Abstract
Objective
To examine the differential diagnostic significance of cerebrospinal fluid (CSF) biomarkers reflecting Alzheimer’s disease-related amyloid β (Aβ) production and aggregation, cortical neuronal damage, tau pathology, damage to long myelinated axons and astrocyte activation, which hypothetically separates patients with idiopathic normal pressure hydrocephalus (iNPH) from patients with other neurodegenerative disorders.
Methods
The study included lumbar CSF samples from 82 patients with iNPH, 75 with vascular dementia, 70 with Parkinson’s disease, 34 with multiple system atrophy, 34 with progressive supranuclear palsy, 15 with corticobasal degeneration, 50 with Alzheimer’s disease, 19 with frontotemporal lobar degeneration and 54 healthy individuals (HIs). We analysed soluble amyloid precursor protein alpha (sAPPα) and beta (sAPPβ), Aβ species (Aβ38, Aβ40 and Aβ42), total tau (T-tau), phosphorylated tau, neurofilament light and monocyte chemoattractant protein 1 (MCP-1).
Results
Patients with iNPH had lower concentrations of tau and APP-derived proteins in combination with elevated MCP-1 compared with HI and the non-iNPH disorders. T-tau, Aβ40 and MCP-1 together yielded an area under the curve of 0.86, differentiating iNPH from the other disorders. A prediction algorithm consisting of T-tau, Aβ40 and MCP-1 was designed as a diagnostic tool using CSF biomarkers.
Conclusions
The combination of the CSF biomarkers T-tau, Aβ40 and MCP-1 separates iNPH from cognitive and movement disorders with good diagnostic sensitivity and specificity. This may have important implications for diagnosis and clinical research on disease mechanisms for iNPH.
Keywords: idiopathic normal pressure hydrocephalus, Alzheimer’s disease, Parkinson’s disease, Multiple systems atrophy, Progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia, vascular dementia, biomarkers, CSF
Introduction
Idiopathic normal pressure hydrocephalus (iNPH), clinically characterised by disturbance of gait and balance, cognition and continence, is one of few neurodegenerative disorders that can be successfully treated.1 Shunt surgery improves around 80% of the patients, but if left untreated, patients will deteriorate emphasising the need to diagnose and treat patients with iNPH without delay.2 Nevertheless, the condition remains underdiagnosed and undertreated3 4 in part due to lack of specific diagnostic tests.
Diagnosing iNPH can at times be difficult as similar signs and symptoms can be seen in other neurodegenerative conditions such as Alzheimer’s disease (AD), vascular dementia (VAD) of the subcortical type and extrapyramidal disorders such as Parkinson’s disease (PD) and atypical parkinsonian syndromes.5–8
Diagnostic markers for these disorders are few. In patients with iNPH, a characteristic pattern comprising low levels of soluble amyloid precursor protein (APP) α and β, amyloid β (38, 40, 42), and total tau (T-tau) and phosphorylated tau (P-tau) proteins has been identified, which seems to separate iNPH from patients with AD, subcortical VAD and controls.9–13 Furthermore, these cerebrospinal fluid (CSF) protein pattern changes are reversed after shunt surgery.9 11
The pathophysiology of iNPH is related to disturbance of CSF dynamics and thus partly different from aforementioned neurodegenerative disorders. The characteristic CSF protein pattern seen in iNPH might thus, besides being a pathophysiological hallmark, constitute a diagnostic tool. Identification of a pathognomonic CSF protein pattern in iNPH would radically strengthen diagnostic accuracy, thereby leading to earlier identification and treatment of patients with iNPH.
Aim
To validate the differential diagnostic significance of CSF biomarkers reflecting amyloid cascade function, AD-related amyloid β (Aβ) production and aggregation, cortical neuronal damage, tau pathology, damage to long myelinated axons and astrocyte activation. All of which hypothetically separates iNPH from other common neurodegenerative disorders
Methods
Study populations
In all, 82 patients with iNPH were selected retrospectively. Patients that had received the diagnosis of iNPH and had undergone preoperative and postoperative examination were consecutively included. All were diagnosed at the Hydrocephalus Research Unit, Sahlgrenska University Hospital in Gothenburg, Sweden, according to international guidelines.6 The patients were staged according to the iNPH scale, and improvement was defined as an increase in ≥5 points on the iNPH scale on the postoperative exam.14 All patients received a ventriculo-peritoneal shunt with an adjustable valve and were evaluated post-surgery at median 8 months (IQR 6:12) by the same clinical protocol. All shunts were working at the time for re-evaluation.
In all, 70 patients with definite PD according to the UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria15; 34 with probable multiple system atrophy (MSA) according to Gilman’s criteria16 ; 34 with probable or definite progressive supranuclear palsy (PSP) according to the National Institute of Neurological Disorders and Stroke and Society for Progressive Supranuclear Palsy, clinical criteria17 ; and 15 with probable corticobasal degeneration (CBD) according to Armstrong et al 18 diagnosed at the Sahlgrenska University Hospitals’ movement disorders unit were included.
In all, 50 patients with AD and 19 patients with frontotemporal lobar degeneration (FTLD) were diagnosed at the Department of Neurology at Kuopio University Hospital, Kuopio, Finland. The diagnoses of AD and FTLD were set by an experienced neurologist specialised in memory disorders. All patients in the AD group met the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association) criteria for probable AD.19 Patients with FTLD were clinically diagnosed according to Nearys’ criteria.20
In all, 75 patients with VAD were diagnosed at the Department of Geriatric Medicine at Linköping University Hospital, Sweden using the International Classification of Disease 10th Revision criteria and subgrouped according to the Association Internationale pour la Recherche et l'Enseignement en Neurosciences) criteria.21 The patients have been described earlier.22
In total, 54 neurologically healthy individuals (HIs) were included in the analysis. From Kuopio University Hospital, 20 cognitively tested subjects with a mini mental state examination (MMSE) score of 26 or higher undergoing spinal anaesthesia due to hip or knee arthroplasty were selected.23 From the Linkoping University Hospital, Linköping, Sweden, 34 controls >60 years of age and with a MMSE score of 26 points or higher undergoing planned surgical orthopaedic intervention with spinal anaesthesia were selected.22
AD, FTLD, VAD, PD, PSP, MSA and CBD were together denoted non-iNPH disorders. Non- iNPH disorders were further subdivided in terms of primary dysfunction. AD, FTLD and VAD were named cognitive disorders, whereas PD, PSP, MSA and CBD formed movement disorders.
Written informed consent was obtained from all participants or their next of kin in agreement with the Declaration of Helsinki. The study was approved by the Regional Ethical Review Board in Gothenburg/Sweden, Kuopio/Finland and Linköping/Sweden.
CSF analyses
All CSF samples were collected via lumbar puncture with the patient in recumbent position, handled using a standard protocol and kept frozen at −80°C until analysis.
Neurofilament light concentration was measured using an in house ELISA as previously described in detail.24 Amyloid β isoforms (Aβ38, Aβ40 and Aβ42), sAPPα, sAPPβ and monocyte chemoattractant protein 1 (MCP-1) were analysed by electrochemiluminescence assays as described by the kit manufacturer (Meso Scale Discovery, Rockville, MD, USA).25 CSF T-tau and P-tau concentrations were measured using commercially available INNOTEST ELISAs as described by the kit manufacturer (Fujirebio, Ghent, Belgium). All analyses were performed batch-wise in one round of experiments by board-certified laboratory technicians who were blinded to clinical data at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. Between run coefficient of variation was below 12% for all measurements.
Statistics
One-way analysis of covariance (ANCOVA) adjusted for age and sex was used for post-hoc analysis with Dunnett’s multiple comparisons test. All significance tests were two-sided and conducted at the 5% significance level. Univariable logistic regression analysis was performed for each individual CSF variable to separate iNPH versus non-iNPH disorders. Stepwise selection of the significant variables was used to select a multivariable logistic model and the chosen model was then cross-validated. Area under the curve (AUC-statistics) was calculated for description of goodness of models for iNPH versus HI, non-iNPH, cognitive disorders and movement disorders. Statistical analyses were performed using SPSS Statistics for Windows V.25 (IBM Corp. Released 2014. IBM SPSS Statistics for Windows, V.25.0. Armonk, NY, USA: IBM Corp.), SAS V.9 for Windows, SAS Institute, Cary, NC, USA and GraphPad Prism for Windows V.8.0.2 (GraphPad Software, La Jolla, CA, USA, www.graphpad.com).
Data availability statement
Any data not published within the article is available and anonymised data will be shared by request from any qualified investigator.
Results
In all, 48 of the 82 (58.5 %) patients with iNPH improved from shunt placement (≥5 points), 23 (28%) remained unchanged (-5 to 5 points), whereas 11 (13.5 %) deteriorated (≤ points).
Patients with iNPH had, compared with HI, lower concentrations of P-tau and APP-derived proteins (sAPPα, sAPPβ, Aβ38, Aβ40 and Aβ42) in combination with elevated MCP-1 (table 1).
Table 1.
Demographic data and biomarker concentrations
| HI | iNPH | Non-iNPH disorders | AD | FTLD | VAD | PD | MSA | PSP | CBD | |
| n=54 | n=82 | n=295 | n=50 | n=19 | n=75 | n=70 | n=34 | n=34 | n=15 | |
| Female (%) | 32 (59) | 29 (35) | 161 (55) | 29 (63) | 14 (74) | 45 (60) | 23 (33) | 20 (59) | 20 (59) | 10 (67) |
| Age; mean (min-max) | 71 (53–93) | 73 (52–89) | 69 (26–88) | 71 (51–81) | 69 (55–84) | 79 (58–88) | 60 (26–87) | 65 (49–83) | 70 (54–82) | 68 (49–77) |
| Biomarkers; mean (SD) | ||||||||||
| T-tau (pg/mL) | 346 (181) | 245 (131) | 496 (443)*** # | 980 (333)*** ### | 342 (146) | 651 (594)*** ## | 224 (105) | 315 (200) | 329 (247) | 366 (230) |
| P-tau (pg/mL) | 50 (20)*** | 32 (12) | 54 (36)*** | 96 (27)*** ### | 45 (13) | 63 (41)*** | 33 (12) | 35 (18) | 46 (38)* | 42 (19) |
| NFL (pg/mL) | 1170 (896) | 1717 (1963) | 1960 (2508) | 1977 (3104) | 2089 (1401) | 2646 (3475) | 839 (622) | 2322 (987) | 2219 (2761) | 2137 (1178) |
| Aβ38 (pg/mL) | 2181 (645)*** | 1526 (519) | 2194 (794)*** | 2710 (807)*** ## | 2324 (608)*** | 2136 (672)*** | 2056 (624)*** | 1888 (856)* | 2125 (1076)*** | 2091 (684)* |
| Aβ40 (pg/mL) | 5425 (1510)*** | 3800 (1193) | 5428 (1684)*** | 6541 (1654)*** # | 5801 (1222)*** | 5477 (1540)*** | 5067 (1391)*** | 4650 (1815)* | 5099 (1965)*** | 5197 (1561)** |
| Aβ42 (pg/mL) | 541 (239)*** | 364 (138) | 454 (197)**# | 318 (95)### | 569 (204)*** | 387 (191)### | 548 (187)*** | 489 (203)* | 488 (170)* | 505 (216) |
| sAPPα (pg/mL) | 661 (223)*** | 446 (178) | 693 (282)*** | 865 (310)*** ### | 738 (279)*** | 631 (262)*** | 715 (262)*** | 597 (209) | 650 (309)** | 585 (224) |
| sAPPβ (pg/mL) | 508 (166)*** | 321 (121) | 498 (188)*** | 599 (192)*** | 502 (166)** | 484 (188)*** | 503 (178)*** | 414 (142) | 470 (212)** | 452 (154) |
| MCP-1 (pg/mL) | 410 (110)* | 492 (109) | 416 (138)** | 436 (162) | 400 (101) | 456 (115) | 382 (128)** | 365 (70)** | 410 (121) | 448 (272) |
Number of patients, age and sex in the different neurodegenerative groups and healthy individuals. Sex is presented as n females (%), age as mean and min-max. Significance testing in comparison with iNPH and healthy controls was done by one-way ANCOVA adjusted for age and sex with Dunnett’s multiple comparisons test and shown as *p< 0.05, **p< 0.01, ***p< 0.001 (vs iNPH) and #p< 0.05, ##p< 0.01, ###p< 0.001 (vs healthy controls).
Aβ, AD-related amyloid β; AD, Alzheimer’s disease; ANCOVA, analysis of covariance; CBD, corticobasal degeneration; FTLD, frontotemporal lobe degeneration; MCP-1, monocyte chemoattractant protein 1; MSA, multiple system atrophy; NFL, neurofilament light; Non-iNPH disorders, AD+FTLD+VAD+PD+MSA+PSP+CBD; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; P-tau, phosphorylated tau; T-tau, total tau; VAD, vascular dementia; iNPH, idiopathic normal pressure hydrocephalus; sAPP, soluble amyloid precursor protein.
Compared with the non-iNPH disorders group, iNPH was characterised by the same significant change; low concentration of tau proteins and APP-derived proteins, and elevated MCP-1 (table 1).
The tau proteins were higher in AD and VAD than in both iNPH and HI. In most of the movement disorders (PD, MSA and CBD), the tau proteins were at the same level as in iNPH, except in PSP, where the P-tau was slightly elevated. These results are confirmative of previous studies.8
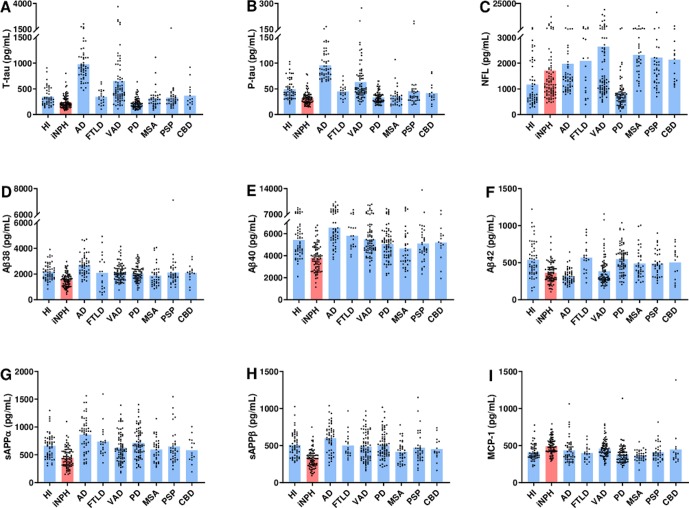
All APP-derived proteins besides Aβ42 were lower in iNPH compared with both the cognitive and the movement disorders groups. Aβ42 concentrations in iNPH were compatible to those in AD, whereas the other APP-derived proteins were lower in iNPH (table 2). In figure 1, the biomarkers are presented as individual observations for each diagnosis.
Table 2.
CSF biomarkers in iNPH compared with non-iNPH disorders, cognitive disorders and movement disorders
| iNPH | Non-iNPH disorders | Cognitive disorders | Movement disorders | |
| n=82 | n=297 | n=144 | n=153 | |
| T-tau (pg/mL) | 245 (131) | 496 (443)*** | 725 (517)*** | 282 (186) |
| P-tau (pg/mL) | 32 (12) | 54 (36)*** | 72 (38)*** | 37 (22)* |
| NFL (pg/mL) | 1717 (1963) | 1960 (2508) | 2340 (3147) | 1603 (1633) |
| Aβ38 (pg/mL) | 1526 (519) | 2194 (794)*** | 2360 (757)*** | 2037 (798)*** |
| Aβ40 (pg/mL) | 3800 (1193) | 5428 (1684)*** | 5889 (1610)*** | 4994 (1641)*** |
| Aβ42 (pg/mL) | 364 (138) | 454 (197)*** | 387 (182) | 517 (190)*** |
| sAPPα (pg/mL) | 446 (178) | 693 (282)*** | 726 (299)*** | 661 (262)*** |
| sAPPβ (pg/mL) | 321 (121) | 498 (188)*** | 527 (193)*** | 471 (179)*** |
| MCP-1 (pg/mL) | 492 (109) | 416 (138)*** | 442 (132)* | 391 (138)*** |
CSF biomarkers in iNPH compared to non-iNPH disorders, cognitive disorders and movement disorders. Concentrations are given as mean and SD. Significance testing was done by one-way ANCOVA corrected for age and sex with Dunnett’s multiple comparisons test and is shown as *p< 0.05, **p< 0.01, ***p<0.001.
Aβ, AD-related amyloid β; AD, Alzheimer’s disease; ANCOVA, analysis of covariance; CBD, corticobasal degeneration; CSF, cerebrospinal fluid; Cognitive disorders, AD+FTLD+VAD; FTLD, frontotemporal lobe degeneration; MCP-1, monocyte chemoattractant protein 1; MSA, multiple system atrophy; Movement disorders, PD, MSA, PSP, CBD; NFL, neurofilament light; Non-iNPH disorders, AD+FTLD+VAD+PD+MSA+PSP+CBD; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; P-tau, phosphorylated tau; T-tau, total tau; VAD, vascular dementia; iNPH, idiopathic normal pressure hydrocephalus; sAPP, soluble amyloid precursor protein.
Figure 1.
Biomarker concentrations in CSF as individual values in each diagnostic group. Biomarker concentrations in CSF plotted as individual values for each group. In each graph, values are given for HIs, iNPH, AD, FTLD, VAD, PD, MSA, PSP and CBD. (A) Concentration of T-tau, (B) P-tau, (C) NFL, (D) Aβ38, (E) Aβ40, (F) Aβ42, (G) sAPPα, (H) sAPPβ and (I) MCP-1. Bar indicates mean value. Y-axes are broken to enhance visibility. Aβ, AD-related amyloid β; AD, Alzheimer’s disease; CBD, corticobasal degeneration; CSF, cerebrospinal fluid; FTLD, frontotemporal lobar degeneration; HIs, healthy individuals; iNPH, idiopathic normal pressure hydrocephalus; MCP-1, monocyte chemoattractant protein 1; MSA, multiple system atrophy; NFL, neurofilament light; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; P-tau, phosphorylated tau; sAPP, soluble amyloid precursor protein; T-tau, total tau; VAD, vascular dementia.
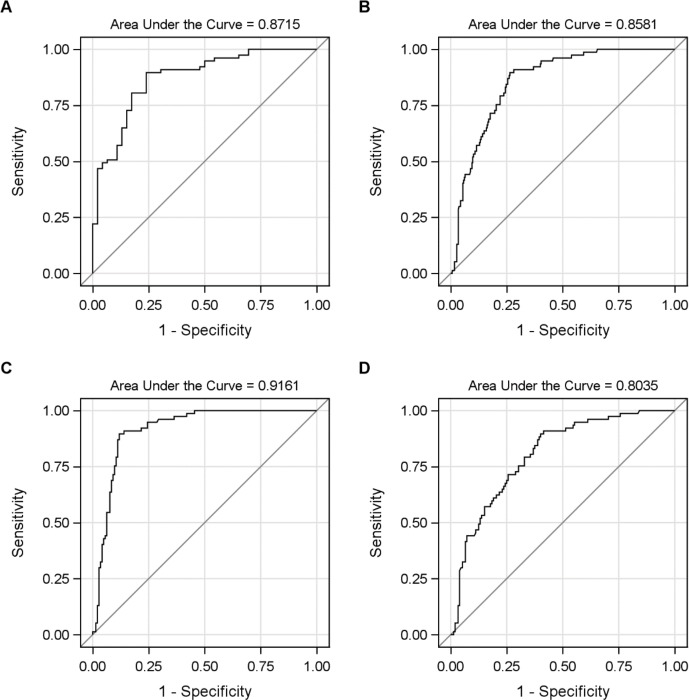
Based on pathophysiological considerations, we combined a tau CSF biomarker with an APP-derived protein and the astroglia marker MCP-1. The model consisting of P-tau, Aβ40 and MCP-1, revealed a high AUC 0.85, and cross-validated 0.84 was achieved. However, after cross-validation, T-tau, instead of P-tau, reached the highest AUC and the final model was T-tau, Aβ40 and MCP-1. Optimisation yielded 0.0.00533*MCP-1–0.00062*Aβ40–0. 000238*T-tau and in its simplified form: 10*MCP-1-Ab40-5*T-tau. The AUC for the model chosen was 0.86 for iNPH versus non-iNPH and the cross-validated AUC was 0.85. Figure 2 presents receiver operating characteristic (ROC) curves for the simplified model and its AUC for iNPH versus HI, versus non-iNPH-disorders, versus dementia and versus movement disorders, respectively. The highest value for separation of iNPH versus non-iNPH was found in the subanalysis of iNPH versus cognitive disorders where AUC reached 0.92.
Figure 2.
ROC curves to separate iNPH with the multivariable model 10*MCP-1 *- Aβ40–5*T-tau. ROC curves for the simplified model 10*MCP-1-Aβ40-5*T-tau. ROC curves are given for (A) iNPH versus HI (AUC = 0.8715), (B) iNPH versus non-iNPH (AUC = 0.8581), (C) iNPH versus cognitive disorders (AUC = 0.9161) and (D) iNPH versus movement disorders (AUC = 0.8035). Aβ, AD-related amyloid β; iNPH, iNPH=idiopathic normal pressure hydrocephalus; HI, healthy control; MCP-1, monocyte chemoattractant protein 1; T-tau; total tau.
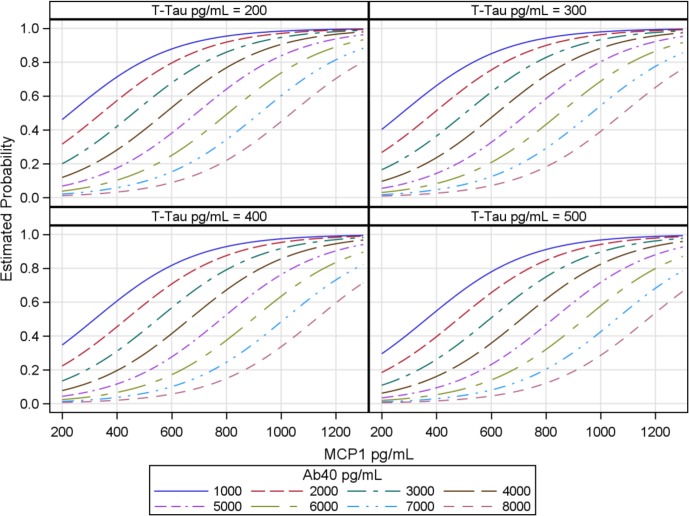
The diagnostic predictability of the combination of the three CSF biomarkers T-tau, Aβ40 and MCP-1 was very high. As evident from the plot, the highest probability of iNPH is for the combination of low T-tau and Aβ40 in combination with an elevated MCP-1.(figure 3).
Figure 3.
Prediction plot of iNPH versus non-iNPH disorders. Prediction plot showing probability of iNPH. T-tau is given in four concentrations, Aβ40 is given in eight different intervals, whereas MCP-1 is shown as a continuous variable on the X-axes. Estimated probability of iNPH is given on the Y-axes. Aβ, AD-related amyloid β; iNPH, iNPH=idiopathic normal pressure hydrocephalus; MCP-1, monocyte chemoattractant protein 1; T-tau, total tau.
Discussion
In this study, we confirm the earlier described characteristic CSF biochemical pattern in iNPH by means of low concentrations of tau proteins and APP-derived proteins in combination with elevated MCP-1.9
The most exciting result in this study is that the typical iNPH profile was not found in the other neurodegenerative disorders supporting the notion that the pathophysiology of iNPH is different from the other conditions. The CSF biomarker profile in iNPH indicates that the pathophysiology is characterised by changed metabolism of APP as well as astrocyte activation, but no major cortical neural damage or tau pathology.
Although not pathognomonic, the biomarker pattern seems to be rather specific for iNPH indicating that iNPH has a specific, from most neurodegenerative disorders different, pathophysiology. Unlike the before mentioned neurodegenerative disorders, iNPH is characterised by a disturbance of CSF dynamics with impaired CSF absorption and enlarged ventricles. The CSF flow is reversed with a caudal-rostral flow through the aqueduct and a possible CSF absorption into the periventricular capillaries. A reduced cerebral perfusion has been documented in the periventricular tissue related to either a reduced metabolism or degenerative changes or both.26 27
The APPs play a role in cell signalling and are important for synaptic formation and repair.28 Roles in cell signalling, long-term potentiation and cell adhesion have also been proposed29 and the low concentrations of APP-derived proteins in CSF could mirror the degree of metabolic changes in the periventricular tissue. The importance of the periventricular region around the third ventricle and frontal horns is also illustrated by symptoms and signs in iNPH being generated from those regions.30 Support for a downregulated periventricular metabolism is the fact that symptomatology in iNPH is reversible and the changes in the APP-derived proteins normalise after surgical treatment of patients with iNPH.9 11
The low concentrations of CSF T-tau and P-tau, which are reversed after treatment,9 11 31 could signal a reduced cortical metabolism or neuronal activity. The biomarker changes seen in iNPH do not support any major cortical degenerative process or tau mis-phosphorylation in iNPH.
MCP-1 expression is involved in cerebral inflammatory processes and the expression in glial cells is increased in traumatic brain injury.32 Pathological examinations of iNPH cases have revealed neurodegenerative changes in the periventricular white matter with demyelination and astrogliosis,13 33 which might be the explanation for the increased MCP-1 concentration seen in iNPH. These degenerative changes could associate with the fact that untreated patients with iNPH will deteriorate over time.2
It has been argued that CSF biomarkers levels may be misleading in iNPH, possibly due to brain compression in iNPH.34 We have reported that amyloid precursor like protein 1 (a substitute marker for APP production) is not affected to the same extent as APP proteins, arguing that it is rather the APPs that are downregulated than a mere reduced clearance from the brain parenchyma to the CSF.35 This does not exclude the possibility of the combination of a downregulated APP production and a reduced clearance from the parenchyma. Micro dialysis studies indicates that Aβ 1–42 levels are within a similar range in CSF and intersititual fluid (ISF) in patients with iNPH36 and a correlation between biopsy-verified Aβ load and CSF Aβ42 is detected in iNPH13 37 arguing that there is a relation between levels of APP-derived proteins in the brain parenchyma and sampled CSF.
There were clear differences between iNPH and the cognitive and movement disorders which is also reflected by the high discriminative power of the CSF biomarker algorithm. The cognitive disorders group had higher tau proteins than both iNPH and HI, whereas none of the movement disorders showed an elevation of tau proteins in comparison with HI. The tau protein levels were compatible between iNPH and the movement disorders (except for in PSP where P-tau was slightly elevated when correcting for age and sex). These findings are in line with the assumption that the cognitive deficits in iNPH are not primarily of the cortical type. We also find the pattern of APP downregulation interesting where we can see that it is only in the cognitive disorders that Aβ42 is affected in isolation, which probably is a reflection of the amyloid plaques.38 This is clearly distinguished from the movement disorders and from iNPH (where there are low concentrations of all APP-derived proteins). There are numerous studies of these biomarkers that corroborate our results as shown in a recent review on the topic.39 Our study is, however, to our knowledge, the first to show this in such a large sample size, with multiple well-defined cohorts and with analysis extending to multiple APP-derived proteins at once. This makes it possible to examine how different the pattern of APP downregulation is in different neurodegenerative disorders.
The pathophysiological profile in iNPH opens up for the possibility of using CSF biomarkers as a tool for diagnosing iNPH in a mixed clinical setting, as proposed in Schirinzi et al.40 For that purpose, we constructed a prediction plot that can complement clinical and radiological examination. The high diagnostic predictive value of the three biomarkers separating iNPH from non-iNPH disorders with an AUC of 0.86 is a result of the different pathophysiology. There are good reasons for further work on the diagnostic predictability including an algorithm like the one presented here and we hope to validate the algorithm in coming studies.
There were several patients diagnosed as iNPH who had low estimated probability of being diagnosed using the algorithm and thus it cannot be used for excluding iNPH. There are several obvious reasons for this dilemma. The most important problem is the high comorbidity seen in patients with iNPH. Clinically, AD and iNPH symptomatology can be found in the same patient that benefits from shunt surgery. Several studies have shown a high frequency, up to 33 %, of typical AD pathology in biopsies from frontal cortex in patients with iNPH.13 The presence of AD pathology with connected CSF biomarker changes will add to the iNPH biomarker pattern blurring the image. Similarly, a coexisting diagnose of VAD is not unusual which will have an impact on the iNPH CSF biomarker pattern, reducing the diagnostic precision. We know that long-term outcome from shunt placement can be affected by the presence of comorbidities and this should be taken into account for the individual patient. There are nevertheless multiple studies that have shown that comorbidity should not be used to exclude patients from surgery.7 12 41 42 This study was not dedicated to examine the prediction of outcome after surgery using the biomarkers profile and to this date there have not been any promising studies on the use of CSF biomarkers to separate responders from non-responders.10 35
Another important problem is the high frequency of vascular risk factors present in patients diagnosed with iNPH. Patients with iNPH have a heavier burden of hypertension, diabetes mellitus, hyperlipidaemia, obesity, ischaemic heart disease and arteriosclerotic cerebrovascular disease than control groups43 which probably will influence the typical iNPH biomarker pattern. The probability that a patient presenting with typical symptoms, signs and MR changes is suffering from iNPH should be close to 1 if T-tau is 200 pg/mL or lower, Aβ40 1000 pg/mL or lower and MCP-1 800 pg/mL or more using the suggested algorithm. Such a patient will probably not have any vascular risk factors and no co-morbidity.
We believe that this study has several strengths. The CSF biomarkers in iNPH are contrasted to a large material of patients from the most common clinical iNPH mimics. For each diagnosis, the patients are diagnosed at single centres and by strict criteria, making them highly representative for their respective disorders. All CSF laboratory analyses were executed at the same time making the CSF analyses solid. Comparisons are corrected for the effect of age and sex. To our knowledge, this is the first time that this can be presented for iNPH.
There are some limitations to this study. We have aimed at analysing distinct disorders. However, the diagnoses are clinical and are as such subject to possible false classification as we do not include post-mortem diagnostic verification. We also know that different comorbidities may be present in the groups. iNPH cases with presumed comorbidity were offered shunt placement if it was believed that the patients’ main symptomatology was caused by the iNPH. This may very well influence the proportion of improved patients in this cohort, which was comparably low. It should be noted that a large amount of patients remained clinically unchanged, whereas we know that patients do deteriorate without shunt placement.2 We therefore think that the disease course is probably slowed by the shunt placement even if not reversed enough to reach the preset limit for improvement.
We are lacking comparative clinical data to stage severity in the different disorders. It would have been beneficial to have access to cognitive measurements for the dementia cases. We believe, nevertheless, that the different groups are clinically representative. Of special note is the large age-span in some of the groups (ie, PD). We made the decision to include all patients from this centre and made the decision that even if this would include cases that would not pose an iNPH-differential diagnostic problem, the span does mirror the clinical reality and the specialised clinics. The results are corrected for the possible age bias but should be taken into consideration when comparing absolute levels. The groups were unequal in numbers which might influence some statistical calculating but the miscalculations would rather be to underestimate possible differences than to exaggerate them. The HIs were from two different cohorts. The choice to merge the groups were made to enhance the group size. We believe that this can be done as the controls were selected using the same criteria but it is of course suboptimal.
Conclusions
The characteristic CSF biochemical pattern in iNPH by means of low concentrations of tau proteins and APP-derived proteins in combination with elevated MCP-1 was confirmed in this study and was shown to separate patients with iNPH from HIs and patients with VAD, PD, MSA, PSP, CBD, AD and FTLD.
The combination of the CSF biomarkers T-tau, Aβ40 and MCP-1 separates iNPH from cognitive and movement disorders with good diagnostic sensitivity and specificity and a prediction plot using these CSF biomarkers was constructed. The findings in this study may have important implications for diagnosis and clinical research on disease mechanisms for iNPH.
Acknowledgments
There are several persons who have contributed to this work. We are grateful for the skilful
work of performing all the CSF biomarker assays in this large set of samples by the technicians
at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Sweden. Kerstin Andrén and Daniel Jaraj contributed enormously with iNPH sample collection. Nils-Gunnar Pehrsson and Anders Pehrsson for the multivariate statistical work and for invaluable comments and critiques on the statistics. Last but not least, we wish to thank all the patients and healthy individuals, whose contribution made it possible to perform this study.
Footnotes
Contributors: Anna Jeppsson: participated in the design of the study; coordinated the study; statistical analysis; interpretation of data; drafted the manuscript for intellectual content. Carsten Wikkelsø: participated in the design of the study; coordination of the study; CSF collection; interpretation of data; drafted the manuscript for intellectual content. Kaj Blennow: participated in the design of the study; coordination of the study; laboratory analyses; interpretation of data; revised the manuscript for intellectual content. Henrik Zetterberg: participated in the design of the study; coordination of the study; laboratory analyses; interpretation of data; revised the manuscript for intellectual content. Radu Constantinescu: patient recruitment; CSF collection; interpretation of data; revised the manuscript for intellectual content. Anne M Remes: patient recruitment; revised the manuscript for intellectual content. Sanna-Kaisa Herukka: patient recruitment, CSF collection; revised the manuscript for intellectual content. Toumas Rauramaa: patient recruitment, CSF collection; revised the manuscript for intellectual content. Katarina Nägga: patient recruitment, CSF collection; revised the manuscript for intellectual content. Ville Leinonen: patient recruitment; interpretation of data; revised the manuscript for intellectual content. Mats Tullberg: participated in the design of the study; coordination of the study; CSF collection; interpretation of data; drafted the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding: This study was supported by grants from the Swedish Research Council (#2017-00915), Swedish State under the agreement between the Swedish government and the country councils, the ALF agreement (2017-04961; ALFGBG-720931, -720121 and ALFGBG715986), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), Kuopio University Hospital VTR-fund and the Göteborg Foundation for Neurological Research. HZ is a Wallenberg Academy Fellow. KB holds the Torsten Söderberg Professorship in Medicine at the Royal Swedish Academy of Sciences.
Disclaimer: Dr Wikkelsø receives honorarium for lecturing by Codman, Integra. Dr Zetterberg has served at scientific advisory boards for Roche Diagnostics, Wave,Samumed and CogRx and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. Dr Blennow has served as a consultant or at advisory boards for Alzheon, CogRx, Biogen, Lilly, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1. Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965;2:307–27. [DOI] [PubMed] [Google Scholar]
- 2. Andrén K, Wikkelsø C, Tisell M, et al. Natural course of idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2014;85:806–10. 10.1136/jnnp-2013-306117 [DOI] [PubMed] [Google Scholar]
- 3. Jaraj D, Rabiei K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 2014;82:1449–54. 10.1212/WNL.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tisell M, Höglund M, Wikkelsø C. National and regional incidence of surgery for adult hydrocephalus in Sweden. Acta Neurol Scand 2005;112:72–5. 10.1111/j.1600-0404.2005.00451.x [DOI] [PubMed] [Google Scholar]
- 5. Molde K, Söderström L, Laurell K. Parkinsonian symptoms in normal pressure hydrocephalus: a population-based study. J Neurol 2017;264:2141–8. 10.1007/s00415-017-8598-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(suppl_3):S2-4–S2-16. 10.1227/01.NEU.0000168185.29659.C5 [DOI] [PubMed] [Google Scholar]
- 7. Tullberg M, Jensen C, Ekholm S, et al. Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. AJNR Am J Neuroradiol 2001;22:1665–73. [PMC free article] [PubMed] [Google Scholar]
- 8. Schirinzi T, Sancesario GM, Ialongo C, et al. A clinical and biochemical analysis in the differential diagnosis of idiopathic normal pressure hydrocephalus. Front Neurol 2015;6 10.3389/fneur.2015.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeppsson A, Zetterberg H, Blennow K, et al. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology 2013;80:1385–92. 10.1212/WNL.0b013e31828c2fda [DOI] [PubMed] [Google Scholar]
- 10. Miyajima M, Nakajima M, Ogino I, et al. Soluble amyloid precursor protein α in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. Eur J Neurol 2013;20:236–42. 10.1111/j.1468-1331.2012.03781.x [DOI] [PubMed] [Google Scholar]
- 11. Moriya M, Miyajima M, Nakajima M, et al. Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the AMYLOID cascade. PLoS One 2015;10 10.1371/journal.pone.0119973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craven CL, Baudracco I, Zetterberg H, et al. The predictive value of t-tau and AB1-42 levels in Idiopathic normal pressure hydrocephalus. Acta Neurochir 2017;159:2293–300. 10.1007/s00701-017-3314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pyykkö OT, Lumela M, Rummukainen J, et al. Cerebrospinal fluid biomarker and brain biopsy findings in Idiopathic normal pressure hydrocephalus. PLoS One 2014;9 10.1371/journal.pone.0091974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellström P, Klinge P, Tans J, et al. A new scale for assessment of severity and outcome in iNPH. Acta Neurol Scand 2012;126:229–37. 10.1111/j.1600-0404.2012.01677.x [DOI] [PubMed] [Google Scholar]
- 15. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4. 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–6. 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Litvan I, Bhatia KP, Burn DJ, et al. Movement disorders Society scientific issues Committee report: sic Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003;18:467–86. 10.1002/mds.10459 [DOI] [PubMed] [Google Scholar]
- 18. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–44. 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 20. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54. 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- 21. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International workshop. Neurology 1993;43:250–60. 10.1212/WNL.43.2.250 [DOI] [PubMed] [Google Scholar]
- 22. Nägga K, Gottfries J, Blennow K, et al. Cerebrospinal fluid phospho-tau, total tau and beta-amyloid(1-42) in the differentiation between Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 2002;14:183–90. 10.1159/000066023 [DOI] [PubMed] [Google Scholar]
- 23. Haapalinna F, Kokki M, Jääskeläinen O, et al. Subtle cognitive impairment and Alzheimer's Disease-Type pathological changes in cerebrospinal fluid are common among neurologically healthy subjects. J Alzheimers Dis 2018;62:165–74. 10.3233/JAD-170534 [DOI] [PubMed] [Google Scholar]
- 24. Gaetani L, Höglund K, Parnetti L, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther 2018;10 10.1186/s13195-018-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zetterberg H, Andreasson U, Hansson O, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 2008;65:1102–7. 10.1001/archneur.65.8.1102 [DOI] [PubMed] [Google Scholar]
- 26. Ziegelitz D, Starck G, Kristiansen D, et al. Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J Magn Reson Imaging 2014;39:1533–42. 10.1002/jmri.24292 [DOI] [PubMed] [Google Scholar]
- 27. Momjian S, Owler BK, Czosnyka Z, et al. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004;127:965–72. 10.1093/brain/awh131 [DOI] [PubMed] [Google Scholar]
- 28. Priller C, Bauer T, Mitteregger G, et al. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 2006;26:7212–21. 10.1523/JNEUROSCI.1450-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener 2006;1 10.1186/1750-1326-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ziegelitz D, Arvidsson J, Hellström P, et al. In patients with idiopathic normal pressure hydrocephalus postoperative cerebral perfusion changes measured by dynamic susceptibility contrast magnetic resonance imaging correlate with clinical improvement. J Comput Assist Tomogr 2015;39:531–40. 10.1097/RCT.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 31. Agren-Wilsson A, Lekman A, Sjöberg W, et al. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol Scand 2007;116:333–9. 10.1111/j.1600-0404.2007.00890.x [DOI] [PubMed] [Google Scholar]
- 32. Semple BD, Bye N, Rancan M, et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J Cereb Blood Flow Metab 2010;30:769–82. 10.1038/jcbfm.2009.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Bigio MR, Cardoso ER, Halliday WC. Neuropathological changes in chronic adult hydrocephalus: cortical biopsies and autopsy findings. Can J Neurol Sci 1997;24:121–6. 10.1017/S0317167100021442 [DOI] [PubMed] [Google Scholar]
- 34. Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 2014;83:1573–5. 10.1212/WNL.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeppsson A, Höltta M, Zetterberg H, et al. Amyloid mis-metabolism in Idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 2016;13 10.1186/s12987-016-0037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herukka S-K, Rummukainen J, Ihalainen J, et al. Amyloid-β and tau dynamics in human brain interstitial fluid in patients with suspected normal pressure hydrocephalus. J Alzheimers Dis 2015;46:261–9. 10.3233/JAD-142862 [DOI] [PubMed] [Google Scholar]
- 37. Abu Hamdeh S, Virhammar J, Sehlin D, et al. Brain tissue Aβ42 levels are linked to shunt response in Idiopathic normal pressure hydrocephalus. J Neurosurg 2018;130:121–9. 10.3171/2017.7.JNS171005 [DOI] [PubMed] [Google Scholar]
- 38. Blennow K, Hampel H, Weiner M, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010;6:131–44. 10.1038/nrneurol.2010.4 [DOI] [PubMed] [Google Scholar]
- 39. Manniche C, Hejl A-M, Hasselbalch SG, et al. Cerebrospinal fluid biomarkers in Idiopathic normal pressure hydrocephalus versus Alzheimer's disease and subcortical ischemic vascular disease: a systematic review. J Alzheimers Dis 2019;68:JAD-180816 10.3233/JAD-180816 [DOI] [PubMed] [Google Scholar]
- 40. Schirinzi T, Sancesario GM, Di Lazzaro G, et al. Cerebrospinal fluid biomarkers profile of idiopathic normal pressure hydrocephalus. J Neural Transm 2018;125:673–9. 10.1007/s00702-018-1842-z [DOI] [PubMed] [Google Scholar]
- 41. Nakajima M, Miyajima M, Ogino I, et al. Preoperative phosphorylated tau concentration in the cerebrospinal fluid can predict cognitive function three years after shunt surgery in patients with idiopathic normal pressure hydrocephalus. J Alzheimers Dis 2018;66:319–31. 10.3233/JAD-180557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kazui H, Kanemoto H, Yoshiyama K, et al. Association between high biomarker probability of Alzheimer's disease and improvement of clinical outcomes after shunt surgery in patients with idiopathic normal pressure hydrocephalus. J Neurol Sci 2016;369:236–41. 10.1016/j.jns.2016.08.040 [DOI] [PubMed] [Google Scholar]
- 43. Andrén K, Wikkelsö C, Sundström N, et al. Long-term effects of complications and vascular comorbidity in Idiopathic normal pressure hydrocephalus: a quality registry study. J Neurol 2018;265:178–86. 10.1007/s00415-017-8680-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article is available and anonymised data will be shared by request from any qualified investigator.