expression of G2/M cell cycle regulatory proteins CDK1 and cyclin B1 were analyzed by qPCR and Western blot assays. As for the mRNA level, CDK1 and cyclin B1 expressions were significantly diminished in NCI-H292 and A549 cells treated with a 12 mg/mL of Se-enriched Cordyceps militaris (Figure 7A and B, P<0.05). Similar results were obtained in the case of the expressions of CDK1 and cyclin B1 at protein level (Figure 7C and D, P<0.05, P<0.01, P<0.001). Collectively, these results indicate the involvement of Se-enriched Cordyceps militaris in G2/M cell cycle arrest in NSCLC cells.
Figure 6.
The effects of Se-enriched Cordyceps militaris on cell cycle of NSCLC cells analyzed by flow cytometric analysis.
Notes: (A) The photos (upper panel) and quantitative analyses (lower panel) of the effects of Se-enriched Cordyceps militaris on cell cycle of NCI-H292. The proportion of G0/G1 and G2/M phase ranged from 82.87% to 77.10% and from 10.28% to 16.10% in NCI-H292 cells, respectively. (B) The photos (upper panel) and quantitative analyses (lower panel) of the effects of Se-enriched Cordyceps militaris on cell cycle of A549. The proportion of G0/G1 and G2/M phase ranged from 92.91% to 84.16% and from 3.10% to 9.01% in A549 cells, respectively. *P<0.05; **P<0.01.
Abbreviations: Se, selenium; NSCLC, non-small cell lung cancer.
Figure 7.
The effects of Se-enriched Cordyceps militaris on the expressions of G2/M cell cycle regulatory proteins CDK1 and cyclin B1.
Notes: (A) The mRNA expression level of CDK1 and cyclin B1 were detected by qPCR assay in NCI-H292 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 4, 8, and 12 mg/mL) (B) and in A549 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 12.5, 25, and 50 mg/mL). (C) The protein expression level of CDK1 and cyclin B1 were detected by Western blot assay in NCI-H292 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 4, 8, and 12 mg/mL) (D) and in A549 cells treated with Se-enriched Cordyceps militaris at varied concentrations (0, 12.5, 25, and 50 mg/mL). *P<0.05; ***P<0.001.Abbreviations: Se, selenium; qPCR, quantitative polymerase chain reaction.
Discussion
Despite the treatments of chemotherapy and radiotherapy for NSCLC are effective, chemo/radiotherapy resistances and some severe side effects always occur.20 In recent years, the potential anti-cancer effects of Chinese Herbal Medicine in tumors have been studied. Because of the effectiveness of herbal medicines with few severe side effects, their application in cancer therapy is now being reconsidered.21 You et al have uncovered that a Chinese Herbal Medicine Baicalin represses cell proliferation, migration, invasion, and induces cell apoptosis in NSCLC cells.22 Liu et al have found that treatment with Chinese Medicine may prolong the survival rate of NSCLC patients.23 The anti-tumor effect of Cordyceps militaris was associated with an induction of cell cycle arrest and mitochondrial-mediated apoptosis.24 In this study, we investigated the anti-cancer effect of Se-enriched Cordyceps militaris in human NSCLC NCI-H292 and A549 cells.
Colony formation assay indicated that Se-enriched Cordyceps militaris plays an important role in the reduction of cell proliferation in NCI-H292 and A549 cells. Many anticancer drugs could target cell apoptosis to induce the death of cancer cells.25 Flow cytometric assay and Hoechst staining analysis demonstrated the promotive efficacy of Se-enriched Cordyceps militaris in NSCLC cell apoptosis. The pro-apoptotic member BAX and the anti-apoptotic member BCL-2 belong to the BCL family.6 BCL-2 could prevent the release of cytochrome c, while the activation of BAX leads to the release of cytochrome C into the cytosol, resulting in activation of caspases 9 and 3, effector caspases, in the mitochondrial pathway of cell apoptosis.25,26 In this study, we found that the ratio of BCL-2/BAX at both mRNA and protein levels was reduced as the concentration of Se-enriched Cordyceps militaris increased in both NCI-H292 and A549 cells, indicating the pro-apoptotic role of Se-enriched Cordyceps militaris in NSCLC cells.
Cell cycle disorder is the main contributor to the formation of tumors.27 The effects on cell cycle progression are one important part of the anti-tumor function of drugs. The normal cell cycle operates in chronological strict order through the G1-S-G2-M phases.28 We found that Se-enriched Cordyceps militaris triggered the changes of cell cycle distribution in NCI-H292 and A549 cells. Cyclin B1 and CDK1 are G2/M cell cycle regulatory proteins.27 The percentage of G2/M phase was increased, and the decreased expression levels of cyclin B1 and CDK1 were obtained in NCI-H292 and A549 cells with Se-enriched Cordyceps militaris. Conjointly, Se-enriched Cordyceps militaris are involved in cell apoptosis and G2/M phase cell cycle arrest in NSCLC cells.
Cordycepin is the major bioactive ingredient of the traditional Chinese medicine herbs Cordyceps sinensis and Cordyceps militaris.29 The anticancer and antimetastatic effects of Cordycepin have been revealed.30 Notably, Cordycepin could repress cell proliferation, migration and invasion as well as tumor metastasis through various signaling pathways, and therefore plays an inhibitory role in various cancers.31–33 The apoptotic effect of Cordycepin has also been revealed in many human cancer cell lines, including ovarian cancer cells,34 pancreatic cancer,25 and renal carcinoma cells.35 The anti-cancer effects of Se have been revealed via involving in cell proliferation and cell invasion.16 Thus, we speculate the importance of combination of Se and Cordycepin in the biological function of Se-enriched Cordyceps militaris, which requires further study. This study investigated the role of Se-enriched Cordyceps militaris in NSCLC cells. It is worth noting that the anti-cancer efficacy between Se-enriched Cordyceps militaris and normal Cordyceps militaris is absent in this work. Cordyceps sinensis has been considered to have anti-tumor effects for many years. However, more recently, literature has emerged that offers contradictory findings that Cordyceps sinensis does not contain cordycepin,36 suggesting it may not have anti-tumor effect. We did not compare the anti-cancer efficacy between Se-enriched Cordyceps militaris and normal Cordyceps militaris because of the conflicts of interest between different commercial varieties. Our work indicated that Se-enriched Cordyceps militaris, as an analogue of Cordyceps sinensis, can be regarded as a kind of health-care plant, supported by its direct lethal effect on tumor cells to some extent in vitro. Despite these promising results, the question remains. Studies relevant to the soluble candidates in the water extract of Se-enriched Cordyceps militaris should be further carried out to evaluate the effective ingredients. In addition, the role of Se-enriched Cordyceps militaris and its functional mechanism in other tumors also requires further investigation.
Conclusion
To conclude, these data indicated that Se-enriched Cordyceps militaris could suppress the viability and induce apoptosis and G2/M phase arrest in NSCLC cell lines, and therefore Se-enriched Cordyceps militaris might be an appealing potential therapeutic agent for NSCLC treatment.
Acknowledgment
We thank the technical support and guidance provided by Wuhan Bojie Biomedical Science and Technology CO., LTD.
Funding Statement
This study is supported by the National Natural Science Foundation of China (no. 81874061) and Enshi Prefecture Science and Technology Bureau Key Support Project (no.XYJ2016000154).
Abbreviations
Se, selenium; SCLC, small cell lung cancer; non-SCLC, non-small cell lung cancer; CCK-8, cell counting kit-8; OD, optical density; PVDF, polyvinylidene difluoride; qPCR, quantitative polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SD, standard deviation.
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Piperdi B, Merla A, Perez-Soler R. Targeting angiogenesis in squamous non-small cell lung cancer. Drugs. 2014;74(4):403–413. doi: 10.1007/s40265-014-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su X, Li Y, Jiang M, et al. Systems pharmacology uncover the mechanism of anti-non-small cell lung cancer for Hedyotis diffusa Willd. Biomed Pharmacother. 2019;109:969–984. doi: 10.1016/j.biopha.2018.10.162 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 5.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology. 2015;20(3):370–378. doi: 10.1111/resp.12490 [DOI] [PubMed] [Google Scholar]
- 6.Lee HH, Lee S, Lee K, Shin YS, Kang H, Cho H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J Pharm Sci. 2015;23:35. doi: 10.1186/s40199-015-0117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng P, Xia Y, Zhang S, Wang C. Genetics of Cordyceps and related fungi. Appl Microbiol Biotechnol. 2013;97(7):2797–2804. doi: 10.1007/s00253-013-4771-7 [DOI] [PubMed] [Google Scholar]
- 8.Yue GG, Lau CB, Fung KP, Leung PC, Ko WH. Effects of Cordyceps sinensis, Cordyceps militaris and their isolated compounds on ion transport in Calu-3 human airway epithelial cells. J Ethnopharmacol. 2008;117(1):92–101. doi: 10.1016/j.jep.2008.01.030 [DOI] [PubMed] [Google Scholar]
- 9.Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–493. doi: 10.1111/j.2042-7158.2012.01601.x [DOI] [PubMed] [Google Scholar]
- 10.Park SE, Yoo HS, Jin CY, et al. Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem Toxicol. 2009;47(7):1667–1675. doi: 10.1016/j.fct.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Reis FS, Barros L, Calhelha RC, et al. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 12.Bizarro A, Ferreira IC, Sokovic M, et al. Cordyceps militaris (L.) link fruiting body reduces the growth of a non-small cell lung cancer cell line by increasing cellular levels of p53 and p21. Molecules. 2015;20(8):13927–13940. doi: 10.3390/molecules200813927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinceti M, Grill P, Malagoli C, et al. Selenium speciation in human serum and its implications for epidemiologic research: a cross-sectional study. J Trace Elem Med Biol. 2015;31:1–10. doi: 10.1016/j.jtemb.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Hu T, Liang Y, Zhao G, Wu W, Li H, Guo Y. Selenium biofortification and antioxidant activity in Cordyceps militaris supplied with Selenate, Selenite, or Selenomethionine. Biol Trace Elem Res. 2019;187(2):553–561. doi: 10.1007/s12011-018-1386-y [DOI] [PubMed] [Google Scholar]
- 15.Chomchan R, Puttarak P, Brantner A, Siripongvutikorn S. Selenium-rich ricegrass juice improves antioxidant properties and nitric oxide inhibition in macrophage cells. Antioxidants (Basel). 2018;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng H, Combs GF Jr. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008;19(1):1–7. doi: 10.1016/j.jnutbio.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 17.Nan JX, Park EJ, Yang BK, Song CH, Ko G, Sohn DH. Antifibrotic effect of extracellular biopolymer from submerged mycelial cultures of Cordyceps militaris on liver fibrosis induced by bile duct ligation and scission in rats. Arch Pharm Res. 2001;24(4):327–332. doi: 10.1007/bf02975101 [DOI] [PubMed] [Google Scholar]
- 18.Hileman CO, Dirajlal-Fargo S, Lam SK, et al. Plasma Selenium concentrations are sufficient and associated with protease inhibitor use in treated HIV-infected adults. J Nutr. 2015;145(10):2293–2299. doi: 10.3945/jn.115.214577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun T, Dong W, Jiang G, et al. Cordyceps militaris improves chronic kidney disease by affecting TLR4/NF-kappaB redox signaling pathway. Oxid Med Cell Longev. 2019;2019:7850863. doi: 10.1155/2019/7850863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang BH, Wang BC, Tong F, et al. Application of dynamic monitoring of genomic profiles in non-small cell lung cancer: a case report. Medicine (Baltimore). 2018;97(51):e13192. doi: 10.1097/MD.0000000000013192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wada T, Sumardika IW, Saito S, et al. Identification of a novel component leading to anti-tumor activity besides the major ingredient cordycepin in Cordyceps militaris extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061–1062:209–219. doi: 10.1016/j.jchromb.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 22.You J, Cheng J, Yu B, Duan C, Peng J. Baicalin, a Chinese herbal medicine, inhibits the proliferation and migration of human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med Sci Monit. 2018;24:2126–2133. doi: 10.12659/msm.909627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Lin HS, Hou W, et al. Comprehensive treatment with Chinese medicine in patients with advanced non-small cell lung cancer: a multicenter, prospective, cohort study. Chin J Integr Med. 2017;23(10):733–739. doi: 10.1007/s11655-016-2737-1 [DOI] [PubMed] [Google Scholar]
- 24.Xie H, Li X, Chen Y, Lang M, Shen Z, Shi L. Ethanolic extract of Cordyceps cicadae exerts antitumor effect on human gastric cancer SGC-7901 cells by inducing apoptosis, cell cycle arrest and endoplasmic reticulum stress. J Ethnopharmacol. 2019;231:230–240. doi: 10.1016/j.jep.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhang XX, Yuan RY, et al. Cordycepin induces apoptosis in human pancreatic cancer cells via the mitochondrial-mediated intrinsic pathway and suppresses tumor growth in vivo. Onco Targets Ther. 2018;11:4479–4490. doi: 10.2147/OTT.S164670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cain K, Brown DG, Langlais C, Cohen GM. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J Biol Chem. 1999;274(32):22686–22692. doi: 10.1074/jbc.274.32.22686 [DOI] [PubMed] [Google Scholar]
- 27.Fu XJ, Li HX, Yang K, Chen D, Tang H. The important tumor suppressor role of PER1 in regulating the cyclin-CDK-CKI network in SCC15 human oral squamous cell carcinoma cells. Onco Targets Ther. 2016;9:2237–2245. doi: 10.2147/OTT.S100952 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8(6):832–837. doi: 10.4161/cc.8.6.7869 [DOI] [PubMed] [Google Scholar]
- 29.Holbein S, Wengi A, Decourty L, Freimoser FM, Jacquier A, Dichtl B. Cordycepin interferes with 3ʹ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA. 2009;15(5):837–849. doi: 10.1261/rna.1458909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127(1):53–56. doi: 10.1016/j.jphs.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Jeong JW, Jin CY, Park C, et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int J Oncol. 2012;40(5):1697–1704. doi: 10.3892/ijo.2012.1332 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Yang SH, Hueng DY, Syu JP, Liao CC, Wu YC. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem Biol Interact. 2014;216:17–25. doi: 10.1016/j.cbi.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 33.Pan BS, Wang YK, Lai MS, Mu YF, Huang BM. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci Rep. 2015;5:13372. doi: 10.1038/srep13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui ZY, Park SJ, Jo E, et al. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-kappaB signaling pathway. Cell death discovery. 2018;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang IH, Oh SY, Jang HJ, et al. Cordycepin promotes apoptosis in renal carcinoma cells by activating the MKK7-JNK signaling pathway through inhibition of c-FLIPL expression. PloS ONE. 2017;12(10):e0186489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia Y, Luo F, Shang Y, Chen P, Lu Y, Wang C. Fungal Cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem Biol. 2017;24(12):1479–1489.e1474. doi: 10.1016/j.chembiol.2017.09.001 [DOI] [PubMed] [Google Scholar]