Abstract
Background
The incidence and clinical manifestations of cardiovascular disease (CVD) differ between blacks and whites. Biomarkers that reflect important pathophysiological pathways may provide a window to allow deeper understanding of racial differences in CVD.
Methods and Results
The study included 2635 white and black participants from the Dallas Heart Study who were free from existing CVD. Cross‐sectional associations between race and 32 biomarkers were evaluated using multivariable linear regression adjusting for age, traditional CVD risk factors, imaging measures of body composition, renal function, insulin resistance, left ventricular mass, and socioeconomic factors. In fully adjusted models, black women had higher lipoprotein(a), leptin, d‐dimer, osteoprotegerin, antinuclear antibody, homoarginine, suppression of tumorigenicity‐2, and urinary microalbumin, and lower adiponectin, soluble receptor for advanced glycation end products and N‐terminal pro‐B‐type natriuretic peptide versus white women. Black men had higher lipoprotein(a), leptin, d‐dimer, high‐sensitivity C‐reactive protein, antinuclear antibody, symmetrical dimethylarginine, homoarginine, high‐sensitivity cardiac troponin T, suppression of tumorigenicity‐2, and lower adiponectin, soluble receptor for advanced glycation end products, and N‐terminal pro‐B‐type natriuretic peptide versus white men. Adjustment for biomarkers that were associated with higher CVD risk, and that differed between blacks and whites, attenuated the risk for CVD events in black women (unadjusted hazard ratio 2.05, 95% CI 1.32, 3.17 and adjusted hazard ratio 1.15, 95% CI 0.69, 1.92) and black men (unadjusted hazard ratio 2.39, 95% CI 1.64, 3.46, and adjusted hazard ratio 1.21, 95% CI 0.76, 1.95).
Conclusions
Significant racial differences were seen in biomarkers reflecting lipids, adipokines, and biomarkers of endothelial function, inflammation, myocyte injury, and neurohormonal stress, which may contribute to racial differences in the development and complications of CVD.
Keywords: biomarker, endothelial dysfunction, inflammation
Subject Categories: Race and Ethnicity
Short abstract
See Editorial Suzuki et al
Clinical Perspective
What Is New?
In this large evaluation of circulating cardiovascular biomarkers among individuals from the general population, significant racial differences were seen in multiple biomarkers reflecting different pathological pathways, including lipids, adipokines, endothelial function, inflammation, myocyte injury, and neurohormonal stress.
When biomarkers that differed by race and associated with cardiovascular disease events were accounted for, the associations of black race with incident cardiovascular disease events were attenuated and no longer significant.
What Are the Clinical Implications?
Biological pathways reflected by circulating biomarkers may contribute to or mediate racial differences in cardiovascular disease.
Introduction
The incidence and clinical manifestations of cardiovascular diseases (CVD) differ substantially between blacks and whites. While blacks in the United States experience higher overall rates of CVD compared with whites,1 these differences vary substantially based on specific subtypes of CVD. Black men and women have higher rates of heart failure, peripheral vascular disease, and cerebrovascular disease than whites.2, 3, 4 Blacks have increased incidence and mortality of coronary artery disease compared with whites, despite a lower burden of coronary atherosclerosis.5 Conversely, whites experience higher rates of atrial fibrillation.6
Factors that contribute to these racial differences in CVD include a higher burden of risk factors among blacks, including increased prevalence and worse control of hypertension,7 excess rates of smoking and diabetes mellitus, and among black women, substantially higher rates of obesity.8 The interplay between socioeconomic status, risk factors and risk factor control, and racial disparities in CVD has been studied extensively.5 In contrast, there has been relatively little focus on pathophysiological mechanisms underlying these racial disparities, beyond known differences in traditional risk factors. Comprehensive assessment of biomarkers that reflect important pathophysiological pathways may provide a window to allow deeper understanding of racial differences in CVD and may facilitate investigation into development of novel precision medicine approaches to CVD prevention and treatment. Thus, we performed an analysis investigating the associations between race and a panel of potentially informative cardiac and metabolic biomarkers, after accounting for traditional risk factors, racial differences in body composition and cardiac structure, and socioeconomic factors.
Methods
The data, methods used in the analysis, and materials used to conduct the research will not be made available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Population
The DHS (Dallas Heart Study) is a multiethnic, population‐based cohort study of residents in Dallas County. Self‐identified black individuals were intentionally oversampled to encompass 50% of the cohort.9 Between July 2000 and September 2002, 3 phases of data collection were performed. Visit 1 (n=6101) was an in‐home visit collecting demographic information, medical history, blood pressure, and anthropometric measurements; Visit 2 (n=3557) was a second home visit collecting fasting blood and urine samples; and Visit 3 (n=2971) was conducted at the University of Texas Southwestern Medical Center, where detailed imaging measurements were performed. The current study, based on blood‐based measurements, includes participants from Visit 2. Participants with cardiovascular disease at baseline, defined as self‐reported history of myocardial infarction, revascularization, stroke, heart failure (n=118), or those with Hispanic ethnicity (n=579) were excluded, resulting in a final study population of 2635 individuals. The Institutional Review Board of the University of Texas Southwestern Medical Center approved the study protocol, and all participants provided written informed consent.
Variable Definitions
Race/ethnicity and sex were self‐reported. Other demographic information including age, insurance status, household income, achieved education and income level, and current smoking status were obtained from participants through self‐report during a structured in‐home interview performed by study staff. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medications.10 Hypercholesterolemia was defined as use of lipid‐lowering medication, fasting and nonfasting low‐density lipoprotein (LDL) ≥160 mg/dL, or total cholesterol ≥240 mg/dL. Diabetes mellitus was defined as use of antihyperglycemic medication, fasting serum glucose ≥126 mg/dL, or nonfasting glucose >200 mg/dL.10 Insulin sensitivity was estimated using the Homeostasis Model Assessment of Insulin Resistance Index calculated by multiplying fasting plasma insulin (mU/L) by fasting plasma glucose (mmol/L) and then dividing by a constant of 22.5.11 Estimated glomerular filtration was calculated using the abbreviated Modification of Diet in Renal Disease calculation: 186×(serum creatinine, mg/dL)×1.154×(age in years)−0.203×0.742 (if female)×1.21 (if black). Education was divided into 3 groups: (1) less than high school, (2) high school graduate, and (3) college graduate or professional school graduate. Household income level was categorized into 4 groups based on amount (<$16K, $16–$29K, $30–$49K, and >$50K). Health insurance status was categorized as having or not having any insurance.
Cardiac Imaging
Measurements of the left atrium and left ventricle were performed using short‐axis, breath‐hold, electrographic‐gated, cine magnetic resonance imaging from 2 similar 1.5‐T magnetic resonance imaging scans (Philips Medical Systems). Manual tracings of endocardial and epicardial borders of slices extending from the apex to the base of the left ventricle were obtained to measure left ventricular wall thickness and cavity volume.12 Maximum left ventricular volume and minimum left atrial volume were measured using the biplane area–length method,13 following the American Society of Echocardiography's guidelines.14 Two consecutive electron‐beam computed tomography scans were performed for measurement of coronary artery calcium. Agatston units were used to quantify coronary artery calcium severity, and the final Agatston score was derived from the mean of 2 consecutive scans.15
Measures of Body Composition
Body surface area was calculated using best‐fit equations for each sex as 128.1×weight (kg)0.44×height (cm)0.60 for men and 147.4×weight (kg)0.47×height (cm)0.55 for women.16 Body mass index was calculated as weight (kg)/height (meters)2. Magnetic resonance imaging measurements of abdominal subcutaneous and visceral fat mass were calculated at the L2‐L3 intervertebral level, using a validated method of fat mass prediction with a single magnetic resonance imaging slice.8 Fat mass and fat‐free mass of the trunk, upper and lower extremities, and head were measured using dual‐energy x‐ray absorptiometry (DEXA).8
Measurement of Circulating Biomarkers
Venous blood was collected in standard EDTA tubes and maintained at 4°C for ≤4 hours until centrifuged (1430g for 15 minutes), after which the plasma component was removed and frozen at −70°C until assays were performed.17
Thirty‐two biomarkers, representing 6 pathophysiological categories (lipids, adipokines, markers of inflammation, endothelial injury, myocyte injury and stress, and kidney function) were included in the analyses. Assay methods and characteristics have been previously reported,18 and are reproduced as Table S1.
Clinical Outcomes
Participants were followed for 10 years, via an annual health survey regarding interval cardiovascular events, and through quarterly tracking for hospital admissions using the Dallas‐Fort Worth Hospital Council Data Initiative database.19 The outcome for the present study was incident global cardiovascular disease, comprising cardiovascular death, myocardial infarction, stroke, coronary or peripheral revascularization, hospitalization for heart failure, and atrial fibrillation. Events were adjudicated by a panel of cardiovascular specialists.19
Statistical Analysis
Cumulative 10‐year rates of global CVD were estimated using the Kaplan‐Meier method and compared across race and sex groups using the log rank test. Biomarkers are reported as median (25th, 75th percentile). Linear regression analyses were performed to assess the association of race with log‐transformed biomarker concentrations in unadjusted models. Multiplicative race × sex interactions were tested in the overall cohort. Because highly significant race × sex interactions were identified for multiple biomarkers, all analyses were stratified by sex. Multivariable linear regression modeling was performed, adjusting for age, traditional risk factors (diabetes mellitus, systolic blood pressure, blood pressure medications, current smoking status, and statin use), Homeostasis Model Assessment of Insulin Resistance Index, estimated glomerular filtration, body composition (lean mass, fat mass, body surface area, visceral fat, subcutaneous fat, and lower body fat), left ventricular measurements (left ventricular mass, ejection fraction, and end‐diastolic volume), and socioeconomic factors (education, income, and healthcare insurance). We tested for collinearity among related variables using the Variation Inflation Factor in the regression models. Variation Inflation Factor was <4.8 for all variables, suggesting no influence of collinearity. Beta coefficients for race are reported for each biomarker in each model, with positive values representing higher levels in blacks and negative values demonstrating lower levels in blacks. Standardized beta coefficients are reported to allow comparison of the magnitude of association of race with different biomarkers. The standardized, log‐transformed biomarkers all have a mean of 0 with a standard deviation of 1. The magnitude of the association of race with each biomarker can be interpreted from the absolute value of the beta coefficient.
To assess whether racial differences in biomarkers potentially mediate racial differences in CVD outcomes, we performed exploratory analyses using sex‐stratified Cox proportional hazards models. We considered biomarkers that were associated with higher rates of CVD events in univariable analyses, and also were higher in black versus white participants in the fully adjusted models described above. We also considered biomarkers that were associated with lower risk for CVD if levels were lower in blacks. We created 2 Cox models within each sex: the first model included only a term for race and the second included terms for race and each biomarker associated with both race and higher risk for CVD. A reduction in the hazard ratio after accounting for the biomarkers suggests a potential contribution of the biomarkers (or pathways they reflect) to racial differences in arteriosclerotic cardiovascular disease (ASCVD).20, 21
Statistical analyses were completed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). All P values are 2‐sided and adjusted for multiple testing using the False Discovery Rate methodology, with P<0.05 considered significant.
Results
Characteristics of the study population stratified by sex and race are presented in Table 1. Age was similar in each race/sex group. Rates of diabetes mellitus and hypertension were higher among black women and men, with associated higher systolic and diastolic blood pressure among blacks. Rates of hypercholesterolemia were similar across racial groups, and black men were more often current smokers than white men. Furthermore, in comparison with white women, black women had higher body mass index, lean mass, fat mass, subcutaneous abdominal fat, and lower body fat. Whereas black men had higher lean mass than white men, they had lower fat mass, visceral fat, subcutaneous fat, and lower body fat. Black women and men had higher Homeostasis Model Assessment of Insulin Resistance Index (indicating more insulin resistance) and estimated glomerular filtration than white women and men. Black women and men had significantly larger left ventricular mass and lower aortic compliance. Black women also had larger left atrial size and higher coronary calcium scores than white women. Other cardiac measurements were similar across racial groups.
Table 1.
Baseline Characteristics
| Covariate | Women | Men | ||
|---|---|---|---|---|
| Black (n=950) | White (n=525) | Black (n=688) | White (n=472) | |
| Age, y | 43 (36, 52)a | 45 (37, 53) | 44 (37, 52) | 44 (37, 51) |
| Diabetes mellitus | 117 (12%)b | 31 (6%) | 90 (13%)b | 28 (6%) |
| Hypertension | 376 (40%)b | 132 (25%) | 277 (41%)b | 112 (24%) |
| Systolic BP, mm Hg | 123 (113, 137)b | 115 (106, 124) | 132 (120, 141)b | 123 (114, 129) |
| Diastolic BP, mm Hg | 79 (74, 86)b | 74 (68, 80) | 81 (74, 87)b | 77 (72, 83) |
| Hypercholesterolemia | 101 (11%) | 72 (14%) | 79 (12%) | 60 (13%) |
| Current smoking | 262 (28%) | 144 (28%) | 266 (39%)b | 130 (28%) |
| Socioeconomic factors | ||||
| Did not finish high school | 155 (16%)b | 34 (6%) | 110 (16%)b | 31 (7%) |
| High school graduate | 386 (41%)b | 138 (26%) | 285 (41%)b | 90 (19%) |
| College, graduate or professional school graduate | 127 (13%)b | 179 (34%) | 92 (13%)b | 211 (45%) |
| Income level: <$16 000 | 243 (31%)b | 36 (8%) | 153 (28%)b | 29 (7%) |
| Income level: $16 000–29 999 | 227 (29%)b | 75 (16%) | 132 (24%)b | 42 (10%) |
| Income level: $30 000–49 999 | 195 (25%) | 131 (27%) | 139 (25%) | 100 (23%) |
| Income level: ≥$50 000 | 112 (14%)b | 236 (49%) | 128 (23%)b | 264 (61%) |
| No healthcare insurance | 129 (14%) | 55 (10%) | 76 (11%) | 45 (10%) |
| Insulin resistance | ||||
| HOMA‐IR, units | 3.6 (2, 5.7)b | 2.2 (1.3, 4) | 2.9 (1.5, 5)a | 2.4 (1.4, 4.3) |
| Kidney function | ||||
| eGFR, mL/min per 1.73 m2 | 102 (89, 118)b | 90 (79, 100) | 101 (89, 115)b | 90 (82, 101) |
| Body composition | ||||
| Body mass index, kg/m2 | 31 (26, 37)b | 27 (23, 32) | 27 (24, 31) | 28 (25, 31) |
| Body surface area, m2 | 1.9 (1.8, 2.1)b | 1.8 (1.7, 2) | 2.0 (1.9, 2.2)a | 2.0 (2, 2.2) |
| Lean mass, kg | 50 (45, 56)b | 45 (40, 50) | 66 (60, 73)c | 64 (60, 70) |
| Fat mass, kg | 33 (25, 43)b | 28 (21, 38) | 19 (13, 27)b | 22 (17, 28) |
| Visceral fat, kg | 1.7 (1.3, 2.2) | 1.7 (1.2, 2.3) | 2.0 (1.5, 2.8)b | 2.8 (2, 3.5) |
| Subcutaneous abdominal fat, kg | 5.9 (4, 8.4)b | 4.4 (2.9, 6.4) | 3.2 (2, 4.8)a | 3.6 (2.6, 4.5) |
| Lower body fat, kg | 12 (10, 16)b | 11 (8, 13) | 6.4 (4.4, 9)a | 6.7 (5.2, 8.8) |
| Cardiac imaging | ||||
| Left ventricular mass, g | 146 (127, 169)b | 128 (111, 143) | 194 (171, 226)b | 181 (160, 204) |
| LVEF, % | 75 (70, 79) | 74 (70, 78) | 70 (65, 75) | 70 (65, 74) |
| LVEDV, mL | 93 (81, 106) | 93 (80, 104) | 114 (97, 130) | 112 (97, 126) |
| LAEDV, mL | 70 (58, 82)a | 65 (54, 76) | 75 (62, 86) | 73 (60, 86) |
| Coronary calcium, Agatston U | 0.5 (0, 3.6)b | 0 (0, 1.2) | 1.0 (0, 14) | 1.0 (0, 13) |
Values are means for continuous variables and percentages for categorical variables. BP indicates blood pressure; eGFR, estimated glomerular filtration rate; HOMA‐IR, Homeostatic Model of Insulin Resistance; LAEDV, left atrial end‐diastolic volume; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; U, units.
P<0.05.
P<0.001.
P<0.01.
Ten‐year rates of incident CVD were 13.2% in black men, 7.0% in white men, 6.6% in black women, and 3.7% in white women (P<0.001 for comparisons between black and white men, and black and white women) (Figure 1).
Figure 1.
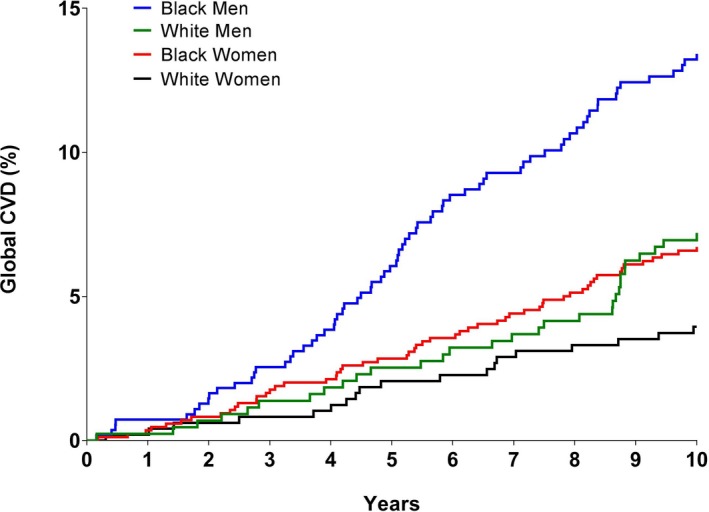
Race and sex differences in incident cardiovascular disease. Comparison of cardiovascular disease events by race and sex. P<0.001 for comparison between black and white men, and P<0.001 for comparison between black and white women. CVD indicates cardiovascular disease.
Racial differences among the 32 biomarkers studied are presented in Table 2. Significant sex×race interactions were observed for multiple biomarkers (Table 2). Thus, all biomarker comparisons are reported stratified by sex. Results ordered by strength of association are shown in Figure 2.
Table 2.
Univariable and Multivariable Association of Biomarkers With Race
| Women | Men | P Value for Interaction Race×Sexa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Black | White | Unadjusted Beta | Fully Adjusted Beta | Black | White | Unadjusted Beta | Fully Adjusted Beta | ||
| Lipid biomarkers | |||||||||
| TC, mg/dL | 174 | 180 | −0.17b | −0.07 | 174 | 181 | −0.23c | −0.19d | 0.56 |
| LDL‐C, mg/dL | 102 | 104 | −0.03 | −0.03 | 101 | 111 | −0.26c | −0.14 | 0.38 |
| LDL‐p, mg/dL | −0.11d | −0.19d | −0.39c | −0.34c | 0.007 | ||||
| HDL‐C, mg/dL | 51 | 52 | −0.02 | 0.15 | 47 | 41 | 0.58c | 0.51c | <0.0001 |
| HDL‐p, mg/dL | 33 | 35 | −0.37c | −0.29c | 32 | 32 | 0.10 | 0.16 | 0.001 |
| TG, mg/dL | 78 | 100 | −0.42c | −0.55c | 91 | 121 | −0.47c | −0.53c | <0.0001 |
| Lp(a), nmol/L | 83 | 28 | 0.82c | 0.85c | 71 | 24 | 0.74c | 0.63c | 0.001 |
| Cholesterol efflux, normalized ratio | 1.0 | 1.0 | −0.23c | −0.12 | 1.0 | 1.0 | 0.06 | 0.10 | <0.0001 |
| Adipokines | |||||||||
| Leptin, μg/L | 28 | 19 | 044c | 0.37c | 5.6 | 5.7 | −0.11 | 0.21c | <0.0001 |
| Adiponectin, ng/mL | 6 | 10 | −075c | −0.57c | 5.0 | 6.6 | −0.48c | −0.63c | <0.0001 |
| Inflammatory biomarkers | |||||||||
| hs‐CRP, mg/L | 4.4 | 2.9 | 0.24c | 0.002 | 2.2 | 1.7 | 0.28c | 0.20d | <0.0001 |
| OPG, pg/mL | 1343 | 1219 | 0.22c | 0.20d | 1158 | 1074 | 0.11 | −0.06 | <0.0001 |
| sRAGE, ng/mL | 1.1 | 1.6 | −0.66c | −0.48c | 1.0 | 1.6 | −0.60c | −0.62c | 0.93 |
| LP‐PLA2 activity, nmol/min per mL | 125 | 143 | −0.53c | −0.52c | 144 | 178 | −0.81c | −0.84c | <0.0001 |
| LP‐PLA2 mass, μg/L | 167 | 196 | −0.55c | −0.51c | 186 | 219 | −0.54c | −0.65c | <0.0001 |
| IL‐18, pg/mL | 482 | 513 | −0.18b | −0.26d | 513 | 644 | −0.31c | −0.40b | 0.02 |
| MCP‐1, pg/mL | 159 | 175 | −0.20c | −0.35c | 161 | 182 | −0.28c | −0.39c | 0.86 |
| sTNFR, ng/mL | 0.6 | 0.6 | −0.11 | −0.05 | 0.6 | 0.6 | −0.05 | 0.11 | 0.35 |
| ANA | 15 | 12 | 0.26c | 0.33c | 13 | 11 | 0.24c | 0.22d | <0.0001 |
| d‐dimer, μg/mL | 0.3 | 0.2 | 0.42c | 0.24b | 0.2 | 0.1 | 0.42c | 0.32c | <0.0001 |
| Endothelial biomarkers | |||||||||
| SDMA, μmol/L | 0.39 | 0.41 | −0.15b | 0.07 | 0.42 | 0.43 | 0.10 | 0.28b | <0.0001 |
| ADMA, μmol/L | 0.47 | 0.49 | −0.15b | −0.15 | 0.47 | 0.50 | −0.20c | −0.39c | 0.56 |
| Homoarginine, μmol/L | 2.9 | 2.3 | 0.57c | 0.48c | 3.0 | 2.6 | 0.32c | 0.38c | 0.10 |
| sESAM, ng/mL | 33 | 34 | −0.15b | −0.15 | 33 | 35 | −0.21c | −0.26b | 0.15 |
| sICAM, ng/mL | 607 | 590 | 0.02 | −0.03 | 606 | 604 | 0.09 | −0.12 | 0.84 |
| sVCAM, ng/mL | 974 | 975 | −0.04 | −0.11 | 986 | 1015 | −0.02 | −0.17 | 0.34 |
| Biomarkers of myocyte injury/stress | |||||||||
| hsTnT, ng/L | 1.5 | 1.5 | 0.14d | 0.12 | 3.0 | 1.5 | 0.40c | 0.34c | <0.001 |
| NT‐proBNP, pg/mL | 31 | 47 | −0.39c | −0.45c | 15 | 20 | −0.11 | −0.41c | <0.0001 |
| GDF‐15, ng/L | 0.7 | 0.7 | −0.07 | −0.09 | 0.7 | 0.7 | 0.24c | 0.13 | <0.0001 |
| ST2, μg/L | 0.4 | 0.4 | 0.37c | 0.21d | 0.4 | 0.4 | 0.54c | 0.59c | <0.001 |
| Biomarkers of kidney function | |||||||||
| Cystatin C, mg/L | 0.8 | 0.8 | −0.21c | −0.07 | 0.9 | 0.9 | −0.14d | 0.00 | <0.0001 |
| Microalbuminuria | 0.4 | 0.3 | 0.39c | 0.16d | 0.5 | 0.3 | 0.43c | 0.16 | 0.006 |
Values are medians for continuous variables and percentages for categorical variables. Variables adjusted for: Age, diabetes mellitus, systolic blood pressure, diastolic blood pressure, blood pressure medication use, smoking, statin use, Homeostasis Model Assessment of Insulin Resistance Index, estimated glomerular filtration rate, lean mass, fat mass, body surface area, visceral fat, subcutaneous fat, lower body fat, left ventricular mass, left ventricular ejection fraction, left ventricular end‐diastolic volume, left atrial size, education, income, and healthcare insurance. ADMA indicates asymmetric dimethyl arginine; ANA, antinuclear antibody; GDF, growth differentiating factor; HDL‐C, high‐density lipoprotein concentration; HDL‐p, HDL particle number; hs‐CRP, high‐sensitivity C‐reactive protein; hscTnT, high‐sensitivity cardiac troponin T; IL, interleukin; LDL‐p, low‐density lipoprotein particle number; LDL‐C, low‐density lipoprotein cholesterol concentration; Lp(a), lipoprotein (a); LP‐PLA2, lipoprotein‐associated phospholipase A2; MCP, monocyte chemoattractant protein; NT‐proBNP, N terminal B‐type natriuretic peptide; OPG, osteoprotegerin; SDMA, symmetric dimethyl arginine; sESAM, soluble endothelial cell adhesion molecule; sICAM, intercellular adhesion molecule; sRAGE, soluble receptor for advanced glycation end products; ST2, suppression of tumorigenicity (ST)‐2; sTNFR, soluble tumor necrosis factor receptor; sVCAM, soluble vascular cell adhesion molecule; TC, total cholesterol; TG, triglyceride.
P value for interaction is from the entire cohort (both men and women) and is unadjusted.
P<0.01.
P<0.001.
P<0.05.
Figure 2.
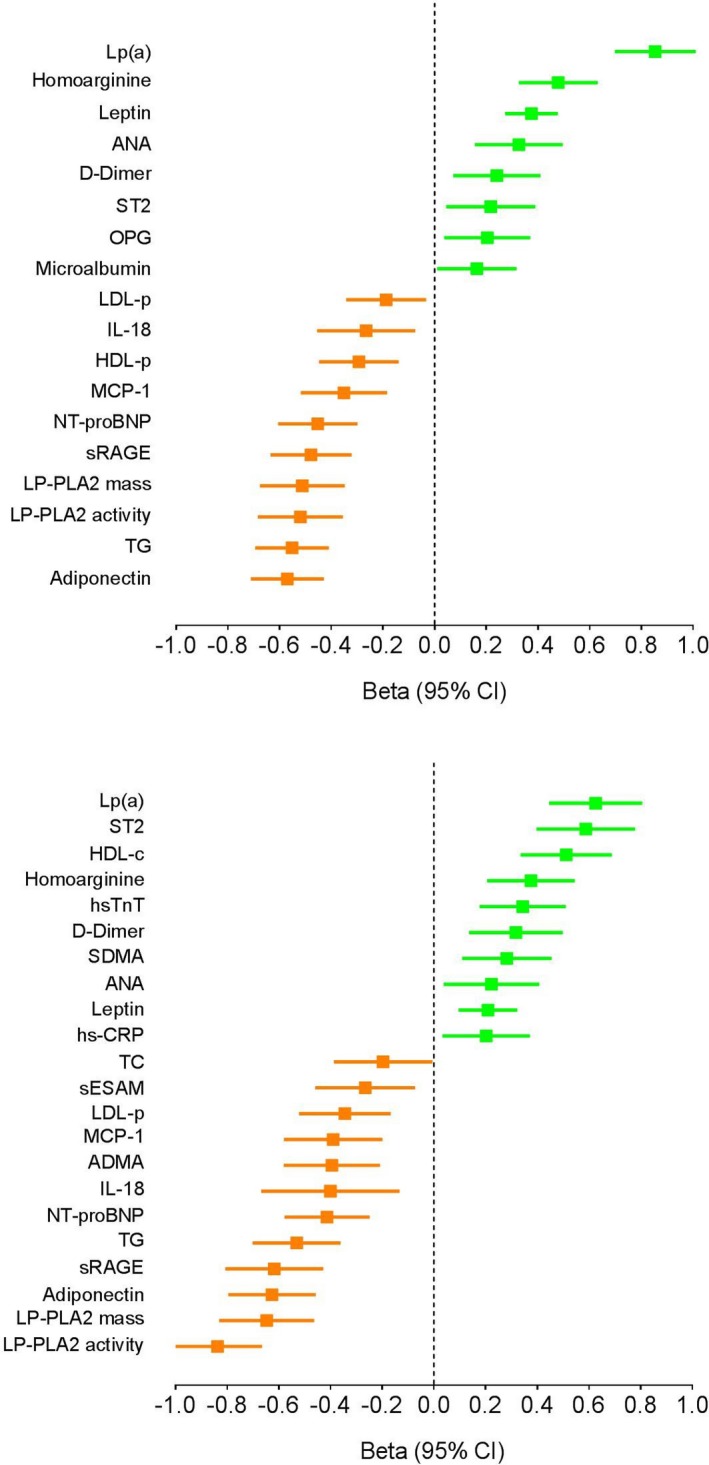
Multivariable association of biomarkers with race. Associations of black race with biomarker concentrations among women (top panel) and men (bottom panel). Fully adjusted standardized beta coefficients are presented, ordered by strength of association. Positive beta coefficients reflect biomarkers higher in black participants and negative beta coefficients identify biomarkers lower in black individuals. Biomarkers not significantly different between black and white participants in fully adjusted analyses are not shown. ANA indicates antinuclear antibodies; HDL‐c, high‐density lipoprotein cholesterol; HDL‐p, high‐density lipoprotein particle concentration; hs‐TnT, high‐sensitivity troponin T; LDL‐p, LDL particle number; LP(a), lipoprotein a; LP‐PLA2, lipoprotein phospholipase A2; MCP‐1, monocyte chemoattractant protein‐1; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OPG, osteoprotegerin; SDMA, symmetrical dimethylarginine; sRAGE, soluble receptor for advanced glycation end products; ST, suppression of tumorigenicity; TC, total cholesterol; TG, triglyceride.
Lipid Biomarkers
In unadjusted models, black women had lower total cholesterol, triglycerides, high‐density lipoprotein particle concentration (HDL‐p), and cholesterol efflux capacity than white women, without significant differences in HDL‐cholesterol (C), low‐density lipoprotein cholesterol (LDL‐C), and LDL‐p. The difference in cholesterol efflux capacity was no longer significant, whereas LDL‐p became significantly lower among black women after multivariable adjustment. Black men had higher HDL‐C and lower total cholesterol, LDL‐C, LDL‐p, and triglycerides than white men, with only LDL‐C losing significance after full adjustment. No significant differences in HDL‐p or cholesterol efflux capacity were observed between black and white men. The most notable lipid differences were in lipoprotein a (Lp(a)) concentrations, which were markedly higher among both black women and men in adjusted analyses.
Adipokines
Black women and men had higher leptin and lower adiponectin than white women and men in adjusted analyses, with associations stronger for adiponectin than leptin.
Inflammatory Biomarkers
Multiple racial differences were observed with regard to inflammatory biomarkers. Black women had higher unadjusted levels of antinuclear antibodies (ANA), d‐dimer, high‐sensitivity CRP (hs‐CRP) and osteoprotegerin and lower levels of soluble receptor for advanced glycation end products (sRAGE), lipoprotein phospholipase A2 (LP‐PLA2) activity and mass, interleukin 18, and monocyte chemoattractant protein‐1 in comparison with white women. After full adjustment, all differences remained statistically significant except for hs‐CRP. No significant racial difference was seen in soluble tumor necrosis factor receptor levels.
Black men had higher levels of d‐dimer, hs‐CRP, and ANA in unadjusted and fully adjusted models compared with white men. Furthermore, in unadjusted and fully adjusted models, black men also had lower levels of sRAGE, LP‐PLA2 activity and mass, interleukin‐18, and monocyte chemoattractant protein‐1 with no significant difference in soluble tumor necrosis factor receptor.
Endothelial Biomarkers
Unadjusted models showed significantly lower levels of soluble endothelial cell selective adhesion molecule, symmetrical dimethylarginine (SDMA) and asymmetrical dimethylarginine (ADMA), and higher levels of homoarginine in black women versus white women. After full adjustment, the racial differences seen in soluble endothelial cell selective adhesion molecule, SDMA, and ADMA were attenuated, while homoarginine remained significantly higher in black women. No racial differences were seen in soluble intercellular adhesion molecule or soluble vascular cell adhesion molecule among women.
Lower levels of soluble endothelial cell selective adhesion molecule and ADMA and higher levels of homoarginine were seen in unadjusted and adjusted models in black versus white men. Additionally, SDMA was noted to be significantly higher in black men after full adjustment. Similar to women, no racial differences were seen in soluble intercellular adhesion molecule and soluble vascular cell adhesion molecule among men.
Biomarkers of Myocyte Injury and Stress
Black women had higher levels of hs‐TnT in unadjusted models; however, full adjustment resulted in loss of statistical significance. A higher level of soluble suppression of tumorigenicity 2 (sST2) was noted in black women in unadjusted and fully adjusted analysis. Black women also had lower N‐terminal pro‐B‐type natriuretic peptide concentrations in comparison with white women. Among men, hs‐TnT, ST2, and growth differentiation factor‐15 were higher in blacks than whites. The racial difference in growth differentiation factor‐15 attenuated with mutivariable adjustment, whereas N‐terminal pro‐B‐type natriuretic peptide became significantly lower in black men after adjustment and high‐sensitivity cardiac troponin T (hs‐cTnT) and sST2 differences remained significantly higher in black men.
Biomarkers of Kidney Function
For both black women and men, significantly lower cystatin C values were observed in unadjusted models that lost significance after full adjustment. Urinary microalbumin was higher among black women and men than white women and men in unadjusted models, and remained significantly higher in black women but not black men after full adjustment.
Exploratory Mediation Analyses
In women, higher Lp(a), ANA, osteoprotegerin, and urinary microalbumin and lower sRAGE were associated with CVD events in univariable analyses (Table 3). The unadjusted hazard ratio (HR) for CVD events in black women was 2.05 (95% CI 1.32, 3.17). Adjusting for these biomarkers attenuated the association of black race with global CVD such that it was no longer significant (HR 1.15, 95% CI 0.69, 1.92) (Table 3). In men, higher Lp(a), leptin, hs‐CRP, d‐dimer, SDMA, and hs‐cTnT and lower sRAGE were associated with CVD events. The unadjusted HR for CVD events in black men was 2.39 (95% CI 1.64, 3.46). After adjusting for these biomarkers, the HR in black men was attenuated and no longer significant (HR 1.21, 95% CI 0.76, 1.95) (Table 3).
Table 3.
Association of Black Race and Biomarkers With Incident Cardiovascular Disease
| Variable | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Women | ||
| Black race | 2.05 (1.32, 3.17) | 1.15 (0.69, 1.92) |
| Lp(a) | 1.23 (1.02, 1.47) | 1.15 (0.94, 1.41) |
| ANA | 1.35 (1.10, 1.65) | 1.25 (1.02, 1.55) |
| Osteoprotegerin | 1.51 (1.16, 1.98) | 1.48 (1.10, 1.99) |
| Urinary microalbumin | 1.73 (1.40, 2.15) | 1.67 (1.32, 2.12) |
| sRAGE | 0.38 (0.19, 0.73) | 0.43 (0.21, 0.87) |
| Men | ||
| Black race | 2.39 (1.64, 3.46) | 1.21 (0.76, 1.95) |
| Lp(a) | 1.33 (1.15, 1.56) | 1.15 (0.96, 1.38) |
| Leptin | 1.21 (1.00, 1.45) | 1.01 (0.84, 1.22) |
| hsCRP | 1.61 (1.34, 1.90) | 1.23 (1.00, 1.52) |
| d‐dimer | 2.13 (1.69, 2.68) | 1.54 (1.17, 2.01) |
| SDMA | 14.28 (3.20, 63.8) | 1.64 (0.32, 8.53) |
| sRAGE | 0.42 (0.23, 0.75) | 0.65 (0.36, 1.17) |
| hs‐cTnT | 1.92 (1.62, 2.26) | 1.53 (1.23, 1.91) |
All biomarkers were log transformed. ANA indicates antinuclear antibodies; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; hs‐cTnT, high‐sensitivity cardiac troponin T; Lp(a), lipoprotein (a); SDMA, symmetric dimethyl arginine; sRAGE, soluble receptor for advanced glycation end products.
Discussion
In a multiethnic population‐based cohort without known CVD, we observed rates of incident CVD that were more than twice as high in black versus white men and women. We found racial differences in multiple biomarkers that reflect distinct biological pathways relevant to the development of CVD or its complications. When biomarkers that differed by race and associated with CVD events were accounted for in exploratory analyses, the associations of black race with incident ASCVD and global CVD were attenuated and no longer significant, suggesting that biological pathways reflected by some of these biomarkers may contribute to or mediate racial differences in CVD.
There has been inadequate study of racial differences in cardiovascular biomarkers, and to our knowledge this study represents the largest comparison of multiple biomarkers linked with CVD risk between black and white women and men without known CVD in a population‐based cohort. Strengths of this study include the large sample size and the deep phenotyping performed, which allowed us to compare biomarkers between black and white individuals independent of racial differences in traditional risk factors, socioeconomic status, body composition, and cardiac structure and function. The prospective follow‐up with adjudicated events also allowed us to document important racial disparities in CVD outcomes and explore potential biological pathways contributing to these differences.
Traditional risk factors are thought to play a major role in racial differences in CVD. The prevalence and mortality attributable to hypertension and diabetes mellitus is higher in blacks than whites,7, 22 and we also observed a higher proportion of these risk factors among blacks in our study. Additionally, black women had more total and regional adiposity than white women; in contrast, the opposite was seen among men, with black men having less total and regional adiposity than white men. Consistent with well‐documented socioeconomic differences between blacks and whites,23 black participants in our study reported lower education and income compared with white participants. Higher risk factor burden, greater visceral adiposity,24, 25 and lower socioeconomic status26 have all been associated with incident CVD and likely contribute to higher rates of CVD observed among blacks.
While it is generally known that blacks have a more favorable standard lipid profile than whites, blacks have higher rates of coronary events,27 suggesting that additional lipid parameters may contribute to ASCVD risk in blacks. We observed lower triglycerides and LDL‐p and HDL‐p among black women than white women, findings partially reported previously in a subset of DHS.28 Among men, findings were generally similar except HDL‐C was significantly higher among black versus white men. Of interest, however, cholesterol efflux capacity, a measure of HDL function reflecting reverse cholesterol transport, did not differ between blacks and whites. These findings suggest that the higher HDL concentrations observed in black men are not accompanied by improved HDL function. Among the lipid biomarkers, the strongest association with race was observed with Lp(a), which was higher in both black men and women than their white counterparts. Lp(a) has been identified as a causal and independent risk factor for CVD, thought to be mediated through its proatherogenic and prothrombotic mechanism.29 Several prior studies have demonstrated higher Lp(a) levels in blacks compared with whites, including a report from the DHS,30 in which higher Lp(a) was associated with increased CVD risk in black, white, and Hispanic race/ethnic groups.30 In the large ARIC (Atherosclerosis Risk in the Community) study, the association between Lp(a) and CV events was also consistent in blacks and whites. In fact, there was a similar HR for cardiovascular events using the same Lp(a) cut point (>30 mg/dL), but a larger proportion of blacks had Lp(a) above this level,31 indicating a larger proportion of risk explained by Lp(a) in blacks versus whites. The Multi‐Ethnic Study of Atherosclerosis investigators suggested that the threshold level of Lp(a) for increased CVD risk may actually be lower in blacks (≥30 mg/dL) than in whites (≥50 mg/dL) despite blacks having higher Lp(a) levels.32 In summary, differences in Lp(a) may contribute to higher coronary disease rates despite a more favorable lipid profile in blacks versus whites.
Unfavorable adipokine profiles may contribute to racial differences in cardiovascular risk. Adiponectin, which increases insulin sensitivity and glucose utilization,33 was lower in both black women and men compared with white counterparts, even after accounting for differences in rates of diabetes mellitus and insulin resistance, and further adjustment for measures of body composition. In a prior DHS study, lower adiponectin was associated with insulin resistance and diabetes mellitus and with higher rates of incident hypertension.34 Additionally, black women and men also had higher levels of leptin in adjusted models. Higher levels of leptin have been associated with numerous adverse cardiometabolic factors.35 Thus, a particularly adverse adipokine profile was seen in blacks, which may contribute to excess diabetes mellitus and metabolic risk.34, 35
Racial differences were observed in multiple inflammatory biomarkers, with some demonstrating adverse associations in blacks and others a more favorable profile. Elevated ANA and d‐dimer demonstrated the strongest associations with black race. Moreover, accounting for ANA in women and for d‐dimer in men contributed to attenuation of the associations of black race with incident CVD, providing preliminary support that these biomarkers may reflect pathways contributing to higher rates of CVD in blacks. ana are autoantibodies that bind to contents of the cell nucleus. Prior studies suggest that atherosclerosis is largely mediated by a chronic inflammatory state.36 In DHS, higher ANA levels were independently associated with all‐cause mortality, cardiovascular death, and ASCVD after adjustment of cardiovascular risk factors, age, sex, and ethnicity.37 d‐dimer, which results from fibrin degradation, is higher in systemic inflammatory and thrombotic conditions and may identify individuals with increased potential for venous thromboembolism in the general population.38
Black women and men had lower levels of sRAGE than their white counterparts. The RAGE is a multiligand cell‐surface protein that binds pro‐inflammatory ligands, including advanced glycation end products. Soluble forms of RAGE (sRAGE) are produced by proteolytic cleavage of membrane‐bound receptor or by alternative splicing and are thought to be protective by acting as a decoy receptor that diminishes RAGE–ligand interactions at the cell surface, diminishing the inflammatory response.39 Lower sRAGE emerged as being associated with CVD risk in both women and men, and adjusting for sRAGE contributed to attenuation of racial differences in CVD. These consistent findings suggest that lower sRAGE could contribute to inflammation‐mediated vascular injury in blacks.
In contrast, other specific inflammatory pathways were lower in blacks. For example, black men and women had significantly lower levels of LP‐PLA2 mass and activity. Inflammatory cells secrete the enzyme LP‐PLA2, which binds to LDL‐C and other lipoproteins, creating pro‐inflammatory lipid particles.40 Although LP‐PLA2 mass and activity have been linked with risk of ASCVD in epidemiological studies,18 therapeutic trials have not found that pharmacological targeting of LP‐PLA2 is of clinical benefit.41
Black women had higher levels of osteoprotegerin than white women, and accounting for these differences contributed to attenuation of the excess CVD risk signal in black women. No significant difference in osteoprotegerin was seen between black and white men. Osteoprotegerin is a soluble decoy receptor for receptor activator of nuclear factor kappa‐B ligand (RANKL) and tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL), inhibiting ligation of these pro‐inflammatory mediators with related receptors. However, circulating levels are thought to reflect activity of the RANK/RANKL pathway.42 Sustained upregulation of osteoprotegerin induces damaging pro‐fibrotic, pro‐inflammatory, pro‐apoptotic, and plaque‐destabilizing effects.43 hs‐CRP is a prototype acute‐phase reactant and has been shown to be independently associated with myocardial infarction and cardiovascular death in prospective cohort studies.44 Black men and women had elevated hs‐CRP levels in unadjusted models, but after full adjustment only black men were found to have significantly higher levels. Adjustment for hs‐CRP contributed to attenuation of excess CVD risk in black men.
A generally more favorable profile of endothelial function biomarkers was seen in blacks versus whites, particularly among men. Levels of homoarginine, which increases nitric oxide availability and thereby enhances endothelial function, were higher in black men and women.45 In black men, lower ADMA but higher SDMA was seen in fully adjusted models. Our exploratory analyses suggest that higher SDMA could contribute to some of the excess CVD risk seen in black versus white men. SDMA and ADMA are endogenous nitric oxide inhibitors, which may contribute to endothelial dysfunction. Black men also had lower levels of soluble endothelial cell selective adhesion molecule than white men. ESAM is a cell adhesion molecule expressed in vascular endothelium and activated platelets, with the soluble form associated with subclinical atherosclerosis and clinical events.46, 47 A more favorable endothelial function profile seen in black men may in part explain why black men have less atherosclerotic burden than whites, despite worse traditional risk factor profiles.
As expected from prior work in this cohort, we found that black men and women had higher levels of hs‐cTnT. Subclinical myocardial injury detected with the hs‐cTnT assay predicts increased risk of death and heart failure events.9 After full adjustment, the association with higher hs‐cTnT persisted in black men but was lost in black women, perhaps because of limited statistical power related to lower hs‐cTnT in women. Moreover, adjustment for hs‐cTnT contributed to attenuation in racial differences in CVD outcomes in men, suggesting that pathways leading to subclinical myocardial injury may contribute to racial differences in CVD. As has been previously reported in DHS,17 natriuretic peptide levels are lower in black versus white men and women after full adjustment. Natriuretic peptides have many favorable physiological effects, including natriuresis, inhibiting the renin‐angiotensin‐aldosterone and sympathetic nervous systems, and promoting cardiac lusitropy. sST2 is a member of the interleukin‐1 receptor family, and is a marker of myocyte strain and fibrosis. A prior general population study has shown that sST2 were higher in blacks and predict increased all‐cause and cardiovascular mortality.48 This biomarker profile of augmented cardiac injury, combined with an impaired natriuretic peptide response, may contribute to the known excess risk of heart failure among blacks.
Various studies have correlated cardiovascular disease with microalbuminuria. Elevated levels have been related to myocardial infarction and left ventricular dysfunction.49 Our analysis showed higher levels of microalbuminuria in black women as well as attenuation of the hazard for CVD in black women in the multivariable model including urinary microalbumin.
Limitations
Race/ethnicity and socioeconomic factors were self‐reported by participants, which may lead to misclassification bias. Furthermore, comparisons of biomarkers by race were limited to cross‐sectional analyses without serial measurements of biomarkers. Our analyses did not account for racial differences in dietary and physical activity patterns, which could influence biomarkers. Although we accounted for multiple testing, replication of positive findings was not performed. Racial differences in some of the biomarkers included in this study have previously been reported in the DHS, but those analyses were less comprehensive than reported here and did not account for racial differences in body composition, insulin resistance, and cardiac structure. Finally, the mediation analyses are exploratory and should only be considered hypothesis generating.
Conclusions
In this population‐based study, significantly higher rates of incident CVD occurred among black compared with white individuals. We observed racial differences in multiple biomarkers associated with cardiovascular disease. Blacks exhibited an adverse adipokine profile but a generally more favorable pattern with regard to endothelial function biomarkers. Inflammatory biomarker relationships were heterogeneous, with some demonstrating adverse and others favorable associations with black race. Black participants were also more likely to have subclinical cardiomyocyte injury and had lower levels of protective natriuretic peptides. In exploratory analyses, adjustment for biomarkers reflecting several of these pathogenic pathways attenuated excess CVD risk among blacks. These findings suggest that, beyond the well‐documented differences in traditional risk factors and obesity, contributions from adipose dysfunction, inflammation, myocyte injury, and neurohormonal pathways may play a role in differences seen in manifestations of CVD between blacks and whites.
Sources of Funding
The Dallas Heart Study has been supported by grants from the Donald W. Reynolds Foundation and the National Center for Advancing Translational Sciences of the NIH (UL1TR001105). Biomarker measurements for the present study were supported by Roche Diagnostics, Alere, Inc, LipoScience, and Siemens Healthcare Diagnostics, Inc. Funding support for Dr Neeland is provided by grant 1K23DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Dedman Family Scholarship in Clinical Care from UT Southwestern. Dr Atzler acknowledges the support of the European Union under a Marie Curie Intra‐European Fellowship for Career Development. Dr Rohatgi is supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number K08HL118131 and by the American Heart Association under Award Number 15CVGPSD27030013.
Disclosures
Dr Neeland has received grant support from Novo Nordisc, speaker fees from Boeringher Ingelheim, and consulting fees from AMRA. Dr Rohatgi has received a research grant from Merck and reports consulting income from Cleveland HeartLab, Vascular Strategies, and CSL Limited. Dr Omland has received research support from Abbott Diagnostics, AstraZeneca, and Thermo Fisher and honoraria from Abbott Diagnostics, Roche Diagnostics, and Novartis. Dr de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics, and consulting income from Roche Diagnostics, Abbott Diagnostics, Siemens Diagnostics, Ortho Clinical Diagnostics, Amgen, Regeneron, Esperion, Jannsen, and Novo Nordisc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Biomarker Assays
(J Am Heart Assoc. 2019;8:e012729 DOI: 10.1161/JAHA.119.012729.)
Preliminary results from this study were presented at the American College of Cardiology Scientific Sessions, March 17 to 19, 2017, in Washington, DC.
References
- 1. Saab KR, Kendrick J, Yracheta JM, Lanaspa MA, Pollard M, Johnson RJ. New insights on the risk for cardiovascular disease in African Americans: the role of added sugars. J Am Soc Nephrol. 2015;26:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. [DOI] [PubMed] [Google Scholar]
- 4. Cruz‐Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, Peterson E, Rosamond W, Trimble B, Valderrama AL; American Heart Association Stroke C, Council on Cardiovascular N, Council on E, Prevention, Council on Quality of C and Outcomes R . Racial‐ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics C and Stroke Statistics S . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 6. Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring). 2013;21:E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powell‐Wiley TM, Ayers CR, de Lemos JA, Lakoski SG, Vega GL, Grundy S, Das SR, Banks‐Richard K, Albert MA. Relationship between perceptions about neighborhood environment and prevalent obesity: data from the Dallas Heart Study. Obesity (Silver Spring). 2013;21:E14–E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qu HQ, Li Q, Rentfro AR, Fisher‐Hoch SP, McCormick JB. The definition of insulin resistance using HOMA‐IR for Americans of Mexican descent using machine learning. PLoS One. 2011;6:e21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. [DOI] [PubMed] [Google Scholar]
- 13. Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging. 2017;10:e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee and European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 15. Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, Morrow DA, Drazner MH, McGuire DK, de Lemos JA. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n‐terminal pro‐brain natriuretic peptide in a multiethnic population‐based sample (the Dallas Heart Study). Am J Cardiol. 2005;96:1284–1289. [DOI] [PubMed] [Google Scholar]
- 16. Tikuisis P, Meunier P, Jubenville CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. 2001;85:264–271. [DOI] [PubMed] [Google Scholar]
- 17. Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial differences in natriuretic peptide levels: the Dallas Heart Study. JACC Heart Fail. 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lew J, Sanghavi M, Ayers CR, McGuire DK, Omland T, Atzler D, Gore MO, Neeland I, Berry JD, Khera A, Rohatgi A, de Lemos JA. Sex‐based differences in cardiometabolic biomarkers. Circulation. 2017;135:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, Drazner MH, Budoff M, Greenland P, Ballantyne CM, Khera A. Multimodality strategy for cardiovascular risk assessment: performance in 2 population‐based cohorts. Circulation. 2017;135:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao D, Post WS, Blasco‐Colmenares E, Cheng A, Zhang Y, Deo R, Pastor‐Barriuso R, Michos ED, Sotoodehnia N, Guallar E. Racial differences in sudden cardiac death. Circulation. 2019;139:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch‐Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, Khera A, Vega GL, McGuire DK, Grundy SM, de Lemos JA. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA, Sperling LS. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am J Cardiol. 2015;115:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saeedi R, Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clin Diabetes Endocrinol. 2016;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SR, Prasad A, Choi YS, Xing C, Clopton P, Witztum JL, Tsimikas S. LPA gene, ethnicity, and cardiovascular events. Circulation. 2017;135:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Azrad M, Gower BA, Hunter GR, Nagy TR. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine. 2013;43:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peri‐Okonny PA, Ayers C, Maalouf N, Das SR, de Lemos JA, Berry JD, Turer AT, Neeland IJ, Scherer PE, Vongpatanasin W. Adiponectin protects against incident hypertension independent of body fat distribution: observations from the Dallas Heart Study. Diabetes Metab Res Rev. 2017;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdullah SM, Khera A, Leonard D, Das SR, Canham RM, Kamath SA, Vega GL, Grundy SM, McGuire DK, de Lemos JA. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. [DOI] [PubMed] [Google Scholar]
- 36. Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, Gabriel SE. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36:2462–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solow EB, Vongpatanasin W, Skaug B, Karp DR, Ayers C, de Lemos JA. Antinuclear antibodies are associated with all‐cause mortality and cardiovascular outcomes in the general population. J Am Coll Cardiol. 2015;65:2669–2670. [DOI] [PubMed] [Google Scholar]
- 38. Folsom AR, Alonso A, George KM, Roetker NS, Tang W, Cushman M. Prospective study of plasma D‐dimer and incident venous thromboembolism: the Atherosclerosis Risk in Communities (ARIC) Study. Thromb Res. 2015;136:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brinkley TE, Leng X, Nicklas BJ, Kritchevsky SB, Ding J, Kitzman DW, Hundley WG. Racial differences in circulating levels of the soluble receptor for advanced glycation endproducts in middle‐aged and older adults. Metabolism. 2017;70:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brilakis ES, Khera A, Saeed B, Banerjee S, McGuire DK, Murphy SA, de Lemos JA. Association of lipoprotein‐associated phospholipase A2 mass and activity with coronary and aortic atherosclerosis: findings from the Dallas Heart Study. Clin Chem. 2008;54:1975–1981. [DOI] [PubMed] [Google Scholar]
- 41. Welsh P, Grassia G, Botha S, Sattar N, Maffia P. Targeting inflammation to reduce cardiovascular disease risk: a realistic clinical prospect? Br J Pharmacol. 2017;174:3898–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Omland T, Drazner MH, Ueland T, Abedin M, Murphy SA, Aukrust P, de Lemos JA. Plasma osteoprotegerin levels in the general population: relation to indices of left ventricular structure and function. Hypertension. 2007;49:1392–1398. [DOI] [PubMed] [Google Scholar]
- 43. Tschiderer L, Willeit J, Schett G, Kiechl S, Willeit P. Osteoprotegerin concentration and risk of cardiovascular outcomes in nine general population studies: literature‐based meta‐analysis involving 26,442 participants. PLoS One. 2017;12:e0183910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kelley‐Hedgepeth A, Lloyd‐Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, Sternfeld B, Pasternak RC, Chae CU; SWAN Investigators . Ethnic differences in C‐reactive protein concentrations. Clin Chem. 2008;54:1027–1037. [DOI] [PubMed] [Google Scholar]
- 45. Marz W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Bohm BO, Ritz E, Wanner C. Homoarginine, cardiovascular risk, and mortality. Circulation. 2010;122:967–975. [DOI] [PubMed] [Google Scholar]
- 46. Rohatgi A, Owens AW, Khera A, Ayers CR, Banks K, Das SR, Berry JD, McGuire DK, de Lemos JA. Differential associations between soluble cellular adhesion molecules and atherosclerosis in the Dallas Heart Study: a distinct role for soluble endothelial cell‐selective adhesion molecule. Arterioscler Thromb Vasc Biol. 2009;29:1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren HY, Khera A, de Lemos JA, Ayers CR, Rohatgi A. Soluble endothelial cell‐selective adhesion molecule and incident cardiovascular events in a multiethnic population. Am Heart J. 2017;191:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community‐dwelling population. J Am Heart Assoc. 2016;5:e003188 DOI: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens. 2004;17:986–993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Biomarker Assays


