Abstract
Background
Prior research has shown higher mortality in women with severe coronary artery disease compared with men, particularly in younger patients. It is unknown if this could be attributable to an adverse risk factor profile.
Methods and Results
In a population‐based cohort study, we included all adults ≤50 years of age (932 women and 4514 men) who underwent coronary artery bypass grafting from 1995 to 2013 from the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) register. Following inverse probability of treatment weighting, we investigated differences between women and men. Women had a higher prevalence of cardiovascular risk factors compared with men. There was no difference in early mortality between women and men (unadjusted: 1.3% versus 0.9%; hazard ratio, 1.42; 95% CI, 0.75–2.70; weighted sample: 1.1% versus 1.0%; hazard ratio, 1.10; 95% CI, 0.52–2.30). During a median follow‐up time of 11.8 years, in the unweighted population, the risk of death was greater in women compared with men (hazard ratio, 1.34; 95% CI, 1.13–1.58). However, in the weighted sample, the risk of death was not significantly different in women compared with men (hazard ratio, 1.02; 95% CI, 0.83–1.26).
Conclusions
Women ≤50 years of age had a higher unadjusted risk of death after coronary artery bypass grafting compared with men, but this was explained by a clustering of cardiovascular risk factors. Female sex per se was not associated with increased mortality or major adverse cardiovascular events. Early mortality was not increased in women compared with men, even though younger women in our study had an increased burden of risk factors known to affect early risk.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02276950.
Keywords: coronary artery bypass graft surgery, coronary artery disease, outcome, women
Subject Categories: Cardiovascular Surgery, Mortality/Survival, Cardiovascular Disease
Clinical Perspective
What Is New?
Women ≤50 years of age had a higher unadjusted risk of death after coronary artery bypass grafting compared with men, but this was explained by a clustering of cardiovascular risk factors.
Female sex per se was not associated with increased mortality or major adverse cardiovascular events in young patients undergoing coronary artery bypass grafting.
Early mortality was not increased in women compared with men, even though younger women in our study had an increased burden of risk factors known to affect early risk.
What Are the Clinical Implications?
The results of this study can be used to inform young women undergoing coronary artery bypass grafting about prognosis and how this is influenced by preoperative risk profile.
Women with coronary artery disease (CAD) are reported to have worse survival compared with men, especially after myocardial infarction.1 It has been debated whether this could be fully accounted for by a more adverse risk factor profile or if female sex or body size per se is associated with increased mortality in patients with CAD.2 Although CAD rarely causes symptoms until later in life,3 the disease process usually starts in young adulthood.4, 5 Previous studies have shown that young adults with symptomatic CAD have a high prevalence of cardiovascular risk factors.6, 7 Younger women with CAD compose an especially interesting and rare entity because of the possible cardiovascular protective effects of estrogen.8 Some previous works have shown that the increased mortality in women compared with men with CAD is mainly apparent in younger patients.9, 10
Because coronary artery bypass grafting (CABG) has proven to be the preferred method of revascularization in the presence of severe CAD, patients undergoing CABG generally represent a cohort with the most advanced form of CAD.11 CABG is one of the most studied surgical procedures, and information about periprocedural risk and long‐term prognosis is generally well characterized. However, younger women compose a minority in both observational and randomized CABG studies,12 and data regarding risk factors as well as short‐ and long‐term outcomes in this population are largely unknown.
We performed a nationwide population‐based cohort study to analyze possible differences between women and men in long‐term survival, major adverse cardiovascular events, and factors associated with mortality in all adults ≤50 years of age undergoing CABG in Sweden during a 19‐year period.
Methods
Study Design
This was an observational, nationwide population‐based cohort study approved by the regional Human Research Ethics Committee, Stockholm, Sweden. The need for informed consent was waived by the Ethics Committee. Study reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology and Reporting of Studies Conducted Using Observational Routinely Collected Health Data guidelines for observational studies using routinely collected data.13, 14 The authors declare that all supporting data are available within the article and its online supplementary files.
Data Sources and Study Population
The SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) register15, 16 was used to identify all adult patients ≤50 years of age who underwent primary isolated CABG in Sweden between 1995 and 2013. Patients who had surgery for congenital coronary malformations were excluded. As previously described, individual‐level data linking to other nationwide healthcare registries used the unique personal identity numbers assigned to all Swedish residents.17, 18 The National Patient Register19 was used to acquire information regarding prior medical history, and the Longitudinal Integration Database for Health Insurance and Labor Market Studies, managed by Statistics Sweden, was used to obtain socioeconomic information.
Outcomes
The main outcome measure was all‐cause mortality. Vital status and date of death was obtained from the Cause of Death Register.20 Additionally, secondary outcomes of myocardial infarction, stroke, and repeat revascularization (percutaneous coronary intervention or CABG) during follow‐up were identified in the National Patient Register.19
Statistical Methods
Baseline characteristics were described as means and standard deviations for continuous variables. Categorical variables were described as frequencies and percentages. Time‐to‐event was calculated as time in days from the date of surgery until the date of the respective event or end of follow‐up, which was March 24, 2014, for death and December 31, 2012, for the secondary outcomes. Because the follow‐up period for the secondary outcome measures ended on December 31, 2012, patients who underwent surgery during 2013 were excluded from the secondary outcome analyses to allow for a reasonable period at risk. Comparisons between men and women with respect to survival and secondary outcomes were made using weighted Cox regression models where the weights were derived from propensity scores estimated using generalized boosted regression modeling.21, 22 All variables reported in Table were used in the estimation of propensity scores. We examined the distribution of weights and found no patients with extreme weights, indicating that trimming was not necessary. Balance between the groups was assessed by standardized mean differences. An absolute standardized difference of ≤0.1 was considered an ideal balance.23 Data management and statistical analyses were performed with the use of Stata 15.1 (Stata Corp, College Station, TX) and R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Baseline Characteristics in 5446 Patients 50 Years or Younger Who Underwent Primary Isolated CABG in Sweden Between 1995 and 2015 Before and After Inverse Probability of Treatment Weighting
| Unweighted | IPTW | |||||
|---|---|---|---|---|---|---|
| Men | Women | SMD | Mena | Womena | SMD | |
| Number of patients | 4514 (83) | 932 (17) | 5372.86 | 4562.74 | ||
| Age, y, mean (SD) | 46.8 (3.76) | 46.1 (4.35) | 0.152 | 46.7 (3.74) | 46.7 (3.70) | 0.006 |
| Non‐Nordic birth region | 695 (15.4) | 69 (7.4) | 0.254 | 768.9 (14.3) | 517.3 (11.3) | 0.089 |
| Education | 0.151 | 0.119 | ||||
| <10 years | 927 (30.7) | 186 (29.4) | 1100.0 (30.7) | 882.6 (29.7) | ||
| 10–12 years | 1463 (48.5) | 347 (54.9) | 1768.8 (49.3) | 1610.6 (54.3) | ||
| >12 years | 625 (20.7) | 99 (15.7) | 719.1 (20.0) | 474.4 (16.0) | ||
| Not married | 3011 (66.7) | 619 (66.4) | 0.006 | 3601.9 (67.0) | 3088.4 (67.7) | 0.014 |
| Disposable income | 0.058 | 0.093 | ||||
| Q1 (lowest) | 801 (26.4) | 159 (25.0) | 951.9 (26.3) | 738.8 (24.7) | ||
| Q2 | 739 (24.3) | 169 (26.5) | 887.3 (24.6) | 850.7 (28.4) | ||
| Q3 | 796 (26.2) | 159 (25.0) | 941.6 (26.1) | 778.6 (26.0) | ||
| Q4 (highest) | 700 (23.1) | 150 (23.5) | 832.2 (23.0) | 626.9 (20.9) | ||
| Body mass index, kg/m2, mean (SD) | 28.0 (4.25) | 27.4 (5.35) | 0.135 | 27.96 (4.40) | 28.13 (4.57) | 0.031 |
| Diabetes mellitus | 771 (17.1) | 312 (33.5) | 0.384 | 1033.9 (19.2) | 949.5 (20.8) | 0.039 |
| Hypertension (%) | 809 (17.9) | 214 (23.0) | 0.125 | 992.4 (18.5) | 889.6 (19.5) | 0.026 |
| Hyperlipidemia (%) | 1060 (23.5) | 180 (19.3) | 0.102 | 1237.0 (23.0) | 982.8 (21.5) | 0.036 |
| Peripheral vascular disease | 115 (2.5) | 51 (5.5) | 0.149 | 156.6 (2.9) | 159.8 (3.5) | 0.033 |
| eGFR, mL/min per 1.73 m2, mean (SD) | 89 (19) | 81 (27) | 0.360 | 87.83 (20.65) | 86.40 (21.25) | 0.070 |
| End‐stage renal disease | 81 (1.8) | 66 (7.1) | 0.259 | 136.1 (2.5) | 142.6 (3.1) | 0.036 |
| Chronic pulmonary disease | 81 (1.8) | 27 (2.9) | 0.073 | 104.4 (1.9) | 117.2 (2.6) | 0.042 |
| Prior myocardial infarction | 2193 (48.6) | 402 (43.1) | 0.110 | 2581.4 (48.0) | 2112.7 (46.3) | 0.035 |
| Prior PCI | 988 (21.9) | 216 (23.2) | 0.031 | 1185.3 (22.1) | 961.2 (21.1) | 0.024 |
| Heart failure | 281 (6.2) | 88 (9.4) | 0.120 | 347.2 (6.5) | 340.0 (7.5) | 0.039 |
| LV ejection fraction <50% | 0.121 | 0.060 | ||||
| >50% | 1265 (71.3) | 286 (76.1) | 1511.9 (71.8) | 1288.9 (73.9) | ||
| 30%–50% | 407 (22.9) | 68 (18.1) | 471.7 (22.4) | 348.2 (20.0) | ||
| <30% | 102 (5.7) | 22 (5.9) | 123.2 (5.8) | 107.4 (6.2) | ||
| Stroke | 129 (2.9) | 36 (3.9) | 0.056 | 162.0 (3.0) | 128.6 (2.8) | 0.012 |
| Atrial fibrillation | 81 (1.8) | 12 (1.3) | 0.041 | 96.2 (1.8) | 53.5 (1.2) | 0.051 |
| Cancer | 48 (1.1) | 26 (2.8) | 0.126 | 66.5 (1.2) | 70.1 (1.5) | 0.026 |
| Alcohol dependency | 117 (2.6) | 15 (1.6) | 0.069 | 135.8 (2.5) | 79.1 (1.7) | 0.055 |
| Off‐pump CABG | 557 (12.3) | 134 (14.4) | 0.060 | 684.3 (12.7) | 565.8 (12.4) | 0.010 |
| Number of grafts | 0.333 | 0.094 | ||||
| 1–2 | 957 (21.2) | 317 (34.0) | 1235.6 (23.0) | 1142.4 (25.0) | ||
| 3–4 | 2741 (60.7) | 482 (51.7) | 3187.5 (59.3) | 2724.4 (59.7) | ||
| >4 | 530 (11.7) | 60 (6.4) | 593.4 (11.0) | 385.0 (8.4) | ||
| Unknown | 286 (6.3) | 73 (7.8) | 356.3 (6.6) | 310.9 (6.8) | ||
| Type of graft | ||||||
| Internal mammary artery | 4225 (93.6) | 848 (91.0) | 0.098 | 5015.8 (93.4) | 4222.9 (92.6) | 0.031 |
| Bilateral mammary arteries | 104 (2.3) | 18 (1.9) | 0.026 | 120.8 (2.2) | 72.2 (1.6) | 0.049 |
| Radial artery graft | 128 (2.8) | 28 (3.0) | 0.010 | 149.6 (2.8) | 143.3 (3.1) | 0.021 |
| >1 arterial graft | 223 (4.9) | 44 (4.7) | 0.010 | 259.6 (4.8) | 210.5 (4.6) | 0.010 |
Numbers are n (%) unless otherwise noted. CABG indicates coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; IPTW, inverse probability of treatment weighting; LV, left ventricular; PCI, percutaneous coronary intervention; SMD, standardized mean difference.
The overall numbers of patients in each group are not integers because of inverse probability of treatment weighting.
Missing Data
The SWEDEHEART register did not include information on ejection fraction before 2002, and there was ≈25% to 55% missing information regarding body mass index and renal function before 2000. We did not have socioeconomic data before 1999. For variables with missing data, the weights were constructed to also balance rates of missingness in both groups. In a separate standard multivariable adjusted Cox regression model in the unweighted sample, missing data were handled as follows: Region of birth and marital status were imputed with the most common category, and missing data regarding left ventricular ejection fraction (61%), educational level (33%), disposable income (33%), renal function (22%), body mass index (21%), and number of grafted vessels (6.6%) were handled by multiple imputation by chained equations.24 The imputation models included all variables in Table, as well as the year of surgery, hospital, the event indicator, and the Nelson‐Aalen estimator of the cumulative baseline hazard.25 Twenty‐five data sets were imputed, and estimates from these data sets were combined. In addition, we used alternative missing data strategies (missing value indicator and complete case analysis).
Results
During the study period, 5446 patients ≤50 years of age underwent primary isolated CABG and were included in the study. Among these patients, there were 4514 (83%) men and 932 (17%) women. During a total follow‐up time of 50 409 patient years (median, 11.9 years), 651 (14%) men died, and during a total follow‐up time of 9883 patient years (median, 11.3 years), 168 (18%) women died. The number of operations per year decreased during the study period, but the proportion of women each year remained fairly stable (Figure S1). The baseline characteristics are shown in Table. In the unweighted sample, women had more diabetes mellitus, hypertension, cancer, and peripheral vascular disease. Women also had worse renal function and more end‐stage renal disease compared with men. After inverse probability of treatment weighting, the distribution of baseline characteristics was well balanced between men and women (Table and Figure S2).
Early Mortality
The unadjusted early mortality (death within 30 days of surgery) in the unweighted sample was 0.9% in men and 1.3% in women (hazard ratio [HR], 1.42; 95% CI, 0.75–2.70). There was no difference in early mortality between men (1.0%) and women (1.1%) (HR, 1.10; 95% CI, 0.52–2.30) after inverse probability of treatment weighting.
Long‐Term Survival
Survival according to sex in the unweighted and the weighted population is shown in Figure 1. In the unweighted population, the risk of death was greater in women compared with men (HR, 1.34; 95% CI, 1.13–1.58). However, after inverse probability of treatment weighting, the risk of death was not significantly different in women compared with men (HR, 1.02; 95% CI, 0.83–1.26). Results were consistent in a standard multivariable adjusted Cox regression model in the unweighted sample (HR, 0.99; 95% CI, 0.80–1.22).
Figure 1.
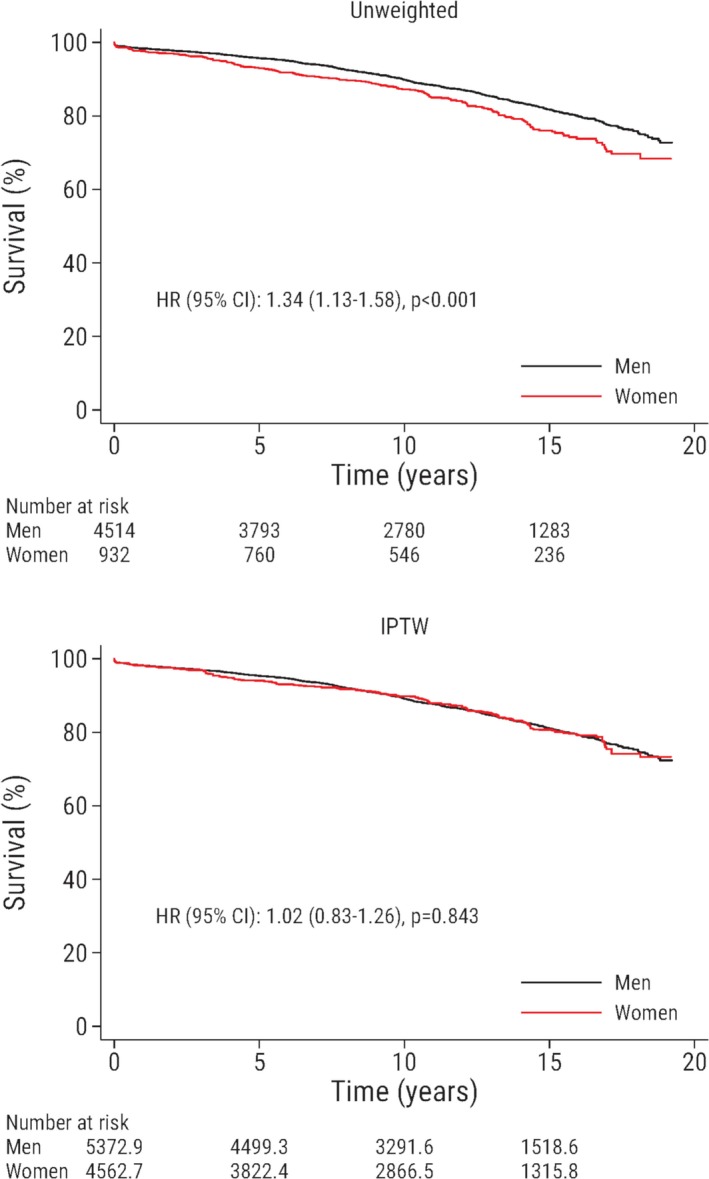
Survival is plotted against time after surgery and stratified according to sex. The upper panel shows the unweighted study population, and the lower panel shows the weighted sample. Male patients are the reference group. The numbers of patients at risk are not necessarily integers in the lower panel because of inverse probability of treatment weighting. HR indicates hazard ratio; IPTW, inverse probability of treatment weighting.
Baseline Characteristics Associated With Mortality
Selected baseline characteristics and their multivariable adjusted association with mortality stratified by sex are reported in Figure 2, and a comprehensive list is provided in Figure S3. Diabetes mellitus, chronic kidney disease, and peripheral vascular disease were significantly associated with an increased risk for death in both men and women. Heart failure, reduced left ventricular ejection fraction, chronic obstructive pulmonary disease, and not being married were significantly associated with an increased risk for death in men, but the association was not statistically significant in women. The direction and magnitude of the point estimates were similar to those in men, indicating that these characteristics should probably be considered risk markers also in women, and the lack of statistical significance was likely related to sample size. We found a strong and statistically significant association between prior cancer and risk of death in women but not in men.
Figure 2.
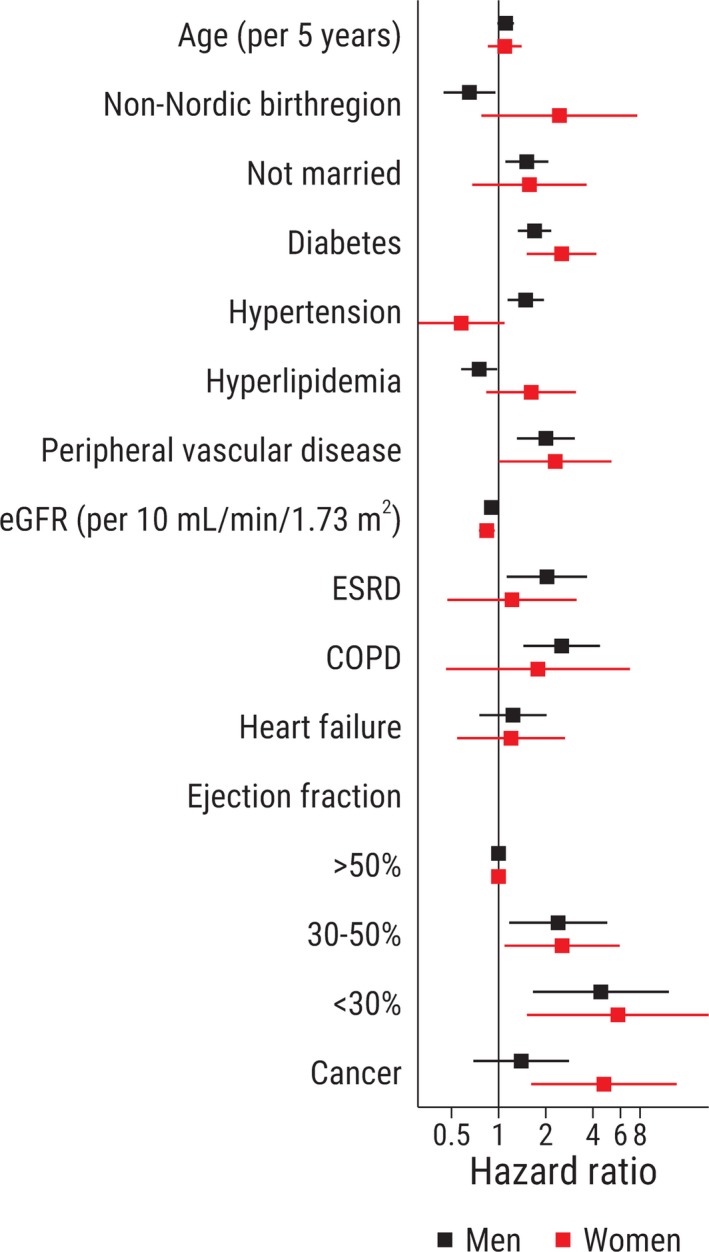
Selected patient characteristics associated with all‐cause mortality. Hazard ratios are adjusted for all variables presented in Table. COPD indicates chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; LVEF, left ventricular ejection fraction.
Myocardial Infarction, Stroke, and Repeat Revascularization
The risk for myocardial infarction, stroke and repeat revascularization, respectively, according to sex in the unweighted and the weighted population is shown in Figure 3. There was a higher rate of myocardial infarctions among women compared with men in the unweighted population (HR, 1.36; 95% CI, 1.08–1.70). After inverse probability of treatment weighting, the risk of the secondary outcome measures was not significantly different in women compared with men: myocardial infarction (HR, 1.21; 95% CI, 0.92–1.58), stroke (HR, 1.16; 95% CI, 0.84–1.61), repeat revascularization (HR, 0.88; 95% CI, 0.71–1.09). For the repeat revascularization outcome, it should be noted that the reported HR was not an optimal representation of the association between sex and repeat revascularization because of nonproportional hazards and should therefore be interpreted with caution. Repeat revascularization was mainly performed by percutaneous coronary intervention. In total, only 29 (0.5%) patients (25 men and 4 women) underwent repeat CABG during follow‐up.
Figure 3.
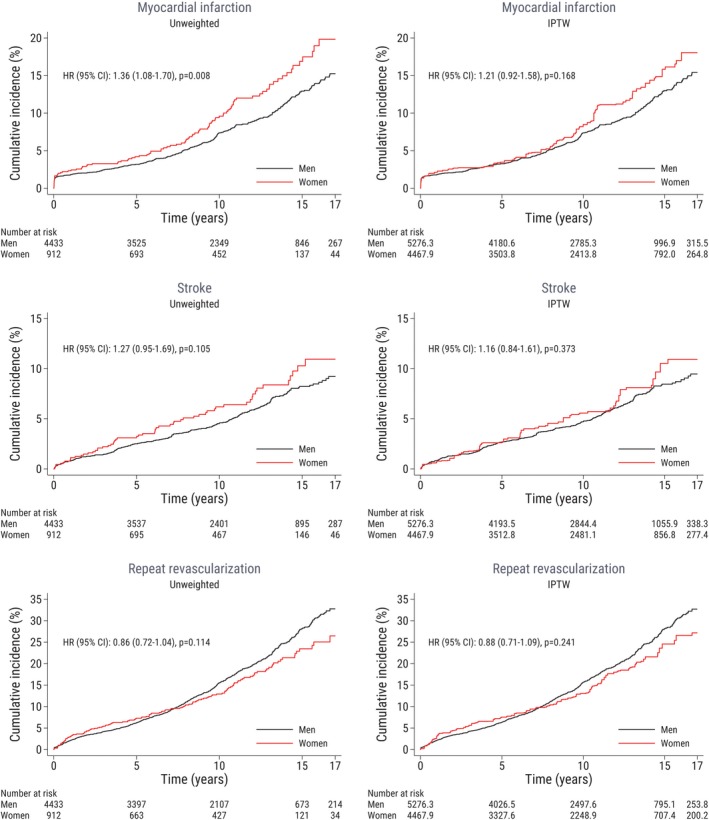
Clinical outcomes are plotted against time after surgery and stratified according to sex. The left‐hand panel shows the unweighted study population, and the right‐hand panel shows the weighted sample. Male patients are the reference group. Repeat revascularization refers to a new coronary intervention (PCI/CABG) following the index CABG. The numbers of patients at risk are not necessarily integers because of inverse probability of treatment weighting (right‐hand panel). CABG indicates coronary artery bypass grafting; HR, hazard ratio; IPTW, inverse probability of treatment weighting; PCI, percutaneous coronary intervention.
We observed that the rate of myocardial infarction was similar in men and women during the first 10 years of follow‐up, but after ≈10 years, the myocardial infarction event rate started to diverge, with an increased rate in women compared with men. At approximately the same time, the rate of repeat revascularization declined among women compared with men.
Discussion
In this nationwide cohort study, we analyzed possible differences between women and men ≤50 years of age undergoing CABG regarding short‐ and long‐term survival, major adverse cardiovascular events, and factors associated with mortality.
Young women who require CABG compose a specific subset of patients with the most advanced form of symptomatic CAD. CABG is one of the most intensively studied surgical procedures, and data regarding periprocedural risk and long‐term prognosis are generally well characterized. However, young women compose a minority of patients in previous CAD and CABG studies, and information about risk factors and prognosis may therefore not be generalizable to this population. The results of this study can be used to inform younger women undergoing CABG about long‐term prognosis and how this is influenced by preoperative risk profile.26
The adverse risk factor profile among young women was evident with diabetes mellitus, hypertension, peripheral vascular disease, chronic kidney disease, end‐stage renal disease, and prior cancer being more frequent among women compared with men. The results of our study confirm that these patient characteristics, which are well‐known risk factors for cardiovascular disease in patients of both sexes and other age groups, were relevant also for young women with advanced CAD undergoing CABG. The finding that young women undergoing CABG carry an adverse burden of risk factors compared with men is in line with previous studies in women of other age groups with less advanced CAD.1, 2, 27 Factors significantly associated with an elevated long‐term risk of death in women were diabetes mellitus, chronic kidney disease, and peripheral vascular disease. This is consistent with previous findings that baseline coronary risk factor status is associated with mortality also in young women.28
Previous studies of coronary revascularization in women have shown contradictory results. While some studies have found a higher mortality in women compared with men undergoing CABG,9, 29, 30 others have shown that this difference did not remain after adjustment for cardiovascular risk factors.31, 32, 33 These previous studies have drawbacks, as they either included a limited number of female patients29, 31, 32, 33 or were of a historical nature with patients who had surgery 15 to 25 years ago.33 Moreover, few have reported outcomes in young women. Two studies found that women younger than 50 years of age had higher mortality than men and that this interaction to some extent remained after risk factor adjustment.9, 30 However, some cardiovascular risk factors that in our study were shown to be more frequent in women compared with men and related to increased mortality were not included in these analyses, and the results could thereby be influenced by residual confounding. Also, only in‐hospital mortality was investigated in these previous studies. It is possible that short‐term mortality after CABG has historically been higher among young women compared with young men, but this may not be true for contemporary CABG. Even though younger women in our study had an increased burden of risk factors known to affect early risk, 30‐day mortality was not increased compared with men. This makes a contemporary difference in short‐term mortality after CABG between young women and men unlikely. This is in line with other previous studies in women of all age groups.29 We adjusted for a large number of baseline characteristics, and by using inverse probability of treatment weighting, we were able to achieve a well‐balanced distribution of characteristics between men and women without excluding patients from the analysis.
We found no differences regarding long‐term risk for stroke between young women and men. This is in line with previous work that demonstrated that stroke incidence is similar between women and men in the general population of younger adults.34 Regarding long‐term risk for repeat coronary revascularization, we found no differences between young women and men in our study. The short‐term incidence of repeat coronary revascularization after percutaneous coronary intervention has been shown to be higher in women compared with men, but the long‐term incidence of repeat revascularization seems to be similar between sexes.35 Although not statistically significant, the short‐term risk of repeat revascularization seemed to be higher in women compared with men in our study.
We observed that the rate of myocardial infarction was similar in men and women during the first 10 years of follow‐up, but after ≈10 years the event rate started to diverge, with an increased rate in women compared with men. At approximately the same time, the rate of repeat revascularization declined among women compared with men. The increased rate of myocardial infarction in women compared with men 10 years after CABG is not in line with findings in the general population, where men have a higher myocardial infarction incidence throughout life.36 It has been suggested that women are less likely to receive guideline‐indicated therapies,1, 37, 38 but other studies have shown that women undergoing CABG receive the same standard of care as men.39 We did not have information about possible changes in risk factor profile during follow‐up, and it is possible that changes could have resulted in altered risk of adverse cardiovascular events. However, it is possible that women received invasive treatment to a lower extent compared with men, and it has previously been shown that in women with acute coronary syndrome, cardiac catheterization and revascularization is lower compared with men.37, 38 Another possible explanation could be that impaired coronary microvascular function and nonobstructive coronary artery disease was more common in women during follow‐up.40, 41, 42 Impaired coronary microvascular function has been shown to predict adverse cardiovascular outcomes including myocardial infarction.40
The strong significant association found between prior cancer and risk of death in women but not in men could possibly be explained by a different cancer type pattern in young women and men and thereby associated difference in cancer‐related risk of death.43
Study Limitations
This study has limitations that need to be considered. By using inverse probability of treatment weighting we accounted for the differences in patient characteristics between women and men, but the possibility remains that residual confounding was still present. The finding that some baseline characteristics were significantly associated with mortality in men but not in women might have been related to the sample size of women and should therefore be interpreted with caution. There was a high proportion of missing data for some preoperative variables. Multiple imputation was used to retain statistical power and reduce the selection bias that may occur when deleting observations with missing covariates. Additionally, 4 alternative strategies to handle missing data were applied: (1) the weights used for inverse probability of treatment weighting were constructed to also balance rates of missing data in both groups, (2) missing value indicator category, (3) exclusion of baseline variables with the largest proportion of missing data from the regression model, and (4) complete case analysis. All approaches gave similar results, indicating that our results were robust in relation to different analytical strategies to handle missing data. Moreover, the registry did not include information regarding the size and quality of the grafted coronary arteries or proportion of incomplete revascularization. Because women have been shown to have smaller coronary arteries than men and that small target vessel diameter has been associated with mortality after CABG,44 it has been hypothesized that smaller coronary arteries might explain the higher mortality among women after CABG seen in some studies. Small target vessel diameter could lead to incomplete revascularization, which has also been associated with impaired survival after CABG.45 Another limitation was that we did not have information regarding secondary prevention measures, and prior research has shown that secondary prevention and success in reaching treatment goals may differ between sexes.46
Acknowledging these limitations, we still think our study has considerable strengths related to the size of the study cohort, which represents a rare patient population of young women with advanced CAD. There was a large number of events during a median follow‐up that exceeded 11 years. We used high‐quality complete coverage national healthcare registers, and therefore there was no loss to follow‐up.
Conclusions
The higher unadjusted risk of death after CABG in young women compared with men did not persist after adjusting for a broad range of risk factors. Female sex per se was not associated with increased mortality in young patients undergoing CABG. The results of this study can be used to inform young women undergoing CABG about prognosis and how this is influenced by preoperative risk profile.
Sources of Funding
This work was supported by the Swedish Heart‐Lung Foundation (grant numbers 20160522, 20160525 to Dr Sartipy and 20170804 to Dr Holzmann); the Mats Kleberg Foundation (grant number 2017‐00096 to Dr Sartipy); Karolinska Institutet Foundations and Funds (grant number 2016fobi47721 to Dr Sartipy); Swedish Heart and Lung Association (grant number E101/16 to Dr Sartipy); Åke Wiberg Foundation (grant number M17‐0089 to Dr Sartipy); Magnus Bergvall Foundation (grant number 2017‐02054 to Dr Sartipy); and the regional ALF agreement between Stockholm County Council and Karolinska Institutet (grant number 20160329 to Dr Sartipy). Dr Dalén was supported by a scholarship provided by the Swedish Women & Health Foundation. Dr Holzmann holds a research position funded by the Swedish Heart‐Lung Foundation (grant 20170804).
Disclosures
Dr Holzmann received consultancy honoraria from Actelion, Idorsia and Pfizer. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Number of primary isolated coronary artery bypass procedures by year and sex in patients ≤50 years of age in Sweden during 1995 to 2013.
Figure S2. Absolute standardized differences before (hollow circles) and after (filled circles) inverse probability of treatment weighting.
Figure S3. Multivariable adjusted associations between patient characteristics and all‐cause mortality. Hazard ratios are adjusted for all variables presented in Table.
Acknowledgments
We thank the SWEDEHEART steering committee for providing data for this study.
(J Am Heart Assoc. 2019;8:e013211 DOI: 10.1161/JAHA.119.013211.)
References
- 1. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow‐up study of 14 786 middle‐aged men and women in Finland. Circulation. 1999;99:1165–1172. [DOI] [PubMed] [Google Scholar]
- 3. Bergstrand R, Vedin A, Wilhelmsson C, Wilhelmsen L. Incidence and prognosis of acute myocardial infarction among men below age 40 in Goteborg, Sweden. Eur Heart J. 1982;3:130–135. [DOI] [PubMed] [Google Scholar]
- 4. Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea: preliminary report. J Am Med Assoc. 1953;152:1090–1093. [DOI] [PubMed] [Google Scholar]
- 5. Tracy RE, Newman WP III, Wattigney WA, Srinivasan SR, Strong JP, Berenson GS. Histologic features of atherosclerosis and hypertension from autopsies of young individuals in a defined geographic population: the Bogalusa Heart Study. Atherosclerosis. 1995;116:163–179. [DOI] [PubMed] [Google Scholar]
- 6. Zimmerman FH, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol. 1995;26:654–661. [DOI] [PubMed] [Google Scholar]
- 7. Jomini V, Oppliger‐Pasquali S, Wietlisbach V, Rodondi N, Jotterand V, Paccaud F, Darioli R, Nicod P, Mooser V. Contribution of major cardiovascular risk factors to familial premature coronary artery disease: the GENECARD project. J Am Coll Cardiol. 2002;40:676–684. [DOI] [PubMed] [Google Scholar]
- 8. Klein LW, Nathan S. Coronary artery disease in young adults. J Am Coll Cardiol. 2003;41:529–531. [DOI] [PubMed] [Google Scholar]
- 9. Regitz‐Zagrosek V, Lehmkuhl E, Hocher B, Goesmann D, Lehmkuhl HB, Hausmann H, Hetzer R. Gender as a risk factor in young, not in old, women undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:2413–2414. [DOI] [PubMed] [Google Scholar]
- 10. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, van der Schaar M, Badimon L, Bugiardini R. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three‐vessel disease and left main coronary disease: 5‐year follow‐up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 12. Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 14. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 16. Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternström L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, Ragnarsson S, Friberg Ö. Validity of the Swedish cardiac surgery registry. Interact Cardiovasc Thorac Surg. 2018;27:67–74. [DOI] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalén M, Ivert T, Holzmann MJ, Sartipy U. Coronary artery bypass grafting in patients 50 years or younger: a Swedish nationwide cohort study. Circulation. 2015;131:1748–1754. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffin BA, Ridgeway G, Morral A, Burgette L, Martin C, Almirall D, Ramchand R, Jaycox LH, McCaffrey DF. Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) Website. 2014. Available at: http://www.rand.org/statistics/twang. Accessed August 1, 2018.
- 22. Ridgeway G, McCaffrey D, Morral A, Griffin BA, Burgette L. Twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. 2017. Available at: https://CRAN.R-project.org/package=twang. Accessed August 1, 2018.
- 23. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 25. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schenck‐Gustafsson K. Risk factors for cardiovascular disease in women. Maturitas. 2009;63:186–190. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde‐Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd‐Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long‐term risk of cardiovascular and all‐cause mortality. JAMA. 2004;292:1588–1592. [DOI] [PubMed] [Google Scholar]
- 29. Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou YB, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 Midwestern hospitals. Circulation. 2005;112:I323–I327. [DOI] [PubMed] [Google Scholar]
- 30. Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–1181. [DOI] [PubMed] [Google Scholar]
- 31. Koch CG, Khandwala F, Nussmeier N, Blackstone EH. Gender and outcomes after coronary artery bypass grafting: a propensity‐matched comparison. J Thorac Cardiovasc Surg. 2003;126:2032–2043. [DOI] [PubMed] [Google Scholar]
- 32. Guru V, Fremes SE, Austin PC, Blackstone EH, Tu JV. Gender differences in outcomes after hospital discharge from coronary artery bypass grafting. Circulation. 2006;113:507–516. [DOI] [PubMed] [Google Scholar]
- 33. Herlitz J, Brandrup‐Wognsen G, Karlson BW, Sjoland H, Karlsson T, Caidahl K, Hartford M, Haglid M. Mortality, risk indicators of death, mode of death and symptoms of angina pectoris during 5 years after coronary artery bypass grafting in men and women. J Intern Med. 2000;247:500–506. [DOI] [PubMed] [Google Scholar]
- 34. Petrea RE, Beiser AS, Seshadri S, Kelly‐Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke. 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Z, Qian J, Ma J, Ge L, Ge J. Effect of gender on repeated coronary artery revascularization after intra‐coronary stenting: a meta‐analysis. Int J Cardiol. 2012;157:381–385. [DOI] [PubMed] [Google Scholar]
- 36. Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bonaa KH. Lifelong gender gap in risk of incident myocardial infarction: the Tromso Study. JAMA Intern Med. 2016;176:1673–1679. [DOI] [PubMed] [Google Scholar]
- 37. Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK; CRUSADE Investigators . Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) national quality improvement initiative. J Am Coll Cardiol. 2005;45:832–837. [DOI] [PubMed] [Google Scholar]
- 38. D'Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with ST‐segment‐elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parolari A, Dainese L, Naliato M, Polvani G, Loardi C, Trezzi M, Fusari M, Beverini C, Tremoli E, Biglioli P, Alamanni F. Do women currently receive the same standard of care in coronary artery bypass graft procedures as men? A propensity analysis. Ann Thorac Surg. 2008;85:885–890. [DOI] [PubMed] [Google Scholar]
- 40. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook‐Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B. Impact of abnormal coronary reactivity on long‐term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crea F, Bairey Merz CN, Beltrame JF, Berry C, Camici PG, Kaski JC, Ong P, Pepine CJ, Sechtem U, Shimokawa H. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J. 2019;40:2455–2462. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova‐Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population‐based study. Lancet Oncol. 2017;18:1579–1589. [DOI] [PubMed] [Google Scholar]
- 44. O'Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O'Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–655. [DOI] [PubMed] [Google Scholar]
- 45. Synnergren MJ, Ekroth R, Oden A, Rexius H, Wiklund L. Incomplete revascularization reduces survival benefit of coronary artery bypass grafting: role of off‐pump surgery. J Thorac Cardiovasc Surg. 2008;136:29–36. [DOI] [PubMed] [Google Scholar]
- 46. Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139:1025–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Number of primary isolated coronary artery bypass procedures by year and sex in patients ≤50 years of age in Sweden during 1995 to 2013.
Figure S2. Absolute standardized differences before (hollow circles) and after (filled circles) inverse probability of treatment weighting.
Figure S3. Multivariable adjusted associations between patient characteristics and all‐cause mortality. Hazard ratios are adjusted for all variables presented in Table.


