Abstract
Pathological high frequency oscillations (HFOs) are putative neurophysiological biomarkers of epileptogenic brain tissue. Utilizing HFOs for epilepsy surgery planning offers the promise of improved seizure outcomes for patients with medically refractory epilepsy. This review discusses possible machine learning strategies that can be applied to HFO biomarkers to better identify epileptogenic regions. We discuss the role of HFO rate, and utilizing features such as explicit HFO properties (spectral content, duration, and power) and phase-amplitude coupling for distinguishing pathological HFO (pHFO) events from physiological HFO events. In addition, the review highlights the importance of neuroanatomical localization in machine learning strategies.
Keywords: : artificial intelligence, epilepsy, epilepsy surgery, epileptiform spike, fast ripple, high-frequency oscillation, HFO, machine learning, phase–amplitude coupling, ripple, seizure, wavelet
In patients with medically refractory epilepsy, localizing epileptogenic regions for subsequent epilepsy surgery requires accurate localization of the seizure-onset zone (SOZ) often using intracranial electroencephalogram (iEEG) evaluation [1]. In the absence of an iEEG evaluation, sometimes intra-operative recordings from subdural electrodes are used to identify regions that exhibit high rates of epileptiform discharges and are presumably epileptogenic [2–4]. This strategy has proven successful for the surgical treatment of certain types of medically refractory epilepsy. For example, the probability of seizure freedom for patients treated with an anterior temporal lobectomy for medically refractory temporal lobe epilepsy is approximately 80% [5]. However, for patients with frontal lobe epilepsy, seizure freedom is only achieved in about half of the patients who undergo resective surgery [6], highlighting the need for improved approaches to planning efficacious epilepsy surgery.
Besides less than ideal seizure outcomes following surgery, another problem with the current clinical approach, using identification of the SOZ alone, is that temporal under-sampling may result in incomplete identification of all epileptogenic sites [7,8]. One solution to this problem of temporal under-sampling for epilepsy surgery planning is to utilize biomarkers of epileptogenic tissue that occur between seizures (i.e., interictal) biomarkers. The clinical gold standard interictal biomarkers of the epileptogenic tissue are spikes (i.e., epileptiform discharges). Although spikes are currently used to guide resections in the intra-operative context, spikes have many short comings as a biomarker. Spikes are relatively nonspecific and also sometimes can lack sensitivity [9].
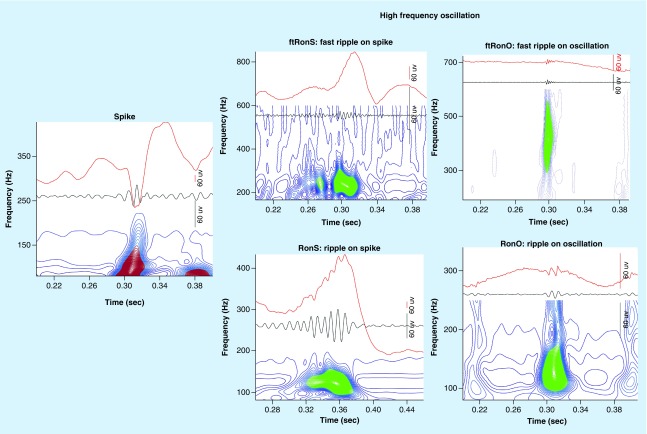
Another type of interictal biomarkers of epileptogenic tissue are high-frequency oscillations (HFOs) [10]. HFOs are brief (15–100 ms) bursts of energy with a spectral content ranging between 80 and 600 Hz. HFOs are further subclassified as ripples (80–200 Hz) and fast ripples (200–600 Hz) on the basis of the spectral content of the event [11]. Ripples and fast ripples can also occur superimposed on epileptiform activity or on the background EEG. This context can be used for further classification of the HFO event (Figure 1). Microelectrode research recordings from patients demonstrated that fast ripple events in the hippocampus and entorhinal cortex occur almost exclusively in epileptogenic tissue [12,13]. Subsequently ripples and fast ripples were identified in clinical EEG recordings and it was found that higher rates of these events occurred in the SOZ sites [14], and also in resected regions of brain for patients with a good postoperative seizure outcome [15]. Furthermore, in patients with a poor postoperative seizure outcome HFO rates were found to be higher outside the resection margins [3,4,15,16].
Figure 1. . Illustration of a spike (left) and the different subtypes of high-frequency oscillation (right) found in the intracranial electroencephalogram.
The raw trace is shown in red (top), the band-pass filtered trace in black and wavelet convolution following topographical analysis (below). Note that the spike (left) does not exhibit a ripple or fast ripple as evident by open-countour loops (red) and no closed contour groups (right, green).
Despite these findings, many perils have prevented the clinical adaptation of HFOs. HFOs are very brief events compared with epileptiform spikes and often their maximal amplitude is only two- to four-times greater than the signal-to-noise ratio of most clinical EEG amplifiers [10]. This fact makes visual identification of HFOs challenging, particularly since most commercially available EEG review software packages are not optimized for HFO visualization [10]. Furthermore, artifactual signals generated by even subtle muscle activity or electrode movement can generate HFO mimics [18]. Another challenge to utilizing HFOs is that sharp transients in the EEG signal, generated by epileptiform spikes, for instance, can generate false HFOs due to filter ringing [19]. Perhaps the most serious shortcoming of HFOs and particularly ripple oscillations, is that these events can also be observed in healthy brain regions and participate in normal cognition [20,21]. Currently no method has yet been devised to completely distinguish the physiological HFOs that occur in healthy brain regions from pathological HFOs (pHFOs) that occur exclusively in epileptogenic brain regions [9].
One promising strategy for distinguishing pathological from physiological HFOs is to characterize properties of individual HFOs and use these properties as features for classifying the events as either pathological or physiological. These properties include the mean or peak spectral content of the HFO, HFO power, HFO duration and the phase relationship between spikes/slower activity and the superimposed HFO. The spectral content, duration and power of a HFO can be precisely defined using a method known as the topographical analysis of the wavelet convolution [2]. This method can also be used to evaluate whether an HFO occurs superimposed on an interictal discharge and whether the HFO consists of a ripple, fast ripple or both a ripple and fast ripple simultaneously [2,22]. In addition, HFO phasors can be used to define the coupling relationship between HFO event amplitude and the phase of interictal discharges [23] or slower oscillations such as slow waves or spindles [24].
The details of the machine learning approaches in which HFO properties are implemented as features in a classifier of pathological and physiological events is beyond the scope of this review. Rather, we hope to provide evidence that this approach has merit for future investigations and may yield results that will promote the adaptation of HFO biomarkers into mainstream clinical practice.
Defining epileptogenic regions using HFO rates
Increased ripple and fast ripple rates (events per min) in epileptogenic human brain electrode sites, relative to electrode sites in healthy brain, were first observed in humans using microelectrode research recordings of the local field potential [12]. These recording from mesial temporal lobe structures demonstrated that ripple rates were elevated in epileptogenic-atrophied tissue, whereas fast ripple rates were elevated in epileptogenic mesial temporal tissue irrespective of whether the tissue was atrophic [13]. Also, epileptogenic mesial temporal lobe tissue exhibited a higher ratio of fast ripples to ripples [13,25].
Subsequent clinical macroelectrode recording studies [14,26,27], and hybrid macro-/microelectrode recording studies [28] demonstrated that ripple and fast ripple event rates were also elevated in electrode sites where seizures were observed to originate (i.e., SOZ). Also, HFO rates were also found to be elevated in resected electrode sites relative to unresected electrode sites for patients who exhibited a good seizure outcome following epilepsy surgery [15,29]; whereas, the opposite pattern was seen for patients who exhibited a poor seizure outcome following epilepsy surgery.
Perhaps the simplest ‘machine learning’ strategy in which HFOs can be used to identify epileptogenic sites is to determine a rate threshold at which a given electrode site is defined as potentially epileptogenic. This approach utilizing receiver operating characteristic (ROC) curves has been applied to HFOs occurring in different lobes of the brain [24], as well as to distinct subtypes of HFOs [18,30]. Using this approach, a threshold can be defined at a fixed specificity [30], or the overall accuracy of a HFO biomarker can be assigned by the area under the ROC curve (AUROC) [18,24], or a partial area under the ROC curve [30] by fixing the minimum specificity.
One special case for using HFO events to classify epileptogenic brain regions are brief (3–10 min) intraoperative recordings. In this case, certain HFO subtypes (i.e., fast ripples) may occur only once in one or a few electrode sites. Thus, for these recording many studies have determined the sensitivity and specificity of a single event observation for defining an epileptogenic brain site [2–4].
Differences in the clinical utility of different HFO subtypes
Contemporary investigations of HFOs using automated or semi-automated approaches have classified ripples and fast ripples as occurring on spikes as distinct from ripples and fast ripples that occur on background EEG (Figure 1) [2,30]. These studies have demonstrated that HFOs that occur on spike have superior specificity for epileptogenic regions and/or the SOZ. One surprising finding using this approach was that spike rates (without HFOs) were noninferior classifiers of the SOZ as compared with the rate of HFOs that were superimposed on spikes and HFOs alone [30]. This observation highlights the need to further refine the identification of HFOs on spikes that have heightened significance using machine learning approaches and combine the measurement of these biomarkers with the rate of spikes alone.
In fact, these same sleep recordings did demonstrate that fast ripples have superior specificity for defining epileptogenic regions as compared with both spikes and ripples; however, the sensitivity was very low and hence they were judged as noninferior [30]. In intraoperative recordings, the presence of a single fast ripple in an unresected electrode site [3,4,16,31,32] was found to predict a poor seizure outcome following surgery. We were able to replicate these findings in an independent investigation [2], but found that a semiautomated approach was required for fast-ripple investigation because artifactual signals masquerading as fast ripple detections were prevalent. We also found that unresected fast ripples on spikes were more specific than fast ripples on background EEG for defining a poor seizure outcome postoperatively.
Ripple rates are almost always higher than fast ripples and although the specificity of ripples for defining epileptogenic sites is less than fast ripples, the sensitivity is greater [2,4,30]. Thus, effort has been focused on trying to improve the specificity of ripples for defining epileptogenic sites. One team of investigators found that in patients with neocortical epilepsy, ripples superimposed on spikes had superior accuracy for defining electrode sites in the seizure onset zone as compared with ripples on background EEG [33,34]. In another cohort of patients, mostly with temporal lobe epilepsy, the mean rate of ripples on spikes in the seizure onset zone relative to other brain regions was found to be greater for ripples on spikes relative to ripples on background EEG [23].
One possible machine learning strategy is to define epileptogenic regions using not just one type of HFO (i.e., ripple or fast ripple), but by determining an optimal rate threshold for a combined measure of different HFO subtype rates. This strategy could optimize the sensitivity by including ripple oscillation and also maximize the specificity by including fast ripple oscillations.
Differences in the clinical utility of HFO rates by neuroanatomical region
Classifying HFOs by the neuroanatomical region in which they are generated may be essential for differentiating physiological from pHFOs. In the hippocampus, ripples superimposed on sharp waves that occur during immobility or sleep are essential for memory consolidation in mammals including humans [35,36]. Similar physiological ripples can also occur in neocortical brain regions [37]. At present, no strategy exists for distinguishing physiological and pathological ripples since they appear morphologically indistinct. Furthermore, in patients with epilepsy, some ripples that occur in certain brain regions serve not to consolidate memory but actually can disrupt memory [22,38]. As compared with ripples, fast ripples in the (sclerotic) mesial temporal lobe are regarded as always pathological [13,25]; however, outside of the mesial temporal lobe, fast ripples can occur, albeit rarely, in healthy brain regions [21].
Since physiological HFOs may have distinct properties that are determined by the neuroanatomical structure in which they are generated one strategy to define pHFOs is to create a brain atlas of physiological HFOs [21]. In this approach, the absolute rate of HFO is less important, rather the HFO rate relative to the normative rate for a specific brain area could indicate whether that area is epileptogenic or not [24,39,40].
From the perspective of building a machine learning classifier of epileptogenic sites, an extension of the brain atlas approach is to determine the combined threshold of HFO subtypes independently for each sampled neuroanatomical region from a large cohort of patients in a training set. To implement this approach requires advanced neuroradiological software to segment, co-register, normalize and then localize the electrode sites into Montreal Neurological Institute (MNI) space [22].
Differences in HFO properties such as power, duration and spectral content in epileptogenic regions
Besides determining optimal rate thresholds by neuroanatomical region for distinguishing physiological from pHFOs, another strategy is to make precise measurements of the HFO events themselves and use machine learning to attempt to distinguish the physiological from the pathological events. This approach may also be useful if HFOs fall along a continuum of pathogenicity with certain events signifying a higher likelihood that a region is epileptogenic.
Commonly measured properties of an HFO are its duration, spectral content and power [2,39,41,42]. The morphology of a single HFO event can also be defined using derived measurements such as entropy [43], or other statistical quantifications [44–47]. Following the quantification of the explicit or derived HFO properties, these properties can be used as features in a machine learning classifier that defines pHFOs.
The potential utility of this approach has been highlighted in several investigations. Explicit HFO properties have been found to differ in the SOZ as compared with the non-SOZ for both ripples [39] and fast ripples [48]. While derived HFO properties have been used to successfully identify epileptogenic regions using intra-operative recordings [44].
Differences in HFO phase–amplitude coupling in epileptogenic regions
Another approach to classify pathological and physiological HFOs is on the basis of phase–amplitude coupling (PAC). PAC or phase–amplitude event coupling (PAEC) refers to the quantified relationship between the phase of a slower frequency modulating oscillation and the amplitude of a faster frequency superimposed oscillation. Coupling between the phase of theta oscillations in the neocortex and superimposed high-γ oscillations is important during cognitive tasks [20], and it has been the subject of speculation that unique forms of PAC could occur in epileptogenic regions [49] or during pathological events [23,50].
Sleep recordings are typically utilized to identify HFOs that can define epileptogenic brain regions. Early work demonstrated that spikes and HFOs were more likely to occur during the UP state in animal models of mesial–temporal lobe epilepsy [51]. Recently, it was demonstrated that ripples in neocortex occur with the highest probability during the transition from UP to DOWN state or from the DOWN to UP state. Careful analysis of the HFO–slow wave relationship [50] established that in certain brain regions [24,52], HFOs in epileptogenic sites were more likely to occur during the transition from UP to DOWN state. Subsequently our group found PAC between sleep EEG oscillations, specifically δ and spindles, and ripples that localized to epileptogenic tissue [24].
Another form of PAC that has been useful to define pHFOs is the relationship between the phase of epileptiform discharges and the amplitude of the ripple superimposed on the epileptiform discharge [23,53]. Detailed analysis of this relationship established that in epileptogenic sites the ripples were more strongly coupled with the phase of the spike [23] and that the phase angle of coupling could vary [23,53].
Other strategies for defining epileptogenic regions using HFOs
Another unique approach for defining epileptogenic regions using HFOs is to examine the temporal relationship between HFOs that co-occur together and propagate. Recently a concept known as the spike-onset zone has been proposed to help identify epileptogenic regions [54]. One solution for identifying the spike onset zone is to measure HFO propagation [55]. By defining these propagation times, or other features, it may be possible to define a network of HFO-generating regions using graph topological measurements [56]. This graph theoretical approach could also be combined with functional MRI (fMRI)-EEG measures in a multimodal strategy for localizing HFO and spike generating regions.
Applying machine learning to HFOs to identifying epileptogenic regions
Herein, we attempt to synthesize the various subtopics of this review of machine-learning applied to the clinical utilization of HFOs using a proof of principle approach. Our group has constructed a database of visually validated HFO events recorded during sleep defined using an automated detector from over 65 patients implanted with stereo-EEG electrodes. The detector defines the explicit HFO properties (spectral content, duration and power) [18,41], and classifies each spike and HFO event into a subtype (Figure 1) [2]. Next, an automated approach is used to quantify the phase–amplitude relationship between each HFO and slow-, δ-, spindle- or spike activity that may coincide with the HFO [23,24]. Finally, each HFO is tagged with neuroanatomical coordinates in MNI space, as well as several neuroanatomical categorical locations based on published brain atlases [22]. We also added clinical metadata to the HFOs entry for use in future investigations.
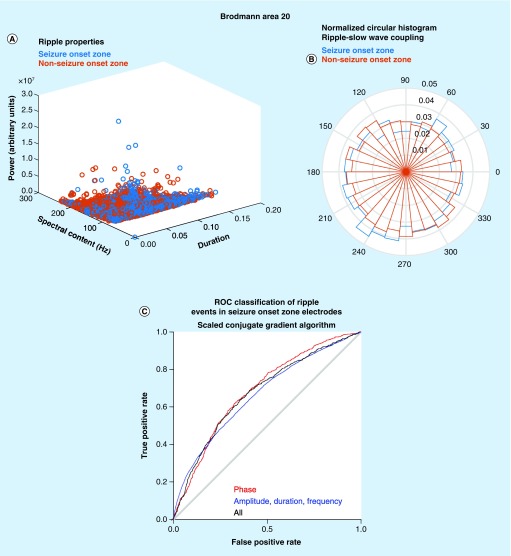
From this database of approximately 3 million HFO entries we randomly selected 29,115 ripples and 4139 ripples on spikes recorded from Brodmann area 20 in the inferior temporal gyrus to illustrate our machine learning approach. A comparison of all these ripples that occur in SOZ electrode sites in Brodmann area 20 with the ripples occurring in non-SOZ sites revealed differences with considerable overlap in cartesian space defined by the explicit ripple properties (Figure 2A). To utilize these differences in the explicit properties to classify pathological Ripples (pRipples) from physiological ripples we used a neural network with a scaled conjugate gradient algorithm. This neural network was able to classify pathological ripples in a distinct test set with a sensitivity of 60% and specificity of 70% at the threshold defined by Youden’s J.
Figure 2. . Proof of principle of the utility of machine learning strategies for identifying pathological ripple events.
(A) Scatter plot of the explicit ripple property values for ripple events recorded from electrode sites in the Brodmann area 20 seizure onset zone (seizure onset zone [SOZ], blue) and non-SOZ (red). (B) Normalized circular histogram of the phase angle of preferred coupling between slow waves and ripple oscillations for events recorded from SOZ sites (blue) and non-SOZ sites (red). (C) ROC curve derived from the classification of a test set of ripple oscillations in Brodmann area 20. The results from a neural network utilizing a scaled conjugate gradient algorithm with phase angle features for slow-, δ- and spindle-coupling (red), explicit ripple property features (blue) and a combined model (black).
ROC: Receiver operating characteristic.
We next compared the phase angle of coupling between ripples and slow oscillations for ripples that occurred in the SOZ with ripples that occurred in the non-SOZ and found subtle differences (Figure 2B). When we constructed a neural network for classifying ripples as pRipples on the basis of the preferred phase angle of coupling with slow-, δ- and spindle oscillations, this neural network exhibited a similar classification accuracy of pRipples as compared with the neural network utilizing explicit ripple properties (Figure 2C). Finally, we combined both the explicit properties and the phase angle measurements into one neural network but did not observe a significant improvement in the classifier’s accuracy.
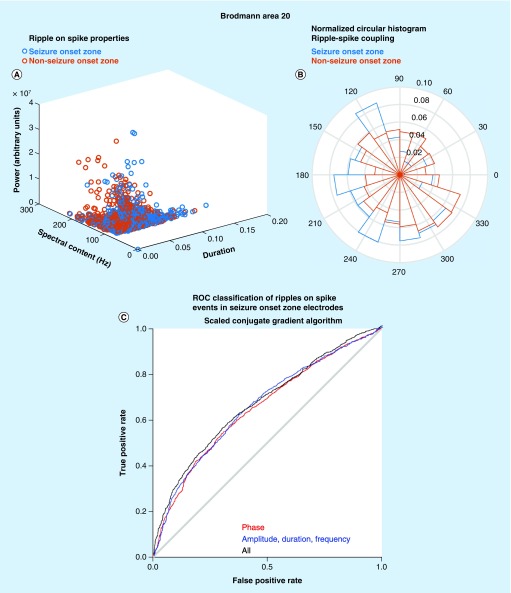
A similar approach was also used to classify the 4139 ripples on spike events in Brodmann area 20. In this case, differences were again seen in the explicit properties of ripples on spikes in the SOZ and in the non-SOZ (Figure 3A). In addition, we examined differences between the phase angle of coupling between ripples and the waveform of the epileptiform discharges (Figure 3B). We found that ripples on spikes in the SOZ exhibited a distinct but overlapping distribution of phase angles as compared with ripples on spikes in the non-SOZ (NSOZ) [23]. Overall, the combined classifier for ripples on spikes performed better than the classifier of ripples with 45% sensitivity and 92% specificity at Youden’s J (Figure 3C).
Figure 3. . Proof of principle of the utility of machine learning strategies for identifying pathological ripple on spike events.
(A) Scatter plot of the explicit ripple property values for ripple on spike events recorded from electrode sites in the Brodmann area 20 (seizure onset zone [SOZ], blue) and non-SOZ (red). (B) Normalized circular histogram of the phase angle of preferred coupling between spike and ripple on spike oscillations for events recorded from SOZ sites (blue) and non-SOZ sites (red). (C) ROC curve derived from the classification of a test set of ripple oscillations in Brodmann area 20. The results from a neural network utilizing a scaled conjugate gradient algorithm with phase angle features for spike coupling (red), explicit ripple property features (blue) and a combined model (black).
ROC: Receiver operating characteristic.
As illustrated by this example it is possible to use machine learning approaches to distinguish pHFO events from physiological events in distinct neuroanatomical locations after categorizing the HFO types (Figure 1). This approach can be individually tailored for each HFO subtype in each neuroanatomical region to identify pHFOs. After constructing a unique machine learning classifier for each pHFO subtype (Figure 1) in each neuroanatomical region, another machine learning classifier can be constructed for each unique neuroanatomical region that combines the rate derivations of each pHFO subtype to determine the optimal rate threshold for classifying that region as epileptogenic. This classifier also could provide a given probability that a region is epileptogenic based on the rate of the pHFOs.
Future perspective
In summary, the optimal machine learning strategy for utilizing HFOs for identifying epileptogenic regions would define pHFOs using explicit properties and PAC for each distinct neuroanatomic region and each HFO subtype, and then use a pHFO rate classifier, in each distinct neuroanatomic region, that combines spike rates with each category of pHFOs to predict epileptogenicity of that region. While the details of the machine learning strategy that will be optimal for this approach remain under study, based on the literature we believe that this approach or similar derivations will yield excellent results.
Footnotes
Financial & competing interests disclosure
SA Weiss is the founder of Fastwave LLC, a neurology software company. ZJ Waldman, F Raimondo and D Slezak are cofounders of Fastwave LLC. SA Weiss and ZJ Waldman both hold more than 5% equity interest in Fastwave LLC. SA Weiss is supported by NIH/NINDS awards 1K23NS094633-01A1 and 1K23NS094633-02A1, and a Junior Investigator Award from the American Epilepsy Society. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Vakharia VN, Duncan JS, Witt J-A, Elger CE, Staba R, Engel J. Getting the best outcomes from epilepsy surgery. Ann. Neurol. 83(4), 676–690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SA, Berry B, Chervoneva I. et al. Visually validated semi-automatic high-frequency oscillation detection aides the delineation of epileptogenic regions during intra-operative electrocorticography. Clin. Neurophysiol. 129(10), 2089–2098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Proof of principle of machine learning to improve utility of high-frequency oscillation (HFOs).
- 3.van ’t Klooster MA, van Klink NEC, Zweiphenning WJEM. et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann. Neurol. 81(5), 664–676 (2017). [DOI] [PubMed] [Google Scholar]
- 4.van ’t Klooster MA, van Klink NEC, Leijten FSS. et al. Residual fast ripples in the intraoperative corticogram predict epilepsy surgery outcome. Neurology 85(2), 120–128 (2015). [DOI] [PubMed] [Google Scholar]
- 5.LoPinto-Khoury C, Sperling MR, Skidmore C. et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia 53(2), 342–348 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Holtkamp M, Sharan A, Sperling MR. Intracranial EEG in predicting surgical outcome in frontal lobe epilepsy. Epilepsia 53(10), 1739–1745 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Ung H, Davis KA, Wulsin D. et al. Temporal behavior of seizures and interictal bursts in prolonged intracranial recordings from epileptic canines. Epilepsia 57(12), 1949–1957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King-Stephens D, Mirro E, Weber PB. et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia 56(6), 959–967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia 50(4), 598–604 (2009). [DOI] [PubMed] [Google Scholar]; • Early review highlighting variable pathogenic significance of HFOs.
- 10.Frauscher B, Bartolomei F, Kobayashi K. et al. High-frequency oscillations: the state of clinical research. Epilepsia 58(8), 1316–1329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus 9(2), 137–142 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J. Neurophysiol. 88(4), 1743–1752 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann. Neurol. 56(1), 108–115 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 49(11), 1893–1907 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs J, Zijlmans M, Zelmann R. et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann. Neurol. 67(2), 209–220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedele T, Burnos S, Boran E. et al. Resection of high frequency oscillations predicts seizure outcome in the individual patient. Sci. Rep. 7(1), 13836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zijlmans M, Worrell GA, Dümpelmann M. et al. How to record high-frequency oscillations in epilepsy: a practical guideline. Epilepsia 58(8), 1305–1315 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Shimamoto S, Waldman ZJ, Orosz I. et al. Utilization of independent component analysis for accurate pathological ripple detection in intracranial EEG recordings recorded extra- and intra-operatively. Clin. Neurophysiol. 129(1), 296–307 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin. Neurophysiol. 121(3), 301–310 (2010). [DOI] [PubMed] [Google Scholar]; •• Highlights that not all HFOs on spikes can be assigned properties.
- 20.Canolty RT, Edwards E, Dalal SS. et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313(5793), 1626–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frauscher B, von Ellenrieder N, Zelmann R. et al. High-frequency oscillations in the normal human brain. Ann. Neurol. 84, 374–385 (2018). [DOI] [PubMed] [Google Scholar]; •• Atlas of normal HFOs highlighting the utility of proposed machine learning strategy.
- 22.Waldman ZJ, Camarillo-Rodriguez L, Chervenova I. et al. Ripple oscillations in the left temporal neocortex are associated with impaired verbal episodic memory encoding. Epilepsy Behav. 88, 33–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss SA, Orosz I, Salamon N. et al. Ripples on spikes show increased phase-amplitude coupling in mesial temporal lobe epilepsy seizure-onset zones. Epilepsia 57(11), 1916–1930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Examines the utility of examining coupling between HFOs and spikes.
- 24.Song I, Orosz I, Chervoneva I. et al. Bimodal coupling of ripples and slower oscillations during sleep in patients with focal epilepsy. Epilepsia 58(11), 1972–1984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Examines the utility of examining coupling between HFOs and slower oscillations.
- 25.Staba RJ, Frighetto L, Behnke EJ. et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia 48(11), 2130–2138 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Jacobs J, Levan P, Châtillon C-E, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain J. Neurol. 132(Pt 4), 1022–1037 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crépon B, Navarro V, Hasboun D. et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain J. Neurol. 133(Pt 1), 33–45 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Worrell GA, Gardner AB, Stead SM. et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain J. Neurol. 131(Pt 4), 928–937 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haegelen C, Perucca P, Châtillon C-E. et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 54(5), 848–857 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roehri N, Pizzo F, Lagarde S. et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann. Neurol. 83(1), 84–97 (2018). [DOI] [PubMed] [Google Scholar]; • Demonstrates that without machine learning HFOs may not provide additional information as compared with interictal spikes.
- 31.Jacobs J, Wu JY, Perucca P. et al. Removing high-frequency oscillations: a prospective multicenter study on seizure outcome. Neurology 91, 1040–1052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedele T, van ’t Klooster M, Burnos S. et al. Automatic detection of high frequency oscillations during epilepsy surgery predicts seizure outcome. Clin. Neurophysiol. 127(9), 3066–3074 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Wang IZ, Bulacio JC. et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia 54(2), 370–376 (2013). [DOI] [PubMed] [Google Scholar]; • Demonstrates that ripple on spike HFOs are superior to ripple on oscillations and highlights the need to better classify HFO subtypes.
- 34.Wang S, So NK, Jin B. et al. Interictal ripples nested in epileptiform discharge help to identify the epileptogenic zone in neocortical epilepsy. Clin. Neurophysiol. 128(6), 945–951 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science 256(5059), 1025–1027 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25(10), 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodagholy D, Gelinas JN, Buzsáki G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358(6361), 369–372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs J, Banks S, Zelmann R, Zijlmans M, Jones-Gotman M, Gotman J. Spontaneous ripples in the hippocampus correlate with epileptogenicity and not memory function in patients with refractory epilepsy. Epilepsy Behav. 62, 258–266 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Guragain H, Cimbalnik J, Stead M. et al. Spatial variation in high-frequency oscillation rates and amplitudes in intracranial EEG. Neurology 90(8), e639–e646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkawadri R, Gaspard N, Goncharova II. et al. The spatial and signal characteristics of physiologic high frequency oscillations. Epilepsia 55(12), 1986–1995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldman ZJ, Shimamoto S, Song I. et al. A method for the topographical identification and quantification of high frequency oscillations in intracranial electroencephalography recordings. Clin. Neurophysiol. 129(1), 308–318 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Method for defining the properties of HFOs accurately.
- 42.Pail M, Halámek J, Daniel P. et al. Intracerebrally recorded high frequency oscillations: simple visual assessment versus automated detection. Clin. Neurophysiol. 124(10), 1935–1942 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Mooij AH, Frauscher B, Amiri M, Otte WM, Gotman J. Differentiating epileptic from non-epileptic high frequency intracerebral EEG signals with measures of wavelet entropy. Clin. Neurophysiol. 127(12), 3529–3536 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Gurses C, Sha Z. et al. Stereotyped high-frequency oscillations discriminate seizure onset zones and critical functional cortex in focal epilepsy. Brain J. Neurol. 141, 713–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Sha Z, Sencer A. et al. Exploring the time-frequency content of high frequency oscillations for automated identification of seizure onset zone in epilepsy. J. Neural Eng. 13(2), 026026 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Pearce A, Wulsin D, Blanco JA, Krieger A, Litt B, Stacey WC. Temporal changes of neocortical high-frequency oscillations in epilepsy. J. Neurophysiol. 110(5), 1167–1179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco JA, Stead M, Krieger A. et al. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain J. Neurol. 134(Pt 10), 2948–2959 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pail M, Řehulka P, Cimbálník J, Doležalová I, Chrastina J, Brázdil M. Frequency-independent characteristics of high-frequency oscillations in epileptic and non-epileptic regions. Clin. Neurophysiol. 128(1), 106–114 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Amiri M, Frauscher B, Gotman J. Phase-amplitude coupling is elevated in deep sleep and in the onset zone of focal epileptic seizures. Front Hum. Neurosci. 10, 387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frauscher B, von Ellenrieder N, Ferrari-Marinho T, Avoli M, Dubeau F, Gotman J. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain J. Neurol. 138(Pt 6), 1629–1641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bragin A, Benassi SK, Engel J. Patterns of the UP-DOWN state in normal and epileptic mice. Neuroscience 225, 76–87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Ellenrieder N, Frauscher B, Dubeau F, Gotman J. Interaction with slow waves during sleep improves discrimination of physiologic and pathologic high-frequency oscillations (80–500 Hz). Epilepsia 57(6), 869–878 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Lévesque M, Salami P, Shiri Z, Avoli M. Interictal oscillations and focal epileptic disorders. Eur. J. Neurosci. 48, 2915–2927 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Khoo HM, von Ellenrieder N, Zazubovits N, He D, Dubeau F, Gotman J. The spike onset zone: the region where epileptic spikes start and from where they propagate. Neurology 91(7), e666–e674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamilia E, Park E-H, Percivati S. et al. Surgical resection of ripple onset predicts outcome in pediatric epilepsy. Ann. Neurol. 84, 331–346 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Lagarde S, Roehri N, Lambert I. et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain J. Neurol. 141, 2966–2980 (2018). [DOI] [PubMed] [Google Scholar]