Abstract
Background
Varying degrees of co‐occurrence of intracranial aneurysms (IA) and aortic aneurysms (AA) have been reported. We sought to compare the risk for AA in fusiform intracranial aneurysms (fIA) and saccular intracranial aneurysms (sIA) disease and evaluate possible genetic connection between the fIA disease and AAs. Additionally, the characteristics and aneurysms of the fIA and sIA patients were compared.
Methods and Results
The Kuopio Intracranial Aneurysm Database includes all 4253 sIA and 125 fIA patients from its Eastern Finnish catchment population, and 13 009 matched population controls and 18 455 first‐degree relatives to the IA patients were identified, and the Finnish national registers were used to identify the individuals with AA. A total of 33 fIA patients were studied using an exomic gene panel of 37 genes associated with AAs. Seventeen (14.4%) fIA patients and 48 (1.2%) sIA patients had a diagnosis of AA. Both fIA and sIA patients had AAs significantly more often than their controls (1.2% and 0.5%) or relatives (0.9% and 0.3%). In a competing risks Cox regression model, the presence of fIA was the strongest risk factor for AA (subdistribution hazard ratio 7.6, 95% CI 3.9–14.9, P<0.0005). One likely pathogenic variant in COL5A2 and 3 variants of unknown significance were identified in MYH11,COL11A1, and FBN1 in 4 fIA patients.
Conclusions
The prevalence of AAs is increased slightly in sIA patients and significantly in fIA patients. fIA patients are older and have more comorbid diseases than sIA patients but this alone does not explain their clinically significant AA risk.
Keywords: abdominal aortic aneurysm, aortic aneurysm, fusiform intracranial aneurysm, genetics, intracranial aneurysm, saccular intracranial aneurysm, thoracic aortic aneurysm
Subject Categories: Cerebral Aneurysm, Aneurysm, Aortic Dissection, Epidemiology
Clinical Perspective
What Is New?
Prevalence of diagnosed aortic aneurysms in patients with intracranial aneurysms, assessed through the Finnish nationwide diagnosis register, is increased in comparison to the matched population controls and first‐degree relatives of the patients with intracranial aneurysms.
Patients with fusiform intracranial aneurysms, constituting 3% of all patients with intracranial aneurysms, are older and have a greater burden of comorbid diseases and cardiovascular risk factors in comparison with patients with saccular intracranial aneurysms.
Presence of a fusiform intracranial aneurysm is strongly associated with aortic aneurysms, independent of other investigated cardiovascular risk factors.
What Are the Clinical Implications?
Fusiform intracranial aneurysms are associated with a clinically significant co‐occurrence rate of aortic aneurysms, and we recommend screening patients with fusiform intracranial aneurysms for aortic aneurysms.
Saccular intracranial aneurysm patients share risk factors with aortic aneurysm patients, but routine screening does not seem to be indicated because of low co‐occurrence rate.
Introduction
Aneurysmal subarachnoid haemorrhage at an incidence of 7.9 per 100 000 and in an average age of 50 to 60 years,1 is a devastating form of stroke: 20% of aneurysmal subarachnoid haemorrhage patients die instantly2 and, when admitted alive, up to 30% within 12 months.3 In most cases, the cause is rupture of a saccular intracranial aneurysm (sIA), formed during life in some 3% of general population.4 Risk factors for sIA include age, female sex, smoking, hypertension, sIA family history, and polycystic kidney disease.5 Fusiform IAs (fIAs), like aortic aneurysms (AA), are dilatations of arterial segments6, 7: fIAs are uncommon, and remain poorly characterized and difficult to treat.7
Aortic aneurysms (AA) are increasingly prevalent amongst aging population especially in aging, smoking, hypertensive men.8, 9, 10 The incidence varies from 6 to 10.4 per 100 000 for thoracic aortic aneurysms (TAA)8 to 0.89 to 176.08 per 100 000 for abdominal aortic aneurysms (AAA), the prevalence increasing steeply towards the most aged groups.11 Familial background precedes 20% of thoracic aortic aneurysms (TAA) and positive family history is a known risk factor for abdominal aortic aneurysm (AAA).8, 9, 10
Increased prevalence of IA in AA patients, as well as AA in IA patients, has been suggested in several studies.12, 13, 14, 15, 16, 17, 18, 19 However, these cohorts did not contain patients and controls from defined catchment populations and, in most cases, fIAs were not distinguished from sIAs.
We analyzed, in retrospect, the characteristics of the 125 fIA patients with 134 fIAs as compared with the 4253 sIA patients with 6097 sIAs from a defined population in the Kuopio Intracranial Aneurysm Patient and Family Database, which comprises all the IA patients of Eastern Finland diagnosed or treated in Kuopio University Hospital (KUH) since 1980. We studied the prevalence of AA in these fIA patients and sIA patients and in their first‐degree relatives, and in the patients’ matched population controls, using the Finnish national clinical registries. Hypothesizing that fIAs and AAs, both fusiform in shape, share genomic risk factors, we systematically reviewed the literature for family trees containing IA and AA patients and for patient cohorts with patients with both IA and AA. Finally, we sequenced 33 fIA patients with an aortic gene panel of 37 AA‐related genes.
Methods
Data that support the findings of this study are available to qualified researchers who meet the criteria provided by the board of the Kuopio Intracranial Aneurysm Patient and Family Database.20 The Finnish National Institute for Health and Welfare provides data for all researchers who meet the criteria set by the institute.
KUH Catchment Population
KUH Neurosurgery has been a sole provider of full‐time acute and elective neurosurgical services for the KUH catchment population in Eastern Finland since 1977. Between 1980 and 2015, the geographic area of the KUH hospital districts did not change, the population decreased from 882 671 to 815 021, the median age increased from 37 to 42 years in men and from 40 to 45 years in women whereas the proportion of men has remained at 49%.21, 22
Kuopio IA Patient and Family Database
The database includes all cases of unruptured and ruptured IA patients admitted to KUH since 1980 for angiography and treatment, unless moribund or very aged. The database has been prospective since 1990. The database is administrated by a full‐time nurse coordinator who interviews all new patients and codes this information, including family history, and the clinical data from hospital periods and follow‐up visits into an extensive list of variables. The criterion for familial IA disease was at least 2 affected first‐degree relatives. The genealogy of the verified IA patients has been extensively mapped through parish records up to the 17th century.22 The phenotype, concomitant diseases, genetics, and outcome of Eastern Finnish sIA patients have been analyzed in several studies.21, 22, 23, 24, 25
Study Population
The Kuopio IA Database includes only patients with intracranial aneurysm(s) in 4‐vessel angiography (digital subtraction angiography, computed tomography angiography, or magnetic resonance angiography), initially verified by both a neurovascular neurosurgeon and a neuroradiologist. The 125 fIA patients were agreed to carry a fusiform or dolichoectatic aneurysm in a re‐review by a neurosurgeon (B.R.J.) and an interventional neuroradiologist (O.T.) (Figure 1). Intracranial artery dissections were excluded. The size of the fIAs was defined as the greatest length or largest perpendicular diameter, whichever larger.
Figure 1.
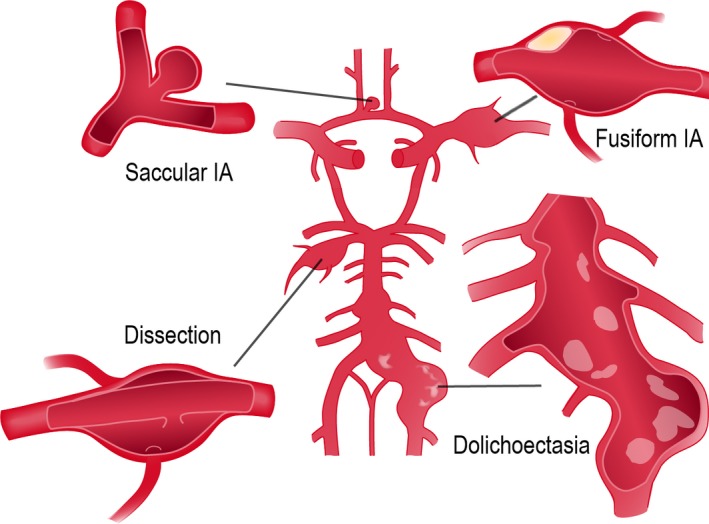
The major intracranial extracerebral arteries with the circle of Willis (middle). The saccular intracranial aneurysm (left upper) forms at the branching sites of the arteries, whereas the uncommon fusiform intracranial aneurysm (right upper) mainly involves the arterial trunks (see Figure 5). The dolichoectasia (right lower) usually involves the vertebrobasilar trunk. The acute dissection with false lumen (left lower), more frequent in the cervical arteries, is also shown. Note the atheromatous plaque of the fusiform intracranial aneurysm wall and the calcification of the dolichoectatic artery. IA indicates intracranial aneurysm.
A total of 450 and 17 825 first‐degree relatives were identified to the 125 fIA patients and the 4253 sIA patients, respectively, using the personal identity codes and the Finnish Population Register Centre (Figure 2).22, 25 A total of 340 and 12 669 matched population controls were assigned to the 125 fIA patients and the 4253 sIA patients, matched by age, sex, year, and municipality at the IA diagnosis. The index date for the matching was the date of the admission for IA or aneurysmal subarachnoid haemorrhage, with all controls alive at that point. Although we aimed at a 3:1 ratio (controls:patients), this ratio was not achieved in 35 fIA and 90 sIA patients.
Figure 2.
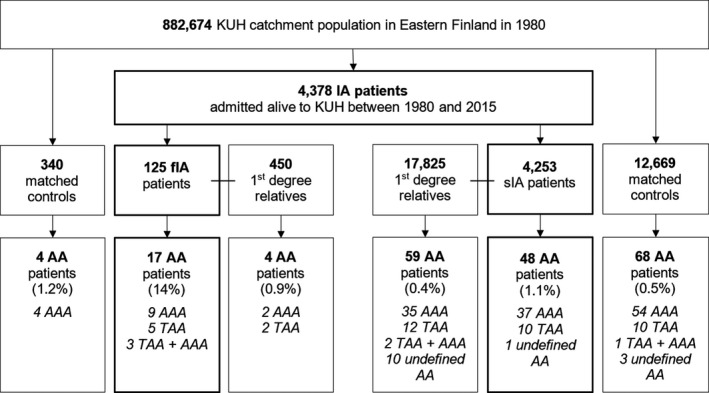
Flowchart of the study population: 4378 patients with intracranial aneurysm (IA) disease admitted to the Kuopio University Hospital with an unruptured IA or first aneurysmal subarachnoid hemorrhage from the Eastern Finnish catchment population from 1980 to 2015. A total of 450 and 17 825 first‐degree relatives were identified to the 125 patients with fusiform IA and the 4253 patients with saccular IA, respectively, using the personal identity codes and the Finnish Population Register Centre. A total of 340 and 12 669 matched population controls were assigned to the 125 fusiform IA patients and the 4253 saccular IA patients, matched by age, sex, year, and municipality at the IA diagnosis. In 35 fusiform IA patients and 90 saccular IA patients, the 3:1 ratio was not achieved. AA indicates aortic aneurysm; AAA, abdominal aortic aneurysm; IA, intracranial aneurysm; KUH, Kuopio University Hospital; TAA, thoracic aortic aneurysm; undefined AA, AA of undefined site.
Data Fusion From National Registries With Personal Identification Codes
The drugs prescribed for the patients, the family members and the controls, purchased from apothecaries, as well as the hospital diagnoses and the causes of death were imported from the Finnish national registries.20 The Finnish electronic hospital diagnosis registry (Care Register for Health Care HILMO, managed by the Finnish Institution for Health and Welfare), which was cross‐linked with the Kuopio IA Database, covers all secondary and tertiary centers in Finland and includes all medical specialties. The hospital diagnoses from 1969 to 2014 and medication reimbursement statistics from 1995 to 2014 were available.
The prevalence of hypertension and atherosclerotic disease were investigated with both the hospital discharge register and the national medicine reimbursement statistics. Hypertension was defined as either: (1) diagnosis of hypertension or hypertensive disease in the diagnosis registry; or (2) use of prescribed antihypertensive medication (ATC codes C02, C03A, C04, C08 and C09). The general limit for diagnosis of hypertension has been ≥ 140/ ≥ 90 mm Hg during the time period covered by the study. Atherosclerotic disease and hyperlipidemia were defined as either: (1) diagnosis of atherosclerotic disease (peripheral atherosclerosis, atherosclerotic heart disease, atherosclerotic disease of cerebral arteries); or (2) use of prescribed lipid‐lowering drugs (ATC code C10).
Diagnosed Aortic Aneurysms in the Study Population
The diagnosed aortic aneurysms in the 4378 IA patients and their 17 278 first‐degree relatives and 13 009 population controls were obtained from the Finnish national hospital discharge register (Care Register for Health Care). The International Classification of Diseases, Ninth Revision (ICD‐9) and ICD‐10 codes for aortic dissections and aneurysm (441.0–441.9 and I71.00–I71.9.) were used. Furthermore, the chest radiographs, as well as thoracic and abdominal computed tomography (CT) or ultrasound imaging available for 80 (64%) fIA patients were re‐reviewed by a neurosurgeon (B.R.J.) and an interventional radiologist (H.M.) for presence of aortic aneurysms. The patient records of the IA patients with AA or dissection were obtained whenever possible to ascertain the diagnosis and the type of AA.
Genotyping With Aortic Aneurysm Gene Panel
Of the 41 fIA patients alive, 33 were available for genotyping with informed and signed consent. Four of these 33 patients were diagnosed with AA. First‐degree relatives of the patients were not available for screening. Targeted sequencing was performed using OS‐seq technology described earlier26, 27 using a commercial Aorta Panel consisting of 37 AA genes: ABCC6, ACTA2, ADAMTS2, CBS, COL1A1, COL1A2, COL2A1, COL3A1, COL5A1, COL5A2, COL9A1, COL9A2, COL11A1, COL18A1, EFEMP2, ELN, ENPP1, FBLN5, FBN1, FBN2, FKBP14, FLNA, GATA5, MFAP5, MYH11, NOTCH1, PLOD1, SKI, SLC2A10, SLC39A13, SMAD3, TGFB2, TGFB3, TGFBR1, TGFBR2, TNXB, ZNF469 (www.blueprintgenetics.com). All protein coding exons and exon‐intron boundaries (±15 bps) are covered in each targeted gene and the panel has high sensitivity to detect single nucleotide variants, small insertions and deletions up to 50 bps as well as exon level copy‐number variations. Variant classification followed the 2015 ACMG (American College of Medical Genetics and Genomics) guideline.28
Shortly, pathogenicity of the identified variants were assessed by considering the predicted consequence, the biochemical properties of the codon change, the degree of evolutionary conservation as well as a number of reference population databases and mutation databases such as, but not limited, to the 1000 Genomes Project, gnomAD, ClinVar and the Human Gene Mutation Database. For missense variants, in silico variant prediction tools such as SIFT, PolyPhen, MutationTaster were used to assist with variant classification.
Statistical Analysis
Categorical variables were compared between groups with χ2 and Fisher exact tests as appropriate. Continuous variables were tested with the Student t‐test or Mann‐Whitney U test as appropriate. As aortic aneurysms and death might share risks factors, competing risks multivariate Cox regression was performed to determine risk factors for the diagnosis of aortic aneurysms among patients with IA. The variables used in the model were sex, hypertension, any diagnosed atherosclerotic disease or prescribed lipid‐lowering drug, type 2 diabetes mellitus, IA rupture status, and IA morphology. Schoenfeld residuals were used to confirm the model's assumption of proportionality. The results are expressed as subdistribution hazard ratios (SHRs) with 95% CIs. Two‐tailed P<0.05 was considered significant.
Analyses were performed using IBM SPSS Statistics, version 22.0 (IBM SPSS, Armonk, NY) and the R environment for statistical computing, including cmprsk library.
Literature Review
Two literature searches were performed in September 2018 for English articles published between 1995 and 2016, a period of non‐invasive IA imaging by CT and magnetic resonance (MR). Firstly, all cohorts with concurrent IA and AA were searched with the terms: (aneurysm OR dolichoecta*) AND (aorta OR aortic OR abdominal OR thoracic) AND (intracranial OR cerebral OR cns OR brain OR subarachnoid*) AND (prevalen* OR inciden* OR epidemiology OR population). This gave 3969 hits). All abstracts were reviewed, and the case reports and duplicates were excluded. The remaining articles were searched for cohorts that reported the angiographically identified IAs in the patients with diagnosed AA and cohorts that reported diagnosed AAs in the patients with IA. The final 8 cohorts presented the proportion of IAs among the AA patients or AAs among the IA patients (Figure 3, Table 1).12, 13, 14, 15, 16, 17, 18, 19
Figure 3.
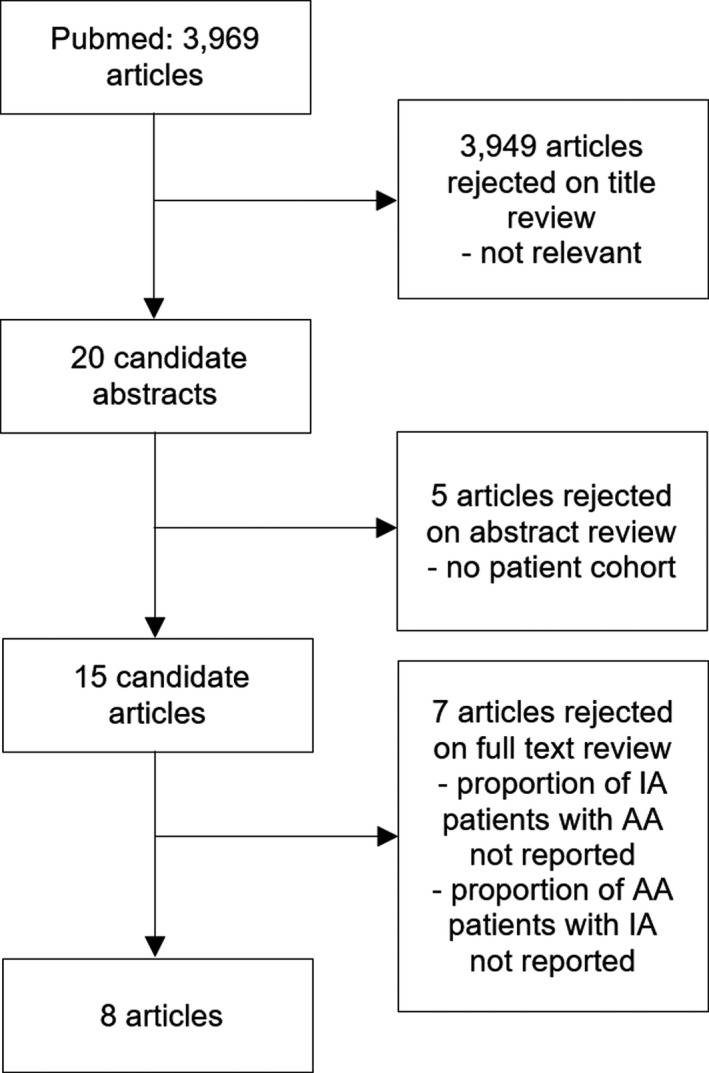
Literature search for patient cohorts with concurrent intracranial aneurysms and aortic aneurysms. AA indicates aortic aneurysm; IA, intracranial aneurysm.
Table 1.
Published Relevant Cohorts of Patients With Intracranial Aneurysms and Aortic Aneurysms
| Reference | Number of Patients | Imaging Modality | Proportion of Patients With Both IA and AA |
|---|---|---|---|
| IA patients with AAs | |||
| Flemming et al12 2005 | 159 patients with fIA or dolichoectasiaa | Not reported |
AAA 29/159 (18%) sIA 16/159 (10%) |
| Miyazawa et al13 2007 | 181 IA patientsb | Abdominal ultrasound | AAA 13/181 (7%) |
| Goyal et al15 2015 | 317 IA patientsb | TTE or TEE | TAA 15/317 (5%) |
| Brinjikji et al18 2016 |
139 patients with vertebrobasilar dolichoectasiab
25 with diffuse intracranial dolichoectasiab |
Not reported | AAA 12/139 (14%) AAA 10/25 (63%) |
| AA patients with IAs | |||
| Kuzmik et al14 2010 | 212 TAA patients 52 retrospective and 160 prospective | MRA or CTA | IAb 9/52 (17%) and IAb 10/160 (6%) |
| Lee et al19 2017 | 133 AA patients 25 aortic dissection patients | MRA or CTA | sIA 25 and fIA 2/133 (20%) sIA 8/25 (30%) |
| Shin et al16 2015 | 660 AA patients | MRA or CTA | IA 71/660 (12%) fIAs excluded |
| Rouchaud et al17 2016 | 1081 AA patients | CTA or MRA or DSA | sIA 128/1081 (12%) 20 dissections or fIAs excluded |
CTA indicates computed tomography angiography; fIA, fusiform intracranial aneurysm; DSA, digital subtraction angiography; MRA, magnetic resonance angiography; sIA, saccular intracranial aneurysm; TEE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
Partially same patient cohort.
Fusiform and saccular intracranial aneurysm were not distinguished.
Secondly, all illustrated IA family trees with co‐occurrence of AA and AA family trees with co‐occurrence of IA were searched with the terms: (intracranial OR cerebral OR cns OR brain OR subarachnoid*) AND (aortic OR aorta) AND aneurysm* AND (gene OR genetic* OR genome OR family OR famili* OR kindred OR generation OR hereditary OR heritable OR inherited OR phenotyp* OR mutation* OR variant* OR polymorphism OR linkage OR chromosomal). This gave 314 articles. Some family trees seemed to be duplicates but were not separable by the available data, and may consequently be counted twice, and some did not differentiate saccular IAs from other types of IAs. The final 8 cohorts presented 27 family trees (Figure 4, Table 2).29, 30, 31, 32, 33, 34, 35, 36, 37
Figure 4.
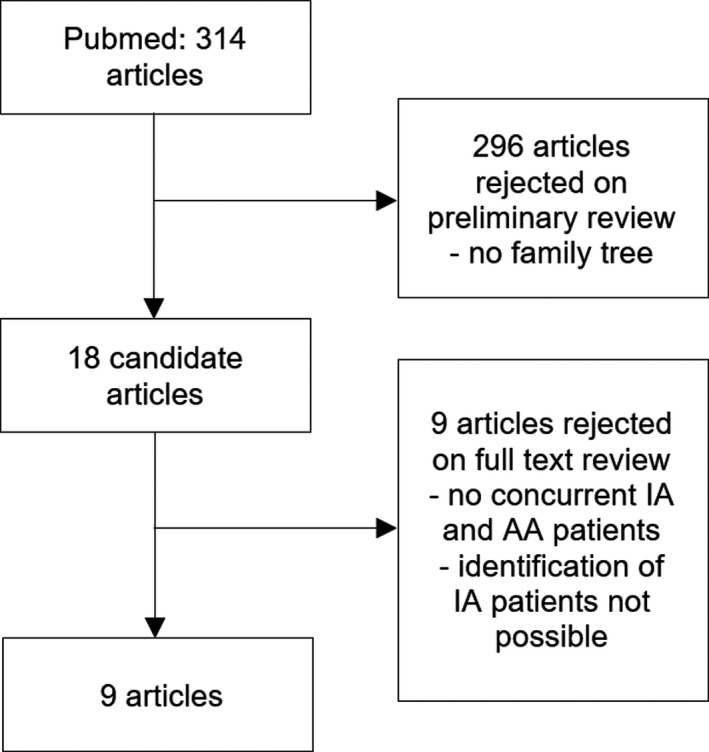
Literature search for family trees with both intracranial aneurysm and aortic aneurysm patients. AA indicates aortic aneurysm; IA, intracranial aneurysm.
Table 2.
Published Family Trees Including Patients With Both Intracranial and Aortic Aneurysms
| Family Members | IA Patients With No AA | IA Patients With AA | AA Patients With No IA | Implicated Genes | |
|---|---|---|---|---|---|
| Schievink et al29 1997 | 19 | 2 IAa | 1 IAa + TAA | 0 | |
| Cannon‐Albright et al30 2003 | 69 | 7 IAa | 0 | 4 AAa | |
| Nahed et al31 2005 | 19 | 2 IAa | 1 IAa + TAA | 0 | |
| Kim et al32 2005 | 28 | 4 IAa | 0 | 4 AAa | |
| 16 | 3 IAa | 0 | 3 AAa | ||
| 24 | 4 IAa | 0 | 2 AAa | ||
| 22 | 3 IAa | 1 IAa + AAa | 1 AAa | ||
| 25 | 5 IAa | 0 | 1 AAa | ||
| 16 | 1 IAa | 0 | 10 AAa | ||
| Regalado et al33, 34 | 19 | 1 IAa | 1 IAa + TAA | 6 TAA | |
| 32 | 1 IAa | 0 | 7 TAA | ||
| 8 | 2 sIA | 0 | 2 TAA, 3 AAA | ||
| 17 | 1 IAa | 1 IAa + TAA | 4 TAA, 1 AAA | ||
| 14 | 4 IAa | 0 | 3 TAA | ||
| 10 | 1 IAa | 1 IAa + TAA | 1 TAA, 1 AAA | ||
| 6 | 1IAa | 0 | 2 TAA | ||
| 6 | 1 IAa | 1 IAa + TAA | 2 TAA | ||
| 19 | 2 sIA | 0 | 6 TAA | ||
| 17 | 2 IAa | 0 | 2 TAA | ||
| 13 | 2 sIA | 0 | 2 TAA | ||
| 7 | 1 IAa | 0 | 2 TAA, 1 AAA | ||
| 11 | 1 IAa | 0 | 4 TAA | ||
| 24 | 3 sIA | 0 | 6 TAA | ||
| 17 | 1 IAa | 1 IAa + TAA | 4 TAA, 1 AAA | SMAD3 | |
| Luukkonen et al35 2012 | 20 | 1 IA | 0 | 3 AAa | NTM |
| Bertoli‐Avella et al36 2015 | 23 | 0 IA | 1 IAa + AAa | 2 AAa | TGFB3 |
| Mazzella et al37 2017 | 22 | 1 fIA | 0 | 5 TAA | TGFB2 |
| Total | 523 | 57 IA | 9 IA + AA | 95 AA |
AA indicates aortic aneurysm; AAA, abdominal aortic aneurysm; fIA, fusiform intracranial aneursym; sIA, saccular intracranial aneurysm; TAA, thoracic aortic aneurysm
The distinction between fIA vs sIA or TAA vs AAA not reported.
Ethical Approvals
The Kuopio IA Patient and Family Database has been approved by the Research Ethics Committee the KUH, the Finnish Ministry of Social Affairs and Health, and the National Institute for Health and Welfare. Written informed consent was obtained from all the IA patients registered to the database.
Results
Our Study Population
There were 125 patients with 134 fIAs and 4253 patients with 6097 sIAs (Figures 2 and 5, Tables 3 and 4). Of the 125 fIA patients, 22 (18%) carried also sIAs. For the 125 fIA patients, there were 450 first‐degree relatives and 340 matched population controls, and for the 4253 sIA patients 17 825 first‐degree relatives and 12 669 matched controls (Figure 2, Table 3).
Figure 5.
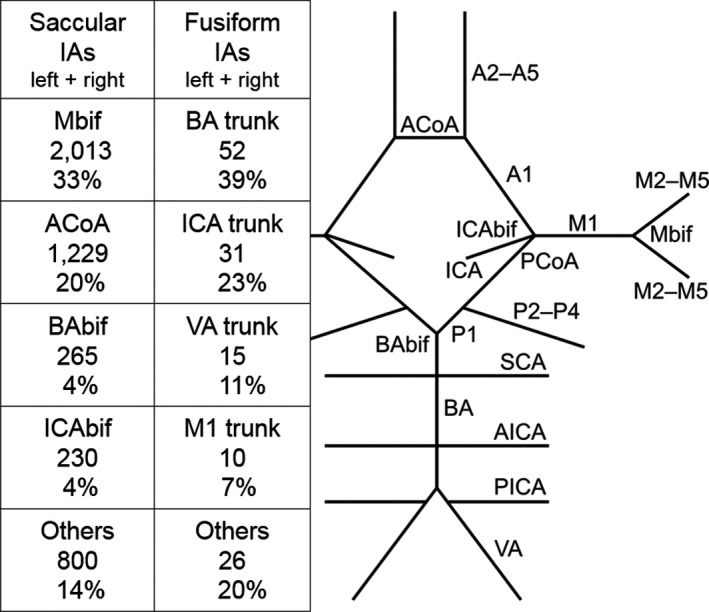
The most common locations of the 134 fusiform intracranial aneurysms and the 6097 saccular intracranial aneurysms in descending order of frequency (the left side and the right side combined). The schematic illustration of the major intracranial arteries with the circle of Willis shows the major arterial bifurcations and segments (compare with Figure 1.). Anterior circulation: the internal carotid artery up to the internal carotid artery bifurcation; A1 the proximal segment of the anterior cerebral artery; the anterior communicating artery; A2 to A5, the distal segments of the anterior cerebral artery; M1, the proximal segment of the middle cerebral artery; the bifurcation of the middle cerebral artery; M2 to M5, the distal segments of the middle cerebral artery. Posterior circulation: the vertebral artery; the posterior inferior cerebellar artery; the basilar artery; the anterior inferior cerebellar artery; the superior cerebellar artery; the basilar tip bifurcation; P1, the proximal segment of the posterior cerebral artery; P2–P4, the distal segments of the posterior cerebral artery; the posterior communicating artery. A1 indicates the proximal segment of the anterior cerebral artery; A2 to A5, the distal segments of the anterior cerebral artery; ACoA, the anterior communicating artery; AICA, the anterior inferior cerebellar artery; BA, the basilar artery; BAbif, the basilar tip bifurcation; IA, intracranial aneurysm; M1, the proximal segment of the middle cerebral artery; Mbif, the bifurcation of the middle cerebral artery; M2 to M5, the distal segments of the middle cerebral artery; P1, the proximal segment of the posterior cerebral artery; P2–P4, the distal segments of the posterior cerebral artery; PCoA, the posterior communicating artery; PICA, the posterior inferior cerebellar artery; SCA, the superior cerebellar artery; VA, the vertebral artery.
Table 3.
Characteristics of the 4378 Patients With Intracranial Aneurysms
| Variables for 4378 IA Patients From 1980 to 2015 | 125 Fusiform IA Patients Including 22 fIA Patients With Concomitant sIAs | 450 First Degree Relatives to the fIA Patients | 340 Matched Controls to the fIA Patients | 4253 Saccular IA Patients With No fIAs | 17 825 First Degree Relatives to the sIA Patients | 12 669 Matched Controls to the sIA Patients | P Value—Total fIA Vs Total sIAa | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unruptured fIA n=93 (64%) | Ruptured fIA n=32 (26%) | Total fIA n=125 | 0 fIA 1 sIA | 0 fIA 1 sIA | Unruptured sIA n=1330 (31%) | Ruptured sIA n=2923 (69%) | Total sIA n=4253 | 0 fIA 224 sIA (76 aSAH) | 0 fIA 58 sIA (33 aSAH) | <0.0005 | |
| Median age at IA diagnosis, y (25 and 75 percentiles) | 66 (57 to 72) | 59 (48 to 73) | 64 (55 to 72) | 47 at sIA | 75 at sIA | 57 (48 to 66) | 52 (43 to 62) | 54 (45 to 63) | 47 (42 to 54) at sIA | 56 (52 to 73) at sIA | <0.0005 |
| Presentation | |||||||||||
| Incidental | 64 (69%) | … | … | … | … | 1164 (88%) | … | … | … | … | <0.0005 |
| Symptomatic | 29 (31%) | 54 (4%) | |||||||||
| sIA family screening | … | 112 (8%) | |||||||||
| Women, n (%) | 39 (42%) | 21 (66%) | 60 (48%) | 222 (49%) | 160 (47%) | 754 (57%) | 1598 (55%) | 2352 (55%) | 8706 (49%) | 7029 (55%) | 0.106 |
| sIA family, n (%) | 5 (5%) | 2 (6%) | 7 (5.7%) | … | … | 259 (20%) | 314 (11%) | 573 (14%) | … | … | 0.007 |
| Concomitant sIA, n (%) | … | … | 22 sIA (18%) | … | … | … | … | … | … | … | |
| Multiple fIAs (≥2), n (%) | 7 (8%) | 1 (3%) | 8 (6%) | … | … | … | … | … | … | … | |
| Multiple sIAs (≥2), n (%) | 9 (10%) | 0 (0%) | 9 (7%) | … | … | 380 (29%) | 811 (28%) | 1191 (28%) | … | … | |
| AAA total | 9 (10%) | … | 9 (7%) | 2 (0.4%) | 4 (0.6%) | 24 (2%) | 13 (0.4%) | 37 (1%) | 35 (0.2%) | 54 (0.4%) | <0.0005 |
| Ruptured | 2 (2%) | 2 (2%) | … | … | 2 (0.2%) | 6 (0.2%) | 8 (0.2%) | 11 (0.1%) | 12 (0.1%) | ||
| Confirmed TAA total | 1 (1%) | … | 1 (1%) | 2 (0.4%) | … | 8 (0.6%) | 2 (0.1%) | 10 (0.2%) | 12 (0.1%) | 10 (0.1%) | 0.273 |
| Ruptured | … | … | … | … | … | … | 4 (0.0%) | 1 (0.0%) | |||
| Suspected TAA | 3 (4%) | 1 (3%) | 4 (4%) | … | … | … | … | … | … | … | |
| AAA + TAA total | 3 (3%) | … | 3 (2%) | … | … | … | … | … | 2 (0.0%) | 1 (0.0%) | |
| Ruptured | 1 (1%) | 1 (1%) | … | … | |||||||
| Unspecified AA | … | … | … | … | … | … | … | 1 (0.0%) | 10 (0.1%) | 3 (0.0%) | |
| Dissection | 1 (1%) | … | 1 (1%) | 2 (0.4%) | … | … | 2 (0.1%) | 2 (0.0%) | 5 (0.0%) | 18 (0.1%) | 0.08 |
| AA or dissection | 17 (18%) | 1 (3%) | 18 (14%) | 6 (1%) | 4 (1%) | 27 (2%) | 16 (0.1%) | 50 (1%) | 64 (0.4%) | 86 (0.7%) | <0.0005 |
| Ruptured | 3 (3%) | … | 3 (2%) | … | … | 2 (0.0%) | 5 (0.0%) | 8 (0.0%) | 15 (0.1%) | 13 (0.1%) | |
| Median age at AA diagnosis, y (25 and 75 percentiles) | … | … | 63 (58 to 73) | 50 (46 to 54) | 76 (73 to 81) | … | … | 65 (59 to 73) | 66 (58 to 72) | 70 (63 to 76) | 0.61 |
| Comorbid diseases in IA patients from 1995 to 2014 | n=69 | n=20 | n=89 | n=405 | n=251 | n=1142 | n=1580 | n=2722 | n=15 652 | n=10 391 | |
| ADPKD, n (%) | 1 (1%) | 1 (3%) | 2 (2%) | 0 | 0 | 19 (1%) | 32 (1%) | 51 (1%) | 33 (0.2%) | 7 (0.1%) | 0.663 |
| Marfan, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.0%) | 1 (0.0%) | |
| Loeys‐Dietz, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ehlers‐Danlos, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypertension, n (%) | 63 (91%) | 18 (90%) | 81 (91%) | 146 (45%) | 245 (97%) | 921 (81%) | 1123 (71%) | 2044 (75%) | 8780 (56%) | 7989 (77%) | 0.001 |
| Type 2 diabetes mellitus, n (%) | 15 (22%) | 5 (25%) | 20 (22%) | 34 (8%) | 61 (24%) | 174 (15%) | 187 (12%) | 361 (13%) | 2270 (14%) | 1742 (17%) | 0.012 |
| Atherosclerotic disease or hyperlipidemia | 23 (25%) | 3 (9%) | 37 (30%) | 98 (24%) | 172 (68%) | 187 (16%) | 158 (10%) | 345 (13%) | 5764 (37%) | 5132 (49%) | <0.0005 |
AA indicates aortic aneurysm; AAA, abdominal aortic aneurysm; ADPKD, autosomal dominant polycystic kidney disease; aSAH, aneurysmal subarachnoid haemorrhage; fIA, fusiform intracranial aneurysm; IA, intracranial aneurysm; sIA saccular intracranial aneurysm; TAA, thoracic aortic aneurysm.
Statistical significance calculated for the difference of the total fIA and sIA groups.
Table 4.
Site and Size Distribution of the 134 Fusiform and the 6097 Saccular Intracranial Aneurysms
| Variables | 134 fIAs | 6097 sIAs | ||||
|---|---|---|---|---|---|---|
| Unruptured n (median size)a | Ruptured n (median size)a | Total n (median size)a | Unruptured n (median size)a | Ruptured n (median size)a | Total n (median size)a | |
| Total | 102 (17 mm) | 32 (10 mm) | 134 (15 mm) | 3145 (4 mm) | 2952 (7 mm) | 6097 (6 mm) |
| 25 and 75 percentiles | 9 to 35 mm | 6 to 19 mm | 8 to 28 mm | 3 to 7 mm | 5 to 11 mm | 4 to 9 mm |
| A1 | 0 | 2 (15 mm) | 2 (15 mm) | 21 (3 mm) | 14 (5 mm) | 35 (3 mm) |
| ACoA | 1 (15 mm) | 1 (7 mm) | 2 (11 mm) | 390 (4 mm) | 909 (7 mm) | 1299 (6 mm) |
| A2 to A5 | 0 | 0 | 0 | 158 (3 mm) | 153 (6 mm) | 311 (4 mm) |
| ICA to ICAbif | 23 (10 mm) | 8 (9 mm) | 31 (10 mm) | 790 (4 mm) | 626 (7 mm) | 1416 (6 mm) |
| M1 to Mbif | 9 (9 mm) | 1 (30 mm) | 10 (9 mm) | 1424 (4 mm) | 963 (9 mm) | 2387 (6 mm) |
| M2 to M5 | 4 (7 mm) | 3 (23 mm) | 7 (8 mm) | 113 (3 mm) | 25 (6 mm) | 138 (3 mm) |
| PCoA | 0 | 0 | 0 | 3 (11 mm) | 2 (8 mm) | 5 (8 mm) |
| P1 | 1 (9 mm) | 0 | 1 (9 mm) | 14 (3 mm) | 11 (6 mm) | 25 (3 mm) |
| P2 to P4 | 4 (8 mm) | 1 (7 mm) | 5 (8 mm) | 2 (13 mm) | 8 (5 mm) | 10 (5 mm) |
| BA | 46 (25 mm) | 6 (8 mm) | 52 (25 mm) | 131 (7 mm) | 134 (9 mm) | 265 (8 mm) |
| SCA | 1 (8 mm) | 0 | 1 (8 mm) | 54 (3 mm) | 25 (7 mm) | 79 (4 mm) |
| AICA | 0 | 0 | 0 | 0 | 1 (8 mm) | 1 (8 mm) |
| PICA | 6 (10 mm) | 2 (9 mm) | 8 (10 mm) | 33 (4 mm) | 67 (5 mm) | 100 (5 mm) |
| VA | 7 (28 mm) | 8 (10 mm) | 15 (19 mm) | 9 (12 mm) | 10 (9 mm) | 19 (10 mm) |
| Others | 0 | 0 | 0 | 3 (2 mm) | 4 (8 mm) | 7 (3 mm) |
A1 indicates the proximal segment of the anterior cerebral artery; A2–A5, the distal segments of the anterior cerebral artery; ACoA, the anterior communicating artery; AICA, the anterior inferior cerebellar artery; BA, the basilar artery; ICA, the internal carotid artery; ICAbif, the ICA bifurcation; M1, the proximal segment of the middle cerebral artery; M2–M5, the distal segments of the middle cerebral artery; Mbif, the bifurcation of the middle cerebral artery; others, not classified; P1, the proximal segment of the posterior cerebral artery; P2–P4, the distal segments of the posterior cerebral artery; PCoA, the posterior communicating artery; PICA, the posterior inferior cerebellar artery; SCA, the superior cerebellar artery; VA, the vertebral artery.
Median of largest sIA diameter or median of largest fIA length or diameter.
Comparison of fIA Patients and sIA Patients
Aneurysmal subarachnoid haemorrhage had occurred in 26% of the 125 fIA patients and 69% of the 4253 sIA patients (P<0.0005) (Table 3). Other clinically notable differences between the fIA and sIA patients were: age at diagnosis of IA (median 64 and 54 years, P<0.0005); male predominance (52% versus 45%, P=0.11); multiple fIAs versus multiple sIAs (6% versus 28%, P<0.0005); hypertension (91% versus 75%, P=0.001); and type 2 diabetes mellitus (22% versus 13%, P=0.012). However, autosomal dominant polycystic kidney disease occurred equally often (2% versus 1%, P=0.663). None of the fIA patients with autosomal dominant polycystic kidney disease had concurrent sIA. There were both fIA and sIA patients who belonged to an sIA family (6% versus 14%, P=0.007). No patients with a diagnosis of Marfan syndrome, Loeys‐Dietz syndrome, or Ehlers‐Danlos syndrome were identified in either patient group.
Comparison of fIAs and sIAs
In a comparison of the 134 fIAs and 6097 sIAs, including the sIAs of the 22 fIA patients with both fIA and sIA, the largest sizes of the 102 unruptured fIAs were significantly larger than those of the 3145 unruptured sIAs (17 mm versus 4 mm, P<0.0005). The 32 ruptured fIAs were significantly larger than the ruptured 2952 sIAs as well (10 mm versus 7 mm, P=0.01). Among the unruptured IAs, the 102 fIAs preferred the arterial trunks of vertebrobasilar tree (59%) and the trunk of the internal carotid artery (ICA) (23%) whereas the 3145 sIAs were most frequent on the middle cerebral artery bifurcation (45%) and the intracranial ICA (25%) (Figure 5, Table 4). Among the ruptured IAs, the 32 fIAs preferred the vertebrobasilar artery trunks (50%) and the ICA trunk (25%) while the 2925 sIAs were most frequent on the middle cerebral artery bifurcation (33%) and the anterior communicating artery (31%) (Figure 5, Table 4).
In the 125 fIA patients, 29 fIAs (23%, median size 32 mm) caused symptoms of brain or cranial nerve compression in contrast to only 54 (1.3%, median size 28 mm) sIAs in the 4253 sIA patients (Table 3). Of all 67 basilar trunk fIAs, 18 (27%, median size 38 mm) caused brainstem compression and 3 obstructive hydrocephalus. In the anterior circulation, 2 symptomatic fIAs were on the middle cerebral artery and 6 on the ICA.
AA in 125 fIA Patients
There were 17 (14%) fIA patients with a diagnosed AA (4 TAA, 12 AAA) or suspected AA (4 widened thoracic aortas in chest radiographs [2 patients] or thoracic echocardiographs [2 patients] indicative of TAAs) and 1 patient with an aortic dissection (Figure 2, Table 3). Of the 17 AAs, 3 (17%) had ruptured. Of the 17 patients, 12 (71%) were men, 16 (94%) had diagnosed hypertension, and 9 (53%) had diagnosed atherosclerotic disease. The median ages at the diagnosis of fIA and AA were 64 and 63 years (Figure 6A); in 8 patients, the AA diagnosis preceded the fIA diagnosis. The 125 fIA patients had significantly more often AA than their 450 first‐degree relatives (14% versus 0.9%, P<0.0005) and their 340 matched population controls (14% versus 1.2%, P<0.0005) (Table 3).
Figure 6.
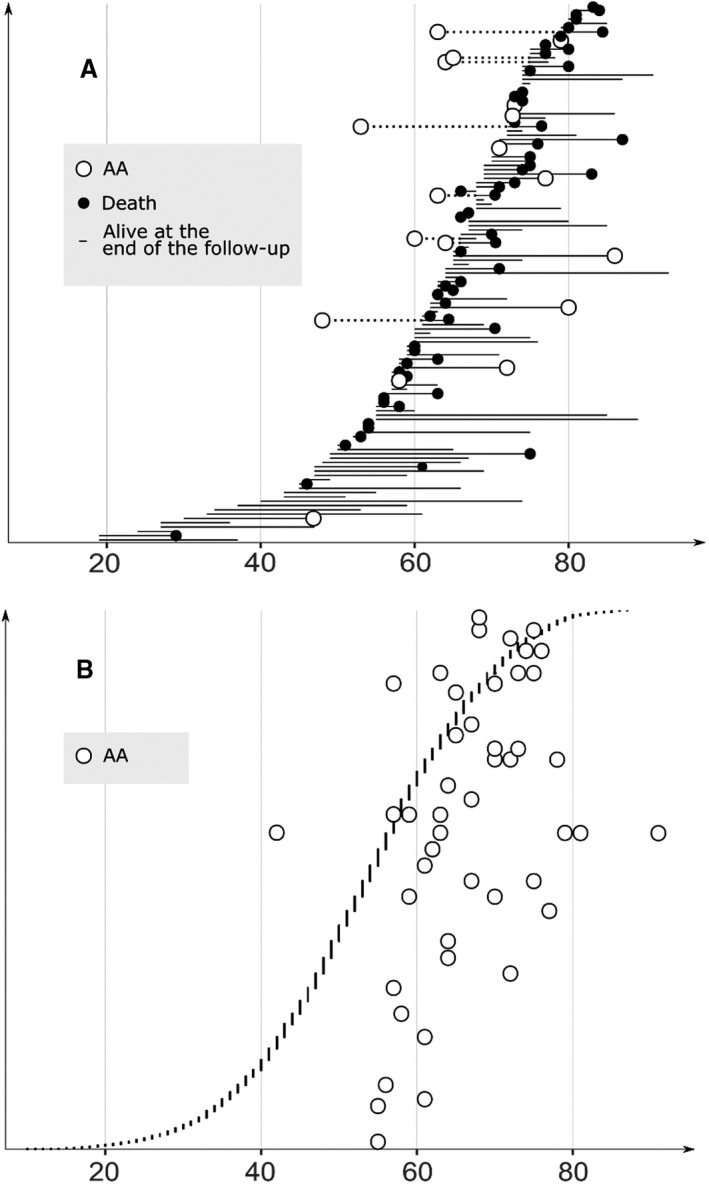
A, Distribution of the ages, in ascending order, of the 125 fusiform intracranial aneurysm patients (32 ruptured, 93 unruptured) at the diagnosis of the fusiform intracranial aneurysm. The ages at the diagnosis their 17 aortic aneurysms are shown (white circles). The lifelines of the fusiform intracranial aneurysm patients from either diagnosis (fusiform intracranial aneurysm or AA) until the AA diagnosis, death (without AA diagnosis, black circles) or the last follow‐up (without AA diagnosis, end of lifeline) are shown. B, Distribution of the ages, in ascending order, of the 4253 saccular intracranial aneurysms patients (2923 ruptured, 1330 unruptured) at the diagnosis of their saccular intracranial aneurysms. The ages at the diagnosis of their 48 aortic aneurysms are shown (white circles). The lifelines of the saccular intracranial aneurysm patients with diagnosed AA are omitted for the sake of clarity. The median ages at the saccular intracranial aneurysm diagnosis (dashed vertical line) and AA diagnosis (continuous vertical line) are shown. AA indicates aortic aneurysm.
AA in 4253 sIA Patients
There were 48 (1.1%) sIA patients with diagnosed AA (37 AAA, 10 TAA, 1 undefined AA) and 2 patients with diagnosed aortic dissection (Figure 2, Table 3). Of the 48 AAs, 8 (17%) had ruptured. Of the 48 patients, 37 (79%) were men, 46 (96%) had diagnosed hypertension, and 22 (48%) had diagnosed atherosclerotic disease. The median ages at the diagnosis of sIA and AA were 57 years and 66 years (P=0.006); in 9 patients, the AA diagnosis preceded the sIA diagnosis (Figure 6B). The 4253 sIA patients had significantly more often AA than their 17 825 first‐degree relatives (1.1% versus 0.3% P<0.0005) and their 12 669 matched population controls (1.1% versus 0.5%, P<0.0005). Diagnosed or suspected AAs were significantly more prevalent in the 125 fIA patients than in the 4253 sIA patients (14% versus 1.1%, P<0.0005).
Multivariate Analysis of Independent Risk Factors for AA in fIA and sIA Patients
In the multivariable regression analysis, fusiform IA morphology (SHR 7.6, 95% CI 3.9–14.9, P<0.0005), diagnosed atherosclerotic disease or use of lipid‐lowering medication (SHR 4.4, 95% CI 2.3–8.2, P<0.0005), and male sex (SHR 3.1, 95% CI 1.5–6.4, P=0.002) were independent risk factors for AA. However, hypertension (SHR 6.4, 95% CI 0.9–47.2, P=0.07), type 2 diabetes mellitus (SHR 1.4, 95% CI 0.7–2.7, P=0.36), and history of IA rupture (SHR 0.4, 95% CI 0.2–1.8, P=0.26) were not.
Sequencing of fIA Patients
Thirty‐three fIA patients were sequenced using an AA gene panel of 37 genes. Six fIA patients each carried a different rare variant (minor allele frequency <0.01): splice‐donor variant COL5A2 c.322+1G>C (NM_000393.3), four missense variants each in one patient: SKI c.1582G>C, p.(Ala528Pro) (NM_003036.3); FBN1 c.3571G>A, p.(Asp1191Asn) (NM_000138.4); MYH11 c.4903T>A, p.Ser1635Thr) (NM_022844.2); COL11A1 c.278G>T, p.Gly93Val) (NM_080629.2) and a synonymous variant COL3A1 c.1038C>T, p.(Ser346=) (NM_000090.3) (Table 5). The splice‐donor variant in COL5A2 was classified as likely pathogenic. There are 21 individuals heterozygous for the COL5A2 c.322+1G>C variant in the Genome Aggregation Database (gnomAD, n>120 000 exomes and >15 000 genomes). This variant affects consensus splice site, and all 5 components of Alamut splicing software (SSF, MaxEnt, NNSPLICE, GeneSplicer, HSF) predict that this variant breaks the wild‐type donor site but does not generate a near‐by in‐frame cryptic splice site. It has been detected in clinical testing in one affected individual by GeneDx (ClinVar 213136), and 2 additional affected patients with either ascending aortic dissection or multiple aneurysms including IA have been identified by Blueprint Genetics (Juha Koskenvuo, MD PhD, unpublished data, 2019). One of the tested healthy family members did not carry the variant, which further supports pathogenicity of the variant. However, variant's allele frequency is relatively high, at least suggesting that it may not be fully penetrant. MYH11, COL11A1, and FBN1 variants were classified as of uncertain significance whereas SKI and COL3A1 were deemed to be likely benign.
Table 5.
Rare Variants Found Among 33 Patients With Fusiform Intracranial Aneurysm in the Genetic Screening of 37 Genes Associated With Aortic Aneurysms
| Gene | Effect on Genomic DNA | Effect on Protein Transcript | Allele Frequency in gnomAD | Predicted Functional Consequence | Clinical Significance |
|---|---|---|---|---|---|
| SKI | c.1582G>C | p.(Ala528Pro) | 7.68e‐05 | Missense variant | Likely benign |
| FBN1 | c.3571G>A | p.(Asp1191Asn) | 1.08e‐05 | Missense variant | VUS |
| COL3A1 | c.1038C>T | p.(Ser346=) | 3.97e‐05 | Synonymous variant | Likely benign |
| MYH11 | c.4903T>A | p.(Ser1635Thr) | 0 | Missense variant | VUS |
| COL11A1 | c.278G>T | p.(Gly93Val) | 0 | Missense variant | VUS |
| COL5A2 | c.322+1G>C | N/A | 7.58e‐05 | Splice donor variant | Likely pathogenic |
gnomAD indicates a reference database of exomic sequences; VUS, variant of unknown significance.
Literature Review
We identified 27 published family trees with co‐occurrence of IAs and AAs (Table 1). In 21 family trees the morphology of the IAs, whether fusiform or saccular, was not reported. There were 9 IA patients with AA and 57 IA patients with no AA. There were 95 AA (58 TAA, 7 AAA, 30 unspecified AA) patients without IA.
Discussion
Comparative Characteristics of fIA and sIA Diseases
To our knowledge, this is the first population‐based study comparing the characteristics of the fIA disease (125 patients with 134 fIAs) and the sIA disease (4253 with 6097 sIAs). Fusiform IAs are rare, comprising 2.2% of all IAs in our study, and sizeable published series are few.7 Like aortic aneurysms, fIAs are dilatations of arterial segments, while sIAs are saccular pouches usually formed at the artery forks (Figure 1). The site distribution of the sIAs and the fIAs differs significantly38, 39: in our study, 56.7% of the fIAs but only 7.6% of the sIAs were located on the vertebrobasilar artery complex (Figure 5, Table 4).
The fIA patients were significantly older than the sIA patients, less likely to present with IA rupture, and more likely diagnosed with hypertension, type 2 diabetes mellitus, hyperlipidemia, or atherosclerotic disease (Table 3). In the fIA patients of this cohort, hypertension and type 2 diabetes mellitus were even more frequent (91% and 22%) than in the previously reported fIA cohorts.12, 40, 41 Autosomal dominant polycystic kidney disease, an unusual monogenic risk factor for the sIA disease,24 previously reported to occur in some 2% of patients with vertebrobasilar fIAs,12 occurred equally in the fIA and the sIA patients (Table 3). Multiple fIAs on arterial trunks were less frequent than multiple sIAs on bifurcations (6% versus 28%) (Table 3). In previous cohorts of patients with vertebrobasilar dolichoectasia, the reported frequency of diffuse dolichoectatic lesions has varied considerably with a range of 16% to 45%.18, 40 Different morphologies make size comparison of the fIAs and sIAs impractical; however, unruptured and ruptured fIAs were larger than unruptured and ruptured sIAs (Table 4), and the pronounced size difference underlines and explains the different rates of aneurysm symptomaticity. Because of their larger size and preference for the posterior fossa, the fIAs often caused symptoms by compression: brainstem compression, cranial nerve symptoms, and obstructive hydrocephalus.
Few studies on the histopathology and molecular biology of the fIA wall, resected during surgery or at autopsy, are available. In 1 study, walls of 8 fIAs displayed fragmentation of internal elastic lamina, neoangiogenesis within thickened intima, intramural haemorrhage and thrombus formation.42 Similar changes are observed not only in sIAs but also in AAAs and TAAs.43, 44, 45 Fusiform IAs have been linked to atherosclerotic risk factors, but the association of atherosclerosis with fIAs or its role as causal factor is complex and unclear.6
The distinctions between and the terminology of the spectrum of conditions that manifest as different forms of non‐saccular dilatations of intracranial arteries are often unclear. Dolichoectasia, elongation and dilatation of intracranial arteries (Figure 1), is most common in the vertebrobasilar complex.6 In neuroimaging (CT, CT angiography, MR imaging, MR angiography, digital subtraction angiography), dolichoectasia can be difficult to distinguish from fusiform IAs, and the two may represent the same disease spectrum and pathogenesis.6 Acute dissection is considerably less frequent in the intracranial arteries than in the cervical arteries, and is a rare cause of subarachnoid haemorrhage or brain infarction.46 There are data indicating that intracranial dissection may develop into a chronic fusiform aneurysm.47
AA in fIA and sIA Patients
To our knowledge, this is also the first population‐based study to investigate the occurrence of aortic aneurysms in both fIA and sIA patients (Figures 2 and 6), and in the first‐degree relatives and the matched population controls for the both patient groups (Figure 2, Table 3). The 125 fIA patients had significantly more often AA than their 450 first‐degree relatives (14% versus 0.9%) and their 340 matched population controls (14% versus 1.2%). In a recent retrospective study of 139 patients with fIA or dolichoectasia, similar proportion of the patients (12% and 14%) had concomitant AAA (Table 1), equal to 14% in this study. In other studies, fIAs may have been excluded or not distinguished from sIAs (Table 1).18 The risk of AA seems to warrant the screening of aortas of all fIA patients with MR angiography or CT angiography.
The 4253 sIA patients had clearly fewer diagnosed AAs during the follow‐up than the fIA patients (1.1% versus 14%) (Figures 2 and 6), but, nevertheless, more than their 17 825 first‐degree relatives (1.1% versus 0.3%) and their 12 669 matched population controls (1.1% versus 0.5%) (Figure 2, Table 3). The 48 sIA patients with AA were, on average, men, diagnosed with hypertension, with a substantial portion carrying atherosclerotic diseases. Despite the older age and higher burden of comorbid diseases in the fIA patients, as compared with the sIA patients, the fusiform IA morphology was the most important risk factor for AA in a multivariate model alongside the recognized risk factors, indicating that the increased AA risk in the fIA patients is not contributable to the traditional risk factors alone. No robust data on smoking were available for the multivariate model. The identified risk factors for AA in IA patients and the equivocal or protective role of diabetes mellitus against AA are well in line with previous research.43
Genetics of fIA Disease in Relation to AA Disease
We hypothesized that the rare fIAs share, in addition to their fusiform shape, genomic and acquired risk factors with AAs. In our review of the literature, we identified 27 families containing both IA patients and AA patients (Table 2). Among the 523 members of these families, 57 IA patients and 95 AA (58 TAA, 7 AAA, 30 unspecified AA) patients but only 9 patients with both IA and AA were identified. In most cases, the morphology of the IAs, whether fusiform or saccular, was not reported. A previous meta‐analysis of genome‐wide association studies (GWAS) of IA, TAA and AAA cohorts did not find evidence for shared genetic risk of IAs, TAAs and AAAs; however, in that study, fIA patients were not distinguished from the sIA patients.48 The genetics of the sIA disease remain elusive.5
In the present study, none of the 125 fIA patients had been diagnosed with inherited traits predisposing to AAs, including Marfan, Loeys‐Dietz, or Ehlers‐Danlos syndromes. We were unable to identify any fIA families, but 6% of the fIA patients belonged to an sIA family. Interestingly, concomitant fIA and sIA were seen in 18% of the fIA patients, suggesting susceptibility of these patients to aneurysms in general. Considerable co‐occurrence of fIAs and sIAs has been reported previously in some studies.18 We sequenced 33 fIA patients with a panel of 37 genes associated to AA, and found only one likely pathogenic variant (COL5A2 c.322+1G>C), which would likely predispose to classic Ehlers‐Danlos syndrome or TAA or thoracic aortic dissection.
The molecular pathologies of TAA and AAA are divergent.49 A substantial proportion of TAAs occur in association with a defined genetic syndrome or an identifiable single‐gene mutation. A focused or genome‐wide testing of the proband and, in the case of a positive finding, the first‐degree relatives, is recommended in patients presenting with syndromic features, positive family history or absence of traditional cardiovascular risk factors. Even though hereditary risk to AAA is well‐established, known causative single‐gene mutations are few.50 Consequently, family history affects the decision threshold of screening for the first degree relatives of AAA patients.9 However, genetic counselling is recommended if the AAA disease cannot be solely explained by a non‐genetic cause.10
Our Strengths
Our study has several strengths. The publicly‐funded Finnish healthcare system is universal, which reduces bias to minimum in population‐based disease databases. Neurosurgery of the Kuopio University Hospital (KUH) is the sole provider of acute and elective neurosurgical services for Eastern Finland, including unruptured and aneurysmal SAH patients. The Kuopio IA Patient and Family Database has incorporated clinical data for both the IA patients and their relatives and matched population controls from the national registries, using the Finnish personal identity codes. The data quality of the Finnish national health registers has been shown to be good.51
Our Limitations
However, the study has some limitations. Aortic aneurysms have not been screened in Finland either on a population level, in patients with intracranial aneurysms or in patients with stroke. Unruptured IAs, with an estimated lifetime prevalence of 2% to 3%, and AAs, with an estimated lifetime prevalence of 1% to 8%, are increasingly diagnosed as incidental findings in the imaging of the head or the body for other reasons in the elderly population. As both sIA and fIA patients may have had shortened survival, their controls may have accrued longer follow‐up with age‐related increasing prevalence of AA, possibly leading to some attenuation in our finding of increased AA prevalence in IA patients. Our data contained 68 AAs in IA patients but only 67 could be accurately verified. Consequently, our available data did not allow exact anatomical classification of all the AAs. Furthermore, the specificity of chest radiograph for TAA, used in 2 fIA patients as the basis diagnosis, is not high enough for definite diagnosis of TAA. The determination of the disease status of the comorbid diseases was in many cases based on medication use without knowledge of the indication of the prescription. Among the 125 fIA patients, only a small subgroup was available for the genotyping. The genotyping included only genes the mutations of which are known to associate with AA.
Suggested Further Research
Fusiform IAs that involve perforators, branches and bifurcations of the cerebral arteries are difficult to treat with the present microsurgical, endovascular and bypass techniques.52 Since fIAs and AAs share the fusiform shape and, possibly, pathogenetic mechanisms, and as fIAs and AAs partially co‐occur, cellular and molecular biology data from AA walls may help to develop pharmacological treatment that would stabilize the fIA wall and reduce its growth. In sIA patients, the risk of AA diagnosis was low in our study, and may not justify screening for AA of sIA patients, but it remains an underestimate in the absence of prospective screening studies of sIA patients for incidental AA.
Conclusions
In our study, without any screening for AA, ≈1% of the sIA patients had diagnosis for AA, in contrast to 0.3% of their first‐degree relatives and 0.5% of their matched population controls. These data suggest that screening for AA in sIA patients is not generally indicated. In fIA patients the risk of AA was 14%. This warrants screening of aortas of all fIA patients with MR angiography or CT angiography. The AA gene panel with 37 previously known AA‐associated genes in 33 fIA patients identified likely disease‐causing variant only in 1/33 (3%) of the patients. This observation suggests that fIA and AA may share genetic background in some cases, but larger cohorts are needed to validate or dispute this association.
Sources of Funding
This study was funded by The Finnish Cultural Foundation, The North Savo Regional Fund of Finnish Cultural Foundation, The Petri Honkanen Foundation, The Päivikki and Sakari Sohlberg Foundation, The Maire Taponen Foundation, The Olvi Foundation, The Pro Humanitate Foundation, the Academy of Finland, and the Kuopio University Hospital.
Disclosures
Dr Koskenvuo is a cofounder and director of Blueprint Genetics. All other authors declare no disclosure.
(J Am Heart Assoc. 2019;8:e013277 DOI: 10.1161/JAHA.119.013277.)
References
- 1. Etminan N, Chang H, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta‐analysis. JAMA Neurol. 2019;76(5):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Risk factors of sudden death from subarachnoid hemorrhage. Stroke. 2017;48:2399–2404. [DOI] [PubMed] [Google Scholar]
- 3. Karamanakos PN, von und zu Fraunberg M, Bendel S, Huttunen T, Kurki M, Hernesniemi J, Ronkainen A, Rinne J, Jaaskelainen JE, Koivisto T. Risk factors for three phases of 12‐month mortality in 1657 patients from a defined population after acute aneurysmal subarachnoid hemorrhage. World Neurosurg. 2012;78:631–639. [DOI] [PubMed] [Google Scholar]
- 4. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta‐analysis. Lancet Neurol. 2011;10:626–636. [DOI] [PubMed] [Google Scholar]
- 5. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713. [DOI] [PubMed] [Google Scholar]
- 6. Pico F, Labreuche J, Amarenco P. Pathophysiology, presentation, prognosis, and management of intracranial arterial dolichoectasia. Lancet Neurol. 2015;14:833–845. [DOI] [PubMed] [Google Scholar]
- 7. Nasr DM, Flemming KD, Lanzino G, Cloft HJ, Kallmes DF, Murad MH, Brinjikji W. Natural history of vertebrobasilar dolichoectatic and fusiform aneurysms: a systematic review and meta‐analysis. Cerebrovasc Dis. 2018;45:68–77. [DOI] [PubMed] [Google Scholar]
- 8. Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R, Coppi G, Czerny M, Fraedrich G, Haulon S, Jacobs MJ, Lachat ML, Moll FL, Setacci C, Taylor PR, Thompson M, Trimarchi S, Verhagen HJ, Verhoeven EL; Esvs Guidelines Committee n , Kolh P, de Borst GJ, Chakfé N, Debus ES, Hinchliffe RJ, Kakkos S, Koncar I, Lindholt JS, Vega de Ceniga M, Vermassen F, Verzini F; Document Reviewers n , Kolh P, Black JH, Busund R, Björck M, Dake M, Dick F, Eggebrecht H, Evangelista A, Grabenwöger M, Milner R, Naylor AR, Ricco J‐, Rousseau H, Schmidli J. Management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- 9. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(77):e2. [DOI] [PubMed] [Google Scholar]
- 10. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z; Esvs Guidelines Committee , de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F; Document Reviewers , Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein H, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco J, Verhagen H. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the management of abdominal aorto‐iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. [DOI] [PubMed] [Google Scholar]
- 11. Sampson UKA, Norman PE, Fowkes FGR, Aboyans V, Song Y, Harrell FE, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH, Mensah GA, Ezzati M, Murray C. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. 2014;9:159–170. [DOI] [PubMed] [Google Scholar]
- 12. Flemming KD, Wiebers DO, Brown RD, Link MJ, Huston J, McClelland RL, Christianson TJH. The natural history of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis. 2005;20:270–279. [DOI] [PubMed] [Google Scholar]
- 13. Miyazawa N, Akiyama I, Yamagata Z. Risk factors for the association of intracranial and aortic aneurysms. Acta Neurochir (Wien). 2007;149:229. [DOI] [PubMed] [Google Scholar]
- 14. Kuzmik GA, Feldman M, Tranquilli M, Rizzo JA, Johnson M, Elefteriades JA. Concurrent intracranial and thoracic aortic aneurysms. Am J Cardiol. 2010;105:417–420. [DOI] [PubMed] [Google Scholar]
- 15. Goyal M, Gottumukkala R, Bhalla S, Kates A, Zipfel G, Derdeyn C. Bicuspid aortic valves and thoracic aortic aneurysms in patients with intracranial aneurysms. Neurology. 2015;84:46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin Y, Jung K, Moon J, Lee S, Lee SK, Chu K, Roh J. Site‐Specific Relationship between intracranial aneurysm and aortic aneurysm. Stroke. 2015;46:1993–1996. [DOI] [PubMed] [Google Scholar]
- 17. Rouchaud A, Brandt MD, Rydberg AM, Kadirvel R, Flemming K, Kallmes DF, Brinjikji W. Prevalence of intracranial aneurysms in patients with aortic aneurysms. AJNR Am J Neuroradiol. 2016;37:1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brinjikji W, Nasr DM, Flemming KD, Rouchaud A, Cloft HJ, Lanzino G, Kallmes DF. Clinical and imaging characteristics of diffuse intracranial dolichoectasia. AJNR Am J Neuroradiol. 2017;38:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee D, Ahn SJ, Cho E, Kim YB, Song S, Jung WS, Suh SH. High prevalence of intracranial aneurysms in patients with aortic dissection or aneurysm: feasibility of extended aorta CT angiography with involvement of intracranial arteries. J Neurointerv Surg. 2017;9:1017–1021. [DOI] [PubMed] [Google Scholar]
- 20. Kuopio Intracranial Aneurysm and Family Database. Available at: www.kuopioneurosurgery.fi. Accessed July 12, 2019.
- 21. Huttunen T, von und zu Fraunberg M, Frosen J, Lehecka M, Tromp G, Helin K, Koivisto T, Rinne J, Ronkainen A, Hernesniemi J, Jaaskelainen JE. Saccular intracranial aneurysm disease: distribution of site, size, and age suggests different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic eastern Finnish patients. Neurosurgery. 2010;66:8. [DOI] [PubMed] [Google Scholar]
- 22. Kurtelius A, Kurki MI, von und zu Fraunberg M, Väntti N, Kotikoski S, Nurmonen H, Koivisto T, Jääskeläinen JE, Lindgren AE. Saccular intracranial aneurysms in children when both parents are sporadic or familial carriers of saccular intracranial aneurysms. Neuroepidemiology. 2018;52:47–54. [DOI] [PubMed] [Google Scholar]
- 23. Huttunen J, Lindgren A, Kurki MI, Huttunen T, Frosen J, von und zu Fraunberg M, Koivisto T, Kalviainen R, Raikkonen K, Viinamaki H, Jaaskelainen JE, Immonen A. Antidepressant use after aneurysmal subarachnoid hemorrhage: a population‐based case‐control study. Stroke. 2016;47:2242–2248. [DOI] [PubMed] [Google Scholar]
- 24. Nurmonen HJ, Huttunen T, Huttunen J, Kurki MI, Helin K, Koivisto T, von und zu Fraunberg M, Jääskeläinen JE, Lindgren AE. Polycystic kidney disease among 4,436 intracranial aneurysm patients from a defined population. Neurology. 2017;89:1852–1859. [DOI] [PubMed] [Google Scholar]
- 25. Kurtelius A, Kallionpää RA, Huttunen J, Huttunen TJ, Helin K, Koivisto T, Frösen J, von und zu Fraunberg M, Peltonen S, Peltonen J, Jääskeläinen JE, Lindgren AE. Neurofibromatosis type 1 is not associated with subarachnoid haemorrhage. PLoS One. 2017;12:e0178711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Myllykangas S, Buenrostro JD, Natsoulis G, Bell JM, Ji HP. Efficient targeted resequencing of human germline and cancer genomes by oligonucleotide‐selective sequencing. Nat Biotechnol. 2011;29:1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akinrinade O, Ollila L, Vattulainen S, Tallila J, Gentile M, Salmenperä P, Koillinen H, Kaartinen M, Nieminen MS, Myllykangas S, Alastalo T, Koskenvuo JW, Heliö T. Genetics and genotype‐phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur Heart J. 2015;36:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schievink WI, Parisi JE, Piepgras DG. Familial intracranial aneurysms: an autopsy study. Neurosurgery. 1997;41:1252. [DOI] [PubMed] [Google Scholar]
- 30. Cannon Albright LA, Camp NJ, Farnham JM, MacDonald J, Abtin K, Rowe KG. A genealogical assessment of heritable predisposition to aneurysms. J Neurosurg. 2003;99:637–643. [DOI] [PubMed] [Google Scholar]
- 31. Nahed BV, Seker A, Guclu B, Ozturk AK, Finberg K, Hawkins AA, DiLuna ML, State M, Lifton RP, Gunel M. Mapping a Mendelian form of intracranial aneurysm to 1p34.3‐p36.13. Am J Hum Genet. 2005;76:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim DH, Van Ginhoven G, Milewicz DM. Familial aggregation of both aortic and cerebral aneurysms: evidence for a common genetic basis in a subset of families. Neurosurgery. 2005;56:661. [DOI] [PubMed] [Google Scholar]
- 33. Regalado E, Medrek S, Tran‐Fadulu V, Guo D, Pannu H, Golabbakhsh H, Smart S, Chen JH, Shete S, Kim DH, Stern R, Braverman AC, Milewicz DM. Autosomal dominant inheritance of a predisposition to thoracic aortic aneurysms and dissections and intracranial saccular aneurysms. Am J Med Genet A. 2011;155A:2125–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Regalado ES, Guo D, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos‐Cortez RL, Leal SM, Bertoli‐Avella AM, Shendure J, Rieder MJ, Nickerson DA, Milewicz DM. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luukkonen TM, Pöyhönen M, Palotie A, Ellonen P, Lagström S, Lee JH, Terwilliger JD, Salonen R, Varilo T. A balanced translocation truncates Neurotrimin in a family with intracranial and thoracic aortic aneurysm. J Med Genet. 2012;49:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bertoli‐Avella AM, Gillis E, Morisaki H, Verhagen JMA, de Graaf BM, van de Beek G, Gallo E, Kruithof BPT, Venselaar H, Myers LA, Laga S, Doyle AJ, Oswald G, van Cappellen Gert WA, Yamanaka I, van der Helm Robert M, Beverloo B, de Klein A, Pardo L, Lammens M, Evers C, Devriendt K, Dumoulein M, Timmermans J, Bruggenwirth HT, Verheijen F, Rodrigus I, Baynam G, Kempers M, Saenen J, Van Craenenbroeck EM, Minatoya K, Matsukawa R, Tsukube T, Kubo N, Hofstra R, Goumans MJ, Bekkers JA, Roos‐Hesselink JW, van de Laar IMBH, Dietz HC, Van Laer L, Morisaki T, Wessels MW, Loeys BL. Mutations in a TGF‐β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65:1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazzella J, Frank M, Collignon P, Langeois M, Legrand A, Jeunemaitre X, Albuisson J. Phenotypic variability and diffuse arterial lesions in a family with Loeys‐Dietz syndrome type 4. Clin Genet. 2017;91:458–462. [DOI] [PubMed] [Google Scholar]
- 38. Pico F, Labreuche J, Touboul P, Amarenco P. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61:1736–1742. [DOI] [PubMed] [Google Scholar]
- 39. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable‐Beckman GL, Torner JC. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. [DOI] [PubMed] [Google Scholar]
- 40. Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology. 2008;70:66–72. [DOI] [PubMed] [Google Scholar]
- 41. Nasr DM, Brinjikji W, Rouchaud A, Kadirvel R, Flemming KD, Kallmes DF. Imaging Characteristics of Growing and Ruptured Vertebrobasilar Non‐Saccular and Dolichoectatic Aneurysms. Stroke. 2016;47:106–112. [DOI] [PubMed] [Google Scholar]
- 42. Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, Ueki K, Kirino T. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: insight on the mechanism of growth. Stroke. 2000;31:896–900. [DOI] [PubMed] [Google Scholar]
- 43. Sakalihasan N, Michel J, Katsargyris A, Kuivaniemi H, Defraigne J, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. [DOI] [PubMed] [Google Scholar]
- 44. Homme JL, Aubry M, Edwards WD, Bagniewski SM, Shane Pankratz V, Kral CA, Tazelaar HD. Surgical pathology of the ascending aorta: a clinicopathologic study of 513 cases. Am J Surg Pathol. 2006;30:1159–1168. [DOI] [PubMed] [Google Scholar]
- 45. Ollikainen E, Tulamo R, Frösen J, Lehti S, Honkanen P, Hernesniemi J, Niemelä M, Kovanen PT. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. J Neuropathol Exp Neurol. 2014;73:855–864. [DOI] [PubMed] [Google Scholar]
- 46. Debette S, Compter A, Labeyrie M, Uyttenboogaart M, Metso TM, Majersik JJ, Goeggel‐Simonetti B, Engelter ST, Pezzini A, Bijlenga P, Southerland AM, Naggara O, Béjot Y, Cole JW, Ducros A, Giacalone G, Schilling S, Reiner P, Sarikaya H, Welleweerd JC, Kappelle LJ, de Borst GJ, Bonati LH, Jung S, Thijs V, Martin JJ, Brandt T, Grond‐Ginsbach C, Kloss M, Mizutani T, Minematsu K, Meschia JF, Pereira VM, Bersano A, Touzé E, Lyrer PA, Leys D, Chabriat H, Markus HS, Worrall BB, Chabrier S, Baumgartner R, Stapf C, Tatlisumak T, Arnold M, Bousser M. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14:640–654. [DOI] [PubMed] [Google Scholar]
- 47. Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y, Ide T, Kitanaka C, Ueki K, Imai H, Saito N. Symptomatic recurrence of intracranial arterial dissections: follow‐up study of 143 consecutive cases and pathological investigation. Stroke. 2013;44:126–131. [DOI] [PubMed] [Google Scholar]
- 48. van ‘t Hof, F N, Ruigrok YM, Lee CH, Ripke S, Anderson G, de Andrade M, Baas AF, Blankensteijn JD, Bottinger EP, Bown MJ, Broderick J, Bijlenga P, Carrell DS, Crawford DC, Crosslin DR, Ebeling C, Eriksson JG, Fornage M, Foroud T, von und zu Fraunberg M, Friedrich CM, Gaal EI, Gottesman O, Guo DC, Harrison SC, Hernesniemi J, Hofman A, Inoue I, Jaaskelainen JE, Jones GT, Kiemeney LA, Kivisaari R, Ko N, Koskinen S, Kubo M, Kullo IJ, Kuivaniemi H, Kurki MI, Laakso A, Lai D, Leal SM, Lehto H, LeMaire SA, Low SK, Malinowski J, McCarty CA, Milewicz DM, Mosley TH, Nakamura Y, Nakaoka H, Niemela M, Pacheco J, Peissig PL, Pera J, Rasmussen‐Torvik L, Ritchie MD, Rivadeneira F, van Rij AM, Santos‐Cortez RL, Saratzis A, Slowik A, Takahashi A, Tromp G, Uitterlinden AG, Verma SS, Vermeulen SH, Wang GT; Aneurysm Consortium, Vascular Research Consortium of New Zealand , Han B, Rinkel GJ, de Bakker PI. Shared genetic risk factors of intracranial, abdominal, and thoracic aneurysms. J Am Heart Assoc. 2016;5:e002603 DOI: 10.1161/JAHA.115.002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saratzis A, Bown MJ. The genetic basis for aortic aneurysmal disease. Heart. 2014;100:916–922. [DOI] [PubMed] [Google Scholar]
- 50. Verhagen JMA, Kempers M, Cozijnsen L, Bouma BJ, Duijnhouwer AL, Post JG, Hilhorst‐Hofstee Y, Bekkers SCAM, Kerstjens‐Frederikse WS, van Brakel TJ, Lambermon E, Wessels MW, Loeys BL, Roos‐Hesselink JW, van de Laar IMBH; National Working Group on BAV & TAA . Expert consensus recommendations on the cardiogenetic care for patients with thoracic aortic disease and their first‐degree relatives. Int J Cardiol. 2018;258:243–248. [DOI] [PubMed] [Google Scholar]
- 51. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. [DOI] [PubMed] [Google Scholar]
- 52. Safavi‐Abbasi S, Kalani MYS, Frock B, Sun H, Yagmurlu K, Moron F, Snyder LA, Hlubek RJ, Zabramski JM, Nakaji P, Spetzler RF. Techniques and outcomes of microsurgical management of ruptured and unruptured fusiform cerebral aneurysms. J Neurosurg. 2017;127:1353–1360. [DOI] [PubMed] [Google Scholar]


