Abstract
The neuroimmune system is involved in development, normal functioning, aging, and injury of the central nervous system. Microglia, first described a century ago, are the main neuroimmune cells and have three essential functions: a sentinel function involved in constant sensing of changes in their environment, a housekeeping function that promotes neuronal well-being and normal operation, and a defense function necessary for responding to such changes and providing neuroprotection. Microglia use a defined armamentarium of genes to perform these tasks. In response to specific stimuli, or with neuroinflammation, microglia also have the capacity to damage and kill neurons. Injury to neurons in Alzheimer’s, Parkinson’s, Huntington’s, and prion diseases, as well as in amyotrophic lateral sclerosis, frontotemporal dementia, and chronic traumatic encephalopathy, results from disruption of the sentinel or housekeeping functions and dysregulation of the defense function and neuroinflammation. Pathways associated with such injury include several sensing and housekeeping pathways, such as the Trem2, Cx3cr1 and progranulin pathways, which act as immune checkpoints to keep the microglial inflammatory response under control, and the scavenger receptor pathways, which promote clearance of injurious stimuli. Peripheral interference from systemic inflammation or the gut microbiome can also alter progression of such injury. Initiation or exacerbation of neurodegeneration results from an imbalance between these microglial functions; correcting such imbalance may be a potential mode for therapy.
Recent research into microglia provides unprecedented insight into their roles in health, aging, and neurodegenerative diseases. These advances started 100 years ago in 1918, when Pio del Rio Hortega published a method for staining microglia and distinguishing them from neighboring cells of the CNS1. Hortega named microglia the ‘third element’ of the CNS, describing their phagocytic function, plasticity, regional distribution, and heterogeneity. For a century, microgliologists have been validating Hortega’s observations.
Development of methods to isolate and culture neonatal microglia2 ascertained their functions, including phagocytosis and response to amyloid-β (Aβ), and supported their roles in neurodegeneration. Generation of mice with GFP-labeled microglia3 allowed in vivo visualization by two-photon microscopy and showed that microglia continually survey and sense their microenvironment, respond rapidly to focal injury4, are involved in synaptic pruning and remodeling5, and contribute to various neurodegenerative diseases. Novel methods to isolate adult microglia6 allowed transcriptomic analyses by RNA sequencing, thus identifying expression signatures that help define these cells7. Recently, single-cell RNA-seq has provided insight into potential microglial subpopulations in neurodegenerative diseases8.
In this Review, we summarize the current knowledge of the roles of microglia in neurodegeneration. To better understand such roles, we introduce a revised functional and transcriptomic definition of microglia, discuss their roles in individual neurodegenerative diseases, and review common pathways involved in neurodegeneration.
A functional and molecular definition of microglia
Microglia constitute 5–12% of CNS cells, depending on the region9. They are the principal resident immune cells of the brain and are involved in homeostasis and in host defense against pathogens and CNS disorders10,11. Ontological studies of microglia confirmed Hortega’s suspicion that they are mesenchymal, myeloid12, originating in the yolk sac, and capable of self-renewal independent of hematopoietic stem cells13. Microglial survival and maintenance depend on cytokines, including CSF1 and interleukin (IL)-3414, and on transcription factors such as IRF812. Reprograming stem cells or monocytes to develop into microglia-like cells is possible15–17 and is dependent on their environment18.
Until recently, a simplistic definition of microglia describes them as innate immune cells of the CNS of myeloid origin that express Cx3cr1, CD11b, Iba1, and F4/8011. Based on comprehensive gene expression profiling and functional studies7,11, we propose a functional and molecular definition of microglia that correlates their gene expression with their functions. RNA-seq analysis identified a new set of microglia-specific markers in the healthy brain that include HexB, P2ry12, S100A8, S100A9, Tmem119, Gpr34, SiglecH, TREM2, and Olfml37. Microglial transcriptomes allow them to perform three essential functions: (i) sense their environment, (ii) conduct physiological housekeeping, and (iii) protect against modified-self and non-self injurious agents. These normal functions are important in various stages of development from embryonic stages to adulthood and aging.
Sensing
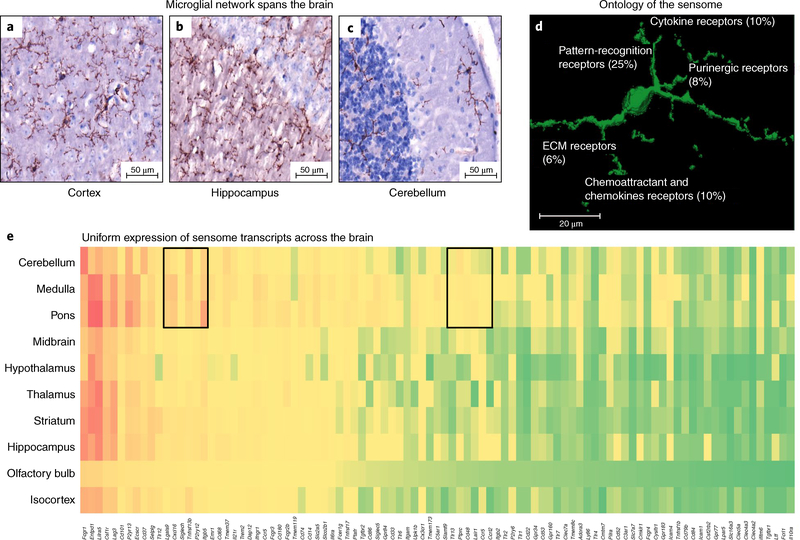
Microglia form a network spanning the CNS9. Their thin processes are dynamic and in constant motion, allowing them to scan the area surrounding their cell body every few hours and rapidly polarize toward focal injury (Fig. 1a–c and Supplementary Video 1). They use the products of nearly 100 genes to sense changes in their microenvironment (their sensome) including P2yr12, AXL, and MER4,7,19 (Fig. 1d,e). Sensome mRNAs are uniformly expressed in microglia in various areas of the brain, suggesting that all microglia are capable of performing their sensing function (Fig. 1e). Sensing is a prerequisite for microglia to perform their housekeeping and host defense functions.
Fig. 1 |. Microglia in a normal mouse brain.
a–c, Mouse microglia, stained here with anti-CD11b, have distinct processes that are constantly moving in the area around the cell body, and form a network of cells that spans most of the CNS, including the (a) cortex (b) hippocampus, and (c) cerebellum. d, Three-dimensional image of a mouse microglia with summary of gene ontology analysis of the sensome genes. e, Heatmap showing comparative expression of microglial sensome genes identified by RNA-seq data using the Allen Brain Atlas in situ hybridization dataset. Most of the genes are similarly expressed in most areas of the brain, except for two small clusters that appear to have differential expression in the brain stem. ECM, extracellular matrix.
Housekeeping
Physiological housekeeping functions include synaptic remodeling (a function critical for CNS development, homeostasis, and neurodegeneration20–22), migration to sites of neuronal death to phagocytose dead or dying cells23,24 or debris, and maintaining myelin homeostasis25. Interacting with astrocytes is another important microglial function involved in homeostasis, inflammation, and possibly neurodegeneration26. Among the genes involved in housekeeping are those encoding chemokine and chemoattractant receptors, genes involved in phagocytosis (scavenger receptors and Trem2), and genes involved in synaptic pruning and remodeling (C1q and Cx3cr1; Fig. 2)7. Aberrant housekeeping can lead to neurodegeneration.
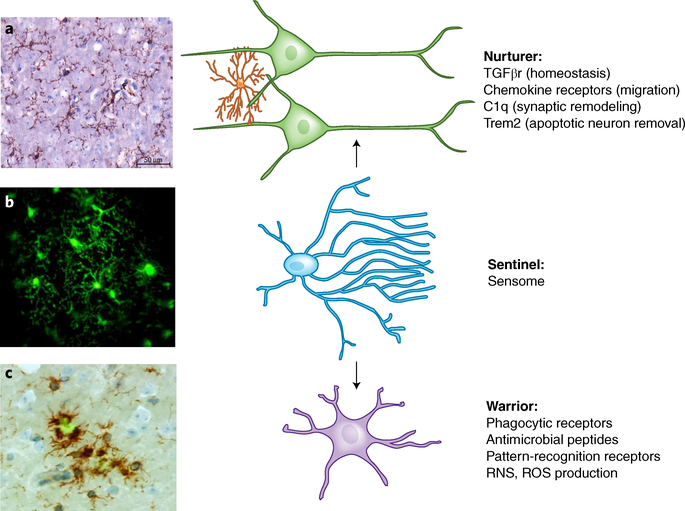
Fig. 2 |. Three proposed functional states of microglia.
a, Nurturer state: microglia (left) stained for Cd11b (brown) in a normal brain are highly ramified and evenly spaced throughout the brain parenchyma. In their nurturer role they maintain milieu homeostasis, participate in synaptic remodeling and migration, and remove apoptotic neurons, all mediated by specific receptors and receptor-linked pathways. b, Sentinel state: micrograph taken from a video using two-photon microscopy from a Cx3cr1-GFP mouse with a cranial window shows a cluster of green microglia with abundant processes. The video from which this micrograph was taken (Supplementary Video 1) shows that microglia (green) processes are in constant motion, surveilling their surroundings. Focal laser-induced injury initiates microglia response, with those microglia closest to the site of injury displaying polarization of surveilling processes toward the area of injury. Microglia sensing is mediated by proteins encoded by sensome genes, which are portals for microglia to perform their housekeeping and host-defense functions. c, Warrior state: microglia (left) stained for Cd11b (brown) accumulate around Aβ deposits stained with thioflavin-S (green), where they are observed to be two- to fivefold denser than in neighboring areas. The warrior morphology becomes stockier and less ramified, and defense against infectious pathogens and injurious-self proteins including Aβ is mediated through microglial Fc receptors, TLRs, viral receptors, and antimicrobial peptides. Sensing is a prerequisite for microglia to perform their housekeeping and host-defense functions.
Protection against injurious self and non-self stimuli
Microglia mediate host defense against infectious pathogens, injurious self-proteins such as Aβ, aggregated α-synuclein, mutant huntingtin, mutant or oxidized superoxide dismutase (SOD), or prions, as well as primary or metastatic CNS tumors. To perform these functions, microglia express Fc receptors, Toll-like receptors (TLRs), viral receptors, and antimicrobial peptides (Fig. 2)7. In response to such stimuli, microglia can initiate a neuroinflammatory response which, like peripheral inflammation, includes production of cytokines such as TNF and IL-16,27, and possibly chemokines such as Ccl228, to recruit additional cells and induce them to clear injurious agents and maintain brain homeostasis. Neuroinflammation, however, unlike peripheral inflammation, can also be limited to microglia without recruiting circulating leukocytes. Persistent neuroinflammation in turn induces neurotoxicity, leading to neurodegeneration.
A take-home message is that there are no resting microglia (Fig. 2). Their sensing, housekeeping, and protecting functions keep them constantly engaged, and most microglia in healthy brains are capable of performing such functions. Dysregulation of any of these functions results in an imbalance that initiates or propagates neurodegeneration. Here we summarize what we know about microglia and what happens to their functions in various neurodegenerative diseases.
Alzheimer’s disease
Alzheimer’s disease (AD) is characterized by formation of Aβ-containing plaques, neurofibrillary tangles comprising intracellular hyperphosphorylated tau protein, and neuronal loss29. An accepted sequence of events is that accumulation of Aβ leads to a microglial response, which promotes tau hyperphosphorylation and formation of neurofibrillary tangles, leading to neurodegeneration and cognitive impairment. In AD patients and animal models, microglia accumulate around senile plaques (Fig. 2c), where their density is two- to fivefold higher than in normal parenchyma30. They contain intracellular Aβ, suggesting phagocytosis31, show proinflammatory morphological changes such as somatic swelling and process shortening (Fig. 2c), and have increased proinflammatory markers including major histocompatibility complex II, CD36, IL-1, IL-6, and TNF32,33. So how do microglia contribute to AD pathogenesis?
Genome-wide association studies
Evidence for a direct microglial role in AD came from genome-wide association studies. Mutations in triggering receptor expressed on myeloid cells 2 (Trem2) were associated with a 3.0- to 4.5-fold increased AD risk, almost as high as that associated with ApoE ε434,35. Mutations in other microglial genes, such as CR1, HLA–DRB1, CD33, MS4A6A, and BIN1, were associated with more modest AD risks34. Since these genes regulate key microglial functions, understanding how they affect AD will impact all AD patients whether they have these mutations or not.
Aβ clearance
Aβ deposition is regulated by equilibrium between Aβ production and clearance. Small changes in this equilibrium result in abnormal accumulation. Aβ clearance involves, in part36, phagocytosis and endocytosis via microglial scavenger receptors (SRs)37,38 and extracellular degradation by Aβ-degrading enzymes6,36. Decreased clearance contributes to Aβ accumulation in late-onset AD. In support of this concept, microglia from a mouse model of Aβ deposition (Aβ-mice) have reduced expression of Aβ-phagocytic receptors and Aβ-degrading enzymes, but their ability to produce proinflammatory cytokines was maintained6. These results suggest that Aβ accumulation is in part due to failure of microglia to clear this toxic peptide.
Aβ-induced inflammation
Microglia–Aβ interactions lead to early synapse loss39, production of neurotoxic reactive oxygen and nitrogen species (ROS and RNS), NLRP3 inflammasome activation, and production of proinflammatory cytokines and TNF27,40–42. This requires Aβ interaction with microglial pattern recognition receptors (PRRs) including TLRs, SRs, and complement receptor 3 (CR3)7,43.
Microglia in AD, a double-edged sword
Based on these findings, microglial–Aβ interaction is a double-edged sword. While monitoring the brain environment, microglial sensing of Aβ peptides results in Aβ clearance and removal of the injurious agent (Fig. 3). However, persistent production of Aβ and its chronic interaction with microglia drive further amyloid deposition. Indeed, Aβ-induced proinflammatory cytokines reduce microglial Aβ clearance ability, and NLRP3 activation releases microglial apoptosis-associated speck-like protein containing a CARD (ASC) which binds Aβ, causing its aggregation and leading to further amyloid ‘seeding’ and spreading of amyloid pathology42. Similarly, Aβ-induced cytokines promote tau hyperphosphorylation and pathology, thus initiating a self-perpetuating loop that culminates in worsening disease44,45. The double-edged sword metaphor refers to various stages of a single microglia in AD. At a certain timepoint during disease progression, microglia assume a useful role, then progress into a dysfunctional cell which ultimately becomes deleterious. In support of this concept, recent transcriptomic studies of microglia in normal and Aβ-mice identified subpopulations defined as disease-associated microglia (DAM)8,24. DAMs are located around Aβ plaques and have dysregulated expression of sensing, housekeeping, and host-defense genes. It is not clear how DAMs differ from ‘dark microglia’ associated with Aβ deposits, which exhibit condensed cytoplasms and nucleoplasms and express high levels of CD11b and Trem246. These findings support a direct link between aberrant microglial functions and AD and suggest that a subset of microglia transition from a homeostatic to DAMs in AD.
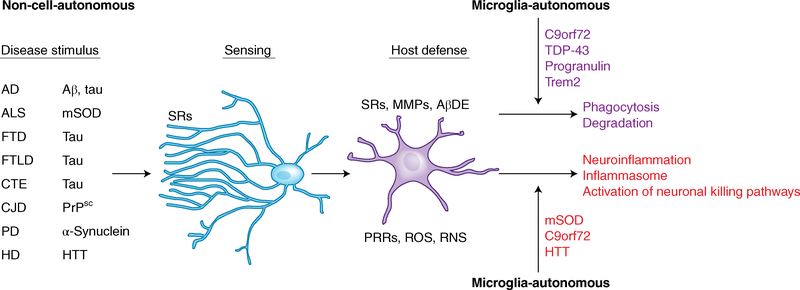
Fig. 3 |. Effectors of microglia function associated with neurodegeneration.
Two common themes for microglia’s roles in neurodegenerative diseases emerge. As microglia perform their normal sentinel function, they encounter aberrant or misfolded proteins such as Aβ, aggregated α-synuclein, oxidized or mSOD1, or PrPsc. In response to these toxic stimuli, microglia perform their host-defense function, attempting to clear these agents via SRs and other PRRs. The nature of the aberrant proteins or their persistent production disrupts microglial housekeeping functions and dysregulates microglial host-defense functions, leading to an exaggerated proinflammatory response, neurotoxicity, and neurodegeneration. A second theme is that in some neurodegenerative diseases, such as AD, ALS, and HD, mutations in specific genes cause self-autonomous dysregulation of host defense, thereby initiating or exaggerating proinflammatory responses, resulting in neurotoxicity and neurodegeneration. For example, mutations in TDP-43, progranulin, and Trem2 affect phagocytosis and associated degradation pathways (purple), whereas mutations in mSOD and HTT affect inflammasome activation and neuronal killing pathways (red). Mutations in C9orf72 affect both phagocytosis and inflammasome pathways. MMPs, matrix metalloproteases; AβDE, Aβ-degrading enzymes.
Tauopathies
Tauopathies are neurodegenerative diseases characterized by hyperphosphorylated and aggregated tau and neurofibrillary tangles. Tauopathies include AD, progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia (FTD), and chronic traumatic encephalopathy (CTE) associated with repetitive concussive head injuries. Most tauopathies have neuronal and glial accumulations of toxic insoluble tau, neuronal loss, and proinflammatory microglia47.
Microglia can engulf, degrade, and clear tau48,49. In contrast, when activated, proinflammatory microglia increase tau phosphorylation50 and drive the spread of tau pathology48. This is supported by human studies showing elevated levels of microvesicle-associated tau in the cerebrospinal fluid and blood of AD patients and by brain imaging studies showing proinflammatory microglia in FTD, progressive supranuclear palsy, and CTE51–53. Proinflammatory microglia precede visible tau pathology in transgenic mice, and their activation is attenuated by the immunosuppressant drug FK506, which attenuates tau pathology and extends lifespan in these mice, suggesting that microglia can mediate tau neurotoxicity54.
The double-edged sword concept of microglia’s role in AD also applies to tauopathies (Fig. 3). Microglia first sense and clear tau in an attempt to protect from tau toxicity, but dysregulation of microglial sensing and housekeeping pathways, such as the Cx3cr1 and Trem2 pathways, lead to dysregulation of the host-defense pathway, resulting in a neuroinflammatory response gone awry, causing neuronal damage and loss55–57.
Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting 1.2% of individuals over age 65. Most cases are sporadic and 5–10% are inherited58. Neurodegeneration occurs in the substantia nigra, with dopaminergic denervation of the striatum and accumulation of Lewy bodies containing aggregated α-synuclein59. Reactive microglia expressing HLA-DR are abundant in the substantia nigra of PD patients60. Positron-emission tomography (PET) studies show widespread proinflammatory microglia, but this response does not correlate with clinical severity, suggesting it occurs early in the disease61.
The mechanism(s) by which microglia participate in PD may be similar to those in AD (Fig. 3). Microglia internalize and degrade α-synuclein, possibly to clear it. A defect in this process leads to accumulation of extracellular α-synuclein similar to Aβ62. Microglia accumulate near α-synuclein deposits and become proinflammatory in a manner dependent on receptors that also bind Aβ, such as CD36 and TLR263–65. These findings, which need to be validated in animal models of PD, suggest that AD and PD have similar pathogenic pathways, raising the possibility that microglia may also be a double-edged sword in PD.
Multiple sclerosis
Multiple sclerosis (MS) patients have demyelinated plaques in the white and gray matter. Ongoing disease leads to progressive neurodegeneration, resulting in brain atrophy. Neuroinflammation is present in all stages of MS, and a proposed classification of MS lesions relies in part on the presence or absence of microglia in the lesions66.
Microglia in MS may be detrimental or beneficial. In experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, microglia release proteases, proinflammatory cytokines, ROS, and RNS, and they recruit reactive T lymphocytes, thereby causing toxicity to neurons and oligodendrocyte precursors. Targeted deletion of the transforming growth factor (TGF)-β-activated kinase 1 in microglia in EAE reduced CNS inflammation and axonal and myelin damage by cell-autonomous inhibition of the NF-κB, JNK, and ERK1/2 pathways11. These results suggest that microglia promote tissue injury in EAE. However, at disease onset, microglia promote axonal regeneration, remyelination, clearance of inhibitory myelin debris, and the release of neurotrophic factors, suggesting a beneficial role67.
These observations are likely to be relevant to MS stages, as microglia are closely associated with lesions with active demyelination68. It is possible that the detrimental and beneficial roles of microglia in MS depend on the stage of the disease or of specific lesions. What is clear is that essential microglial functions are altered in EAE and possibly in MS, including their ability to sense and clear debris and mount a neuroprotective response.
Huntington’s disease
Huntington’s disease (HD) is characterized by progressive atrophy of the striatum and cortex in advanced disease69. Microscopically, there are intranuclear inclusions containing the protein huntingtin (HTT) and neurodegeneration of medium-size spiny, encephalin-containing inhibitory neurons69. HD is caused by mutations in HTT (mHTT) that lead to expansion of the trinucleotide CAG stretch, which translates into a polyglutamine stretch in the HTT protein69.
HTT mRNA is expressed in microglia at a relatively high level, similarly to TLR47. Proinflammatory microglia are seen early in HD by PET. In presymptomatic HD gene carriers, their presence correlates with a higher probability of developing HD in 5 years70. In HD patients, the presence of proinflammatory microglia correlates with HD severity71,72.
HD is associated with altered microglial function and mRNA profile. Expression of mHTT in microglia confers a cell-autonomous increase in proinflammatory genes73 (Fig. 3). Levels and transcriptional activities of the myeloid lineage-determining factors PU.1 and CCAAT/enhancer-binding protein (C/EBP)-α,β are increased and exhibit enhanced binding at thousands of genomic locations in HD patients and mouse models73. This correlates with increased expression of IL6 and TNF. These changes are unique to microglia and not observed in other myeloid cells73. Functionally, several of the genes that are increased in mHTT microglia are involved in sensing their milieu, such as Tlr2, Cd14, Fcgr1, Clec4d, Adora3, Tlr9, Tnfrsf1b, and others7,73, suggesting a possible increase in capacity to sense extracellular stimulants. This was associated with increased microglial neurotoxicity73. Upregulation of IL6 and TNF mRNA73 suggest that the microglial response is an exaggerated host-defense response to rid the brain of mHTT that went awry, thereby exacerbating neurodegeneration.
Amyotrophic lateral sclerosis
Most patients with amyotrophic lateral sclerosis (ALS; also called Lou Gehrig’s disease) have sporadic ALS but ~10% of patients have mutations in specific genes including SOD1, C9orf72, TDP43, and FUS74. The disease presents loss of motor neurons in the cortex, brainstem, and spinal cord. Microglia expressing proinflammatory markers are found near injured neurons in autopsies75 and seen by PET in the brains of live ALS patients76.
Transgenic mice overexpressing mutant human SOD1 (mSOD) develop a progressive motor neuron disease similar to ALS77. mSOD1 expression in microglia accelerates disease onset78, and microglial activation exacerbates motor neuron death79. Microglia change their phenotype with disease progression. Some proinflammatory microglia are seen in the spinal cord before clinical disease develops, increase with disease progression, and persist into end-stage disease80. Microglia isolated from mSOD1 mice at disease-onset were neuroprotective, in contrast to microglia isolated at end-stage disease81. Neurotoxicity of mSOD1 microglia is NF-κB-dependent82 and partly mediated by IL-1β83. These findings directly implicate microglia with mSOD1 in ALS progression.
Pathways leading to microglial activation and neurotoxicity in ALS are both cell-autonomous and dependent on exogenous stimuli (Fig. 3). Expression of mSOD1 in microglia disrupts regulation of NADPH oxidase, leading to excessive neurotoxic superoxide production84. Intraneuronal or extracellular misfolded SOD1 is sensed by microglia similarly to their sensing of Aβ or α-synuclein through TLRs and SRs, rendering them proinflammatory85. These findings suggest that the two major microglial functions altered in ALS include the sensing of exogenous stimuli and danger signals and the host response. They also point to shared neurodegenerative pathways between ALS, AD, and PD, as ALS microglia also change their phenotype with disease progression from neuroprotective to neurotoxic81.
Transgenic mice carrying the human C9orf72 gene with disease-associated expansion repeats display pathologic features of ALS, without behavioral abnormalities or neurodegeneration86. In contrast, mice deficient in C9orf72 exhibited enhanced TNF and IL-1 production when stimulated and defective maturation of phagosomes to lysosomes87. While these findings seem contradictory, they suggest that C9orf72 is required for normal microglial function and that altering microglial ability to clear aggregated proteins by altering phagosomes-to-lysosomes maturation, an important step in host defense, may contribute to neurodegeneration in patients carrying the C9orf72 expansion (Fig. 3). Future functional studies with microglia from C9orf72 patients would help clarify their complex role in this subset of ALS.
Transgenic mice expressing inducible human TDP-43 (hTDP-43) exhibit progressive motor neuron loss but only subtle microglial changes88. Following suppression of hTDP-43 transgene expression, microglia selectively cleared the existing neuronal hTDP-43. When microgliosis was blocked during the early recovery phase using a CSF1R and c-Kit inhibitor, these mice failed to regain full motor function, suggesting a neuroprotective role for microglia88. Interestingly, conditional deletion of TDP43 in microglia promotes their phagocytic functions and leads to enhanced synapse loss89. While additional work is required to establish a clear pathway linking TDP-43, microglia, and ALS pathogenesis, these findings suggest dysregulation of microglial phagocytic function in ALS patients with TDP-43 mutations.
We propose that several genes with ALS-associated mutations regulate microglial host defense functions, including production of ROS (mutant SOD1), cytokines (C9orf72), and phagocytosis (C9orf72 and TDP43; Fig. 3). These findings support a key role for microglia in ALS pathogenesis but indicate that targeting microglia for potential ALS therapy should be tailored to the specific pathway(s) affected and that a gunshot approach is not a useful therapeutic strategy.
Prion diseases
Prion diseases are genetic, sporadic, or acquired neurodegenerative disorders resulting from sustained aggregation of PrPsc, the proteinase-resistant form of the prion protein. Examples of prion diseases include Creutzfeldt–Jakob disease, scrapie, and chronic wasting disease. Prion-related neurodegeneration includes neuronal loss, increased proinflammatory microglia, and spongiform changes90. Microglia phagocytose PrPsc as early as 60 days postinfection91, and their depletion increases prion titers and susceptibility to prion infection92, suggesting they help control prion disease.
The double-edged sword metaphor also applies to microglia in prion diseases. Microglia produce ROS in response to the PrP106–126 fragment and enhance its neurotoxicity. Ablation of superoxideproducing enzymes protected mice from PrP toxicity, further suggesting that microglia mediate prion neurodegeneration93. Upregulation of proinflammatory IL-1β, IL-6, inducible nitric oxide synthase (iNOS), NF-κB, cyclophilin A, matrix metalloproteinases, and NLRP3 inflammasome components have all been demonstrated in prion disease microglia94,95. Whether this proinflammatory response affects disease progression is unclear, since deletion of NLRP3 or the inflammasome adaptor Pycard did not markedly affect the clinical course of scrapie in mice94.
Prion infection affects microglial sensing and housekeeping ability, in part by disrupting the Cx3cr1–fractalkine pathway96. PrPsc also reduces microglial phagocytosis of aberrant proteins, including PrPsc and apoptotic debris or cells, despite production of proinflammatory mediators, suggesting dysregulated host defense97. As in AD, PD, and ALS, the effects of PrPsc on microglia appear to be mediated by SRs and TLRs in an Src-kinase-dependent manner98,99. It is plausible that microglia try initially to clear PrPsc, leading to their persistent activation and dysregulated functions in another example of a host-defense response gone awry, resulting in neurotoxicity and subsequent disease progression.
Two common themes for microglia’s roles in neurodegenerative diseases emerge (Fig. 3). First, while microglia are performing their normal sentinel function, they sense the presence of an aberrant or misfolded protein such as Aβ, aggregated α-synuclein, oxidized or mutant SOD1, or PrPsc. In response to these toxic stimuli, microglia perform their host-defense function, attempting to clear these agents via SRs and other PRRs. The nature of the aberrant proteins or their persistent production disrupts microglial housekeeping functions and dysregulates microglial immune checkpoints and pathways that keep inflammation in check, such as the Cx3cr1 or progranulin pathways, leading to an exaggerated proinflammatory response, neurotoxicity, and neurodegeneration. A second theme is that in some neurodegenerative diseases, mutations in specific genes, such as Trem2, HTT, and TDP43, cause a self-autonomous dysregulation of sensing, housekeeping, or host defense, thereby initiating or exaggerating proinflammatory responses and leading to neurotoxicity and neurodegeneration.
Common pathways to neurodegeneration: microglial immune checkpoints
How do microglia damage and kill neurons?
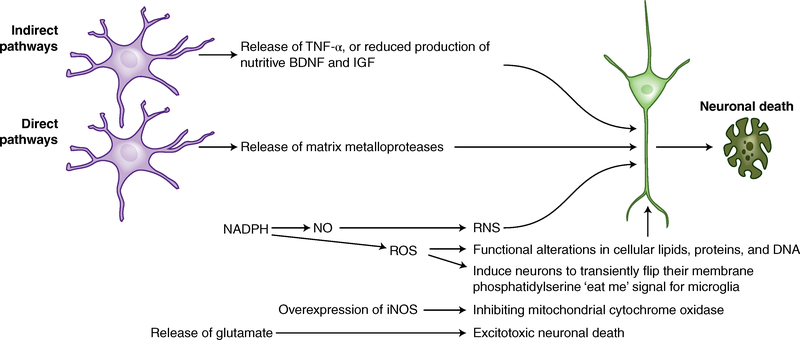
A recurring theme in neurodegenerative diseases is the damaging and killing of neurons by microglia using several direct and indirect tools (Fig. 4). When activated by ligands such as an infectious pathogen, Aβ, PrPSc, aggregated α-synuclein, or mSOD1, NADPH produces superoxide, which is released and either transformed into H2O2 by extracellular SOD or reacts with NO to produce peroxynitrite100. These cause cellular necrosis100 or apoptosis101 (Fig. 4). Microglia also cause excitotoxic neuronal death either by overexpressing iNOS or directly releasing glutamate101,102. Microglial proteases such as cathepsins are released in response to Aβ, leading to neuronal apoptosis103, and matrix metalloproteases can cause neuronal injury in hypoxia-ischemia (Fig. 4)104. Microglia can also damage neurons indirectly, either by releasing TNF or by reducing production of nutritive or neuroprotective brain-derived neurotrophic factor (BDNF) and insulin-like growth factor (IGF), thereby increasing neuronal apoptosis (Fig. 4)101.
Fig. 4 |. How microglia damage or kill neurons: There are several direct and indirect tools used by microglia to perform this task.
When microglia interact with ligands such as an infectious pathogen, Aβ, PrPSc, aggregated α-synuclein, or mSOD1, several pathways are activated. NADPH oxidase produces superoxide and derivative oxidants. Nitric oxide and its derivatives are produced by iNOS. Glutamate, cathepsin B, and other proteases are released, or phagocytic killing of stressed neurons occurs. Oxidative lipid damage reduces membrane fluidity and membrane potential and increases ion permeability, resulting in organelle swelling, loss of membrane depolarization, and rupture of the plasma membrane, leading to necrosis. Microglia also utilize indirect means to kill and damage neurons, including release of TNF, which stimulates NMDA receptor activity, or reduced production of nutritive BDNF and IGF.
It is evident that the microglial host-defense function provides microglia with the tools for fratricide to become neuronal killers. This does not happen continuously because microglia have several immunological checkpoints or pathways that prevent their overreaction to external stimuli. These include the Trem2, Cx3cr1–fractalkine sensing, and housekeeping pathways, the progranulin pathways that keep their inflammatory response in check, and the SR pathways that promote clearance of injurious stimuli. Dysregulation of any of these pathways or disruption of the sentinel and housekeeping functions initiates or exacerbates neurodegeneration (Box 1).
Box 1 |. Microglia immune checkpoints disrupted in neurodegeneration.
As with lymphocytes, microglia have several immunological checkpoints that prevent their overreaction to external stimuli. These checkpoints are different from those of lymphocytes. They include the microglial Trem2, Cx3cr1–fractalkine sensing, and housekeeping pathways and the progranulin pathways, which keep their inflammatory response in check. Dysregulation of any of these pathways or disruption of the sentinel, defense, or housekeeping functions initiates or exacerbates neurodegeneration.
Trem2 regulates all three microglial functions (sensing, housekeeping, and host defense) and dysregulation of Trem2 increases the risk for AD, FTLD, FTD, and possibly ALS.
Cx3cr1 regulates sensing and housekeeping functions. However, disruption of this pathway has not been shown to increase the risk for neurodegenerative diseases, but it alters disease courses in animal models.
The progranulin pathway regulates housekeeping and its disruption may increase risk for AD, FTLD, FTD, and ALS.
As with T cell immune checkpoints, microglial immune checkpoints may be important targets for therapy.
Trem2
The Trem2 gene encodes an innate immune receptor of the immunoglobulin family located on chromosome 6 in humans and chromosome 17 in mouse105. Trem2 is expressed on macrophages, dendritic cells, osteoclasts, and microglia and is part of the microglial sensome7. Trem2 ligands include ApoE, phosphatidylserine, sphingomyelin, Aβ, dead neurons, and damaged myelin106–108.
Trem2 forms a signaling complex with the adaptor tyrosine kinase-binding protein (TyroBP or DAP12)105. Ligand binding to Trem2 triggers phagocytosis and chemotaxis and negatively regulates TLR-induced inflammatory responses105. The extracellular domain of Trem2 can be released as a soluble protein (sTrem2) and increases with age and with MS, AD, and FTD109. How is Trem2 involved in specific neurodegenerative disease?
AD
Genome-wide association studies identified mutations in Trem2 as major risk factors in late-onset AD35. Trem2 expression increases in plaque-associated microglia and infiltrating monocytes, suggesting a role in the microglial response to Aβ105. Trem2 deletion decreased microglial phagocytosis, proliferation, and survival, and increased proinflammatory cytokines and RNS110. Trem2−/−Aβ-mice have increased Aβ deposition, reduced numbers of myeloid cells around plaques, reduced Aβ phagocytosis, and greater neuritic dystrophy in early disease111. This reduction of microglia around plaques was attributed to lower proliferation, decreased metabolic fitness112, and increased death111. Since microglia form a physical barrier to prevent plaque expansion and protect neurons113, increased accumulation of dystrophic neurons in Trem2−/−Aβ-mice was attributed to decreased clearance by microglia rather than increased neuronal death. Collectively, these results implicate Trem2 in microglial recruitment to Aβ plaques and restricting exposure of neurons to toxic Aβ.
Trem2 variants comprise amino acid substitutions, frameshift and nonsense mutations, and splice site alterations that likely result in loss of function114. The Trem2 variant with the strongest AD association is R47H (three to four times increased risk), a single amino acid substitution in the extracellular domain114. In transgenic Aβ-mice in which endogenous Trem2 was replaced with the human common variant of normal Trem2 or the variant R47H115, RH47 was associated with reduced Aβ-induced microglial responses, further supporting that R47H reduces Trem2 function in vivo.
Single-cell RNA-seq identified three microglial subpopulations in AD: homeostatic, intermediate, and DAM8. Transition from homeostatic to the DAM state appears to be a two-step process characterized by a Trem2-independent homeostatic-to-intermediate state and a Trem2-dependent intermediate-to-DAM state. DAMs are neuroprotective and clear Aβ. Another subtype of DAM identified in aged mice, models of AD, ALS, and MS, is dependent on a Trem2–ApoE pathway and is induced by phagocytosis of apoptotic neurons24. This subtype exhibits decreased housekeeping and sensing genes and increased neurodegeneration-associated genes, suggesting that the Trem2–ApoE pathway regulates a switch from the homeostatic to a neurodegenerative phenotype. More work is needed to reconcile the role of Trem2 in each DAM subtype. However, there is consensus that Trem2 is a key regulator of microglia functions and phenotype in AD.
Nasu–Hakola disease
Trem2 variants are also associated with increased risk for polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy, also called Nasu–Hakola disease (NHD)116. NHD patients develop axonal degeneration, loss of white matter, and cortical atrophy. They exhibit increased microglial density and activation suggesting a dysregulated proinflammatory response117.
Tauopathies, ALS, and PD
Trem2 may regulate tau pathology. sTrem2 correlates with tau levels in cerebrospinal fluid early in clinical AD118 and with tangle score and paired helical filament levels in postmortem AD brains119. Trem2 variants were identified in families with FTD or frontotemporal lobar degeneration (FTLD; Supplementary Table 1). Studies assessing the risk of FTD or FTLD in nonfamilial cases are conflicting, though Trem2 variants appear to influence clinical manifestations of these diseases120. One study found R47H to be a significant risk for ALS121, but this needs to be replicated. Attempts to correlate R47H with increased PD risk have had mixed results120.
Studies in mice are also conflicting. In one study, Trem2 deficiency exacerbated tau pathology, neurodegeneration, and spatial learning deficits122. In another study, Trem2 deficiency reduced microgliosis, brain atrophy, and levels of inflammatory cytokines without affecting tau levels123. Similarly, in a CTE model, injured Trem2−/− mice showed less hippocampal atrophy, reduced inflammatory transcripts, and improved behavioral deficits compared with controls. Additional studies are needed to definitively assess the role of Trem2 in tauopathies and reconcile conflicting results.
The association of Trem2 variants with AD, NHD, FTD, and possibly ALS and PD suggest that Trem2 is a major immune checkpoint that regulates an important microglial homeostatic pathway. Disruption of this pathway by Trem2 variants leads to neurodegeneration (Box 1). However, the diversity of pathologies associated with these diseases suggests that additional genetic and/or epigenetic factors interact with Trem2 to cause a specific disorder. Therefore, understanding the biology of Trem2 and how it regulates microglial functions will be useful not only for helping patients with Trem2 variants but for understanding the role of microglia in neurodegeneration in general. Ongoing work should determine whether Trem2 is a realistic therapeutic target for neurodegenerative diseases, at what stage would therapy work best, and whether sTrem2 is a potential biomarker for efficacy.
Cx3cr1–fractalkine
Interactions between neuronal fractalkine (Cx3cl1) and its microglial receptor, Cx3cr1, define another immune checkpoint and pathway that regulates microglial function, likely because fractalkine and Cx3cr1 promote reciprocal neuron–microglial signaling. Membrane-bound fractalkine or its soluble form, (s)fractalkine, are the only known ligands for Cx3cr1124.
Fractalkine–Cx3cr1 interactions regulate microglial homeostatic functions and temper microglial response to inflammatory and injurious stimuli. Blocking such interactions upregulates microglial TNF production, causing neurotoxicity125. In neurons, these interactions regulate synaptic maturation22 and promote neuronal survival125. However, as Cx3cr1 is also expressed on perivascular macrophages, studies using Cx3cr1−/− mice should be cautiously interpreted; the effects observed may also be attributed to perivascular macrophages.
AD
In Aβ and tau mouse models, Cx3cr1 deficiency enhances Aβ clearance126 with a gene-dosage effect, but worsens tau pathology55. In support of such dual role, overexpression of (s) fractalkine in tau mice but not in Aβ-mice led to substantial improvement127. The reason for this dichotomy is unclear, but since Cx3cr1 is important for microglial sensing and homeostatic functions, dysregulation of these functions is implicated. Because of this dichotomy, the role of fractalkine–Cx3cr1 in AD remains ambiguous and needs to be tested in mice with combined Aβ and tau pathology.
PD
In the MPTP (a dopaminergic neurotoxic compound) model of PD, Cx3cr1−/− mice showed more extensive neuronal loss than Cx3cr1+/− mice128. In support of this neuroprotective role, overexpression of (s)fractalkine reversed MPTP toxicity for dopaminergic neurons127. In contrast, in transgenic mice overexpressing α-synuclein, Cx3cr1−/− microglia were less activated by aggregated α-synuclein, leading to reduced loss of dopaminergic neurons129. In mice deficient in LRRK2, another PD-associated gene130, Cx3cr1 was upregulated and microglia had reduced responsiveness to lipopolysaccharide. These studies suggest that altered Cx3cr1-mediated microglial sensing and housekeeping functions play a direct effect in PD.
ALS
In the mSOD1G93A mouse model of ALS, Cx3cr1−/− mice showed more neuronal loss than Cx3cr1+/− or Cx3cr1+/+ mice, indicating a gene-dosage neuroprotective effect128. In support of this role, the loss of function of Cx3cr1 seen with the V249I rs3732379 Cx3cr1 variant was associated with accelerated progression and reduced survival in some ALS patients131, but not in others132. In both studies, Cx3cr1 variants did not increase risk for ALS. Though these opposing findings are confusing, they suggest that Cx3cr1 is a disease-modifying factor in ALS and raise the need for studies with more patients.
Prion diseases
Scrapie-infected hamsters’ brains show progressive downregulation of fractalkine96. The same effect is observed in response to PrP106–126 in mixed neuron–glia cultures96. Infection of Cx3cr1−/− mice reduced their time to disease-onset compared to wild-type mice, suggesting that Cx3cr1 may be protective in prion disease. No differences were seen in the pattern and localization of microglia or in chemokine and cytokine levels133. This was not fully duplicated in Cx3cr1−/− mice with a different genetic background134. Repeating these studies and using fractalkine-deficient (Cx3cl1−/−) mice would help clarify the discrepancy.
There is consensus that the Cx3cr1–fractalkine immune checkpoint is overall a neuroprotective pathway that regulates sensing, housekeeping, and host-defense functions of microglia (Box 1). However, there are multiple knowledge gaps in our understanding of the roles of this pathway in neurodegeneration.
Scavenger receptors
SRs are innate immune PRRs that promote removal of non-self or altered-self ligands and elimination of degraded or harmful substances135. They have roles in AD, PD, ALS, and prion diseases.
AD
SR-A1 is an Aβ-phagocytic receptor37,38 expressed on microglia surrounding Aβ plaques in humans and Aβ-mice. SR-A1-deficiency decreases microglial Aβ uptake by 60%37,136 and increases mortality and Aβ accumulation37,136, whereas upregulation of SR-A leads to reduced Aβ burden in Aβ-mice37. Therapeutically, it would be beneficial to upregulate microglial expression of SRA1 to increase Aβ clearance.
SR-B2 (also called CD36) is also a microglial Aβ receptor that forms a complex with TLR-4 and TLR-6, leading to cytokine, chemokine, and ROS production, as well as microglial migration, inflammasome activation, and neurotoxicity27,40. Polymorphism (rs3211892) that increases CD36 levels is associated with increased AD risk137. Therapeutically, it may be advantageous to inhibit CD36–Aβ binding or signaling to reduce microglial neurotoxicity138.
Binding of Aβ to the microglial SR-J1 (also called RAGE) activates MAPK and NF-κB and contributes to synaptic dysfunction through IL-1β release in 2- to 3-month-old animals139. Six-monthold RAGE−/− Aβ-mice have reduced Aβ deposition and more Aβ-degrading enzymes140. However, no change in cognition or microglial recruitment to plaques was seen in 12-month-old mice, suggesting that RAGE is not essential for microglial recruitment, but could affect Aβ processing in early disease140.
These reports indicate that various SRs play complementary roles in microglia–Aβ interactions that may have therapeutic implications for AD. Pharmacologic upregulation of SR-A1 expression or function may be helpful for AD treatment, whereas blocking CD36– or RAGE–Aβ interactions or reducing expression of these SRs may stop or delay AD progression.
PD, ALS, and prion diseases
Similarly to the roles they play in AD, SRs may also be involved in PD, ALS, and prion diseases through their interactions with misfolded proteins. SR-B2 promotes α-synuclein–microglial interactions64 and senses misfolded mutant or oxidized SOD1 in diseased motor neurons or in the extracellular space, rendering these cells proinflammatory85. Aggregated prion peptides also interact with microglial SRs, leading to uptake and/or neurotoxin production99.
Cumulatively, these reports suggest a potential common pathway for handling misfolded proteins in neurodegenerative diseases, involving SRs (Fig. 3). Because of their ability to bind a diverse class of ligands, SRs are important sensors of misfolded proteins including Aβ, mSOD1, aggregated α-synuclein, and PrPsc, possibly to clear them. With disease progression, SR expression decreases, leading to defective clearing and subsequent accumulation of these misfolded proteins. Some of these receptors, such as CD36, form receptor complexes with other PRRS such as TLRs and initiate an inflammatory response that leads to neurotoxicity and neurodegeneration.
Progranulin
Progranulin is a secreted glycoprotein with neuro-immunomodulatory properties and autocrine neurotrophic functions important for long-term neuronal survival141. Progranulin deficiency leads to age-dependent, progressive upregulation of lysosomal and innate immunity genes, increased complement production, and enhanced C1q-dependent synaptic pruning by microglia, suggesting that progranulin is an important immune checkpoint that suppresses aberrant microglial activation in aging21. Dysregulation of this pathway in microglia may occur in neurodegenerative diseases. Interestingly, similar results were obtained in a mouse model of adrenomyeloneuropathy. Deficiency in the very-long-chain fatty-acid transporter ABCD1 increased complement activation and synapse loss and aggravated microglial phagocytosis of neurons, suggesting a novel pathway for ‘death by microglia’142.
FTLD and AD
Haploinsufficiency caused by autosomal dominant mutations in the progranulin gene leads to FTLD141. Progranulin polymorphism is also linked to late-onset AD143. In Aβ-mice, progranulin overexpression inhibits Aβ deposition and protects from Aβ toxicity. Selective reduction of microglial expression of progranulin in Aβ-mice also impairs phagocytosis, increases plaque load, and exacerbates cognitive deficits. Thus, increasing progranulin expression is proposed as a potential therapy for FTLD and AD144.
PD
Progranulin polymorphism is associated with increased PD risk but the mechanism is not clear143. In the MPTP mouse model of PD, upregulation of progranulin expression in nigrostriatal neurons was accompanied by reductions in markers of MPTP-induced inflammation and apoptosis, and it protected these neurons from MPTP toxicity and preserved striatal dopamine content and turnover. This was associated with preservation of locomotor function. Dysregulation of the progranulin pathway may therefore contribute to PD pathogenesis, and restoring progranulin levels may have therapeutic potential for PD145.
ALS
Five progranulin mutations (four missense and one 5′ regulatory variant) are associated with reduction in age at onset and shorter survival after onset of ALS, suggesting that progranulin modifies ALS disease course146. The mechanism of this association is not clear. Based on mouse and human studies, progranulin appears to be an important immune checkpoint that modulates the microglial response to external stimuli and prevents them from overreacting to such stimuli and thereby causing neurodegeneration (Box 1).
Peripheral regulation of microglia in neurodegeneration
Despite the anatomical separation between the CNS and the gastrointestinal system, a bidirectional connection between the two, known as the ‘brain–gut axis’ exists. Intestinal bacterial colonization regulates immune system maturation and development in the CNS. Eradication of gut microbiota also alters microglial numbers, size, transcriptomes, and surveillance functions and downregulates host-defense genes147. Recolonization with a complex microbiota or with microbiota-derived products partially restores microglial properties147.
Human studies show associations between gut microbiota and neurodegenerative diseases148. In Aβ-mice, manipulating gut microbiota influences Aβ deposition and alters microglial reactivity and morphology by unknown mechanisms149. In a PD model, antibiotic treatment ameliorates PD pathology, whereas oral administration of specific microbial metabolites promotes neuroinflammation and motor symptoms150. These studies add another layer to how microglial functions are regulated in normal and diseased states, suggesting that gut-to-brain signaling modulates neurodegeneration.
Conclusions
Understanding of microglial biology has increased exponentially in the past decade. Microglial gene expression profiles are being defined at the single-cell level and correlated with specific functions. We are also beginning to understand microglial roles in neurodegeneration and to explore pathways that regulate their response to injury, including neuroprotective immune checkpoint pathways that keep the microglial proinflammatory response in check and pathways that promote clearance of injurious stimuli. We are also exploring how peripheral influences from the gut microbiome can alter progression of such injury. Yet significant knowledge-gaps exist. Analyzing the transcriptomes and epigenetic profiles in various disease states, understanding how aging and disease progression alter these profiles at the single-cell level, and correlating such changes with microglial behavior are necessary. Expanding the studies from mouse models to human patients remains a major limiting factor and requires development of new reliable in vitro cellular models derived from patient samples as well as additional technologies for in vivo imaging and analysis. In this regard, the ability to isolate primary microglia from fresh postmortem brain tissues or to reprogram human stem cells or monocytes to develop into microglia-like cells, as well as the ability to generate such cells from patients with various neurodegenerative diseases, are steps in the right direction15–17. However, since correct programming is dependent on the cell’s environment18, new techniques to incorporate these cells into three-dimensional organoids or improving existing techniques for organotypic brain slice culture while maintaining their in vivo transcriptomic and epigenetic profiles are crucial next breakthroughs waiting to be achieved. These steps are necessary for any successful effort to harness the power of microglia for treatment of neurodegenerative disease.
Supplementary Material
Acknowledgements
This work was supported by NIH grant RF1 AG051506 to J.E.K.
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41593-018-0242-x.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Río Hortega P Noticia de un nuevo y fácil método para la coloración de la neuroglia y el tejido conjuntivo. Trab. Lab. Invest. Biol 15, 367–378 (1918). [Google Scholar]
- 2.Giulian D & Baker TJ Characterization of ameboid microglia isolated from developing mammalian brain. J. Neurosci 6, 2163–2178 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung S et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol 20, 4106–4114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes SE et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci 9, 1512–1519 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Parkhurst CN, Hayes S & Gan WB Peripheral elevation of TNF-α leads to early synaptic abnormalities in the mouse somatosensory cortex in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 110, 10306–10311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickman SE, Allison EK & El Khoury J Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci 28, 8354–8360 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickman SE et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci 16, 1896–1905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keren-Shaul H et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Lawson LJ, Perry VH, Dri P & Gordon S Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 (1990). [DOI] [PubMed] [Google Scholar]
- 10.El Khoury J Neurodegeneration and the neuroimmune system. Nat. Med 16, 1369–1370 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM & El Khoury J Microglia in health and disease. Cold Spring Harb. Perspect. Biol 8, a020560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kierdorf K et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci 16, 273–280 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Tay TL et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci 20, 793–803 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Wang Y et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol 13, 753–760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abud EM et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muffat J et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med 22, 1358–1367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan KJ et al. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci. Transl. Med 9, eaai7635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourgeaud L et al. TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasek MJ et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui H et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci 17, 400–406 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Fuhrmann M et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci 13, 411–413 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krasemann S et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healy LM et al. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol 196, 3375–3384 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Khoury JB et al. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med 197, 1657–1666 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Khoury J et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med 13, 432–438 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Selkoe DJ & Hardy J The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med 8, 595–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frautschy SA et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol 152, 307–317 (1998). [PMC free article] [PubMed] [Google Scholar]
- 31.D’Andrea MR, Cole GM & Ard MD The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol. Aging 25, 675–683 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Tooyama I, Kimura H, Akiyama H & McGeer PL Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res. 523, 273–280 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Boucher C, Fontaine B & Delarasse C Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer’s disease models: effects of aging and amyloid pathology. Aging Cell 16, 27–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert JC et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45, 1452–1458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson T et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med 368, 107–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Khoury J & Hickman SE Mechanisms of amyloid-beta clearance in Alzheimer’s disease in Research Progress in Alzheimer’s Disease and Dementia, vol. 4 (ed. Sun M-K) 37–66 (Nova Science Publishers, Hauppauge, NY, USA, 2009). [Google Scholar]
- 37.Frenkel D et al. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun 4, 2030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Khoury J et al. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 382, 716–719 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Hong S et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coraci IS et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol 160, 101–112 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold M & El Khoury J β-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin. Immunopathol 37, 607–611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venegas C et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Hickman SE & El Khoury J The neuroimmune system in Alzheimer’s disease: the glass is half full. J. Alzheimers Dis 33(Suppl 1), S295–S302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oddo S, Caccamo A, Kitazawa M, Tseng BP & LaFerla FM Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 24, 1063–1070 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Villemagne VL et al. Aβ-amyloid and tau imaging in dementia. Semin. Nucl. Med 47, 75–88 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Bisht K et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrer I et al. Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J. Neuropathol. Exp. Neurol 73, 81–97 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci 18, 1584–1593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolós M et al. Direct evidence of internalization of tau by microglia in vitro and in vivo. J. Alzheimers Dis 50, 77–87 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Lee DC et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J. Neuroinflammation 7, 56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saman S et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem 287, 3842–3849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiandaca MS et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11, 600–7.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherry JD et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun 4, 112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshiyama Y et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Bhaskar K et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nash KR et al. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol. Aging 34, 1540–1548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bemiller SM et al. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener 12, 74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng H, Wang P & Jankovic J The genetics of Parkinson disease. Ageing Res. Rev 42, 72–85 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Dickson DW Neuropathology of Parkinson disease. Parkinsonism Relat. Disord 46(Suppl 1), S30–S33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeer PL, Itagaki S, Boyes BE & McGeer EG Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291 (1988). [DOI] [PubMed] [Google Scholar]
- 61.Gerhard A et al. In vivo imaging of microglial activation with [11C] (R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis 21, 404–412 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Halliday GM & Stevens CH Glia: initiators and progressors of pathology in Parkinson’s disease. Mov. Disord 26, 6–17 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Croisier E, Moran LB, Dexter DT, Pearce RK & Graeber MB Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J. Neuroinflammation 2, 14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su X et al. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 29, 1690–1701 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim C et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun 4, 1562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhlmann T et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Yamasaki R et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med 211, 1533–1549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zrzavy T et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh R & Tabrizi SJ Huntington disease. Handb. Clin. Neurol 147, 255–278 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Tai YF et al. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 130, 1759–1766 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Pavese N et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 66, 1638–1643 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Sapp E et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol 60, 161–172 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Crotti A et al. Mutant huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci 17, 513–521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lall D & Baloh RH Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J. Clin. Invest 127, 3250–3258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henkel JS et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol 55, 221–235 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Turner MR et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol. Dis 15, 601–609 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Gurney ME et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Yamanaka K et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc. Natl. Acad. Sci. USA 105, 7594–7599 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apolloni S, Amadio S, Montilli C, Volonté C & D’Ambrosi N Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Hum. Mol. Genet 22, 4102–4116 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Hall ED, Oostveen JA & Gurney ME Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 23, 249–256 (1998). [DOI] [PubMed] [Google Scholar]
- 81.Liao B, Zhao W, Beers DR, Henkel JS & Appel SH Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol 237, 147–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frakes AE et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meissner F, Molawi K & Zychlinsky A Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc. Natl. Acad. Sci. USA 107, 13046–13050 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harraz MM et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Invest 118, 659–670 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao W et al. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia 58, 231–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Rourke JG et al. C9orf72 BAC transgenic mice display typical pathologic features of ALS/FTD. Neuron 88, 892–901 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Rourke JG et al. C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spiller KJ et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat. Neurosci 21, 329–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paolicelli RC et al. TDP-43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron 95, 297–308.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iaccarino L et al. An in vivo 11C-(R)-PK11195 PET and in vitro pathology study of microglia activation in Creutzfeldt-Jakob disease. Mol. Neurobiol 55, 2856–2868 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Yamasaki T, Suzuki A, Hasebe R & Horiuchi M Flow cytometric detection of PrPSc in neurons and glial cells from prion-infected mouse brains. J. Virol 92, e01457–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falsig J et al. A versatile prion replication assay in organotypic brain slices. Nat. Neurosci 11, 109–117 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sorce S et al. The role of the NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog. 10, e1004531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguzzi A & Zhu C Microglia in prion diseases. J. Clin. Invest 127, 3230–3239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hafner-Bratkovič I, Benčina M, Fitzgerald KA, Golenbock D & Jerala R NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1β and neuronal toxicity. Cell. Mol. Life Sci 69, 4215–4228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie WL et al. Abnormal activation of microglia accompanied with disrupted CX3CR1/CX3CL1 pathway in the brains of the hamsters infected with scrapie agent 263K. J. Mol. Neurosci 51, 919–932 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Hughes MM, Field RH, Perry VH, Murray CL & Cunningham C Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon LPS stimulation. Glia 58, 2017–2030 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakai K et al. Absence of CD14 delays progression of prion diseases accompanied by increased microglial activation. J. Virol 87, 13433–13445 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kouadir M et al. CD36 participates in PrP(106–126)-induced activation of microglia. PLoS One 7, e30756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simonian NA & Coyle JT Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol 36, 83–106 (1996). [DOI] [PubMed] [Google Scholar]
- 101.Brown GC & Vilalta A How microglia kill neurons. Brain Res. 1628(Pt B), 288–297 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Maezawa I & Jin LW Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci 30, 5346–5356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gan L et al. Identification of cathepsin B as a mediator of neuronal death induced by Abeta-activated microglial cells using a functional genomics approach. J. Biol. Chem 279, 5565–5572 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Leonardo CC, Hall AA, Collier LA, Gottschall PE & Pennypacker KR Inhibition of gelatinase activity reduces neural injury in an ex vivo model of hypoxia-ischemia. Neuroscience 160, 755–766 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hickman SE & El Khoury J TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol 88, 495–498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeh FL, Wang Y, Tom I, Gonzalez LC & Sheng M TREM2 Binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91, 328–340 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Wang Y et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y et al. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron 97, 1023–1031.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suárez-Calvet M et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci. Transl. Med 8, 369ra178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi K, Rochford CD & Neumann H Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med 201, 647–657 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med 213, 667–675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ulland TK et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Condello C, Yuan P, Schain A & Grutzendler J Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun 6, 6176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jay TR, von Saucken VE & Landreth GE TREM2 in neurodegenerative diseases. Mol. Neurodegener 12, 56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song WM et al. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med 215, 745–760 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paloneva J et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med 198, 669–675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Satoh J et al. Immunohistochemical characterization of microglia in Nasu-Hakola disease brains. Neuropathology 31, 363–375 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Suárez-Calvet M et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med 8, 466–476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lue LF et al. TREM2 protein expression changes correlate with Alzheimer’s disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol. 25, 469–480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lill CM et al. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimers Dement. 11, 1407–1416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cady J et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 71, 449–453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang T et al. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiol. Aging 36, 3176–3186 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Leyns CEG et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 114, 11524–11529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hickman SE & El Khoury J Mechanisms of mononuclear phagocyte recruitment in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 9, 168–173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zujovic V, Schussler N, Jourdain D, Duverger D & Taupin V In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J. Neuroimmunol 115, 135–143 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Liu Z, Condello C, Schain A, Harb R & Grutzendler J CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J. Neurosci 30, 17091–17101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morganti JM et al. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J. Neurosci 32, 14592–14601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cardona AE et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci 9, 917–924 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Thome AD, Standaert DG & Harms AS Fractalkine signaling regulates the inflammatory response in an α-synuclein model of Parkinson disease. PLoS One 10, e0140566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin I et al. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell 157, 472–485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lopez-Lopez A et al. CX3CR1 is a modifying gene of survival and progression in amyotrophic lateral sclerosis. PLoS One 9, e96528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calvo A et al. Common polymorphisms of chemokine (C-X3-C motif) receptor 1 gene modify amyotrophic lateral sclerosis outcome: a population-based study. Muscle Nerve 57, 212–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grizenkova J, Akhtar S, Brandner S, Collinge J & Lloyd SE Microglial Cx3cr1 knockout reduces prion disease incubation time in mice. BMC Neurosci. 15, 44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Striebel JF, Race B, Carroll JA, Phillips K & Chesebro B Knockout of fractalkine receptor Cx3cr1 does not alter disease or microglial activation in prion-infected mice. J. Gen. Virol 97, 1481–1487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.PrabhuDas MR et al. A consensus definitive classification of scavenger receptors and their roles in health and disease. J. Immunol 198, 3775–3789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cornejo F et al. Scavenger receptor-A deficiency impairs immune response of microglia and astrocytes potentiating Alzheimer’s disease pathophysiology. Brain Behav. Immun 69, 336–350 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Šerý O et al. CD36 gene polymorphism is associated with Alzheimer’s disease. Biochimie 135, 46–53 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Wilkinson K, Boyd JD, Glicksman M, Moore KJ & El Khoury J A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. J. Biol. Chem 286, 34914–34922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Origlia N et al. Microglial receptor for advanced glycation end product-dependent signal pathway drives beta-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J. Neurosci 30, 11414–11425 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vodopivec I et al. RAGE does not affect amyloid pathology in transgenic ArcAbeta mice. Neurodegener. Dis 6, 270–280 (2009). [DOI] [PubMed] [Google Scholar]
- 141.Chitramuthu BP, Bennett HPJ & Bateman A Progranulin: a new avenue towards the understanding and treatment of neurodegenerative disease. Brain 140, 3081–3104 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Gong Y et al. Microglial dysfunction as a key pathological change in adrenomyeloneuropathy. Ann. Neurol 82, 813–827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Y et al. Association of progranulin polymorphism rs5848 with neurodegenerative diseases: a meta-analysis. J. Neurol 262, 814–822 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Minami SS et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat. Med 20, 1157–1164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Kampen JM, Baranowski D & Kay DG Progranulin gene delivery protects dopaminergic neurons in a mouse model of Parkinson’s disease. PLoS One 9, e97032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sleegers K et al. Progranulin genetic variability contributes to amyotrophic lateral sclerosis. Neurology 71, 253–259 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Erny D et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 18, 965–977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vogt NM et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep 7, 13537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Minter MR et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep 6, 30028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sampson TR et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.