Abstract
Background
There are no available lifetime risk estimates of lower‐extremity peripheral artery disease (PAD).
Methods and Results
Using data from 6 US community‐based cohorts and the vital statistics, we estimated the prevalence and incidence of PAD, defined as an ankle‐brachial index < 0.90, at each year of age from birth to 80 years for white, black, and Hispanic men and women. Then, we used Markov Monte Carlo simulations in a simulated cohort of 100 000 individuals to estimate lifetime risk of PAD. On the basis of odds ratios of PAD for traditional atherosclerotic risk factors (eg, diabetes mellitus and smoking), we developed a calculator providing residual lifetime risk of PAD. In an 80‐year horizon, lifetime risks of PAD were 30.0% in black men and 27.6% in black women, but ≈19% in white men and women and ≈22% in Hispanic men and women. From another perspective, 9% of blacks were estimated to develop PAD by 60 years of age, while the same proportion was seen at ≈70 years for whites and Hispanics. The residual lifetime risk within the same race/ethnicity varied by 3.5‐ to 5‐fold according to risk factors (eg, residual lifetime risk in 45‐year‐old black men was 19.9% when current smoking, diabetes mellitus, and history of cardiovascular disease were absent versus 70.4% when all were present).
Conclusions
In the United States, ≈30% of blacks are estimated to develop PAD during their lifetime, whereas the corresponding estimate is ≈20% for whites and Hispanics. The residual lifetime risk within the same race/ethnicity substantially varies according to traditional risk factors.
Keywords: epidemiology, peripheral artery disease, risk factor, risk score
Subject Categories: Peripheral Vascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
In blacks, ≈30% of men and women were estimated to develop peripheral artery disease (PAD) during their lifetime, whereas in whites and Hispanics the corresponding estimate is ≈20%.
From another perspective, blacks reached a 10% lifetime risk for PAD ≈10 years earlier than whites and Hispanics.
Current and former smoking, diabetes mellitus, and a history of other cardiovascular disease were particularly potent predictors of developing PAD, contributing to ≈5‐fold gradient of residual lifetime risk and ≈10‐fold gradient of the probability of prevalent PAD at a given age, sex, and race/ethnicity.
What Are the Clinical Implications?
Although major guidelines for PAD did not specify black race as a risk factor of PAD, our results suggest the importance for healthcare providers to keep in mind this disproportional risk of PAD in blacks.
Risk calculators for the lifetime risk of PAD as well as prevalent PAD have been developed from this study, which may contribute to comprehensive cardiovascular risk assessment and inform targeted PAD screening.
Lifetime risk is useful for communicating long‐term risk and estimating the future burden of disease in populations.1, 2, 3, 4, 5 Lifetime risk estimation of coronary heart disease and stroke is recommended in the American Heart Association and the American College of Cardiology guidelines for guiding preventive therapies.6, 7 However, to our knowledge, there are no available estimates of lifetime risk of lower‐extremity peripheral artery disease (PAD), despite its impact on prognosis,8 leg amputation,9 and physical function.10 Here, using data from 6 US community‐based cohorts with over 35 000 unique participants, we estimated lifetime risk of PAD by sex and among 3 racial/ethnic groups (whites, blacks, and Hispanics). Subsequently, we extended the lifetime risk estimations by age and other traditional atherosclerotic risk factors, which would complement lifetime risk prediction of coronary disease and stroke in the American Heart Association/American College of Cardiology guidelines6, 7 and facilitate personalized and comprehensive cardiovascular risk discussion between healthcare providers and patients.6, 11
Methods
This study was approved by the institutional review board for use of deidentified data at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD), and the need for informed consent was waived. Each participating cohort has its own policy for data sharing, but some of their data are publicly available (the National Health and Nutrition Examination Survey [NHANES] through the Centers for Disease Control and Prevention's website12 and other cohorts through BioLINCC13).
Lifetime Risk Estimation
There are 2 main approaches to estimate lifetime risk in the literature: (1) estimating long‐term cumulative incidence from a survival analysis of individuals14, 15, 16 and (2) estimating lifetime risk using Monte Carlo simulations with state transition probabilities based on a Markov chain model.1, 2 The former approach may sound more intuitive than the latter, but because there are few cohort studies with lifelong follow‐up, the former approach often needs to stack cumulative incidences from different risk sets with different age categories. For example, to obtain lifetime risk from the age of 40 in a cohort with an age range of 40 to 80 years and follow‐up of 20 years, cumulative incidence before and after the age of 60 years should rely on younger and older study populations, respectively, which potentially induces some biases (eg, birth cohort effects). Also, the former approach is likely to be particularly suboptimal for clinical conditions without evident signs or symptoms, as incidences may not be appropriately captured in any cohort studies. This issue is especially relevant to PAD because the vast majority of disease is asymptomatic or accompanied by atypical leg symptoms.17 Also, very few cohort studies have regularly repeated measurement of ankle‐brachial index (ABI) in a relatively short elapsed time to capture asymptomatic cases to minimize the number of losses to follow‐up or deaths between repeated ABI measurements. Thus, we estimated the lifetime risk of PAD using the Monte Carlo simulations approach.2
A Markov chain model was constructed to simulate progression of an initially PAD free individual of a given baseline age, sex, and race/ethnicity through the mutually exclusive states of no PAD, PAD, and death, with PAD and death treated as “absorbing states” (once entered unable to leave) (Data S1). State transition probabilities of interest were (1) the probability of developing PAD (IPAD) and (2) the probability of dying prior to the development of PAD (QnoPAD). Details for the calculations of each state transition probability are provided subsequently in the section “State Transition Probabilities and Data Sources” below as well as in Data S1. Using these state transition probabilities, Monte Carlo simulations were performed 100 000 times from the age of 0 through 80 years with a cycle length of 1 year, which is equivalent to a simulated cohort of 100 000 individuals of a specified baseline age, sex, and race with a lifetime horizon capped at 80 years. All analyses were performed by Stata 14 (StataCorp LP, College Station, TX).
State Transition Probabilities and Data Sources
QnoPAD (1‐year probability of dying among people free of PAD of a given age, sex, and race) was calculated from age‐, sex‐, and race/ethnicity‐specific estimates of annual mortality in the overall population (ie, populations with and without PAD), the prevalence of PAD, and hazard ratios for mortality associated with PAD. This approach is based on the fact that annual overall mortality can be viewed as a weighted average of the 1‐year probability of dying among people with PAD and that of those without PAD.2 Annual probabilities of death for the US population by each year of age, sex, and race/ethnicity (whites, blacks, and Hispanics) were based on the National Vital Statistics Report.18 The prevalence of PAD according to ABI <0.9019, 20 was based on data from a total of 6 US community‐based cohorts (5 cohorts with ABI data included in the Cardiovascular Lifetime Risk Pooling Project,21 namely, the ARIC [Atherosclerosis Risk in Communities] study,22 the CHS [Cardiovascular Health Study],23 the FHS [Framingham Heart Study],24 the FOF [Framingham Offspring study], and the MESA [Multi‐Ethnic Study of Atherosclerosis],25 as well as NHANES 1999–2004).26 Data collection in each study was conducted according to a standardized protocol as previously reported,22, 23, 24, 25 and details of how ABI was measured in each cohort are summarized in Data S2. The relative risk of mortality for PAD versus no PAD was also obtained from pooled data from these 6 US community‐based cohorts.
IPAD (the probability of developing PAD at a given age) was estimated from age‐, sex‐, and race/ethnicity‐specific estimates of annual mortality in the overall population, QnoPAD (detailed above), age‐, sex‐, and race/ethnicity‐specific prevalence of no PAD (ie, 1‐ the prevalence of PAD). This approach is based on the fact that the prevalence of no PAD at age x+1 is determined by 3 elements, the prevalence of no PAD at age x (denominator) and 2 types of exits from no PAD, annual incidence of PAD during age x or mortality among no PAD during age x (ie, QnoPAD). Data sources for these parameters were the same as those noted above for QnoPAD. More details about state transition probabilities are provided in Data S1.
Estimating the Prevalence of PAD
Logistic regression models with age, sex, and race/ethnicity as independent variables were used to estimate the prevalence of PAD. To improve the precision of age‐, sex‐, and race/ethnicity‐specific estimates of PAD prevalence, we used the pooled data of the 6 US cohort studies (indicators of 6 cohorts were included in logistic models).2 Because NHANES is most representative of US populations among the 6 cohorts (although data were somewhat sparse in some age and race/ethnic groups), we combined the odds of PAD in a base case of 70‐year‐old white men in NHANES and odds ratios for age, sex, and race/ethnicity from the pooled data to estimate the prevalence of PAD for each year of age among sex and race/ethnicity groups (white men and women, black men and women, and Hispanic men and women). In this step, we used sample weights in NHANES. We also incorporated significant product terms between age, race/ethnicity, and sex.
Estimating Relative Risk of Mortality for PAD Versus No PAD
The relative risk of mortality for PAD (ABI <0.90) versus no PAD (ABI ≥0.90) was obtained from pooled data from the 6 US community‐based cohorts using stratified Cox proportional hazards models. Cox models were adjusted for sex and race/ethnicity and incorporated interaction terms between PAD and age with splines (knots at age 65 years). Again, indicator variables for 6 cohorts were included in regression models.
Residual Lifetime Risk by Age and Traditional Atherosclerotic Risk Factors
Lifetime risk estimates from a given age (ie, residual lifetime risk) can be viewed as a weighted average of residual lifetime risk of subgroups determined by combinations of the presence and absence (or higher and lower values if continuous factors) of traditional risk factors at that given age. Using sample weights and actual values of traditional atherosclerotic risk factors in NHANES, as well as odds ratio of PAD for traditional atherosclerotic risk factors from the pooled data of 6 studies, we estimated residual lifetime risk by combinations of age and traditional atherosclerotic risk factors. More details can be found in Data S3.
Logistic regression models to estimate odds ratios of PAD in the pooled data of the 6 US cohort studies included diabetes mellitus, smoking (both current and former), systolic blood pressure, high‐density lipoprotein cholesterol, total cholesterol, and a history of coronary heart disease or stroke as well as age, sex, race/ethnicity, and study indicators.
Results
Study Characteristics
A total of 38 154 participants were included from the 6 cohorts, of whom 2517 participants (6.6%) had PAD based on an ABI <0.90. Whites and blacks were included in 4 cohorts: ARIC, CHS, MESA, and NHANES (Table 1). Hispanics were represented in 2 cohorts, MESA and NHANES. The mean age ranged from 55 to 80 years across the cohorts. Compared with cohorts with a lower average age, cohorts with an older average age tended to have a less favorable risk factor profile (eg, higher blood pressure and prevalence of diabetes mellitus). The prevalence of current smokers was highest in ARIC (26%) and lowest in FHS (6.7%). The prevalence of PAD varied from 3.8% in MESA to 21% in FHS. Generally consistent across cohorts, a history of coronary heart disease and stroke as well as most traditional atherosclerotic risk factors (ie, ever smokers, low high‐density lipoprotein cholesterol, and diabetes mellitus) were more prevalent in men than in women (Table S1).
Table 1.
Study Characteristics
| Study | ||||||
|---|---|---|---|---|---|---|
| ARIC (14 274) | CHS (5662) | FHS (597) | FOF (3520) | MESA (6772) | NHANES (7329) | |
| Total of death, N (%) | 4272 (30%) | 4608 (81%) | 430 (72%) | 380 (12%) | 1144 (17%) | 1516 (21%) |
| Follow‐up time (y), mean (SD) | 18.4 (5.2) | 13.1 (6.5) | 9.1 (3.9) | 10.3 (2.1) | 12.4 (2.5) | 9.0 (2.6) |
| Age (y), mean (SD) | 55 (5.9) | 72 (5.5) | 80 (3.9) | 58 (9.6) | 62 (10) | 60 (13) |
| Female sex, N (%) | 7757 (54%) | 3234 (57%) | 376 (63%) | 1870 (53%) | 3573 (53%) | 3581 (48%) |
| Race/Ethnicity, N (%) | ||||||
| White | 10 719 (75%) | 4790 (85%) | 597 (100%) | 3520 (100%) | 2614 (39%) | 4092 (56%) |
| Black | 3555 (25%) | 837 (15%) | … | … | 1868 (28%) | 1361 (19%) |
| Hispanic | … | 35 (0.62%) | … | … | 2290 (34%) | 1876 (26%) |
| Smoking status, N (%) | ||||||
| Never | 5863 (41%) | 2615 (47%) | 557 (93%) | 2977 (85%) | 3403 (50%) | 3063 (42%) |
| Former | 4699 (33%) | 2369 (42%) | … | … | 2489 (37%) | 3292 (45%) |
| Current | 3712 (26%) | 678 (12%) | 40 (6.7%) | 543 (15%) | 880 (13%) | 967 (13%) |
| SBP (mm Hg), mean (SD) | 121 (19) | 136 (22) | 143 (21) | 128 (18) | 126 (21) | 132 (21) |
| Total cholesterol (mmol/L), mean (SD) | 5.6 (1.1) | 5.5 (1.0) | 5.3 (1.0) | 5.3 (1.0) | 5.0 (0.9) | 5.4 (1.1) |
| HDL cholesterol (mmol/L), mean (SD) | 1.3 (0.4) | 1.4 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.4 (0.4) |
| Diabetes mellitus, N (%) | 1579 (11%) | 837 (15%) | 130 (22%) | 336 (9.6%) | 848 (13%) | 1200 (16%) |
| History of CHD or stroke, N (%) | 1796 (13%) | 893 (16%) | 136 (23%) | 221 (6.3%) | 5 (0.07%) | 914 (13%) |
| ABI, mean (SD) | 1.13 (0.14) | 1.06 (0.18) | 1.01 (0.21) | 1.11 (0.12) | 1.11 (0.12) | 1.09 (0.16) |
| PAD (ABI <0.90), N (%) | 634 (4.4%) | 759 (13%) | 124 (21%) | 112 (3.2%) | 255 (3.8%) | 633 (8.6%) |
ABI indicates ankle‐brachial index; ARIC, Atherosclerosis Risk in Communities study; CHD, coronary heart disease; CHS, Cardiovascular Health Study; FHS, Framingham Heart Study; FOF, Framingham Offspring study; HDL, high‐density lipoprotein; MESA, Multi‐Ethnic Study of Atherosclerosis; NHANES, National Health and Nutrition Examination Survey; PAD, peripheral artery disease; SBP, systolic blood pressure.
Estimates of PAD Prevalence and Mortality Risk Associated With PAD
Pooled results from logistic regression models confirmed age as a strong predictor of prevalent PAD, with an odds ratio of 1.56 (95% CI, 1.50–1.61) per 5‐year increment (Table S2). At 65 years of age, women had slightly higher odds of having ABI <0.90 than men (odds ratio, 1.24 [95% CI, 1.12–1.38]), and this sex‐difference was slightly but significantly attenuated with older age (as represented by odds ratio of 0.90 [per 5‐year increment] for the product term of female and age). Compared with whites, blacks had >2‐fold higher odds of having PAD (eg, 2.16 [95% CI, 1.86–2.52] for black men versus white men), without any difference by age. Hispanics had significantly lower odds of PAD than whites, especially in middle age (eg, 0.76 [95% CI, 0.62–0.93] for men at 65 years of age versus white men), but this difference was attenuated at older ages (1.16 [95% CI, 1.06–1.27] for the product term of age and Hispanic ethnicity). Estimates of PAD prevalence were higher in blacks than the other 2 racial/ethnic groups in either sex at any age (Figure S1).
Pooled Cox models demonstrated a sex‐ and race/ethnicity‐adjusted hazard ratio of 2.43 (95% CI, 2.18–2.72 at age of 65 years) for total mortality comparing PAD (ABI <0.90) versus no PAD (ABI ≥0.90) (Table S3). The strength of association was slightly attenuated in older age (13% [95% CI, 8–17%] smaller hazard ratio per 5 year older age above 65 years) (Table S3).
Lifetime Risk Estimates by Sex and Race/Ethnicity
Using estimates of PAD prevalence and relative risk of mortality for PAD in the prior section as well as national mortality data, we estimated QnoPAD (1‐year probability of dying among people of a given age, sex, and race/ethnicity and free of PAD) and IPAD (the probability of developing PAD at a given age) at each age from birth to 80 years in each sex and racial/ethnic group (Table S4). On the basis of these estimates, the lifetime risk estimate was highest in black men, at 30.0%, followed by 27.6% in black women (Figure). The lifetime risk estimate was similar in white men and women, with a lifetime risk of ≈19%, and Hispanic men and women, with a lifetime risk of ≈22%.
Figure 1.
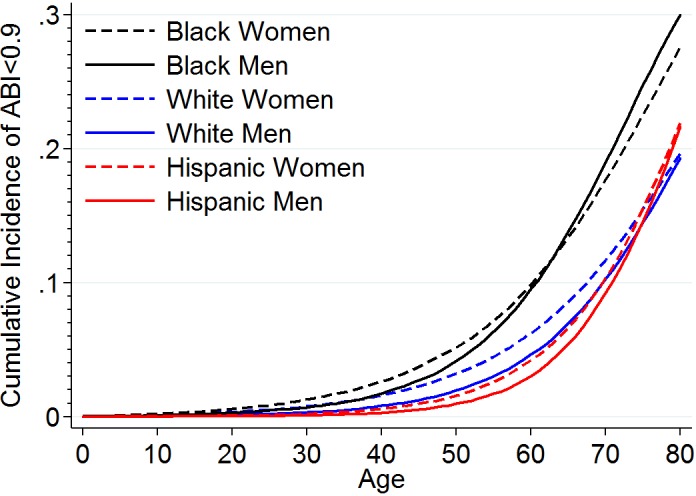
Lifetime cumulative incidence of PAD by race and sex. ABI indicates ankle‐brachial index; PAD, peripheral artery disease.
Residual Lifetime Risk of PAD by Traditional Risk Factors
Even after accounting for other traditional risk factors, age remained a strong predictor of PAD with an adjusted hazard ratio of 1.47 (95% CI, 1.43–1.52) (Table 2). Again, women had slightly higher odds of having an ABI <0.90 than men. Compared with whites, blacks had higher odds and Hispanics had lower odds of having PAD. All of the other traditional risk factors—diabetes mellitus, smoking (both current and former), systolic blood pressure, high‐density lipoprotein cholesterol, total cholesterol, and a history of other cardiovascular disease—demonstrated significant associations with prevalent PAD.
Table 2.
Adjusted Pooled OR of Having ABI <0.90
| Variables | Pooled OR (95% CI) |
|---|---|
| Age, 5 y | 1.47 (1.43, 1.52) |
| Female | 1.31 (1.19, 1.44) |
| Black | 1.65 (1.48, 1.84) |
| Hispanic | 0.78 (0.64, 0.94) |
| Diabetes mellitus | 1.54 (1.38, 1.71) |
| Former smoker | 1.50 (1.34, 1.67) |
| Current smoker | 3.09 (2.74, 3.49) |
| SBP, 20 mm Hg | 1.27 (1.22, 1.32) |
| HDL cholesterol, 1 mmol/L | 0.68 (0.60, 0.76) |
| Total cholesterol, 1 mmol/L | 1.14 (1.09, 1.19) |
| History of coronary disease or stroke | 2.01 (1.81, 2.24) |
All variables were modeled simultaneously. ABI indicates ankle‐brachial index; HDL, high‐density lipoprotein; OR, odds ratio; SBP, systolic blood pressure.
On the basis of these estimates, we developed a calculator for residual lifetime risk as well as probability of prevalent PAD (http://ckdpcrisk.org/padrisk and every step is summarized in Data S4). According to this calculator, we observed a gradient of residual lifetime risk of PAD in the same race/ethnicity exceeding 3.5‐ to 5‐fold according to traditional risk factors. For example, the residual risk varied from 19.9% to 70.4% in black men aged 45 years according to combinations of smoking, diabetes mellitus, and history of cardiovascular disease (Table 3). For an individual at age 65 years, because of the shorter duration to 80 years of age, the residual cumulative incidence was consistently lower than the scenario for an individual at age 45 years, at a given combination of traditional risk factors. Still, there was a 4‐ to 5.5‐fold gradient of residual lifetime risk of PAD according to smoking, diabetes mellitus, and a history of coronary disease or stroke within the same sex and race/ethnicity even in this scenario of a 65‐year‐old individual.
Table 3.
Projected Residual Cumulative Incidence (%) of PAD in the United States Across Different Scenarios of Demographic and Clinical Factors
| Demographic and clinical factors | Male | Female |
|---|---|---|
| Age 45 y | ||
| Whites | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 11.3 | 13.4 |
| Diabetes mellitus | 16.4 | 19.2 |
| History of CHD or stroke | ||
| No diabetes mellitus | 20.5 | 23.7 |
| Diabetes mellitus | 28.4 | 32.4 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 28.4 | 32.4 |
| Diabetes mellitus | 37.8 | 42.4 |
| History of CHD or stroke | ||
| No diabetes mellitus | 44.4 | 49.1 |
| Diabetes mellitus | 55.0 | 59.7 |
| Blacks | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 19.9 | 17.7 |
| Diabetes mellitus | 27.6 | 24.8 |
| History of CHD or stroke | ||
| No diabetes mellitus | 33.3 | 30.2 |
| Diabetes mellitus | 43.4 | 39.9 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 43.4 | 39.9 |
| Diabetes mellitus | 54.1 | 50.5 |
| History of CHD or stroke | ||
| No diabetes mellitus | 60.7 | 57.2 |
| Diabetes mellitus | 70.4 | 67.2 |
| Age 65 y | ||
| Whites | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 8.0 | 8.2 |
| Diabetes mellitus | 11.7 | 12.1 |
| History of CHD or stroke | ||
| No diabetes mellitus | 14.8 | 15.3 |
| Diabetes mellitus | 21.1 | 21.7 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 21.1 | 21.7 |
| Diabetes mellitus | 29.1 | 29.8 |
| History of CHD or stroke | ||
| No diabetes mellitus | 35.0 | 35.8 |
| Diabetes mellitus | 45.3 | 46.1 |
| Blacks | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 16.8 | 12.9 |
| Diabetes mellitus | 23.7 | 18.6 |
| History of CHD or stroke | ||
| No diabetes mellitus | 29.0 | 23.0 |
| Diabetes mellitus | 38.5 | 31.5 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 38.5 | 31.5 |
| Diabetes mellitus | 49.0 | 41.4 |
| History of CHD or stroke | ||
| No diabetes mellitus | 55.8 | 48.1 |
| Diabetes mellitus | 66.0 | 58.7 |
Scenarios are all for a systolic blood pressure of 130 mmHg, high‐density lipoprotein cholesterol of 50 mg/dL, and total cholesterol of 200 mg/dL. CHD indicates coronary heart disease; PAD, peripheral artery disease.
Predicting Prevalent PAD by Traditional Risk Factors
Using odds ratios of PAD according to traditional risk factors in Table 2 as well as the prevalence of PAD in the aforementioned base case in NHANES, we developed a calculator to predict the probability of having PAD as well. To cross‐validate this approach, we split NHANES into 3 data sets (1999–2000, 2001–2002, and 2003–2004), developed a prediction model using 2 data sets, and compared predicted versus observed using the remaining data set. We indeed confirmed decent calibration (particularly in higher risk groups with all P>0.1 in the top 5 deciles for 3 cross‐validation scenarios) and discrimination (cross‐validated c‐statistic=0.75) (Figure S2). To maximize available data for best predicting the probability of having PAD, the final model used the whole NHANES data, which demonstrated good calibration (χ2 of 10.2 with P=0.35) and discrimination (c‐statistic=0.77) (Figure S3). The variation of predicted probability of having PAD in several scenarios is shown in Table 4. The probability of having PAD within the same age, sex, and race/ethnicity varied by 6‐ to 10‐fold by smoking status, diabetes mellitus, and a history of coronary disease or stroke (eg, predicted probability from 1.2% to 10.5% in 45‐year‐old black men).
Table 4.
Estimated Probability of Prevalent PAD (%) in the United States Across Different Scenarios of Demographic and Clinical Factors
| Demographic and clinical factors | Male | Female |
|---|---|---|
| Age 45 y | ||
| Whites | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 0.6 | 1.4 |
| Diabetes mellitus | 0.9 | 2.1 |
| History of CHD or stroke | ||
| No diabetes mellitus | 1.2 | 2.7 |
| Diabetes mellitus | 1.8 | 4.1 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 1.8 | 4.1 |
| Diabetes mellitus | 2.8 | 6.2 |
| History of CHD or stroke | ||
| No diabetes mellitus | 3.6 | 8.0 |
| Diabetes mellitus | 5.5 | 11.8 |
| Blacks | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 1.2 | 1.8 |
| Diabetes mellitus | 1.8 | 2.8 |
| History of CHD or stroke | ||
| No diabetes mellitus | 2.4 | 3.6 |
| Diabetes mellitus | 3.6 | 5.5 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 3.6 | 5.5 |
| Diabetes mellitus | 5.5 | 8.2 |
| History of CHD or stroke | ||
| No diabetes mellitus | 7.1 | 10.4 |
| Diabetes mellitus | 10.5 | 15.2 |
| Age 65 y | ||
| Whites | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 2.7 | 4.3 |
| Diabetes mellitus | 4.1 | 6.4 |
| History of CHD or stroke | ||
| No diabetes mellitus | 5.3 | 8.2 |
| Diabetes mellitus | 7.9 | 12.1 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 7.9 | 12.1 |
| Diabetes mellitus | 11.7 | 17.5 |
| History of CHD or stroke | ||
| No diabetes mellitus | 14.8 | 21.7 |
| Diabetes mellitus | 21.1 | 29.9 |
| Blacks | ||
| Nonsmoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 6.6 | 6.9 |
| Diabetes mellitus | 9.8 | 10.3 |
| History of CHD or stroke | ||
| No diabetes mellitus | 12.4 | 13.1 |
| Diabetes mellitus | 17.9 | 18.8 |
| Current smoker | ||
| No history of CHD or stroke | ||
| No diabetes mellitus | 17.9 | 18.8 |
| Diabetes mellitus | 25.1 | 26.2 |
| History of CHD or stroke | ||
| No diabetes mellitus | 30.5 | 31.7 |
| Diabetes mellitus | 40.3 | 41.7 |
Scenarios are all for a systolic blood pressure of 130 mmHg, high‐density lipoprotein cholesterol of 50 mg/dL, and total cholesterol of 200 mg/dL. CHD indicates coronary heart disease; PAD, peripheral artery disease.
Discussion
Using data from 6 US community‐based cohorts, this study reported lifetime risk estimation of PAD. In blacks, ≈30% of men and women were estimated to develop PAD during their lifetime, whereas in whites and Hispanics the corresponding estimate is ≈20%. From another perspective, blacks reached a 10% lifetime risk for PAD 8 to 11 years earlier than whites and Hispanics. Women had a similar lifetime risk of PAD as men in all racial/ethnic groups. As anticipated, smoking (both current and former), diabetes mellitus, and a history of other cardiovascular disease were particularly potent predictors of developing PAD, contributing to an ≈5‐fold gradient of residual lifetime risk and ≈10‐fold gradient of the probability of prevalent PAD at a given age, sex, and race/ethnicity.
The higher lifetime risk of PAD in blacks compared with whites and Hispanics in our study is consistent with prevalence data shown in previous studies.27, 28 It is possible that this is partially explained by poor risk factor profiles in blacks compared with the other 2 groups, but this pattern remained consistent even after accounting for key traditional atherosclerotic risk factors in the present study (Table 2) as well as in previous studies.29 In this context, a recent study has demonstrated that socioeconomic factors such as education, household income, and neighborhood deprivation are associated with PAD risk beyond traditional risk factors.30 Nonetheless, although major guidelines for PAD did not specify black race as a risk factor for PAD,19 our results suggest the importance for healthcare providers to keep in mind this disproportional risk of PAD in blacks.
We observed a slightly higher prevalence of PAD in women than in men, particularly below 65 years of age (and similar lifetime risk of PAD between women and men). This may seem counterintuitive in light of epidemiology of other atherosclerotic diseases such as coronary disease, but several previous community‐based studies report the same pattern for PAD prevalence.27, 31, 32 Some reports suggest that this may be partially explained by shorter height in women than in men, potentially influencing ankle blood pressure.33, 34 Importantly, this cannot be simply interpreted as misclassification, as ABI <0.90 conferred a 2‐fold higher risk of mortality as compared with the reference category of 1.1 to 1.2 in both men and women.8 Nonetheless, as the American Heart Association called for action,35 further studies are need for PAD in women, including investigations of its similar burden between men and women. In this context, it will be important to evaluate lifetime risk specifically for severe types of PAD requiring leg revascularization or amputation in both sexes.
There seem to be a few ways to use the risk calculators from this study. For example, our lifetime risk calculator can be complementary to the American Heart Association/American College of Cardiology lifetime risk calculator for coronary disease and stroke7 and may contribute to comprehensive cardiovascular risk assessment. Importantly, the awareness of PAD is low, and most PAD patients do not have typical leg symptoms of intermittent claudication.17 Also, diagnostic processes and signs/symptoms are unique for PAD (eg, leg examination, ABI measurement, and reduced leg pulse). Thus, identifying people at high lifetime risk of PAD may have implications on targeted patients’ education about manifestation and complications of PAD. The risk calculator for prevalent PAD may have implications for screening PAD. Although it is still under debate,19, 36 some guidelines recommend screening PAD using ABI among high‐risk individuals.19 Although those clinical guidelines empirically rely on age and the presence of a few key risk factors to identify “high‐risk” populations for PAD (eg, all adults aged ≥65 years or people aged 50 to 64 years with any atherosclerotic risk factors19), our risk calculator for prevalent PAD will allow healthcare providers and patients to quantify the risk objectively on the basis of a combination of risk factors.
Although we tried to use the best readily available data in the United States, we need to acknowledge several limitations. First, most data were collected in the 1990s or early 2000s, and, unfortunately, few contemporary US community‐based cohorts measured ABI in multiple racial/ethnic groups. Since a recent international study reported that the prevalence of PAD increased by 13% in high‐income countries from 2000 to 2010,32 our estimates may be conservative. On the other hand, the increase in prevalent PAD may be attributable to improved survival in people with PAD rather than an increased incidence, and thus a follow‐up investigation with contemporary data would be important. Also, we cannot deny the possibility of birth cohort effects on prevalence of PAD. Second, we could not reliably estimate lifetime risk of PAD over the age of 80 years because of sparse data in this age range in our data sets (eg, NHANES coded all ages older than 80 as age 80). Given extending life expectancy in the United States, this is another element indicating that our estimates are likely to be conservative. Third, as anticipated from the literature,37 the mortality rate in our cohorts tended to be lower than the mortality rate from vital statistics (Table S5). However, our lifetime risk estimation is based on the mortality rate from vital statistics and relative risk from 6 US cohorts. Of note, cardiovascular relative risk of many risk factors including ABI has been shown to be often consistent regardless of the baseline risk of study populations.8, 38 Fourth, we did not have systematic data regarding history of leg revascularization across cohorts, indicating some level of underestimation by not capturing some PAD cases with ABI >0.90 after vascular procedures. Fifth, a few cohorts did not have granular data of smoking status. Sixth, as true for almost any diagnostic tests, ABI is validated to diagnose PAD but is not perfect (sensitivity and specificity >90%–95% in various studies39, 40, 41, 42). For example, a high ABI may mask PAD in some individuals.43 Finally, although our data consisted of the 3 major racial/ethnic groups in the United States, data for other racial/ethnic groups were lacking.
In conclusion, ≈30% of blacks are estimated to develop PAD during their lifetime, whereas the estimate is ≈20% for whites and Hispanics. Unlike other atherosclerotic diseases, lifetime risk and the prevalence of PAD based on ABI <0.90 was similar in women and in men. Among traditional atherosclerotic risk factors, age, smoking, and diabetes mellitus as well as a history of coronary disease or stroke are confirmed to be potent predictors of PAD risk and contributed to substantial variation of lifetime risk of PAD within the same race/ethnicity.
Sources of Funding
This study was supported by the American Heart Association (14CRP20380886).
Disclosures
Dr Matsushita received research funding and personal fees from Fukuda Denshi outside of the work. Dr Wilkins reports consulting for NGM Biopharmaceuticals (modest). The remaining authors have no disclosures to report.
Supporting information
Data S1. State Transition Probabilities
Data S2. Methods for Measuring ABI in Each Cohort
Data S3. Residual Lifetime Cumulative Incidence by Traditional Atherosclerotic Risk Factors
Data S4. Project an Individual's Residual Cumulative Incidence (%) of PAD in the United States
Table S1. Baseline Study Characteristics by Sex
Table S2. Odds Ratio of PAD (ABI <0.9) According to Demographic Factors From the Pooled Data of 6 US Cohorts
Table S3. Sex‐ and Race/Ethnicity‐Adjusted Hazard Ratios (95% CIs) for Mortality According to PAD (ABI <0.9) Vs No PAD (ABI ≥0.9)
Table S4. Estimated QnoPAD (1‐year Probability of Dying Among People of a Given Age, Sex, and Race/Ethnicity and Free of PAD) and IPAD (the Probability of Developing PAD at a Given Age) at Each Age From Birth to 80 Years in Each Sex and Racial/Ethnic Group
Table S5. One‐Year Probability of Dying Among Those With ABI >0.9 by Age Categories
Figure S1. Prevalence estimates of PAD according to odds of PAD at a given demographic variables in NHANES and meta‐analyzed odds ratio for age, sex, and race/ethnicity from six cohorts.
Figure S2. Observed vs predicted risk of PAD by deciles of predicted risk (cross‐validation by splitting NHANES according to 2‐year cycles).
Figure S3. Observed vs predicted risk of PAD by deciles of predicted risk (NHANES 1999–2004).
(J Am Heart Assoc. 2019;8:e012177 DOI: 10.1161/JAHA.119.012177.)
References
- 1. Kiberd BA, Clase CM. Cumulative risk for developing end‐stage renal disease in the US population. J Am Soc Nephrol. 2002;13:1635–1644. [DOI] [PubMed] [Google Scholar]
- 2. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3‐5 in the United States. Am J Kidney Dis. 2013;62:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd‐Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies ML, Hofman A, Ikram MA, Hunink MG, Franco OH, Stricker BH, Witteman JC, Roos‐Hesselink JW. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 7. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 8. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita K, Ballew SH, Sang Y, Kalbaugh C, Loehr LR, Hirsch AT, Tanaka H, Heiss G, Windham BG, Selvin E, Coresh J. Ankle‐brachial index and physical function in older individuals: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2017;257:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin SS, Sperling LS, Blaha MJ, Wilson PWF, Gluckman TJ, Blumenthal RS, Stone NJ. Clinician‐patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CDC/National Center for Health Statistics . Public‐use data files and documentation. https://www.cdc.gov/nchs/data_access/ftp_data.htm. Last updated June 22, 2018. Accessed July 24, 2019.
- 13. National Heart, Lung, and Blood Institute . BioLINCC: biologic specimen and data repository information coordinating center. https://biolincc.nhlbi.nih.gov/home/. Last updated 2019. Accessed July 24, 2019.
- 14. Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D, Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 15. Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle‐aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. [DOI] [PubMed] [Google Scholar]
- 16. Lloyd‐Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. [DOI] [PubMed] [Google Scholar]
- 17. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 18. Arias E. United States life tables, 2008. Natl Vital Stat Rep. 2012;61:1–64. [PubMed] [Google Scholar]
- 19. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola‐Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Rother J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group . 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 21. Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A, Lloyd‐Jones DM. Data resource profile: the cardiovascular disease lifetime risk pooling project. Int J Epidemiol. 2015;44:1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 24. Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. [DOI] [PubMed] [Google Scholar]
- 25. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 26. Dhangana R, Murphy TP, Pencina MJ, Zafar AM. Prevalence of low ankle‐brachial index, elevated plasma fibrinogen and CRP across Framingham risk categories: data from the National Health and Nutrition Examination Survey (NHANES) 1999‐2004. Atherosclerosis. 2011;216:174–179. [DOI] [PubMed] [Google Scholar]
- 27. Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic‐specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. [DOI] [PubMed] [Google Scholar]
- 28. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 29. Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. [DOI] [PubMed] [Google Scholar]
- 30. Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K. Socioeconomic status and incidence of hospitalization with lower‐extremity peripheral artery disease: Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2017;6:e004995 DOI: 10.1161/JAHA.116.004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. [DOI] [PubMed] [Google Scholar]
- 32. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 33. London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension. 1995;26:514–519. [DOI] [PubMed] [Google Scholar]
- 34. Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–1479. [DOI] [PubMed] [Google Scholar]
- 35. Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat‐Jacobson D. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125:1449–1472. [DOI] [PubMed] [Google Scholar]
- 36. Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle‐brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:342–348. [DOI] [PubMed] [Google Scholar]
- 37. Leening MJ, Heeringa J, Deckers JW, Franco OH, Hofman A, Witteman JC, Stricker BH. Healthy volunteer effect and cardiovascular risk. Epidemiology. 2014;25:470–471. [DOI] [PubMed] [Google Scholar]
- 38. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; Group CHDRP . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 39. Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation. 1968;37:624–637. [DOI] [PubMed] [Google Scholar]
- 40. Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969;56:676–679. [DOI] [PubMed] [Google Scholar]
- 41. Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–693. [PubMed] [Google Scholar]
- 42. Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg. 1982;117:1297–1300. [DOI] [PubMed] [Google Scholar]
- 43. Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–1203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. State Transition Probabilities
Data S2. Methods for Measuring ABI in Each Cohort
Data S3. Residual Lifetime Cumulative Incidence by Traditional Atherosclerotic Risk Factors
Data S4. Project an Individual's Residual Cumulative Incidence (%) of PAD in the United States
Table S1. Baseline Study Characteristics by Sex
Table S2. Odds Ratio of PAD (ABI <0.9) According to Demographic Factors From the Pooled Data of 6 US Cohorts
Table S3. Sex‐ and Race/Ethnicity‐Adjusted Hazard Ratios (95% CIs) for Mortality According to PAD (ABI <0.9) Vs No PAD (ABI ≥0.9)
Table S4. Estimated QnoPAD (1‐year Probability of Dying Among People of a Given Age, Sex, and Race/Ethnicity and Free of PAD) and IPAD (the Probability of Developing PAD at a Given Age) at Each Age From Birth to 80 Years in Each Sex and Racial/Ethnic Group
Table S5. One‐Year Probability of Dying Among Those With ABI >0.9 by Age Categories
Figure S1. Prevalence estimates of PAD according to odds of PAD at a given demographic variables in NHANES and meta‐analyzed odds ratio for age, sex, and race/ethnicity from six cohorts.
Figure S2. Observed vs predicted risk of PAD by deciles of predicted risk (cross‐validation by splitting NHANES according to 2‐year cycles).
Figure S3. Observed vs predicted risk of PAD by deciles of predicted risk (NHANES 1999–2004).


