Abstract
Emergency clinicians are on the front lines of responding to the opioid epidemic and are leading innovations to reduce opioid overdose deaths through safer prescribing, harm reduction, and improved linkage to outpatient treatment. Currently, there are no nationally recognized quality measures or best practices to guide emergency department quality improvement efforts, implementation science researchers, or policymakers seeking to reduce opioid-associated morbidity and mortality. To address this gap, in May 2017, the National Institute on Drug Abuse’s Center for the Clinical Trials Network convened experts in quality measurement from the American College of Emergency Physicians’ (ACEP’s) Clinical Emergency Data Registry, researchers in emergency and addiction medicine, and representatives from federal agencies, including the National Institute on Drug Abuse and the Centers for Medicare & Medicaid Services. Drawing from discussions at this meeting and with experts in opioid use disorder treatment and quality measure development, we developed a multistakeholder quality improvement framework with specific structural, process, and outcome measures to guide an emergency medicine agenda for opioid use disorder policy, research, and clinical quality improvement.
INTRODUCTION
Opioid overdose deaths in the United States have nearly doubled in the last decade.1 In 2016, more than 42,000 people died from an opioid overdose, the number of deaths surpassing that from motor vehicle crashes.2 Emergency departments (EDs) are on the front line of the overdose epidemic, caring for increasing numbers of opioid overdose patients. From 2016 to 2017, ED visits for opioid overdose increased 30% nationwide1,3,4; however, a minority of opioid-related ED visits result in linkage to treatment.5–7
The ED’s role in the opioid overdose epidemic is often mischaracterized. Although there are certainly opportunities for improvement in ED opioid prescribing practices, EDs account for a small and declining proportion of overall opioid prescriptions.8–10 EDs are also leading innovative policies and practices to end the overdose epidemic with ED initiatives in harm reduction and opioid use disorder treatment initiation and linkage to care.9–11 As a key point of access to medical care and the primary provider of acute care diagnostic and unscheduled care, the ED is well positioned to improve care quality and outcomes for patients with opioid use disorder.12
Quality measurement and quality improvement initiatives can standardize practice, reduce care inequities,13,14 accelerate implementation and adoption, and improve patient outcomes and resource use.15 Although there is a well-identified need to improve quality of care for patients with opioid use disorder nationally,16 there is a gap between available research evidence and adoption into clinical practice.17 There are some state-18,19 and professional-organization-established best practices20,21 for opioid use disorder primary prevention, treatment, and harm reduction, but there are currently no nationally recognized ED opioid use disorder quality measures or universal quality improvement models for adoption. Care for opioid use disorder is often fragmented, which exacerbates health inequities, produces poor patient outcomes, and wastes resources. To date, most research examining ED opioid use disorder interventions have been institution or state specific,22 in controlled clinical trials,23,24 or limited to feasibility and use evaluations.25,26 Research examining the effect of ED opioid use disorder interventions on patient outcomes is needed to guide quality improvement strategies to reduce overdose deaths and improve linkage to treatment.
Given the paucity of indicators and measures available to guide ED opioid use disorder quality improvement initiatives, in May 2017, the National Institute on Drug Abuse’s (NIDA’s) Center for the Clinical Trials Network convened experts in quality measurement from the American College of Emergency Physicians’ (ACEP’s) Clinical Emergency Data Registry, researchers in emergency and addiction medicine, and representatives from federal agencies, including NIDA and the Centers for Medicare & Medicaid Services (CMS). Drawing from discussions at this meeting and experts in opioid use disorder and quality measure development, we developed a multistakeholder quality framework to guide opioid use disorder emergency care policy, research, and clinical quality improvement programs.
THE ROLE OF THE ED IN ADVANCING QUALITY FOR OPIOID USE DISORDER
During the last 50 years, the ED has evolved to serve as a universal point of medical access, diagnosis, treatment, and linkage to definitive care. Broadly, the ED provides 3 basic functions encompassing a spectrum of patient care (Table 1). First, EDs specialize in the identification, stabilization, and treatment of time-sensitive conditions such as sepsis, stroke, acute myocardial infarction, trauma,27 and toxic ingestions, including opioid overdose. Quality improvement initiatives that have successfully improved emergency care quality and patient outcomes in these domains include door-to-balloon time for acute myocardial infarction,28–30 early goal-directed therapy for sepsis,31–33 and door-to-needle time for stroke.34–36 There are currently no agreed-on standards of care after acute opioid overdose.
Table 1.
Emergency medicine work domains and associated quality indicators: 3 domains of ED clinical care, clinical examples with associated quality care indicators, and opioid use disorder corollaries.
| Emergency Department Work Domain | Clinical Example | Emergency Care Quality Indicators | Application to OUD |
|---|---|---|---|
| Time-sensitive treatment and stabilization | Acute myocardial infarction | Door-to-balloon time | Overdose reversal and clinical stabilization Treatment of severe withdrawal |
| Stroke | Door-to-needle time Door-to-CT time |
||
| Sepsis | Initial sepsis bundle compliance | ||
| Trauma | Appropriate head CT use Protocolized trauma care |
||
| Acute diagnostic center | Chest pain | Timely ECG and cardiac biomarkers | Assessment of undifferentiated altered mental status |
| Abdominal pain | hCG testing in women | Identification of OUD | |
| Headache | Appropriate head CT use | ||
| Interpersonal violence | Intimate partner violence screening | Treatment of opioid withdrawal | |
| Sexual assault | Sexual assault nurse examiner evaluation | ||
| STI testing | STI testing and treatment | ||
| Health care access and treatment linkage | Diabetes | Initiation of antihyperglycemics | |
| Hypertension | Home medication adjustment | Referral to OUD treatment | |
| Early pregnancy | Linkage to reproductive health care provider | ||
| Tobacco use | Provision of smoking cessation resources | Harm reduction (naloxone, | |
| Suicidal ideation | Linkage to psychiatric services | overdose education, syringe access) | |
| Child and elder abuse | Mandatory reporting |
hCG; Human chorionic gonadotropin; OUD, opioid use disorder.
Second, the ED is a center for acute diagnostic testing, where emergency testing is conducted to rule out life-threatening illness or injury in circumstances of diagnostic uncertainty. Common examples of quality indicators for these types of clinical encounters include timely ECG and cardiac enzyme testing to evaluate patients for acute coronary syndromes and appropriate use of head computed tomography (CT) for head trauma (Table 1). The opioid use disorder corollaries in this treatment domain are commonly encountered but lack standardized approaches or indicators of quality, namely, the identification of opioid use disorder and treatment of acute opioid withdrawal.37
Third, the ED is a critical point of access to the health care system and providing linkage to definitive treatment.38 If and when a diagnosis is made in the ED, providers commonly initiate treatment and link patients to definitive care in the form of hospitalization or referral to outpatient providers.39 In the setting of newly diagnosed pregnancy, for example, the ED links patients to appropriate obstetrics follow-up (Table 1). In instances of newly diagnosed hyperglycemia without diabetic ketoacidosis, the ED often initiates antihyperglycemics and facilitates referral to primary care. Similarly, for under- or untreated hypertension, providers may adjust a patient’s antihypertensive medications in coordination with his or her primary care provider or link patients to a community primary care provider for follow-up.38,40 The ED function as an access point to the health care system is especially true for opioid use disorder, but despite evidence supporting the efficacy of ED-initiated treatment for opioid use disorder and linkage to outpatient treatment,41,42 linkage to opioid use disorder treatment has yet to become standard of care in many EDs. This may be in part due to stigma, lack of available outpatient addiction medicine resources, or gaps in provider knowledge and training. However, because the ED encounter may be an individual’s only contact with the medical system, ED visits present an important opportunity to provide patients with opioid use disorder harm reduction services and linkage to definitive opioid use disorder treatment.37,43
FRAMEWORK DEVELOPMENT
Recognizing the crucial role of the ED in addressing the opioid overdose epidemic, in May 2017, the NIDA Center for the Clinical Trials Network invited experts in quality measurement from ACEP’s Clinical Emergency Data Registry, emergency medicine and addiction medicine researchers, and representatives from NIDA, CMS, and other federal agencies to a consensus meeting titled “Exploring Substance Use Relevant Measures in EHRs and Clinical Quality Measurement in Emergency Settings.” Thirty-four people attended the meeting and during the course of 2 days discussed current opioid use disorder quality measures in the electronic health record, electronic health record data science, quality measure development, CMS quality measure priorities, ways to leverage the ACEP Clinical Emergency Data Registry, emergency medicine substance use disorder research, and trends in emergency medicine opioid use disorder clinical practice.
A summary of the meeting proceedings was composed by NIDA and reviewed by a smaller group of meeting attendees that included quality, emergency medicine, and addiction researchers; representatives from NIDA; and 2 emergency medicine researchers who did not attend the meeting. Current opioid use disorder quality literature and the meeting proceeding summary were rereviewed by the study team. Through iterative discussions, the author team refined the meeting summary into the proposed opioid use disorder quality improvement framework. All items included in the framework were finalized by group consensus.
A QUALITY IMPROVEMENT FRAMEWORK
The development and implementation of quality improvement campaigns for emergency care conditions has yielded substantial improvements in patient outcomes, perhaps best exemplified in acute myocardial infarction and stroke. After national quality improvement campaigns for acute myocardial infarction, there was a 21% reduction in mortality from 1990 to 2006.44 Similarly, the adoption of the American Heart Association’s Get With the Guidelines for stroke has been associated with decreased mortality and improved functional status after hospital discharge.45,46 As with acute myocardial infarction and stroke, using a quality improvement framework, we can improve care and patient outcomes for the prevention, treatment, and reduction of harm associated with opioid use.
The Donabedian framework for quality measurement classifies indicators of quality into 3 basic categories: structural, process, and outcome measures.47 Structural measures assess a provider’s or institution’s capacity, systems, and processes.47 For acute myocardial infarction, for example, this would be the presence or absence of cardiac catheterization abilities. Process measures assess what a provider does, such as giving aspirin for chest pain, getting to the catheterization laboratory within 90 minutes of ED arrival for ST-elevation myocardial infarction, or administering broad-spectrum antibiotics in sepsis. Outcome measures assess the effect of the health service or intervention on the health of patients. For acute myocardial infarction, common outcome measures are cardiac function and survival, and in stroke, outcome measures are survival and functional status. Historically, researchers and policymakers have linked structural and process measures to population-level patient outcomes and identified structural and process measures that can be used for quality improvement. Clinicians and hospitals have subsequently implemented system changes to meet structural measure requirements and continually assess process measures. Integrated structure, process, and outcome measures may then be used to guide and define quality improvement and accountability programs that pay for performance. However, outcomes evaluated by quality improvement efforts are different from those used in the course of research. Quality measures, regardless of clinical focus, require several considerations, given the high financial stakes of pay-for-performance and public reporting programs. In the case of opioid use disorder, these considerations warrant particular attention.
First, all quality measures are designed for use at a specific “level of analysis,” meaning they are attributed to a certain type of provider or organization, clinical setting, or geography. Proposed ED opioid use disorder process measures focused on opioid prescribing may be well suited for measuring practice of individual clinicians while other process measures, such as naloxone distribution, may be better suited to an entire group of clinicians in an ED or hospital. Given the multiple organizational structures and resources necessary to affect naloxone distribution, both of which are largely outside the control of individual clinicians, a population-based level of analysis is often superior to measure many harm reduction interventions.
Second, attribution is a common challenge when measuring quality of a clinical entity with either multiple types of clinicians or non–health care influences, both of which hold true for opioid use disorder. Although outcome measures such as successful referral to treatment may be attributed to the care provided in an ED and may resonate with clinicians seeking concrete metrics for improvement, maintenance of medication for opioid use disorder is more attributable to available community resources and outpatient providers, rather than care delivered in the ED. Many of the outcome measures presented in Table 2 therefore may be better suited as community- or health-system-level measurements, of which the ED is just one contributor. Specifying quality metrics at the community and organizational level ensures accountability to improvement of overall health outcomes and supports investments at the organizational level and by the public sector for ED opioid use disorder quality improvement projects.
Table 2.
Quality measurement framework for ED treatment of opioid use disorder: structural, process, and ED and population health outcomes for opioid use disorder primary prevention, harm reduction, and treatment.
| Outcome Measures |
||||
|---|---|---|---|---|
| OUD Domain | Structural Measures | Process Measures | ED | Population |
| Primary prevention | Availability of nonopioid pain management | Patient education about opioid safe storage and disposal on discharge | Adverse events after ED discharge after receiving new opioids | Patients with unintended prolonged opioid use |
| PDMP-EMR integration | Trial of nonopioid analgesics before opioid initiation when indicated | ED revisitation for analgesia-associated adverse medication events | New OUD per-capita incidence | |
| “Safe prescribing” ED policies | Median days opioids prescribed | OUD prevalence | ||
| Median MME/day per ED visit | Opioid overdose incidence | |||
| Frequency of benzodiazepine and opioid coprescribing | ||||
| Harm reduction | ED naloxone distribution policy | Proportion ED OUD patients: a) Provision of overdose prevention and response patient education |
Risk-adjusted inhospital mortality for overdose | Risk-adjusted repeated nonfatal overdose |
| Community syringe access program | b) Discharged with/prescribed naloxone | Risk-adjusted repeated fatal overdose | ||
| c) Referred to a syringe access program | Risk-adjusted 30-day repeated ED visit for nonfatal opioid overdose | Risk-adjusted out-of-hospital overdose mortality | ||
| d) Referred to community resources | HCV and HIV incidence and prevalence | |||
| Buprenorphine-waivered providers (ED and community) | Structured screening and diagnostic questionnaires | Repeated ED visit rates for opioid overdose, opioid withdrawal, or complications of injection drug use | ||
| addiction medicine specialist consultation access | Urine toxicology testing | Opioid withdrawal scale at ED discharge | Proportion patients engaged in formal addiction treatment at 30 days | |
| Treatment | Availability of outpatient providers of medication for OUD | Proportion ED OUD patients: | Proportion patients maintaining receipt of medication for OUD at 30 days | |
| Community opioid treatment programs and providers | a) With initiated medication for OUD | Risk-adjusted inhospital mortality for overdose | Risk-adjusted repeated nonfatal overdose | |
| Hospital or community bridge clinics | b) Prescribed medication for OUD | Risk-adjusted repeated fatal overdose | ||
| c) Linked to outpatient OUD treatment | Risk-adjusted out-of-hospital overdosemortality | |||
| d) Counseled by health promotion advocates, counselors, or social workers | ||||
Third, when designing quality measures, researchers and quality measure developers must consider whether measures need to be risk adjusted. Many ED and community factors will significantly affect the treatment of ED opioid use disorder patients and patient outcomes. Factors affecting ED opioid use disorder care include workforce training and availability, outpatient addiction treatment availability, existing community resources, stigma, and state policies and regulations. Social determinants of health that contribute to opioid use disorder and overdose—specifically, housing, urbanicity, poverty, transportation, and employment—will affect opioid use disorder development and treatment access. To account for these variations in measure development, we will need to identify which measures will need to be risk adjusted and whether social risk factors should be adjusted for in risk models without masking important disparities.48
OPIOID USE DISORDER QUALITY MEASUREMENT
Taking considerations of level of measurement, attribution, and risk-adjustment into account, to evaluate and improve the quality of ED opioid use disorder primary prevention, treatment, and harm reduction, we must develop specific structural, process, and outcome measures (Table 2) that are feasible to implement and measure in the ED. Potential quality measures for opioid use disorder primary prevention, treatment, and harm reduction were identified, developed, and discussed with experts during the NIDA meeting. Some measures are specific to ED quality improvement, whereas others may be more suited for public reporting. Many identified patient outcomes are population-based measures that are affected by multiple factors in addition to treatment provided in the ED. Therefore, ED quality measures and quality improvement initiatives will largely focus on structural and process measures. Many of the measures identified and summarized in Table 2 have already been implemented in US EDs as part of local quality improvement initiatives; however, future research and programmatic evaluations are necessary to determine whether these are valid and reliable measures that can be used to improve the quality of opioid use disorder care and improve patient outcomes nationwide.
Opioid use disorder primary prevention focuses on decreasing excessive opioid prescribing, which has been shown to be associated with the development of opioid use disorder,49 and reducing coprescribing with benzodiazepines. Structural measures EDs and hospitals can implement are the development and expansion of nonopioid pain management services, such as regional anesthesia, the development and implementation of alternatives-to-opioids programs, and integration of prescription drug monitoring program databases within the electronic health record. Process measures include patient education on opioid use, storage, and disposal; trial of nonopioid analgesics before opioid initiation; and presence of and adherence to institutional “safe prescribing” policies. Safe prescribing policies would include limits on number of days prescribed,49 adherence to outpatient care plans for patients with outpatient pain contracts, not providing opioid refills for chronic pain, and avoidance of opioid and benzodiazepine coprescribing. Decreased opioid and benzodiazepine coprescribing to patients with opioid use disorder has been shown to have mortality benefit.50 ED-relevant quality measures in this domain may be focused on structural and process measures because patient outcomes, such as development of opioid use disorder, are distal in relation to the time of the ED visit. Population outcome measures for research, program, and policy evaluation in this arena include prevalence of unintended prolonged opioid use, incidence of new opioid use disorder per capita, and opioid use disorder prevalence.
Opioid use disorder harm reduction goals include reducing opioid overdoses and opioid overdose mortality. Structural measures within this domain include the ability to distribute naloxone or refer patients to a community syringe access program. Process measures would be the provision of patient education for overdose education and response, proportion of patients at risk of overdose with a harm reduction care plan, referral to outpatient services, and either given or prescribed naloxone at discharge. Harm reduction outcome measures for research, program, and policy evaluations include risk-adjusted repeat fatal and nonfatal overdose, risk-adjusted mortality, and hepatitis C and HIV incidence and prevalence. Given the psychosocial aspects of addiction and the criminalization of drug use, there are other broader patient outcomes outside of the ED quality framework that should be considered markers of higher-quality opioid use disorder treatment, including incarceration, employment, and education. Community harm reduction goals include these, as well as prevention and reduction of HIV and HCV transmission.
Opioid use disorder treatment goals include reduction of opioid overdose mortality and opioid overdose and increased engagement in addiction treatment. Structural measures for opioid use disorder treatment are the presence of ED buprenorphine-waivered providers, access to addiction medicine specialists for consultation, and community availability of medication for opioid use disorder and opioid treatment programs for outpatient referral. Other structural measures EDs could implement are evaluation by a patient navigator, such as a community health worker, peer navigator or coach, or social worker. Opioid use disorder treatment process measures, then, would assess the use of structured screening and diagnostic questionnaires,51 use of ED-outpatient opioid use disorder treatment care plans, urine toxicology testing, proportion of patients with opioid use disorder initiating medication for opioid use disorder, and proportion of patients linked to a program of community medication for opioid use disorder.52 The National Quality Forum and the Agency for Healthcare Research and Quality National Quality Measure Clearinghouse have specified similar process measures in regard to initiation of medication for opioid use disorder and referrals to outpatient treatment.52 Psychosocial treatment and quarterly physician visits are other potential process measures that have been shown to have mortality benefit among patients with opioid use disorder.50 Outcome measures for research, program, and policy evaluations include proportion of individuals with opioid use disorder engaged in an opioid treatment program, proportion of people maintaining medication use for opioid use disorder at 30 days, risk-adjusted fatal and nonfatal repeated overdose, and risk-adjusted inhospital and out-of-hospital overdose mortality.
OPIOID USE DISORDER QUALITY IMPROVEMENT OPPORTUNITIES
Changes in ED clinical practice are driven by research evidence, benefit to patients, cost-effectiveness, decreased provider burden, financial incentives for policy compliance, alignment with national goals, available resources, and fit with local values. The changing needs of our patient population, current gaps in care, emerging clinical evidence about ED opioid use disorder treatment initiation and linkage,23 potential patient benefit, and national goals and priorities to reduce opioid overdose deaths are well aligned to expand the scope of ED care to include not only opioid use disorder prevention, treatment, and harm reduction but also the development of quality improvement initiatives to accelerate implementation and adoption of high-quality practices.
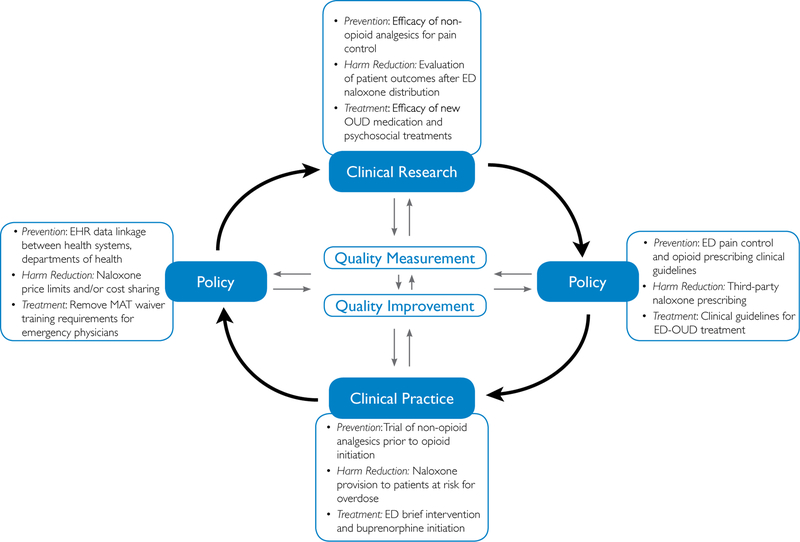
Clinical guidelines can help change clinical practice and improve care quality in combination with or in the absence of quality measurements. Guidelines, in addition to consensus recommendations and clinical protocols, are useful tools for care standardization and rapid dissemination. Although guidelines are not quality measures, they are an important underpinning in setting professional standards of care.53–55 ED clinical practice guidelines for management of opioid use disorder in emergency medicine settings are currently limited. Establishing practice guidelines and best practices can guide care improvement initiatives while patient outcome research and opioid use disorder quality measures are in development. To develop quality measures, numerous research, health policy, and clinical practice opportunities in each opioid use disorder care domain were identified during the meeting at NIDA (Figure).
Figure.
Learning health care system for research, policy, and practice to improve outcomes for ED patients with opioid use disorder. EHR, Electronic health record; MAT, medication-assisted treatment.
Opioid Use Disorder Primary Prevention
Currently, we lack the necessary ED opioid use disorder quality measure “building blocks” that link primary prevention, treatment, and harm reduction initiatives to patient outcomes. Opioid use disorder primary prevention research opportunities should focus on the efficacy of nonopioid analgesics for pain control; patient education about safe opioid use, storage, and disposal; opioid prescribing guideline adherence; development and use of care plans for patients with outpatient pain contracts; and evaluation of population trends in the development of unintended prolonged opioid use or opioid use disorder. Policy opportunities include development and implementation of ED pain control and prescribing guidelines, electronic health record linkage between health systems and departments of health, and increasing coverage of nonpharmacologic and nonopioid pain management alternatives by insurers. In clinical practice, developing and implementing opioid use disorder screening practices37 and using nonopioid pain management strategies such as nerve blocks and nonopioid analgesics can improve care. The implementation of organized systematic efforts to promote nonopioid analgesic pain management programs across EDs (eg, alternatives-to-opioids programs, Colorado Opioid Safety Collaborative) has been associated with significant decreases in ED opioid prescribing.56,57 The electronic health record can also be used to alert providers about unsafe prescribing, either because of coprescribing with benzodiazepines or prescribing opioids at high daily morphine milliequivalents.58
Opioid Use Disorder Harm Reduction
Although evidence shows that community naloxone distribution has resulted in a reduction in overdose deaths42 and is supported by a 2018 advisory from the Office of the Surgeon General,59 patient outcomes of ED naloxone distribution have yet to be thoroughly evaluated.25,26,60,61 Future research should focus on the development and use of harm reduction care plans for patients with opioid use disorder, out-of-hospital patient use of naloxone for overdose reversal, ED transport refusal and associated patient mortality, and repeated overdose. Policy opportunities to expand opioid use disorder harm reduction initiatives include passage of Good Samaritan and syringe-access legislation in states that lack these protections, allowance of third-party naloxone prescribing, centralized overdose reporting, pharmacist naloxone dispensing, and cost containment or cost sharing to decrease naloxone distribution cost. Finally, although any ED clinician can refer patients to a syringe access program or prescribe take-home naloxone, establishing a clinical infrastructure that ensures naloxone provision before hospital discharge and access to syringes requires a structured quality improvement program.
Opioid Use Disorder Treatment
Interventions that have well-established evidence outside of the ED are being adapted in EDs, such as buprenorphine initiation for opioid use disorder,62–64 with linkage to outpatient addiction treatment and harm reduction services.37,65,66 Clinical research and training to be conducted includes pain control for patients receiving medication for opioid use disorder and presenting with acute injury or illnesses, buprenorphine safety and efficacy after overdose and for acute opioid withdrawal, and feasibility of use of newer buprenorphine formulations such as extended-release buprenorphine injections. Implementation research needs to be conducted about ED provider buprenorphine waiver training, acceptance, uptake, and use; ED-outpatient opioid use disorder treatment care plan development and use; barriers and facilitators to brief interventions in the ED; initiation of medication for opioid use disorder and linkage to outpatient treatment; counseling and navigation efficacy and intensity; and evaluation of repeated overdose and mortality among ED patients who have begun receiving medication for opioid use disorder. Policy work should include the development of ED opioid use disorder clinical treatment guidelines, insurance reimbursement for ED patient counseling and navigation services, removal of buprenorphine waiver requirements for emergency physicians, and mandated insurance coverage for medication for opioid use disorder and naloxone without previous authorization. Clinical practice initiatives include trainee, provider, and staff education on opioid use disorder identification, harm reduction, treatment, and referral37; development of streamlined addiction treatment linkage processes; clinical protocols for initiating medication for opioid use disorder; and hospital dispensing of a limited supply of buprenorphine until outpatient care can be established.
Clinical data registries are resources for measure development that can be used for data collection and measuring the effect of an intervention. Nationally, emergency medicine is adopting electronic clinical quality measurement for participation in the CMS Quality Payment Program through the ACEP Clinical Emergency Data Registry. The registry is a CMS-designated Qualified Clinical Data Registry, which includes information from EDs across the United States and is used to improve quality measure development and quality reporting efficiency. Including common opioid use disorder–relevant data elements in this registry would aid in the development of ED opioid use disorder quality measures and align ED efforts to reduce opioid-associated harm with existing financial and regulatory incentives.
Inclusion of opioid use disorder–relevant elements in the Clinical Emergency Data Registry, and therefore in the electronic health record, for data collection and clinical quality measurement will embed data collection in routine ED work flow, minimizing requirements of additional work or documentation. This will enable standardized data collection; the development and monitoring of ED opioid use disorder structural, process, and outcome measures; and, ultimately, the evaluation of ED quality improvement intervention effectiveness. In addition to data collection, the electronic health record can also be used for decision support to promote current best practices and reduce variability in care. Actualizing the potential of digitally based quality improvement, however, will require the participation and coordination of numerous parties, including clinicians, hospitals, and electronic health record vendors. Improving the accessibility or availability of scalable data-collection tools, decision support, and platforms by electronic health record vendors such as Epic App Orchard or the Cerner App Gallery/Open Platform may present new opportunities to rapidly scale and standardize tools that previously did not exist.
Beyond quality measurement, the creation of national quality improvement initiatives will also be essential to engaging clinicians in opioid use disorder–focused quality measures and identifying best practices for quality improvement across each domain of ED opioid use disorder care. Recently, ACEP launched the Emergency Quality Network (E-QUAL) Opioid Initiative to engage emergency physicians in a quality improvement-based approach to reducing opioid-associated harm, and the American Hospital Association has also released a Stem the Tide toolkit including quality improvement tools for hospital-based EDs. The coordination of these types of efforts has the potential to normalize the implementation of local initiatives to improve ED opioid use disorder care, using well-established QI methods such as the Plan-Do-Study-Act cycle, lean engineering, or the Institute for Healthcare Improvement’s Model for Improvement in a manner and language familiar to emergency clinicians.
CONCLUSION
As the primary provider of acute illness stabilization, timely diagnosis, and linkage to appropriate care, the ED is uniquely positioned to improve the quality of care for patients with opioid use disorder and has an essential role in addressing the opioid epidemic and preventing overdose deaths. The quality framework described above provides a clear outline for future research and policy approaches to ED opioid use disorder quality improvement. Successful initiatives will use cross-sector partnerships and national learning collaboratives to develop an emergency care learning health care system capable of developing and disseminating safe and effective ED opioid use disorder care.
Acknowledgments
The authors acknowledge the National Institute on Drug Abuse’s (NIDA’s) Clinical Trials Network and participants in the NIDA Clinical Trials Network Meeting “Exploring Substance Use Relevant Measures in EMRs & Clinical Quality Measurement in Emergency Department Settings”: James Augustine, MD, Quandra Blackeney, Olivier Bodenreider, MD, PhD, Katharine Bradley, MD, MPH, Jeremy Brown, MD, Ron A. Dobbins, MBA, Sarah Duffy, PhD, Reena Duseja, MD, Udi Ghitza, PhD, Robert Gore-Langton, PhD, Pawan Goyal, MD, MHA, Yu-Hsiang Hsieh, MSc, PhD, Petra Jacobs, MD, Chad Kessler, MD, MPH, Robert Lindblad, MD, David Liu, MD, Raul N. Mandler, MD, Ryan P. McCormack, MD, Carmen L. Rosa, MS, Richard Rothman, MD, John Rotrosen, MD, Cynthia Singh, MS, Steven Sparenborg, PhD, Geetha Subramaniam, MD, Constance Weisner, DrPH, MSW, Ashley Wilder Smith, PhD, MPH, and Li-Tzy Wu, ScD, RN.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. Dr. Venkatesh is supported by the Yale Center for Clinical Investigation KL2 TR000140 from the National Center for Advancing Translational Science, a component of the National Institutes of Health, and also works under contract with the Centers for Medicare & Medicaid Services (CMS) in the development of hospital outcome and efficiency quality measures. Drs. Schuur and Venkatesh are supported by CMS for leading the quality development work of the American College of Emergency Physicians’ (ACEP’s) Support and Alignment Network of the Transforming Clinical Practice Initiative. Drs. Schuur and Venkatesh are also supported by the Addiction Policy Forum in the development of a national quality improvement collaborative for emergency department–based quality improvement focused on opioids.
Footnotes
Disclaimer: The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy, or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
Contributor Information
Elizabeth A. Samuels, Department of Emergency Medicine, Alpert Medical School of Brown University, Providence, RI; Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Gail D’Onofrio, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Kristen Huntley, Center for the Clinical Trials Network, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD.
Scott Levin, Department of Emergency Medicine, Johns Hopkins University, Baltimore, MD.
Jeremiah D. Schuur, Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, MA.
Gavin Bart, Addiction Medicine, Hennepin Healthcare, University of Minnesota, Minneapolis, MN.
Kathryn Hawk, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Betty Tai, Center for the Clinical Trials Network, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD.
Cynthia I. Campbell, Division of Research, Kaiser Permanente Northern California, Oakland, CA.
Arjun K. Venkatesh, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
REFERENCES
- 1.NIDA. Overdose death rates. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates/. Accessed March 6, 2018.
- 2.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. [DOI] [PubMed] [Google Scholar]
- 3.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016–September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss A, Elixhauser A, Barrett M, et al. Opioid-Related Inpatient Stays and Emergency Department Visits by State, 2009–2014. Rockville, MD: Agency for Healthcare Research & Quality; 2016; HCUP Statistical Brief 219. [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013; HHS Publication No. (SMA) 13–4760, DAWN Series D-39. [Google Scholar]
- 6.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;33:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazier W, Cochran G, Lo-Ciganic W, et al. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA. 2017;318:750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose AJ, Bernson D, Chui KKH, et al. Potentially inappropriate opioid prescribing, overdose, and mortality in Massachusetts, 2011–2015. J Gen Intern Med. 2018. [DOI] [PMC free article] [PubMed]
- 9.Axeen S, Seabury SA, Menchine M. Emergency department contribution to the prescription opioid epidemic. Ann Emerg Med. 2018;71:659–667e3. [DOI] [PubMed] [Google Scholar]
- 10.Schneberk T, Raffetto B, Kim D, et al. The supply of prescription opioids: contributions of episodic-care prescribers and high-quantity prescribers. Ann Emerg Med. 2018;668–690e3. [DOI] [PubMed]
- 11.Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: characteristics of prescriptions and association with long-term use. Ann Emerg Med. 2018;71:326–336.e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesh AK, Goodrich K. Emergency care and the national quality strategy: highlights from the Centers for Medicare & Medicaid Services. Ann Emerg Med. 2015;65:396–399. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal AR. Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA. 2003;289:996–1000. [DOI] [PubMed] [Google Scholar]
- 14.Schmittdiel JA, Gopalan A, Lin MW, et al. Population health management for diabetes: health care system-level approaches for improving quality and addressing disparities. Curr Diab Rep. 2017;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 16.Houry DE, Haegerich TM, Vivolo-Kantor A. Opportunities for prevention and intervention of opioid overdose in the emergency department. Ann Emerg Med. 2018;71:688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharfstein JM. The opioid crisis from research to practice. Milbank Q. 2017;95:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhode Island Department of Health and Rhode Island Department of Behavioral Healthcare Developmental Disabilities, and Hospitals. Levels of Care for RI Emergency Departments and Hospitals for Treating Overdose and Opioid Use Disorder. March 2017. Available at: http://health.ri.gov/publications/guides/LevelsOfCareForTreatingOverdoseAndOpioidUseDisorder.pdf. Accessed October 4, 2018.
- 19.Broida RI, Gronowski T, Kalnow AF, et al. State emergency department opioid guidelines: current status. West J Emerg Med. 2017;18:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwood-Ericksen MB, Poon SJ, Nelson LS, et al. Best practices for prescription drug monitoring programs in the emergency department setting: results of an expert panel. Ann Emerg Med. 2016;67:755–764. e754. [DOI] [PubMed] [Google Scholar]
- 21.Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60:499–525. [DOI] [PubMed] [Google Scholar]
- 22.Patrick SW, Fry CE, Jones TF, et al. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood). 2016;35:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313: 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnert AS, Bonar EE, Cunningham R, et al. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug Alcohol Depend. 2016;163:40–47. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer K, Walley AY, Langlois BK, et al. Opioid education and nasal naloxone rescue kits in the emergency department. West J Emerg Med. 2015;16:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels E. Emergency department naloxone distribution: a Rhode Island department of health, recovery community, and emergency department partnership to reduce opioid overdose deaths. R I Med J (2013). 2014;97:38–39. [PubMed] [Google Scholar]
- 27.Celso B, Tepas J, Langland-Orban B, et al. A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma. 2006;60:371–378; discussion 378. [DOI] [PubMed] [Google Scholar]
- 28.Bradley EH, Nallamothu BK, Herrin J, et al. National efforts to improve door-to-balloon time: results from the Door-to-Balloon Alliance. J Am Coll Cardiol. 2009;54:2423–2429. [DOI] [PubMed] [Google Scholar]
- 29.Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008;1:97–104. [DOI] [PubMed] [Google Scholar]
- 30.Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–2947. [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen HB, Rivers EP, Abrahamian FM, et al. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48:28–54. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–1112. [DOI] [PubMed] [Google Scholar]
- 34.Kamal N, Holodinsky JK, Stephenson C, et al. Improving door-to-needle times for acute ischemic stroke: effect of rapid patient registration, moving directly to computed tomography, and giving alteplase at the computed tomography scanner. Circ Cardiovasc Qual Outcomes. 2017;10:e003242. [DOI] [PubMed] [Google Scholar]
- 35.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsberg PJ, Happola O, Kallela M, et al. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology. 2006;67:334–336. [DOI] [PubMed] [Google Scholar]
- 37.Duber HC, Barata IA, Cioe-Pena E, et al. Identification, management, and transition of care for patients with opioid use disorder in the emergency department. Ann Emerg Med. 2018;72:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein S, D’Onofrio G. Public health in the emergency department: Academic Emergency Medicine consensus conference executive summary. Acad Emerg Med. 2009;16:1037–1039. [DOI] [PubMed] [Google Scholar]
- 39.Schuur JD, Baugh CW, Hess EP, et al. Critical pathways for post-emergency outpatient diagnosis and treatment: tools to improve the value of emergency care. Acad Emerg Med. 2011;18:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemhoefer C, Rabe GL, Wellmann J, et al. Emergency department–initiated tobacco control: update of a systematic review and meta-analysis of randomized controlled trials. Prev Chronic Dis. 2017;14:E89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein SL, D’Onofrio G. Screening, treatment initiation, and referral for substance use disorders. Addict Sci Clin Pract. 2017;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change In: Glanz K. BKRFML, ed. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. San Francisco, CA: Wiley; 2002. [Google Scholar]
- 44.Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1045–1055. [DOI] [PubMed] [Google Scholar]
- 45.Song S, Fonarow GC, Olson DM, et al. Association of Get With the Guidelines–Stroke program participation and clinical outcomes for Medicare beneficiaries with ischemic stroke. Stroke. 2016;47:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ormseth CH, Sheth KN, Saver JL, et al. The American Heart Association’s Get With the Guidelines (GWTG)–Stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–1748. [DOI] [PubMed] [Google Scholar]
- 48.Krumholz HM, Bernheim SM. Considering the role of socioeconomic status in hospital outcomes measures. Ann Intern Med. 2014;161:833–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnett M, Olenski A, Jena A. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins KE, Paddock SM, Hudson TJ, et al. Association between process measures and mortality in individuals with opioid use disorders. Drug Alcohol Depend. 2017;177:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNeely J, Wu LT, Subramaniam G, et al. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) tool for substance use screening in primary care patients. Ann Intern Med. 2016;165:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams AR, Nunes EV, Bisaga A, et al. Developing an opioid use disorder treatment cascade: a review of quality measures. J Subst Abuse Treat. 2018;91:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8 pt 2):642–646. [DOI] [PubMed] [Google Scholar]
- 54.Walter LC, Davidowitz NP, Heineken PA, et al. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291:2466–2470. [DOI] [PubMed] [Google Scholar]
- 55.Nothacker M, Stokes T, Shaw B, et al. Reporting standards for guideline-based performance measures. Implement Sci. 2016;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.American Hospital Association. Stem the Tide: Addressing the Opioid Epidemic. Chicago, IL: American Hospital Association; 2017. [Google Scholar]
- 57.Colorado Opioid Safety Collaborative. Colorado Opioid Safety Pilot Results Report. Greenwood Village, CO: Colorado Hospital Association; 2017. [Google Scholar]
- 58.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams J. Increasing naloxone awareness and use. JAMA. 2018;319:2073–2074. [DOI] [PubMed] [Google Scholar]
- 60.Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuels EA, Hoppe J, Papp J, et al. Emergency Department Naloxone Distribution: Key Considerations and Implementation Strategies. Irving, TX: American College of Emergency Physicians; 2015. [Google Scholar]
- 62.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–2066. [DOI] [PubMed] [Google Scholar]
- 64.Berg ML, Idrees U, Ding R, et al. Evaluation of the use of buprenorphine for opioid withdrawal in an emergency department. Drug Alcohol Depend. 2007;86:239–244. [DOI] [PubMed] [Google Scholar]
- 65.Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2018;113:545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sawangjit R, Khan TM, Chaiyakunapruk N. Effectiveness of pharmacy-based needle/syringe exchange programme for people who inject drugs: a systematic review and meta-analysis. Addiction. 2017;112:236–247. [DOI] [PubMed] [Google Scholar]