Abstract
Background
Our objective was to estimate receipt of preconception health care among women with congenital heart defects (CHD), according to 2017 American Heart Association recommendations, as a baseline for evaluating recommendation implementation.
Methods and Results
Using 2007 to 2013 IBM MarketScan Commercial Databases, we identified women with CHD diagnosis codes ages 15 to 44 years who became pregnant and were enrolled in health insurance for ≥11 months in the year before estimated conception. We assessed documentation of complete blood count, electrolytes, thyroid‐stimulating hormone, liver function, ECG, comprehensive echocardiogram, and exercise stress test, using procedural codes, and outpatient prescription claims for US Food and Drug Administration category D and X cardiac‐related medications. Differences were examined according to CHD severity, age, region of residence, year of conception, and documented encounters at obstetric and cardiology practices. We found 2524 pregnancies among 2003 women with CHD (14.4% severe CHD). In the 98.3% of women with a healthcare encounter in the year before conception, <1% received all and 22.6% received no American Heart Association–recommended tests or assessments (range: 54.4% for complete blood count to 3.1% for exercise stress test). Women with the highest prevalence of receipt of recommended care were 35 to 44 years old, pregnant in 2012 to 2013, or had a documented obstetric or cardiology encounter in the year before conception (P<0.05 for all). In 9.0% of pregnancies, ≥1 prescriptions for US Food and Drug Administration category D or X cardiac‐related medications were filled in the year before conception.
Conclusions
A low percentage of women with CHD received American Heart Association–recommended preconception health care in the year before conception.
Keywords: congenital cardiac defect, preconception, pregnancy
Subject Categories: Epidemiology, Pregnancy, Women, Health Services, Congenital Heart Disease
Clinical Perspective
What Is New?
During 2007 to 2013, <1% of women with congenital heart defects who became pregnant received all American Heart Association–recommended preconception healthcare tests and assessments in the year before conception.
In 9.0% of pregnancies among women with congenital heart defects, 1 or more prescriptions for US Food and Drug Administration category D or X cardiac‐related medications were filled in the year before conception.
What Are the Clinical Implications?
There may be missed opportunities to provide preconception health care to women with congenital heart defects since, even among women who had documented healthcare encounters at obstetric and cardiology practices, >1 in 5 had not received any American Heart Association–recommended tests and assessments and <1% had documentation of all of them.
Introduction
Pregnant women with congenital heart defects (CHD) are at increased risk of cardiovascular complications, including arrhythmias and heart failure,1 as well as adverse pregnancy and infant outcomes.2, 3 Because of these increased risks during pregnancy for women with CHD, in 2017 the American Heart Association (AHA) recommended that clinicians counsel all women with CHD about pregnancy soon after sexual maturity.4 The AHA also recommended that women with complex CHD get treatment for repairable lesions or clinical problems and receive specific tests and assessments to determine maternal risk before becoming pregnant. According to the AHA recommendations, clinicians should assess a woman's arterial oxygen saturation, complete blood count (CBC), electrolytes, and thyroid and liver function, and discuss any current medications to determine the risks and benefits before conception. Additionally, clinicians should conduct an ECG, comprehensive echocardiogram, cardiopulmonary or exercise stress test for the woman, and provide genetic counseling, depending on the woman's risk of recurrence in offspring. While certain recommendations are for women with complex CHD, the authors note that designating women as having simple or complex CHD is inadequate when considering pregnancy, since some women with simple CHDs may have comorbid conditions, requiring a higher level of prenatal care.
Little is known about whether women with CHD receive the AHA‐recommended preconception tests and assessments. To our knowledge, only 1 hospital‐based study from London has examined receipt of preconception counseling among women with CHD, reporting that in 62% of pregnancies, women with CHD received preconception counseling.5 However, in that study the authors examined 102 pregnancies among women with CHD attending an adult CHD clinic with an established joint heart disease in pregnancy service and did not examine specific tests and assessments received. Therefore, the results are likely not generalizable to the broader US population of women with CHD.
To assess implementation over time of AHA recommendations, baseline information from the United States is needed. Therefore, we used 2007 to 2013 healthcare claims data on privately insured women with CHD to assess the percentage who had 1 or more healthcare encounters, had documentation of receipt of AHA‐recommended preconception tests and assessments, and took cardiac‐related medications that were potentially teratogenic, causing birth defects, or fetotoxic, causing harm to a developing fetus later in pregnancy, in the year before conception.
Methods
This analysis used the IBM MarketScan Commercial Databases, which include inpatient and outpatient medical claims data, as well as outpatient pharmacy records, from a convenience sample of employer‐sponsored, privately insured individuals and their dependents. The data that support the findings of this study are available directly from IBM MarketScan at https://www.ibm.com/us-en/marketplace/marketscan-research-databases. Information is included on all people enrolled in the included insurance plans, even if no healthcare claims are filed for the individual. Between 2008 and 2013, IBM MarketScan had information on ≈50 million people per year enrolled in ≈100 different insurance companies and residing in all US census regions. We included in the analytic sample pregnant women ages 15 to 44 years whose estimated last menstrual period was between January 1, 2008 and December 31, 2013, and who were enrolled in a private health insurance plan including prescription drug coverage for at least 11 months in the year before last menstrual period (hereafter, referred to as “continuously enrolled”). Timing of pregnancy was based on a previously published algorithm using end of pregnancy‐related procedures or diagnostic codes.6 In that algorithm, gestational age at the end of the pregnancy was estimated based on these codes and used to calculate an estimated date of last menstrual period (hereafter referred to as “date of conception”). More than 1 pregnancy from the same woman could be included.
We considered a woman to have CHD if between January 1, 2007 and December 31, 2014 she had ≥1 inpatient CHD International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code(s) or ≥2 outpatient codes, separated by >30 days. For all pregnancies, we required the CHD codes to be documented before the woman's estimated 15th week of gestation or after 90 days postpartum, to discern women with CHD from their infants screened for or diagnosed with CHD during pregnancy and early postpartum and recorded on the mother's health record. CHD codes used and their categorization as severe and nonsevere were based on a previously published algorithm incorporating information on hemodynamic severity and basic anatomy, with severe CHD defined as a CHD generally requiring surgery or catheterization in the first year of life.7
Since women's date of conception occurred during 2008 to 2013, we used data from 2007 to 2013 to assess outcomes related to preconception health care received 1 to 366 days before conception. In the year before conception, we assessed the percentage of pregnancies in which the woman had a healthcare encounter with any provider, received AHA‐recommended preconception tests and assessments, and filled a prescription for potentially teratogenic cardiac‐related medications from an outpatient pharmacy.
We defined components of preconception health care according to the AHA recommendations and assessed Current Procedural Terminology codes for the following blood tests and assessments: CBC, electrolytes, thyroid‐stimulating hormone, liver function, ECG, comprehensive echocardiogram, and exercise stress test.4 For CBC, electrolytes, and liver function, we examined both Current Procedural Terminology codes for panel tests and Current Procedural Terminology codes for all individual tests comprising the panel (Table S1). We considered thyroid‐stimulating hormone sufficient to assess thyroid function. We were unable to examine whether a woman received a test for arterial oxygen saturation because of the lack of a relevant Current Procedural Terminology code. Additionally, since genetic counseling is recommended only for women with risk of recurrence in offspring, and may only be documented if provided by a genetic counselor, we did not examine its receipt.
Among women with any healthcare encounter in the year before conception, we assessed the percentage documented in claims as receiving each AHA‐recommended preconception test and assessment. We examine prevalence estimates by CHD severity (severe and nonsevere), age at conception (15–24, 25–34, and 35–44 years), US Census region of residence at month of conception (Northeast, Midwest, South, and West), year of conception (2008–2009, 2010–2011, and 2012–2013), and occurrence of a patient encounter at an obstetric billing practice or cardiology billing practice in the year before conception.
Among all pregnancies in the analytic sample, we also examined the percentage in which women filled a prescription for a potentially teratogenic cardiac‐related medication from an outpatient pharmacy in the year before conception, defined as medications with a US Food and Drug Administration pregnancy category of D or X (Table S2; https://www.pdr.net/). However, we did not examine aspirin, a US Food and Drug Administration category D medication, because it is commonly obtained over the counter and is recommended for use during pregnancy in women at high risk of preeclampsia.8
The unit of analysis for all outcomes was the pregnancy. We compared estimates across subgroups using χ2 tests generated from logistic regression models using generalized estimating equations to account for multiple pregnancies per woman. For ease of interpretation in some cases, we refer to the percent of women, rather than pregnancies, receiving AHA‐recommended preconception health care. In a sensitivity analysis to examine whether preconception health care occurred more frequently in earlier pregnancies, we conducted all analyses limited to the first pregnancy occurring in the analytic sample. We also conducted all analyses excluding women identified as having CHD based solely on ICD‐9 code 745.5, which can be used to denote presence of a secundum atrial septal defect, a type of CHD, but also for patent foramen ovale, a normal variant of the heart.9 All data from the IBM MarketScan Commercial Databases are de‐identified and Institutional Review Board review is not needed.
Results
We found 187 769 women with CHD diagnosis codes that met our inclusion criteria (Figure 1). After applying inclusion and exclusion criteria, the final sample included 2003 women with 2524 pregnancies (mean number of pregnancies: 1.2). Of included pregnancies, 14.4% were among women with severe CHD and 85.6% among women with nonsevere CHD (Table 1). The majority of pregnancies among women with severe CHD (59.3%) and nonsevere CHD (57.5%) were among 25 to 34‐year‐olds. Overall among women with severe and nonsevere CHD, >30% of pregnancies were among women residing in the South and 39% of pregnancies began in 2010 to 2011. In 98.3% of pregnancies, women had 1 or more healthcare encounters in the year before conception.
Figure 1.
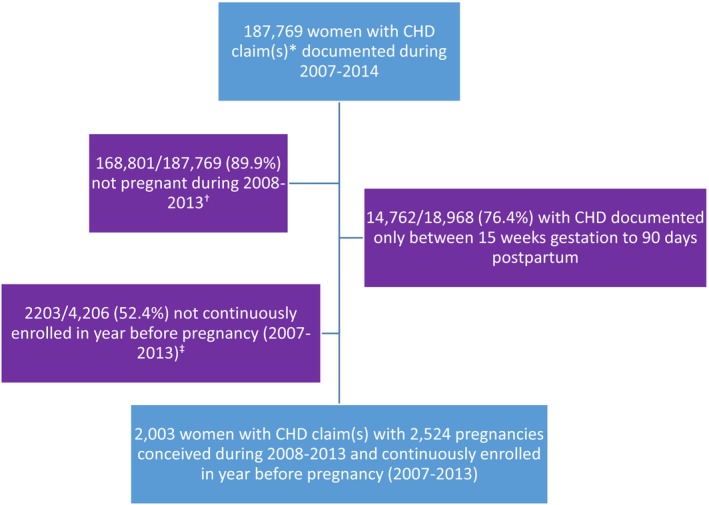
Exclusion criteria and final sample size of women with healthcare claim(s) for congenital heart defect (CHD)* who became pregnant†, IBM MarketScan Commercial Databases. *1 inpatient and/or ≥2 outpatient claims ≥30 days apart; †Pregnancy based on documentation of end of pregnancy procedure and diagnostic codes and estimated last menstrual period occurring 2008 to 2013; ‡Enrolled for ≥11 months on plan with prescription drug coverage.
Table 1.
Characteristics of Pregnancies Among Women With CHDa, by Severity, in the Year Before Conception, IBM MarketScan Commercial Databases, 2007–2013
| Total | Severe CHD | Nonsevere CHD | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total | 2524 (100%) | 364 (14.4) | 2160 (85.6) |
| Age at conception, y | |||
| 15–24 | 472 (18.7) | 97 (26.6) | 375 (17.4) |
| 25–34 | 1459 (57.8) | 216 (59.3) | 1243 (57.5) |
| 35–44 | 593 (23.5) | 51 (14.0) | 542 (25.1) |
| Region of residenceb | |||
| Northeast | 403 (17.0) | 73 (20.1) | 357 (16.5) |
| Midwest | 630 (25.0) | 81 (22.3) | 549 (25.4) |
| South | 796 (31.5) | 119 (32.7) | 677 (31.3) |
| West | 649 (25.7) | 86 (23.6) | 563 (26.1) |
| Year of conception | |||
| 2008–2009 | 650 (25.8) | 81 (22.3) | 569 (26.3) |
| 2010–2011 | 976 (38.7) | 147 (40.4) | 829 (38.4) |
| 2012–2013 | 898 (35.6) | 136 (37.4) | 762 (35.3) |
| Any healthcare encounter in the year before conception | |||
| Yes | 2480 (98.3) | 360 (98.9) | 2120 (98.1) |
| No | 44 (1.7) | 4 (1.1) | 40 (1.9) |
CHD indicates congenital heart defects.
One inpatient and/or ≥2 outpatient claims ≥30 days apart.
Nineteen pregnancies missing information on woman's region of residence.
Among the 2480 pregnancies in women with 1 or more healthcare encounters in the year before conception, documented receipt of any individual AHA‐recommended test or assessment in the year before conception ranged from 54.4% for CBC to 3.1% for exercise stress test (Figure 2). While prevalence of tests and assessments varied, in general, prevalence estimates for blood tests (CBC, electrolytes, thyroid‐stimulating hormone, and liver function) were highest among women 35 to 44 years of age, those residing in the Northeast and South, and those with documented obstetric or cardiology encounters (Table 2). ECGs and echocardiograms were highest among women with severe CHD, those <35 years of age, women residing in areas other than the West, and those with documented obstetric or cardiology encounters. In <5% of pregnancies, women received an exercise stress test (overall: 3.1%; range: 1.4–5.0%, depending on subgroup) in the year before conception. Over a fifth (22.6%) of women had not received any of the tests or assessments and only 0.7% of women received all AHA‐recommended preconception tests and assessments (excluding exercise stress test, this figure rose to 7.5%).
Figure 2.
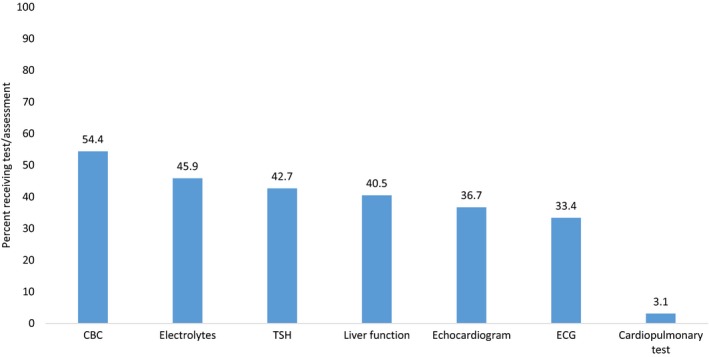
Receipt of American Heart Association–recommended tests and assessments in the year before conception among women with congenital heart defects (CHD) and 1 or more healthcare encounters in the year before conception, IBM MarketScan Commercial Databases, 2007–2013. CBC indicates complete blood count; TSH, thyroid‐stimulating hormone.
Table 2.
Receipt of AHA‐Recommended Tests and Assessments in the Year Before Conception Among Women With CHD and ≥1 Healthcare Encounter in the Year Before Conception, by CHD Severity and Demographic Characteristics at Conception, IBM MarketScan Commercial Databases, 2007–2013
| Total | CBC | Electrolytes | Thyroid Stimulating Hormone | Liver Function | Comprehensive Echocardiogram | ECG | Cardiopulmonary Test | |
|---|---|---|---|---|---|---|---|---|
| N | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| CHD severity | * | * | * | * | * | |||
| Nonsevere | 2120 | 1165 (55.0) | 995 (46.9) | 929 (43.8) | 878 (41.4) | 736 (34.7) | 681 (32.1) | 59 (2.8) |
| Severe | 360 | 185 (51.4) | 142 (39.4) | 130 (36.1) | 127 (35.3) | 174 (48.3) | 146 (40.6) | 18 (5.0) |
| Age at conception, y | * | * | * | * | * | |||
| 15–24 | 465 | 238 (51.2) | 191 (41.1) | 124 (26.7) | 172 (37.0) | 195 (41.9) | 169 (36.3) | 12 (2.6) |
| 25–34 | 1431 | 750 (52.4) | 633 (44.2) | 605 (42.3) | 543 (37.9) | 532 (37.2) | 471 (32.9) | 43 (3.0) |
| 35–44 | 584 | 362 (62.0) | 313 (53.6) | 330 (56.3) | 290 (49.7) | 183 (31.3) | 187 (32.0) | 22 (3.8) |
| Region of residence† | * | * | * | * | * | |||
| Northeast | 421 | 254 (60.3) | 214 (50.8) | 192 (45.6) | 194 (46.1) | 154 (36.6) | 170 (40.4) | 17 (4.0) |
| Midwest | 623 | 298 (47.8) | 249 (40.0) | 250 (40.1) | 204 (32.7) | 260 (41.7) | 207 (33.2) | 20 (3.2) |
| South | 788 | 473 (60.0) | 417 (52.9) | 344 (43.7) | 371 (47.1) | 322 (40.9) | 311 (39.5) | 28 (3.6) |
| West | 629 | 314 (49.9) | 247 (39.3) | 266 (42.3) | 225 (35.8) | 168 (26.7) | 133 (21.1) | 11 (1.7) |
| Year of conception | * | |||||||
| 2008–2009 | 636 | 345 (54.2) | 283 (44.5) | 263 (41.4) | 244 (38.4) | 218 (34.3) | 200 (31.4) | 26 (4.1) |
| 2010–2011 | 958 | 513 (53.3) | 433 (45.2) | 418 (43.6) | 389 (40.6) | 344 (35.9) | 311 (32.5) | 28 (2.9) |
| 2012–2013 | 886 | 492 (55.5) | 421 (47.5) | 378 (42.7) | 372 (42.0) | 348 (39.3) | 316 (35.7) | 23 (2.6) |
| Documented obstetric encounter | * | * | * | * | * | |||
| Yes | 972 | 625 (64.3) | 500 (51.4) | 521 (53.6) | 444 (45.7) | 362 (37.2) | 347 (35.7) | 31 (3.2) |
| No | 1508 | 725 (48.1) | 637 (42.4) | 538 (35.7) | 561 (37.2) | 548 (36.3) | 480 (31.8) | 46 (3.1) |
| Documented cardiology encounter | * | * | * | * | * | |||
| Yes | 696 | 409 (58.8) | 356 (51.1) | 304 (43.7) | 291 (41.8) | 486 (69.8) | 430 (61.8) | 52 (7.5) |
| No | 1784 | 941 (52.7) | 781 (43.8) | 755 (42.3) | 714 (40.0) | 424 (23.8) | 397 (22.3) | 25 (1.4) |
AHA indicates American Heart Association; CBC, complete blood count; CHD, congenital heart defects.
* Chi square P<0.05 from generalized estimating equation approach to logistic regression, accounting for multiple pregnancies per woman; assesses distribution of test/assessment by levels of maternal characteristic (eg, receipt of CBC differentially distributed by maternal age at conception).
†Nineteen pregnancies among women with no information on region of residence.
Nine percent of women in our sample filled a prescription for a potentially teratogenic cardiac‐related medication from an outpatient pharmacy in the year before conception (Table 3). Women with documented cardiology healthcare encounters (16.4%), severe CHD (14.0%), who conceived in 2008–2009 (10.3%), and resided in the Midwest (10.3%) or South (10.2%) had the highest point prevalence for filling a prescription for a potentially teratogenic cardiac‐related medication in the year before pregnancy. The most commonly filled medications from outpatient pharmacies during the year before pregnancy were lisinopril (5.0%), among women with severe CHD, and warfarin (5.0%), in women with nonsevere CHD. Of the 228 pregnancies in women who filled a prescription for a potentially teratogenic cardiac‐related medication in the year before conception, 35.5% had a documented encounter at an obstetric practice and 50% had a documented encounter at a cardiology practice in the year before conception. For all analyses, no results changed substantially when limited to first pregnancies in the analytic sample (n=491 pregnancies excluded) nor when excluding 408 pregnancies in women with only an ICD‐9 CHD diagnosis code of 745.5.
Table 3.
Percent of Pregnancies Among Women With CHD With Filled Prescriptions for a Potentially Teratogenic Cardiac‐Related Medicationa in the Year Before Conception, IBM MarketScan Commercial Databases, 2007–2013
| ≥1 Filled Prescriptions for Potentially Teratogenic or Fetotoxic Cardiac‐Related Medicationa | |
|---|---|
| N (%) | |
| Total | 228 (9.0) |
| CHD severityb | |
| Nonsevere | 177 (8.2) |
| Severe | 51 (14.0) |
| Age, y | |
| 15–24 | 45 (9.5) |
| 25–34 | 126 (8.6) |
| 35–44 | 57 (9.6) |
| Region of residenceb , c | |
| Northeast | 37 (8.6) |
| Midwest | 65 (10.3) |
| South | 81 (10.2) |
| West | 44 (6.8) |
| Year of conception | |
| 2008–2009 | 67 (10.3) |
| 2010–2011 | 80 (8.2) |
| 2012–2013 | 81 (9.0) |
| Any inpatient or outpatient healthcare encounterb | |
| Yes | 227 (9.2) |
| No | 1 (2.3) |
| Documented obstetric encounterb | |
| Yes | 81 (8.3) |
| No | 147 (9.5) |
| Documented cardiology encounterb | |
| Yes | 114 (16.4) |
| No | 114 (6.2) |
CHD indicates congenital heart defects.
US Food and Drug Administration category D or X medications filled at outpatient pharmacies.
Chi square P<0.05 from generalized estimating equation approach to logistic regression, accounting for multiple pregnancies per woman.
n=19 pregnancies among women with no information on region of residence.
Discussion
We used healthcare claims data from 2007 to 2013 to assess receipt of AHA‐recommended preconception health care in the year before conception among women with CHD. Of the 2033 women in this sample, <1% had documentation of receipt of all AHA‐recommended blood tests and assessments. Additionally, 9% of women filled a prescription for a potentially teratogenic cardiac‐related medication in the year before conception. Although patterns varied, women 35 to 44 years of age at conception, pregnant in 2012 to 2013, and those with a documented obstetric or cardiology encounter in the year before conception had the highest prevalence of receipt of AHA‐recommended tests and assessments. We do not have information on reasons women did not receive AHA‐recommended tests and assessments, which may vary based on the woman's pregnancy intention, clinician judgment, and the woman's preference for receipt of a test or assessment.
To our knowledge, no other studies have examined healthcare encounters, receipt of specific preconception tests and assessments, and use of potentially teratogenic cardiac‐related medications among women with CHD in the year before conception. One hospital‐based study from London examined receipt of preconception counseling in 2015 and 2016 among women with CHD.5 In that study, researchers evaluated attendance at a specific adult CHD clinic with an established joint heart disease in pregnancy service. Of 102 pregnancies among women with modified World Health Organization classification of maternal cardiovascular risk of III or IV, 62% of women received individualized preconception counseling provided by a cardiologist and obstetrician when considering pregnancy. The counseling included risks to the woman and fetus, genetic syndromes, in vitro fertilization, potentially teratogenic drugs, level of prenatal care needed, and modifiable risk factors in general (eg, smoking cessation). Aside from counseling and review of medications, the authors did not mention specific tests or assessments the women received. These results are likely not generalizable to the US population of women with CHD.
Other studies have examined, more generally, the percent of women with CHD reporting ever having a discussion with their providers about pregnancy, but these studies have been largely limited to small numbers of women receiving care in pediatric or adult CHD clinics.10, 11 In 1 study of 83 women with CHD receiving care at a single tertiary care center in Nebraska between 2005 and 2010, 65% reported having a discussion with a provider (often a pediatric cardiologist or an obstetrician) about the risks of pregnancy to the woman's health.10 In another study of women with CHD, published in 2008, one third said they had never been informed by a clinician about an increased risk of pregnancy complications and did not know their child may be at increased risk of CHD.11
Receipt of preconception counseling among women in general may help women adopt behaviors that improve pregnancy outcomes, such as using folic acid; however, only one third of US women reported receiving preconception counseling.12 Although our analysis could not examine preconception counseling, we found that, almost all women had 1 or more healthcare encounters in the year before pregnancy. These may be opportunities for other clinicians, including primary care providers, to encourage women with CHD to receive cardiac and obstetric–gynecologic care.
Almost 1 in 10 women with CHD filled a prescription for a US Food and Drug Administration pregnancy categories D and X cardiac‐related medication from an outpatient pharmacy in the year before conception, most commonly lisinopril (category D) and warfarin (category X). Though these medications may be indicated for some women, such as those with chronic heart failure or valvular heart disease,4 these statistics highlight the importance of preconception counseling and patient–provider discussions on risks and benefits of specific medications before a woman with CHD becomes pregnant. In this sample, it is unknown what percentage of women were using effective contraception concurrently with these medications, whether the pregnancy was planned, and whether women changed medications or doses before becoming pregnant. However, in a previous study, among sexually active women with CHD receiving care at an adult CHD clinic, ≈25% reported using no contraceptive method and 16% reported using less effective methods (ie, failure rates of 18–28% per year).13 Reports show that 25% to 45% of pregnancies in women with CHD may be unintended.13, 14 It is important to understand the unmet need for contraception among women with CHD who do not desire pregnancy, as well as provide risk‐based counseling for women taking potentially teratogenic cardiac‐related medications.
This is the first study to examine baseline data on preconception healthcare practices for women with CHD before publication of the 2017 AHA statement. We were able to examine outcomes among a large convenience sample of women with CHD diagnosis claims from across the nation. However, this sample comprises privately insured women, enrolled for ≥11 months in the year before conception, which limits generalizability to all US women with CHD. We could not distinguish whether women had “complex” CHD; however, the AHA recommendations note that “a designation of simple versus complex is not adequate when referring to patients with CHD considering pregnancy.” We did examine prevalence by CHD severity, based on ICD‐9 codes. Additionally, all data on CHD, pregnancy, and tests and assessments were from healthcare claims. Based on 1 report, grouping inpatient and outpatient claims together, the positive predictive value of a single claim with a documented CHD may be ≈50%, although it is higher (77%) for moderate and severe forms of CHD.15 For this analysis, we implemented an algorithm to increase the positive predictive value of the sample; however, doing so would have excluded women with CHD from the sample whose CHD may not have been documented in claims data, was documented only once in an outpatient setting, or was documented only during pregnancy. Pregnancy status and gestational age at end of pregnancy were based on pregnancy‐related diagnostic and procedure codes, and were used to calculate an estimated date of last menstrual period. Pregnancies that ended early, without a healthcare encounter, would have been missed. Additionally, it is unknown how well information on the billing practice (eg, obstetric practice) estimates visits with that type of provider (eg, obstetrician) and how well this information is documented in claims data. It is also unknown whether a woman used the prescribed medication filled or the dose prescribed.
One study comparing administrative data to administrative and medical record data from 283 commercial health plans found that the percentage of individuals with documented receipt of laboratory tests (cervical cancer screening, hemoglobin A1c testing, and cholesterol screening) were underestimated by 8% to 14% on average in 2006.16 If these findings are generalizable to our sample and the laboratory tests assessed in this analysis, our findings on the prevalence of receipt of laboratory tests are likely underestimated by 1 to 7 percentage points. Given that our prevalence estimates were <55% for each individual laboratory test and <1% of women had documentation of receipt of every test and assessment, this level of misclassification does not largely change the conclusion that a substantial percentage of women with CHD did not receive AHA‐recommended tests and assessments in the year before conception.
In summary, our findings show that low percentages of women with CHD received all evaluated AHA‐recommended preconception healthcare tests and assessments before 2014; however, percentages tended to increase over time. There may be missed opportunities to provide preconception health care to women with CHD since, even among women who had documented healthcare encounters at obstetric and cardiology practices, >1 in 5 had not received any AHA‐recommended tests and assessments and <1% had documentation of all of them. According to those recommendations, clinicians caring for women with complex CHD should discuss the pregnancy‐related risks and benefits of any new and current medications and encourage patients to seek care by both an obstetrician and cardiologist before a pregnancy. With these baseline data, which estimate preconception healthcare practices for women with CHD prior to the 2017 publication of the AHA statement, we can track implementation of recommendations over time.
Disclosures
None.
Supporting information
Table S1. CPT Codes for American Heart Association‐Recommended Preconception Tests and Assessments
Table S2. Potentially Teratogenic or Fetotoxic Cardiac‐Related Medications (US Food and Drug Administration pregnancy category D or X)
Acknowledgments
The authors acknowledge April Summers, MPH, for replicating the tables and figures and confirming all results. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. *IBM MarketScan are trademarks of IBM Corporation in the United States, other countries, or both.
(J Am Heart Assoc. 2019;8:e013608 DOI: 10.1161/JAHA.119.013608.)
References
- 1. Balci A, Sollie‐Szarynska KM, van der Bijl AG, Ruys TP, Mulder BJ, Roos‐Hesselink JW, van Dijk AP, Wajon EM, Vliegen HW, Drenthen W, Hillege HL, Aarnoudse JG, van Veldhuisen DJ, Pieper PG; ZAHARA‐II investigators . Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart. 2014;100:1373–1381. [DOI] [PubMed] [Google Scholar]
- 2. Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, Wald RM, Colman JM, Siu SC. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71:2419–2430. [DOI] [PubMed] [Google Scholar]
- 3. Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research . Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e50–e87. [DOI] [PubMed] [Google Scholar]
- 5. Cauldwell M, Gatzoulis M, Steer P. Congenital heart disease and pregnancy: a contemporary approach to counselling, pre‐pregnancy investigations and the impact of pregnancy on heart function. Obstet Med. 2017;10:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ailes EC, Gilboa SM, Gill SK, Broussard CS, Crider KS, Berry RJ, Carter TC, Hobbs CA, Interrante JD, Reefhuis J; The National Birth Defects Prevention S . Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, national birth defects prevention study 1997 to 2011. Birth Defects Res A Clin Mol Teratol. 2016;106:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glidewell J, Book W, Raskind‐Hood C, Hogue C, Dunn JE, Gurvitz M, Ozonoff A, McGarry C, Van Zutphen A, Lui G, Downing K, Riehle‐Colarusso T. Population‐based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res. 2018;110:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeFevre ML; Force USPST . Low‐dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;161:819–826. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez FH III, Ephrem G, Gerardin JF, Raskind‐Hood C, Hogue C, Book W. The 745.5 issue in code‐based, adult congenital heart disease population studies: relevance to current and future ICD‐9‐CM and ICD‐10‐CM studies. Congenit Heart Dis. 2018;13:59–64. [DOI] [PubMed] [Google Scholar]
- 10. Hinze A, Kutty S, Sayles H, Sandene EK, Meza J, Kugler JD. Reproductive and contraceptive counseling received by adult women with congenital heart disease: a risk‐based analysis. Congenit Heart Dis. 2013;8:20–31. [DOI] [PubMed] [Google Scholar]
- 11. Kovacs AH, Harrison JL, Colman JM, Sermer M, Siu SC, Silversides CK. Pregnancy and contraception in congenital heart disease: what women are not told. J Am Coll Cardiol. 2008;52:577–578. [DOI] [PubMed] [Google Scholar]
- 12. Williams L, Zapata LB, D'Angelo DV, Harrison L, Morrow B. Associations between preconception counseling and maternal behaviors before and during pregnancy. Matern Child Health J. 2012;16:1854–1861. [DOI] [PubMed] [Google Scholar]
- 13. Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126:363–369. [DOI] [PubMed] [Google Scholar]
- 14. Miner PD, Canobbio MM, Pearson DD, Schlater M, Balon Y, Junge KJ, Bhatt A, Barber D, Nickolaus MJ, Kovacs AH, Moons P, Shaw K, Fernandes SM. Contraceptive practices of women with complex congenital heart disease. Am J Cardiol. 2017;119:911–915. [DOI] [PubMed] [Google Scholar]
- 15. Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS. Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc. 2018;7:e007378 DOI: 10.1161/JAHA.117.007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawlson LG, Scholle SH, Powers A. Comparison of administrative‐only versus administrative plus chart review data for reporting HEDIS hybrid measures. Am J Manag Care. 2007;13:553–558. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CPT Codes for American Heart Association‐Recommended Preconception Tests and Assessments
Table S2. Potentially Teratogenic or Fetotoxic Cardiac‐Related Medications (US Food and Drug Administration pregnancy category D or X)


