Abstract
Background
Long‐term corticosteroid therapy is the standard of care for treatment of cardiac sarcoidosis (CS). The efficacy of long‐term corticosteroid‐sparing immunosuppression in CS is unknown. The goal of this study was to assess the efficacy of methotrexate with or without adalimumab for long‐term disease suppression in CS, and to assess recurrence and adverse event rates after immunosuppression discontinuation.
Methods and Results
Retrospective chart review identified treatment‐naive CS patients at a single academic medical center who received corticosteroid‐sparing maintenance therapy. Demographics, cardiac uptake of 18‐fluorodeoxyglucose, and adverse cardiac events were compared before and during treatment and between those with persistent or interrupted immunosuppression. Twenty‐eight CS patients were followed for a mean 4.1 (SD 1.5) years. Twenty‐five patients received 4 to 8 weeks of high‐dose prednisone (>30 mg/day), followed by taper and maintenance therapy with methotrexate±low‐dose prednisone (low‐dose prednisone, <10 mg/day). Adalimumab was added in 19 patients with persistently active CS or in those with intolerance to methotrexate. Methotrexate±low‐dose prednisone resulted in initial reduction (88%) or elimination (60%) of 18‐fluorodeoxyglucose uptake, and patients receiving adalimumab‐containing regimens experienced improved (84%) or resolved (63%) 18‐fluorodeoxyglucose uptake. Radiologic relapse occurred in 8 of 9 patients after immunosuppression cessation, 4 patients on methotrexate‐containing regimens, and in no patients on adalimumab‐containing regimens.
Conclusions
Corticosteroid‐sparing regimens containing methotrexate with or without adalimumab is an effective maintenance therapy in patients after an initial response is confirmed. Disease recurrence in patients on and off immunosuppression support need for ongoing radiologic surveillance regardless of immunosuppression regimen.
Keywords: immunosuppression, sarcoidosis, ventricular arrhythmia
Subject Categories: Arrhythmias, Cardiomyopathy, Inflammatory Heart Disease, Nuclear Cardiology and PET
Clinical Perspective
What Is New?
A corticosteroid‐sparing strategy using methotrexate with or without adalimumab is an effective maintenance therapy for treatment of active cardiac sarcoidosis.
Discontinuation of immunosuppression is associated with a high rate of radiologic relapse and ventricular tachycardia.
What Are the Clinical Implications?
Treatment of cardiac sarcoidosis with immunosuppression remains a cornerstone of therapy, and these data provide evidence that stable immunosuppression can be achieved with low doses of prednisone combined with other agents.
These findings can be used to guide future prospective and randomized studies to investigate the optimal immunosuppression regimen and duration of therapy.
Introduction
Cardiac sarcoidosis (CS) is an infiltrative cardiomyopathy characterized by granulomatous inflammation that can occur in patients without overt clinical manifestations, but often presents with ventricular tachyarrhythmia (VT), conduction system disease, and/or heart failure.1, 2 Although diagnosis of CS can be challenging, with wider use of cardiac magnetic resonance imaging and 18‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET), CS is now increasingly recognized as an important cause of previously unexplained atrioventricular block, VT, and non‐ischemic cardiomyopathy.3, 4 Furthermore, as more patients with systemic sarcoidosis are actively screened for subclinical cardiac involvement, CS is increasingly diagnosed in asymptomatic patients.5
Immunosuppression with corticosteroids or other agents is currently the standard of care for treatment of symptomatic active CS, with some centers also recommending treatment of asymptomatic CS patients, since suppression of active inflammation may prevent the development of myocardial fibrosis.1, 6, 7, 8, 9, 10 While retrospective studies have provided evidence that immunosuppression reduces mortality and is associated with reduction in VT burden, reversal of Atrioventricular block, and improvement in left ventricular ejection fraction,4, 7, 11, 12, 13, 14, 15, 16 prolonged use of high‐dose corticosteroids confers significant risks and impairs quality of life.17, 18, 19, 20, 21 In addition, there are no data investigating the efficacy and long‐term outcomes of immunosuppressive therapy in those with asymptomatic CS or quiescent disease, so aggressively treating this group of patients could theoretically expose them to medication side effects without providing clear benefits.22 For these reasons, balancing the desire for long‐term disease suppression with the risks of medication side effects can be challenging in clinical practice, particularly for patients with long‐term quiescent disease on immunosuppressive therapy. Although the current consensus is that immunosuppression is indicated for treatment of CS, there is no consensus on the appropriate dosage and the optimal duration of treatment with corticosteroids, nor on potential roles of steroid‐sparing agents in CS.4, 19, 23, 24, 25, 26, 27, 28 Thus far, no randomized controlled trials have been performed comparing different immunosuppressive treatment regimens for CS, resulting in substantial heterogeneity among different treatment sites and the lack of evidence‐based guidelines on duration and type of treatment (Table 1).27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39
Table 1.
Previously Studied Immunosuppressive Regimens in Cardiac Sarcoidosis
| Authors | Year | n | Country | High‐Dose Immunosuppression | Taper Duration | Low‐Dose Immunosuppression | Corticosteroid‐Sparing Agent | Immunosuppression Discontinuation |
|---|---|---|---|---|---|---|---|---|
| Ahmadian et al29 | 2017 | 17 | United States | Not reported | Not reported | Not reported | No | Noa |
| Banba et al11 | 2007 | 15 | United States | Variable | 6 to 12 mo | Prednisone 5 to 10 mg/d | No | No |
| Ballul et al40 | 2016 | 36 | France | Prednisone 60 mg/d | Not reported | Not reported | Multiple | No |
| Chapelon‐Abric et al24, 30 | 2004, 2017 | 41, 59 | France | IV prednisolone 15 mg/kg×3 d | Variable | Not reported | Multiple | Yes |
| Chiu et al25 | 2005 | 43 | Japan | Prednisone 60 mg QOD | 2 mo | Prednisone 10 mg/d | No | No |
| Fussner et al31 | 2018 | 91 | United States | Prednisone 40 to 60 mg/d | Variable | Variable | Multiple | No |
| Futamatsu et al26 | 2006 | 21 | Japan | 5 to 60 mg prednisone/d | Variable | Prednisone 0 to 20 mg/d | No | No |
| Hiramatsu et al41 | 2005 | 49 | Japan | Prednisone 30 to 60 mg/d | 0.5 to 1 mo | Prednisone 5 to 10 mg/d | No | No |
| Kato et al32 | 2003 | 20 | Japan | Variable | Variable | Variable | No | No |
| Kudoh et al33 | 2010 | 10 | Japan | Not reported | Not reported | Not reported | No | No |
| Lee et al34 | 2017 | 16 | United States | Not reported | Variable | Not reported | No | No |
| Muser et al39 | 2018 | 20 | United States | Not reported | Variable | Not reported | Methotrexate | No |
| Nagai et al4, 35, 36 | 2014, 2015, 2016 | 17 to 61 | Japan | Prednisone 30 to 40 mg/d | Variable | Prednisolone 5 to 15 mg/d | Methotrexate | Yes |
| Osborne et al37 | 2014 | 23 | United States | Not reported | Variable | Not reported | No | No |
| Padala et al38 | 2016 | 30 | United States | Variable | Variable | Variable | No | No |
| Yazaki et al27 | 2001 | 95 | Japan | Prednisone 30 to 60 mg/d | Variable | Prednisone 5 to 15 mg/d | No | No |
| Yodogawa et al28, 42 | 2011, 2013 | 31, 15 | Japan | Prednisone 30 mg/d | 6 mo | Prednisone 5 to 10 mg/d | No | No |
Prior studies investigating the efficacy of specific immunosuppression regimens. IV indicates intravenous.
4 of 6 subjects in the study by Ahmadian et al demonstrated worsening qualitative FDG uptake in those who reduced immunosuppression.
In this context, answering 2 key questions could help to clarify the utility of different treatment regimens and ultimately to design appropriate randomized trials: (1) Are steroid‐sparing immunosuppressive agents effective for maintaining long‐term disease quiescence in CS? (2) In those patients with adequate disease control, what is the likelihood of disease recurrence after discontinuation of immunosuppression? The goal of this study is to address these 2 questions using a retrospective, single center series of patients who were treated for newly diagnosed CS with short‐duration high dose corticosteroids followed by long‐term methotrexate with or without adalimumab. A subset of these patients chose to discontinue immunosuppression after achieving remission, permitting us to evaluate the frequency of clinical and radiologic CS recurrence after cessation of immunosuppression therapy.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. Patients were referred for diagnosis and management of CS to the University of California San Francisco Medical Center between January 2009 and November 2018. A histologic or clinical CS diagnosis was ascertained per 2017 Japanese Society of Cardiology expert consensus diagnostic criteria.43 Inclusion criteria were as follows: age ≥18 years with a diagnosis of confirmed CS; initial treatment with 4 to 8 weeks of high dose prednisone (>30 mg/day), followed by maintenance therapy with oral methotrexate±low‐dose prednisone (LDP, <10 mg/day)±adalimumab; at least 2 consecutive pre‐ and post‐treatment PET scans ≈6 months apart. Those with unavailable or insufficient studies, follow‐up <6 months, and those treated with immunosuppression before presentation to our institution were excluded. The requirement for subject informed consent was waived.
Clinical Characteristics
Baseline demographics, medical history, and ECG characteristics at the time of CS diagnosis were gathered by utilizing the electronic medical record. Demographics investigated included age, sex, and body mass index. Medical history included cardiovascular risk factors such as hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, as well as the presence of extra‐cardiac sarcoidosis. Baseline ECG characteristics were determined from available ECGs, ambulatory cardiac monitors, and device interrogations. Prevalent cardiac disease included sustained atrial arrhythmias (atrial fibrillation, atrial flutter, or supraventricular tachycardia requiring treatment), high‐grade atrioventricular block (defined as Mobitz II or higher), clinical heart failure, and sustained ventricular arrhythmias (defined as VT/VF lasting >30 seconds, appropriate implanted cardioverter defibrillator therapy, or VT storm). High‐grade atrioventricular block was identified by chart review, ECG, or review of underlying rhythm on device interrogation. In addition, the minimum and maximum percent pacing was recorded for each patient. Symptomatic heart failure was defined as an inpatient hospitalization for acute decompensated heart failure, worsening functional status consistent with heart failure, or decline in left ventricular ejection fraction by at least 10% on consecutive echocardiograms. VT storm was defined as ≥2 VT/VF episodes within 24 hours. VF was defined as disorganized ventricular electrical activity resulting in cardiac arrest. This study is in accordance with requirements approved by the University of California, San Francisco Institutional Review Board, and all procedures were performed per University of California, San Francisco institutional guidelines (#14‐15430).
18F‐FDG PET
Serial 18F‐FDG PET scans were performed at ≈6‐month intervals until patients were clinically stable, and after that were performed annually for surveillance or in response to a change in therapy or clinical status. More specifically, PET 1 was performed before initiation of immunosuppression at the time of CS diagnosis, and PET 2 was obtained on maintenance immunosuppression (ie, following taper of initial high‐dose prednisone). Subsequent PET scans were obtained in patients after any change in maintenance immunosuppressive regimen (including switch to adalimumab‐containing regimen), after immunosuppression discontinuation in applicable patients, or in patients with a clinical change in status on relapse. Patients were studied using a whole‐body PET scanner with 3D acquisition (Siemens HR plus, Erlangene, Germany). External germanium‐68 sources were used for attenuation correction. All patients prepared for the scan by consuming a high‐fat, limited or non‐carbohydrate diet for a minimum of 24 hours before the exam and were advised to fast for 12 hours before the procedure per recent guideline recommendations.44 Resting myocardial perfusion was assessed after using the intravenous administration of 40 mCI of 82‐rubidium. Blood glucose level was confirmed before the injection of FDG (7–10 mCi) to ensure euglycemia. Approximately 10 mci of 18F‐FDG was injected intravenously. Metabolic images were acquired after ≈45 to 60 minutes of injection of 18F‐FDG, which resulted in adequate diagnostic images. Limited whole body 18F‐FDG images were taken from the vertex to mid‐thigh in all the patients. The maximal standardized uptake value (SUVmax) was reported as the highest voxel value with the region of interest, and the ratio myocardial SUVmax to liver SUVmax was determined (reported as “normalized SUVmax” to facilitate comparison between studies). All 3 image sets were reconstructed into transaxial images and resliced into standard data sets for image display. The radiologist was masked to patient treatment and clinical status.
Statistical Analysis
Patients were retrospectively divided into those who did and did not discontinue immunosuppression during clinical follow‐up, and baseline characteristics were reported. Differences in radiologic disease activity (PET 1) and cardiac manifestations present at the time of CS diagnosis were reported in each group. Following CS diagnosis, the incidence of persistent radiologic disease activity (PET 2) and adverse cardiac events including VT, symptomatic heart failure, and atrioventricular block were reported and compared between groups. In addition, CS activity (PET 3) and clinical manifestations were compared in patients with continuous immunosuppression to those after immunosuppression discontinuation.
Each PET scan was qualitatively reported as positive (18F‐FDG avidity in a pattern consistent with CS), improved (myocardial 18F‐FDG uptake present but qualitatively reduced compared with the prior scan), or negative (no myocardial 18F‐FDG uptake). In addition, a subset of PET scans were subject to quantification in which software calculated global and regional cardiac metabolic activity. Active myocardial disease sites were separated from mediastinal blood pool activity. Each scan was interpreted by an experienced radiologist (M.H.P.). A Wilcoxon rank sum test was used for comparison between groups because of non‐normally distributed data. A P value of <0.05 was considered statistically significant. Statistical analyses were performed on Stata (College Station, TX).
Results
Baseline Characteristics
CS was diagnosed in 34 patients referred per 2017 Japanese Society of Cardiology expert consensus diagnostic criteria,45 and 28 patients ultimately met inclusion criteria for this study. Patients not included did not have 2 consecutive PET scans for analysis (n=4), or were initially treated at an outside facility (n=2). Three patients met clinical criteria for clinical CS, and 25 patients met histologic criteria. Mean follow‐up was 4.1 years (SD 1.5 years) after CS diagnosis. Patients were predominantly young (mean age 52 years), non‐black, had few medical comorbidities, and had frequent baseline ECG abnormalities at the time of CS diagnosis (Table 2). Three subjects were black, 3 were Asian, 1 Hispanic, and the remainder white. The study cohort was highly enriched for symptomatic patients at time of CS diagnosis, with the majority (n=22) presenting with cardiac symptoms, 8 presenting with pulmonary symptoms (6 later developed cardiac symptoms), and only 2 had incidental diagnosis of CS. Two patients had isolated CS while the remainder had extra‐cardiac sarcoidosis, predominantly pulmonary. Ultimately 9 of 26 patients discontinued immunosuppression after a mean 1.97 years (SD 0.96 years) of continuous therapy because of medication side effect (n=3) and/or patient preference (n=6). No significant differences in baseline demographics or history were observed between patients who discontinued versus continued immunosuppression, with the exception of a female predominance of patients who discontinued immunosuppression (67% versus 32%, Table 2).
Table 2.
Baseline Demographics of Patients With Cardiac Sarcoidosis
| Total (n=28) | Immunosuppression Discontinued (n=9) | No Immunosuppression Discontinuation (n=19) | |
|---|---|---|---|
| Demographics | |||
| Age, y | 52.2 | 51.3 | 53.0 |
| Women | 12 (42.8%) | 6 (66.7%) | 6 (31.6%) |
| Body mass index, kg/m2 | 28.8 | 27.4 | 21.1 |
| Medical history | |||
| Hypertension | 5 (17.8%) | 1 (11.1%) | 4 (21.1%) |
| Hyperlipidemia | 3 (10.7%) | 1 (11.1%) | 2 (10.5%) |
| Diabetes mellitus | 2 (3.6%) | 0 | 2 (10.5%) |
| Coronary artery disease | 1 (3.6%) | 0 | 1 (5.3%) |
| PPM/ICD | 26 (92.9%) | 9 (100%) | 17 (89.5%) |
| Extracardiac sarcoidosis | 26 (92.9%) | 9 (100%) | 17 (89.5%) |
| Lung | 21 (72.4%) | 5 (55.6%) | 16 (84.2%) |
| Spleen | 5 (17.8%) | 1 (11.1%) | 4 (21.1%) |
| Lymph nodes | 18 (64.3%) | 4 (44.4%) | 14 (73.7%) |
| ECG | |||
| LBBB | 3 (10.7%) | 1 (11.1%) | 2 (10.5%) |
| RBBB | 12 (42.9%) | 5 (55.6%) | 7 (36.8%) |
| 1st degree atrioventricular block | 11 (39.3%) | 3 (33.3%) | 8 (42.1%) |
| Frequent PVCs | 16 (57.1%) | 6 (66.7%) | 10 (52.6%) |
| Non‐sustained VT | 10 (35.7%) | 3 (33.3%) | 7 (36.8%) |
| Prevalent cardiac manifestations | |||
| Atrial arrhythmia | 8 (28.6%) | 4 (44.4%) | 4 (21.1%) |
| High‐grade atrioventricular block | 11 (39.2%) | 3 (33.3%) | 8 (42.1%) |
| Sudden cardiac arrest | 4 (14.3%) | 2 (22.2%) | 2 (10.5%) |
| Heart failure | 11 (39.3%) | 4 (44.4%) | 7 (36.8%) |
| Sustained VT or VF | 18 (64.3%) | 6 (66.7%) | 12 (63.2%) |
ICD indicates implanted cardioverter defibrillator; LBBB, left bundle branch block; PPM, permanent pacemaker; RBBB, right bundle branch block; VF, ventricular fibrillation; VT, ventricular tachycardia; PVCs, premature ventricular contractions.
Immunosuppression Regimens
Of the 28 patients included in the study, 27 treatment‐naïve patients who had positive 18F‐FDG‐PET scans at baseline (PET 1) received a period of high‐dose prednisone (40–60 mg daily, mean 46.6 mg, SD 9.3 mg) for 4 to 8 weeks (mean 6.8 weeks, SD 2.2 weeks) followed by a taper to <10 mg of prednisone daily, typically 5 mg daily. Methotrexate was started at 10 to 15 mg per week and increased by 5 mg increments every 2 weeks until a dose of 20 mg per week was reached. One patient was treated with adalimumab monotherapy without methotrexate or concomitant corticosteroids because of previous intolerance both corticosteroids and methotrexate. By the time of the repeat 18F‐FDG PET scan (median 34.3 weeks, SD 14.5 weeks, range 11.0–73.8 weeks) after initiating treatment (PET 2), 18 of 28 patients were on LDP (mean 6.4 mg daily, SD 4.6 mg daily) with methotrexate 20 mg weekly, 7 were on methotrexate 20 mg weekly alone, and 3 were on an adalimumab‐containing regimen. In those with intolerance or contraindications to methotrexate, the next line agent used was adalimumab (40 mg subcutaneous injection biweekly) except in patients with New York Heart Association class III or IV heart failure. In those patients, once adalimumab was added, the methotrexate dose was concomitantly reduced to 7.5 or 10 mg weekly (n=8). In patients with persistent disease as assessed by PET despite methotrexate, the same dose of adalimumab (40 mg subcutaneous injection biweekly) was added along with methotrexate 15 mg weekly (n=11). While 19 of 28 patients maintained indefinite immunosuppression using ≥1 steroid‐sparing agents, immunotherapy was discontinued entirely in 9 of 28 patients after a period of disease quiescence, per patient preference, and/or because of medication intolerance or side effects.
Minor side effects of immunosuppression requiring medication discontinuation or additional medications (eg, insulin or metformin) occurred in 16 of 28 patients. The most common side effects were elevated transaminase levels (n=9) attributed to or exacerbated by methotrexate. All resolved with reduced dose or cessation of methotrexate. Four patients on prednisone developed hyperglycemia that required treatment. One patient on methotrexate monotherapy developed recurrent episodes of diverticulitis although such episodes both preceded and succeeded methotrexate treatment, and a patient on methotrexate with adalimumab developed a presumed lung infection (on imaging) requiring a temporary cessation of immunosuppression. In addition, there was 1 possible case of pulmonary toxicity (acutely reduced diffusion capacity on PFTs, but normal chest CT) for which methotrexate was empirically stopped.
Corticosteroid Sparing Therapy and Radiologic CS Suppression
We defined corticosteroid‐sparing therapy as methotrexate and/or adalimumab with or without concomitant low‐dose prednisone (<10 mg daily). As described above, patients were preferentially treated initially with prednisone taper and methotrexate. At the time of first follow‐up PET scan, 15 of 25 patients treated primarily with methotrexate achieved complete resolution of 18F‐FDG uptake, 7 experienced partial resolution of 18F‐FDG uptake, and 3 patients experienced persistent 18F‐FDG uptake (Figure 1).
Figure 1.
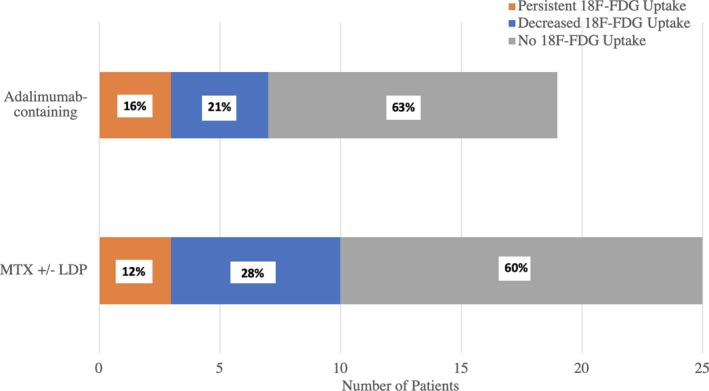
Association between immunosuppression regimen and complete resolution of 18‐fluorodeoxyglucose uptake on positron emission tomography in patients with cardiac sarcoidosis. All patients were initially treated with a methotrexate‐containing regimen except for 2 patients, who were initially treated with an adalimumab‐containing regimen. Persistent, decreased, or no 18‐fluorodeoxyglucose resolution in each patient stratified on maintenance immunosuppression. FDG indicates fluorodeoxyglucose; LDP, low‐dose prednisone; MTX, methotrexate.
Those patients with partial resolution or persistent 18F‐FDG avidity on methotrexate (refractory disease) were treated with addition of adalimumab, as described above, and further PET scans were obtained to assess response to treatment change. Of the remaining 15 complete responders, 4 eventually experienced radiographic relapse while on stable regimens of methotrexate±LDP. Six additional patients changed immunosuppression regimens because of side effects and/or personal preference. For these reasons, adalimumab was used in a total of 19 patients; 12 of 19 experienced complete resolution on PET, another 4 of 19 exhibited partial response, and 3 of 19 remained unresponsive (Figure 1). No subject receiving adalimumab demonstrated a radiologic CS relapse to date although average duration of follow‐up for adalimumab‐treated patients is shorter than for methotrexate‐treated patients as methotrexate was used first line in our cohort.
During cumulative follow‐up, 117 total PET scans were analyzed, 76 of which were obtained on immunosuppression. Four studies were excluded because of poor patient dietary preparation resulting in non‐diagnostic image quality. Overall, 69.7% (53/76) of PET scans on immunosuppression demonstrated complete 18F‐FDG resolution, and an additional 15.8% (12/76) demonstrated partial 18F‐FDG resolution, compared with positive 18F‐FDG uptake in 37/41 (90.2%) of PET scans obtained off immunosuppression. PETs obtained after immunosuppression discontinuation demonstrated positive 18F‐FDG uptake in 11of 12 scans.
Discontinuation of Immunotherapy for Recurrent CS
During follow‐up, 9 patients ultimately discontinued immunosuppression after achieving initial complete resolution of cardiac inflammation on PET because of medication intolerance or patient preference (Table 3). Three of these patients discontinued therapy twice. Immunosuppression was stopped after a mean 32.0 months (SD 9.7 months) of continuous therapy. Each of these patients underwent a repeat 18F‐FDG‐PET scan to assess for disease recurrence (Figure 2). Patients who discontinued immunosuppression subsequently experienced radiologic CS recurrence by PET in 8 of 9 (88.9%) patients an average of 8.4 months (SD 2.4 months) after discontinuation, compared with recurrence in 3 of 19 (15.8%) patients with uninterrupted immunosuppression.
Table 3.
Radiologic Relapse or Cardiac Event in Cardiac Sarcoidosis Patients Stratified by Immunosuppression Regimen
| Total Subjects | Incomplete Treatment Response | Radiologic Relapse | Cardiac Event | |
|---|---|---|---|---|
| After immunosuppression discontinuation | 9 | N/A | 8 | 2 |
| Methotrexate | 25 | 10 | 4 | 3 (2 AVB, HF) |
| Adalimumab‐containing | 19 | 7 | 0 | 0 |
Incomplete treatment response is defined as persistent or decreased 18F‐FDG uptake after initial treatment with immunosuppression. Radiologic relapse is defined as initial complete resolution of 18F‐FDG on PET, followed by serial increase in 18F‐FDG uptake on follow‐up scan. HF indicates heart failure; AVB indicates atrioventricular block.
Figure 2.
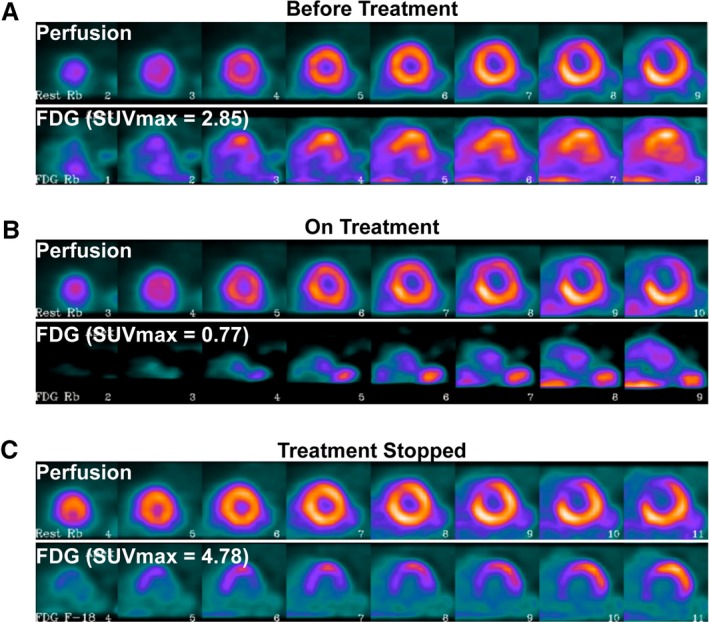
Cardiac sarcoidosis (CS) disease activity determined by 18‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET) throughout treatment course. Serial positron emission tomography scans obtained in a single patient with different immunosuppression regimens, with the short‐axis view visualized, from apex to base. The image on top is perfusion imaging, and the bottom demonstrates 18F‐FDG uptake. A, De novo CS: Initial positron emission tomography demonstrates 18F‐FDG uptake in the anteroseptum with a corresponding perfusion defect (myocardial maximal standardized uptake value [SUVmax]=2.85, SUVmax/standardized uptake value(liver)=1.08). B, CS suppression: Complete resolution of 18F‐FDG uptake on methotrexate maintenance therapy, with persistent perfusion defect, consistent with treated CS with residual scar. There is only mild 18F‐FDG uptake seen in the blood pool (myocardial SUVmax 0.77, SUVmax/standardized uptake value (liver)=0.31). C, CS recurrence: After immunosuppression discontinuation, there is new 18F‐FDG uptake in the basal to mid‐anterolateral wall with a new perfusion defect (myocardial SUVmax 4.78, SUVmax/SUV(liver)=1.53). FDG indicates fluorodeoxyglucose; SUVmax, maximal standardized uptake value.
18/28 patients in our study experienced VT during the course of their disease before starting immunosuppression. The pre‐treatment incidence of VT was comparable between patients who continued immunosuppression and those who discontinued therapy (Table 2). After initial treatment, 3 of 19 (15.8%) patients who remained on uninterrupted immunosuppression subsequently experienced VT, while 3 of 9 (33.3%) with interrupted immunosuppression experienced VT after discontinuation of therapy (Table 4). Of note, the time of observation for patients after discontinuation of immunosuppression was much less than for the group with uninterrupted immunosuppression; similarly, the length of time over which patients had VT before treatment was greater still.
Table 4.
Ventricular Tachycardia in Cardiac Sarcoidosis by Immunosuppression Status
| VT Incidence | |
|---|---|
| Before immunosuppression | 18/28 (64.3%) |
| On continuous immunosuppression | 3/19 (15.7%) |
| After discontinuation of immunosuppression | 3/9 (33.3%) |
Of the 28 subjects in this study, 6 had VT before starting immunosuppression. After starting immunosuppression, 3 of 19 who remained on uninterrupted immunosuppression developed VT, while 3 of 9 who discontinued immunosuppression developed VT after interruption of therapy. VT indicates ventricular tachycardia.
One patient developed heart failure while off immunosuppression, but overall there was no significant difference in LVEF assessed by TTE after starting immunosuppression (53.4% [SD 12.3%] versus 48.7% [SD 11.4%], P=0.14) or after immunosuppression discontinuation (49.9% [SD 10.2%] versus 44.4% [SD 13.4%], P=0.77). One patient with an implantable pacemaker developed permanent atrioventricular block during the study period, and one patient developed transient complete heart block following VT ablation that resolved within 2 weeks. After immunosuppression discontinuation, maximal percent pacing was similar (34.8%, SD 43.0%) to that before immunosuppression discontinuation (32.1%, SD 36.4%, P=0.71).
Quantification of Myocardial 18F‐FDG Uptake
18F‐FDG quantification before starting immunosuppression and after discontinuing immunosuppression was available in 18 of 28 subjects (Figure 3). The remaining subjects were unavailable because of inaccessible primary data (n=6) and PET scans performed at outside institutions (n=4). Initiation of immunosuppression was associated with a decrease in normalized myocardial SUVmax from a mean of 2.15 (SD 0.73) to 1.07 (SD 0.57, P<0.01). In the 9 subjects who discontinued immunosuppression, normalized myocardial SUVmax increased from 0.78 (SD 0.24) to 1.73 (SD 1.07, P<0.01). Subjects with an initial clinical presentation of atrioventricular block demonstrated a decrease in mean SUVmax of 2.81 (SD 0.96) before immunosuppression to 1.53 (SD 1.19) after starting immunosuppression, and those presenting with VT demonstrated a decrease in mean SUVmax of 2.03 (SD 0.41) to 0.94 (SD 0.18) after starting immunosuppression.
Figure 3.
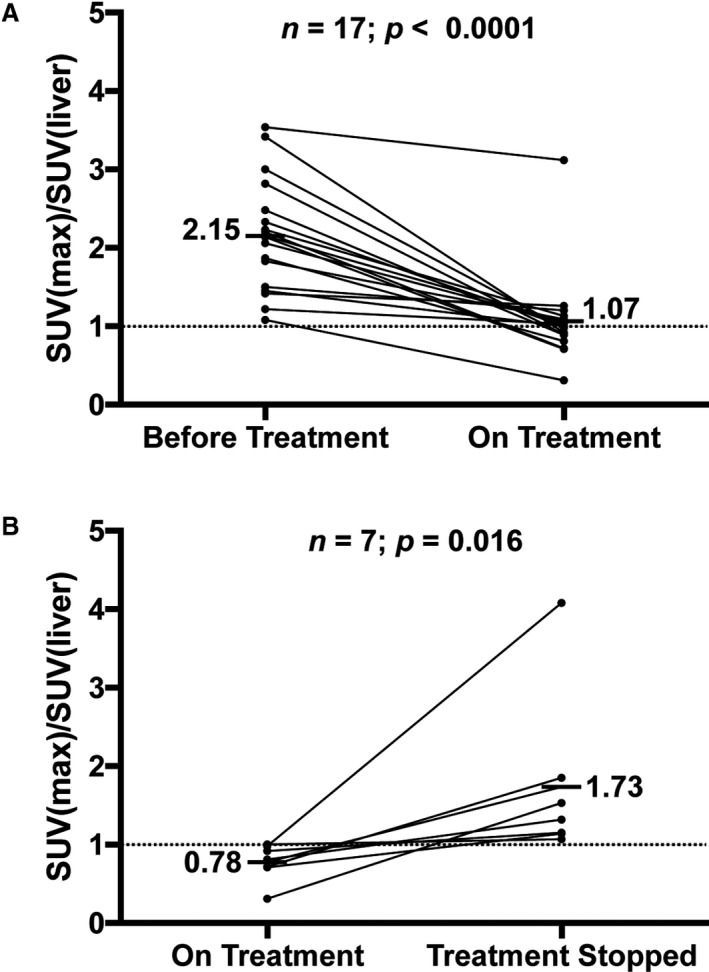
FDG quantification and immunosuppression status in cardiac sarcoidosis. A, Normalized maximal standardized uptake valuefor each subject at the time of cardiac sarcoidosis diagnosis and after initial immunosuppression treatment. B, In those subjects who discontinued immunosuppression, normalized maximal standardized uptake value is reported before and after immunosuppression discontinuation. SUV indicates standardized uptake value; SUVmax, maximal standardized uptake value.
Discussion
In this report, we present outcomes of corticosteroid‐sparing immunosuppression and frequency of disease recurrence after cessation of immunosuppression in a single‐center cohort of CS patients followed for several years. Our main findings are that steroid‐sparing therapies utilizing methotrexate and/or adalimumab are effective to maintain radiographic disease quiescence after taper of prednisone (at least 60% for either regimen), and that patients with quiescent disease who discontinue immunosuppression have a significantly increased risk of exhibiting radiologic recurrence as well as recurrent VT.
Efficacy of Corticosteroid‐Sparing Treatment for Cardiac Sarcoidosis
A strategy using LDP, methotrexate, and/or adalimumab is associated with a high likelihood of complete resolution or marked improvement of 18F‐FDG uptake in active CS. The optimal induction therapy, dose, and duration of immunosuppressive agents in CS remains incompletely understood and currently needs to be determined on a case‐by‐case basis because of the complexity of achieving immunosuppression while minimizing adverse medication effects. However, this study demonstrates that each combination regimen has substantial efficacy for achieving complete CS remission.
Previously, corticosteroid monotherapy was the mainstay of treatment for CS.19 However, a recent study demonstrated high rates of CS recurrence, increased steroid doses, and medication side effects in those treated with corticosteroid monotherapy compared with immunosuppression with prednisone and methotrexate.40 Patients in the aforementioned study were not routinely surveilled by PET, and exact treatment regimens and doses were not presented. However, the results of the current and aforementioned study suggest that immunosuppression in addition to corticosteroids may be required for persistent CS suppression. We found that 60% of patients on LDP and methotrexate therapy exhibited an initial complete radiologic response, with a mean LDP dose of 6.4 mg/day. However, 4 patients developed radiologic relapse while on methotrexate therapy despite dosing 20 mg once weekly (consistent with doses used for treatment of other rheumatologic diseases such as rheumatoid arthritis).46, 47, 48 Furthermore, almost half of patients treated with methotrexate experienced side effects, often resulting in dosage decrease and/or discontinuation of the medication. Given long‐term reported relapse rates of ≈40% in sarcoidosis patients treated with corticosteroid monotherapy,49 these data collectively support the use of methotrexate as an initial corticosteroid‐sparing agent, albeit with serial surveillance for side effects and CS relapse.
The efficacy of adalimumab‐containing regimens in this study is notable. Adalimumab is a monoclonal antibody to tumor necrosis factor‐alpha, an important mediator of granulomatous inflammation, and is an effective treatment for selected patients with systemic sarcoidosis.50, 51, 52, 53 Concerns over the association between high dose infliximab and worsened heart failure in the ATTACH (Anti‐TNF Therapy Against Congestive Heart Failure) trial have limited the use of tumor necrosis factor‐alpha blockers in cardiac sarcoidosis.54 Indeed, use of tumor necrosis factor inhibitors in New York Heart Association class III and IV heart failure is contraindicated, and high doses of infliximab (>3 mg/kg) should be used with caution in all patients with heart failure or reduced EF. However, adalimumab (dosed at 40 mg every 2 weeks) has potential advantages because of shorter half‐life, lower adverse drug events, and improved safety signal for heart failure in large cohorts of patients treated for rheumatoid arthritis compared with infliximab.55, 56
In the current study, 84% of those who received adalimumab‐containing regimens demonstrated at least partial reduction in 18F‐FDG uptake, and 63% experienced complete 18F‐FDG resolution without drug toxicity. We treated only patients with New York Heart Association class 1 or 2 symptoms with adalimumab. Although we did not observe a significant decline in EF or increase in clinical heart failure among adalimumab‐treated patients in our cohort, our duration of follow‐up on this drug was not long and we strongly favor cautious use of this pharmacologic agent with close monitoring of heart failure and EF. These findings cannot be directly compared with the efficacy of those receiving LDP and methotrexate in this study because several of those patients who received adalimumab had previously failed methotrexate. Longer‐term follow‐up with surveillance PET scans will be needed to establish whether relapse on adalimumab occurs and at what rate relative to methotrexate. In addition, it will be critical to determine long‐term effect on EF and New York Heart Association class, although separating effects of adalimumab from natural history of heart failure will be challenging without a clinical trial or a reasonable comparison group (eg, methotrexate‐treated patients). Overall, our data support the use of adalimumab to maintain CS quiescence. Further studies are needed to determine whether adalimumab can be effective as part of a first‐line treatment strategy.
Immunosuppression Discontinuation in CS
Few studies have investigated clinical outcomes in CS patients following discontinuation of immunosuppression. Nagai et al recently published the first data suggesting that discontinuation of immunosuppression in CS patients was associated with a reduction of systolic function and increased mortality during long‐term follow‐up of almost 10 years.4 Our data demonstrates that immunosuppression discontinuation is associated with an increase in clinical and radiographic CS recurrence even after over 2 years of continuous treatment with immunosuppression. In conjunction with previous research demonstrating increased cardiac morbidity and mortality in patients with active CS,11, 57 these data support prolonged and possibly indefinite use of immunosuppression in CS patients to prevent disease reactivation, or alternatively meticulous surveillance with PET scans in those who do choose to stop immunosuppression.58
Immunosuppression Status and Clinical End Points in CS
Our results also highlight the increased risk of VT in CS patients after immunosuppression discontinuation. While immunosuppression is associated with prevention of left ventricular remodeling and recovery of atrioventricular nodal function in active CS,25, 28, 59 a paucity of data exists examining long‐term follow‐up of CS patients who ultimately discontinue therapy because of high rates of medication side effects, interactions, and intolerance.21 Patients with continuous immunosuppression experienced less VT compared with those after immunosuppression discontinuation, corroborating previous findings.42 The majority of VT episodes were temporally associated with positive PET scans, suggesting that the risk of VT after immunosuppression discontinuation is mediated largely by the presence of active CS.
While VT was more common during periods of active disease, multiple events were noted during periods of inactive disease. These findings are consistent with the hypothesis that VT associated with inactive disease is mediated by steroid‐unresponsive scar‐mediated reentry, while VT occurring during active or reactivated CS may be because of steroid‐responsive areas of active inflamed and healing myocardium.7, 15, 41, 60, 61 VT in both active and inactive CS frequently requires interventions in addition to immunosuppression including implanted cardioverter defibrillator implantation, anti‐arrhythmic medications and radiofrequency ablation.61, 62 Those who do not tolerate immunosuppression represent a population at high VT risk—careful monitoring with surveillance PET imaging, and a low threshold for implanted cardioverter defibrillator implantation should be considered and investigated prospectively.
Despite the majority of patients with frequently monitored devices, one patient developed clinical atrioventricular block while on methotrexate therapy with an associated radiographic relapse. In those with implantable pacemakers, the burden of ventricular pacing was unchanged after stopping immunosuppression, which remains a poor surrogate measure of intermittent atrioventricular block because of heterogeneity of programming settings, high burden of premature ventricular contractions, and use of anti‐arrhythmic drugs. The relatively shorter follow‐up period in the current study likely explains the lack of heart failure seen in CS patients who discontinue immunosuppression, as progressive fibrosis is often a more indolent process.
Limitations and Future Investigations
The major limitations of the current study are the small sample size and retrospective nature of data collection that precludes statistical comparisons between specific immunosuppression regimens, and between those who did and did not discontinue immunosuppression. The statistical tests that were performed in this study were univariate and the cohort size was likely underpowered to detect significant differences between groups due to limited sample size. In addition, the current study represents the experience at a single tertiary care institution and patient selection may suffer from referral bias, supported by the finding that 60% of patients had sustained VT at initial presentation of CS. Future work involving multiple centers will hopefully be able to address specific clinical predictors of response to specific therapies and to identify clinical risk markers that could be harbingers of disease recurrence. While the goal of steroid‐sparing treatment was to minimize the duration of high‐dose steroid administration, individual regimens varied and, as such, the ability to investigate specific agents or duration of treatments in isolation remains limited.19
Future studies should prospectively investigate not only the efficacy of specific steroid‐sparing agents, but also differences in quality‐of‐life in patients treated with this strategy compared with patients treated with escalating doses of steroids. It will be important to clarify the type of induction therapy, duration of maintenance therapy, and preferred agents required to maintain disease quiescence. Further, the patients in the current study overwhelmingly presented with symptomatic CS, and all patients in the study had cardiac or extra‐cardiac evidence of sarcoidosis. It will be important to determine whether our conclusions can be generalized to patients with incidentally identified CS without histologic confirmation, particularly given the recent finding that inflammatory myocarditis is a common and underrecognized cause of ventricular ectopy, and adverse outcomes may be ameliorated by immunosuppression.63, 64 Despite these inherent limitations, the findings in the current study are provocative and warrant future prospective investigation.
Conclusions
A steroid‐sparing immunosuppressive regimen using methotrexate or adalimumab was effective in suppressing myocardial inflammation in CS, and discontinuation of immunosuppression was associated with disease recurrence and VT events. These findings support a strategy of long‐term immunosuppression with steroid‐sparing agents for maintaining disease quiescence in CS. Prospective and randomized data will ultimately be required to determine the optimal regimen and duration of therapy.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010952 DOI: 10.1161/JAHA.118.010952.)
Contributor Information
Julie Zikherman, Email: vasanth.vedantham@ucsf.edu.
Vasanth Vedantham, Email: julie.zikherman@ucsf.edu.
References
- 1. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietila‐Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 2. Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of heart—clinicopathologic study of 35 necropsy patients and review of 78 previously described necropsy patients. Am J Med. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 3. Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, Yang J, Keren A, Beanlands RS, Birnie DH. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–374. [DOI] [PubMed] [Google Scholar]
- 4. Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S, Ogawa H, Anzai T. Effect of corticosteroid therapy on long‐term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J. 2015;79:1593–1600. [DOI] [PubMed] [Google Scholar]
- 5. Mc Ardle BA, Birnie DH, Klein R, de Kemp RA, Leung E, Renaud J, DaSilva J, Wells GA, Beanlands RS, Nery PB. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by (1)(8)F‐fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–626. [DOI] [PubMed] [Google Scholar]
- 6. Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, Ji SY, Feinstein S, Gupta S, Ilg K, Sinno M, Abu‐Hashish S, Al‐Mallah M, Sauer WH, Ellenbogen K, Morady F, Bogun F. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–1115. [DOI] [PubMed] [Google Scholar]
- 7. Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, Greenspon AJ, Ortman M, Delurgio DB, Valadri R, Narasimhan C, Swapna N, Singh JP, Danik S, Markowitz SM, Almquist AK, Krahn AD, Wolfe LG, Feinstein S, Ellenbogen KA. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–354. [DOI] [PubMed] [Google Scholar]
- 8. Kron J, Sauer W, Mueller G, Schuller J, Bogun F, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, Greenspon AJ, Ortman M, Delurgio DB, Valadri R, Narasimhan C, Swapna N, Singh JP, Danik S, Markowitz SM, Almquist AK, Krahn AD, Wolfe LG, Feinstein S, Ellenbogen KA, Crawford T. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol. 2015;43:55–64. [DOI] [PubMed] [Google Scholar]
- 9. Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F‐fluoro‐2‐deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. [DOI] [PubMed] [Google Scholar]
- 10. Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T, Kubo T, Tanaka A, Akasaka T. Comparison of cardiac MRI and 18F‐FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complet heart block. Heart Rhythm. 2015;12:2477–2485. [DOI] [PubMed] [Google Scholar]
- 11. Banba K, Kusano KF, Nakamura K, Morita H, Ogawa A, Ohtsuka F, Ogo KO, Nishii N, Watanabe A, Nagase S, Sakuragi S, Ohe T. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–1299. [DOI] [PubMed] [Google Scholar]
- 12. Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, Shimizu W, Anzai T, Kitakaze M. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–1172. [DOI] [PubMed] [Google Scholar]
- 14. Kusano KF. Effect of corticosteroid on arrhythmic events in patients with cardiac sarcoidosis. J Cardiol. 2013;62:326–327. [DOI] [PubMed] [Google Scholar]
- 15. Stees CS, Khoo MSC, Lowery CM, Sauer WH. Ventricular tachycardia storm successfully treated with immunosuppression and catheter ablation in a patient with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2011;22:210–213. [DOI] [PubMed] [Google Scholar]
- 16. Zipse MM, Sauer WH. Electrophysiologic manifestations of cardiac sarcoidosis. Curr Opin Pulm Med. 2013;19:485–492. [DOI] [PubMed] [Google Scholar]
- 17. Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, Albera C, Brutsche M, Davis G, Donohue JF, Mueller‐Quernheim J, Schlenker‐Herceg R, Flavin S, Lo KH, Oemar B, Barnathan ES, Sarcoidosis I. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 18. Nagai S, Yokomatsu T, Tanizawa K, Ikezoe K, Handa T, Ito Y, Ogino S, Izumi T. Treatment with methotrexate and low‐dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med. 2014;53:427–433. [DOI] [PubMed] [Google Scholar]
- 19. Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. [DOI] [PubMed] [Google Scholar]
- 20. Uthman I, Touma Z, Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol. 2007;26:2001–2003. [DOI] [PubMed] [Google Scholar]
- 21. Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. Health‐related quality of life of persons with sarcoidosis. Chest. 2004;125:997–1004. [DOI] [PubMed] [Google Scholar]
- 22. Blankstein RCL. Management and prognosis of cardiac sarcoidosis. UpToDate 2018.
- 23. Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapelon‐Abric C, de Zuttere D, Duhaut P, Veyssier P, Wechsler B, Huong DL, de Gennes C, Papo T, Bletry O, Godeau P, Piette JC. Cardiac sarcoidosis—a retrospective study of 41 cases. Medicine. 2004;83:315–334. [DOI] [PubMed] [Google Scholar]
- 25. Chiu C‐Z, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long‐term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 26. Futamatsu H, Suzuki J‐i, Adachi S, Okada H, Otomo K, Ohara T, Hashimoto Y, Kakuta T, Iesaka Y, Yamaguchi H, Sakurada H, Sato A, Obayashi T, Niwa A, Hirao K, Isobe M. Utility of gallium‐67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006;22:443–448. [DOI] [PubMed] [Google Scholar]
- 27. Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M; Central Japan Heart Study Group . Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. [DOI] [PubMed] [Google Scholar]
- 28. Yodogawa K, Seino Y, Shiomura R, Takahashi K, Tsuboi I, Uetake S, Hayashi H, Horie T, Iwasaki Y, Hayashi M, Miyauchi Y, Shimizu W. Recovery of atrioventricular block following steroid therapy in patients with cardiac sarcoidosis. J Cardiol. 2013;62:320–325. [DOI] [PubMed] [Google Scholar]
- 29. Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24:413–424. [DOI] [PubMed] [Google Scholar]
- 30. Chapelon‐Abric C, Sene D, Saadoun D, Cluzel P, Vignaux O, Costedoat‐Chalumeau N, Piette JC, Cacoub P. Cardiac sarcoidosis: diagnosis, therapeutic management and prognostic factors. Arch Cardiovasc Dis. 2017;110:456–465. [DOI] [PubMed] [Google Scholar]
- 31. Fussner LA, Karlstedt E, Hodge DO, Fine NM, Kalra S, Carmona EM, Utz JP, Isaac DL, Cooper LT. Management and outcomes of cardiac sarcoidosis: a 20‐year experience in two tertiary care centres. Eur J Heart Fail. 2018;20:1713–1720. [DOI] [PubMed] [Google Scholar]
- 32. Kato Y, Morimoto S, Uemura A, Hiramitsu S, Ito T, Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 33. Kudoh H, Fujiwara S, Shiotani H, Kawai H, Hirata K. Myocardial washout of 99mTc‐tetrofosmin and response to steroid therapy in patients with cardiac sarcoidosis. Ann Nucl Med. 2010;24:379–385. [DOI] [PubMed] [Google Scholar]
- 34. Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017;24:19–28. [DOI] [PubMed] [Google Scholar]
- 35. Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–1072. [DOI] [PubMed] [Google Scholar]
- 36. Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S, Ogawa H, Anzai T. Effect of discontinuation of prednisolone therapy on risk of cardiac mortality associated with worsening left ventricular dysfunction in cardiac sarcoidosis. Am J Cardiol. 2016;117:966–971. [DOI] [PubMed] [Google Scholar]
- 37. Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in (1)(8)F‐fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. [DOI] [PubMed] [Google Scholar]
- 38. Padala SK, Peaslee S, Sidhu MS, Steckman DA, Judson MA. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–570. [DOI] [PubMed] [Google Scholar]
- 39. Muser D, Santangeli P, Castro SA, Liang JJ, Enriquez A, Werner TJ, Nucifora G, Magnani S, Hayashi T, Zado ES, Garcia FC, Callans DJ, Dixit S, Desjardins B, Marchlinski FE, Alavi A. Prognostic role of serial quantitative evaluation of (18)F‐fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45:1394–1404. [DOI] [PubMed] [Google Scholar]
- 40. Ballul T, Borie R, Crestani B, Daugas E, Descamps V, Dieude P, Dossier A, Extramiana F, van Gysel D, Papo T, Sacre K. Treatment of cardiac sarcoidosis: a comparative study of steroids and steroids plus immunosuppressive drugs. Int J Cardiol. 2019;276:208–211. [DOI] [PubMed] [Google Scholar]
- 41. Hiramastu S, Tada H, Naito S, Oshima S, Taniguchi K. Steroid treatment deteriorated ventricular tachycardia in a patient with right ventricle‐dominant cardiac sarcoidosis. Int J Cardiol. 2009;132:E85–E87. [DOI] [PubMed] [Google Scholar]
- 42. Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fumio Terasaki KY. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol. 2017;3:42–45. [Google Scholar]
- 44. Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, White ES, Borges‐Neto S, Di Carli MF, Gropler RJ, Ruddy TD, Schindler TH, Blankstein R. Joint SNMMI‐ASNC expert consensus document on the role of (18)F‐FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–1758. [DOI] [PubMed] [Google Scholar]
- 45. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 46. Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax. 1999;54:742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saketkoo LA, Baughman RP. Biologic therapies in the treatment of sarcoidosis. Exp Rev Clin Immunol. 2016;12:817–825. [DOI] [PubMed] [Google Scholar]
- 48. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–1372. [DOI] [PubMed] [Google Scholar]
- 49. Rizzato G, Montemurro L, Colombo P. The late follow‐up of chronic sarcoid patients previously treated with corticosteroids. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:52–58. [PubMed] [Google Scholar]
- 50. Erckens RJ, Mostard RLM, Wijnen PAHM, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non‐infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burns AM, Green PJ, Pasternak S. Etanercept‐induced cutaneous and pulmonary sarcoid‐like granulomas resolving with adalimumab. J Cutan Pathol. 2012;39:289–293. [DOI] [PubMed] [Google Scholar]
- 52. Denys BG, Bogaerts Y, Coenegrachts KL, De Vriese AS. Steroid‐resistant sarcoidosis: is antagonism of TNF‐alpha the answer? Clin Sci (Lond). 2007;112:281–289. [DOI] [PubMed] [Google Scholar]
- 53. Jamilloux Y, Cohen‐Aubart F, Chapelon‐Abric C, Maucort‐Boulch D, Marquet A, Perard L, Bouillet L, Deroux A, Abad S, Bielefeld P, Bouvry D, Andre M, Noel N, Bienvenu B, Proux A, Vukusic S, Bodaghi B, Sarrot‐Reynauld F, Iwaz J, Amoura Z, Broussolle C, Cacoub P, Saadoun D, Valeyre D, Seve P. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: a multicenter study of 132 patients. Semin Arthritis Rheum. 2017;47:288–294. [DOI] [PubMed] [Google Scholar]
- 54. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐alpha, in patients with moderate‐to‐severe heart failure: results of the anti‐TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 55. Adler BL, Wang CJ, Bui TL, Schilperoort HM, Armstrong AW. Anti‐tumor necrosis factor agents in sarcoidosis: a systematic review of efficacy and safety. Semin Arthritis Rheum. 2019;48:1093–1104. [DOI] [PubMed] [Google Scholar]
- 56. Solomon DH, Rassen JA, Kuriya B, Chen L, Harrold LR, Graham DJ, Lewis JD, Lii J, Liu L, Griffin MR, Curtis JR. Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Ann Rheum Dis. 2013;72:1813–1818. [DOI] [PubMed] [Google Scholar]
- 57. Bravo PE, Raghu G, Rosenthal DG, Elman S, Petek BJ, Soine LA, Maki JH, Branch KR, Masri SC, Patton KK, Caldwell JH, Krieger EV. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol. 2017;241:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bremer W, Sweiss NJ, Lu Y. Serial FDG‐PET/CT imaging in the management of cardiac sarcoidosis. Clin Nucl Med. 2018;43:e50–e52. [DOI] [PubMed] [Google Scholar]
- 59. Takaya Y, Kusano KF, Nakamura K, Ito H. Outcomes in patients with high‐degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol. 2015;115:505–509. [DOI] [PubMed] [Google Scholar]
- 60. Kumar S, Barbhaiya C, Nagashima K, Choi EK, Epstein LM, John RM, Maytin M, Albert CM, Miller AL, Koplan BA, Michaud GF, Tedrow UB, Stevenson WG. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 61. Segawa M, Fukuda K, Nakano M, Kondo M, Satake H, Hirano M, Shimokawa H. Time course and factors correlating with ventricular tachyarrhythmias after introduction of steroid therapy in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9:12. [DOI] [PubMed] [Google Scholar]
- 62. Naruse Y, Sekiguchi Y, Nogami A, Okada H, Yamauchi Y, Machino T, Kuroki K, Ito Y, Yamasaki H, Igarashi M, Tada H, Nitta J, Xu D, Sato A, Aonuma K. Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2014;7:407–413. [DOI] [PubMed] [Google Scholar]
- 63. Barnabe C, McMeekin J, Howarth A, Martin L. Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol. 2008;35:1686–1687. [PubMed] [Google Scholar]
- 64. Toh H, Mori S, Keno M, Yokota S, Shinkura Y, Izawa Y, Nagamatsu Y, Shimoyama S, Fukuzawa K, Doi T, Hirata KI. Serial observation of electrocardiographic responses to corticosteroid therapy in a patient with right ventricular‐predominant cardiac sarcoidosis. J Electrocardiol. 2018;51:658–662. [DOI] [PubMed] [Google Scholar]


