Abstract
Background
Current guidelines recommend the new‐generation P2Y12‐inhibitor ticagrelor for patients with acute ST‐segment–elevation myocardial infarctions (STEMIs). The aim of the present study was to assess efficacy and safety of ticagrelor for elderly patients with STEMI (≥75 years) in an all‐comers STEMI registry.
Methods and Results
Patients with STEMI, aged ≥75 years, treated with primary percutaneous coronary intervention and documented in the Bremen STEMI Registry between 2006 and 2017 entered analysis. The primary efficacy outcome, major adverse cardiac and cerebrovascular events, was defined as a composite of death, myocardial reinfarction, and stroke. The safety outcome was defined as any significant bleeding event within 1 year. To estimate benefit/risk ratio, net adverse clinical events (major adverse cardiac and cerebrovascular events+bleedings) were calculated. Outcomes were estimated in propensity score–matched cohorts to adjust for possible confounders. Of a total of 7466 patients with STEMI, 1087, aged ≥75 years, were selected, of which 552 (51%) received clopidogrel and 535 (49%) received ticagrelor, with similar age (80.9±4.6 versus 80.9±4.6 years) and sex (51% versus 50% female) distributions between treatment arms. The primary efficacy outcome occurred in 32.4% of patients treated with clopidogrel versus 25.5% treated with ticagrelor (P=0.015), with the 1‐year mortality rate at 26.8% versus 21.1% (P=0.035). Because there was no difference in the safety outcome (clopidogrel versus ticagrelor, 4.9% versus 5.1%; not significant), net adverse clinical events were higher for clopidogrel than for ticagrelor: 37.3% versus 30.6% (P=0.028). In a propensity score–matched model, the advantage for ticagrelor on major adverse cardiac and cerebrovascular events remained significant (hazard ratio, 0.69; 95% CI, 0.49‐0.97; P=0.03), whereas 1‐year‐mortality (hazard ratio, 0.89; 95% CI, 0.67–1.27; P=0.5) and 1‐year bleeding events (hazard ratio, 1.1; 95% CI, 0.4–2.3; P=0.8) did not differ.
Conclusions
These results from propensity score–matched registry data show that for elderly patients with STEMI, ticagrelor compared with clopidogrel was associated with a reduction in major adverse cardiac and cerebrovascular events without a significant increase in bleeding events within 1 year.
Keywords: morbidity/mortality, myocardial infarction, P2Y12 receptor, ST‐segment–elevation myocardial infarction
Subject Categories: Percutaneous Coronary Intervention, Mortality/Survival, Quality and Outcomes, Myocardial Infarction
Short abstract
See Editorial Capranzano and Angiolillo
Clinical Perspective
What Is New?
Since 2011 the majority of elderly patients (≥75 years) admitted with ST‐elevation myocardial infarction (STEMI) undergoing emergency percutaneous coronary intervention (PCI) were treated with the more potent P2Y12‐inhibitor ticagrelor.
The use of ticagrelor was associated with a 21% reduction in major adverse cardiac or cerebrovascular events (MACCE) compared to clopidogrel after STEMI in the elderly.
The signficant advantage of ticagrelor remained after adjusting in a propensity score (PPS) matched model.
Ticagrelor was not associated with a significant elevation in bleeding events for the elderly
What Are the Clinical Implications?
Ticagrelor should be preferred to clopidogrel in elderly STEMI‐patients undergoing emergency PCI when no contraindications are present.
Introduction
The key role of dual‐antiplatelet therapy (DAPT) with clopidogrel in addition to aspirin to prevent death or adverse cardiac or cerebrovascular events in patients admitted with acute coronary syndrome (ACS) was first shown in 2001.1 However, the new‐generation P2Y12 inhibitors ticagrelor2 and prasugrel3 have both been shown to be superior to clopidogrel in preventing cardiovascular deaths and adverse cardiovascular events. Current guidelines of the European Society of Cardiology for patients presenting with ACS with4 and without5 ST‐segment elevation, therefore, recommend a DAPT with ticagrelor or prasugrel in addition to aspirin if no contraindications are present. Current guidelines from the American Heart Association/American College of Cardiology likewise recommend ticagrelor or prasugrel over clopidogrel in patients with ACS/ST‐segment–elevation myocardial infarction (STEMI), with the exception that prasugrel is contraindicated for patients with prior stroke or transient ischemic attack.6 Both guidelines recommend the more potent P2Y12 inhibitors prasugrel and ticagrelor with a class of recommendation I, level of evidence A. All guidelines do not recommend prasugrel in patients aged ≥75 years because no net clinical benefit could be shown for this age group, while bleeding rates were generally higher.3 In contrast, a substudy of the PLATO (Platelet Inhibition and Patient Outcomes) trial revealed that the clinical benefit for ticagrelor did not depend on age,7 which led to its recommendation for patients with ACS regardless of age. However, elderly patients were underrepresented in the TRITON TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38) and PLATO trials, composing only 13% and 15% of the study populations, respectively.2, 3 This is especially striking because elderly patients contribute the majority of deaths and adverse events in patients with ACS.8 Furthermore, patient cohorts in randomized trials are often highly selective and may not reflect clinical reality.
The present study, therefore, analyzed data from an all‐comers clinical registry to investigate the impact of ticagrelor on ischemic events, death, and bleeding rates in elderly patients (aged ≥75 years) admitted with STEMI and treated with primary percutaneous coronary intervention (PCI) in a “real‐world” setting.
Methods
The data that support the study findings are not available because of the sensitive nature of the collected clinical data. The analytic methods are available from the corresponding author on reasonable request.
The Bremen STEMI Registry
All patients with STEMI from the metropolitan area of Bremen in northwest Germany (≈1 000 000 inhabitants) are admitted to the Bremen Heart Center and since 2006 documented in the BSR (Bremen STEMI Registry). Emergency services and regional hospitals are closely connected by telephone and fax with the Bremen Heart Center for rapid communication to enable urgent coronary catheterization. Data documentation for the BSR is done via data sheets completed by the responsible interventional cardiologist and/or through patient records after a cardiologist has confirmed the exact diagnosis. Data about age, sex, concomitant diseases, severity of STEMI, acute medical or interventional treatment, and laboratory parameters at admission and during the hospital stay are recorded. After hospital discharge, major adverse cardiac and cerebral events as well as bleeding events are documented. This is done via follow‐up examination performed after 1, 5, and 10 years by a telephone interview. All patients provided written consent on study participation. The study was approved by the ethical committee of the Ärztekammer Bremen, Germany. Studies about methods and results from the BSR have been previously published elsewhere.9, 10, 11, 12 To analyze the effect of ticagrelor on ischemic events and bleedings, an intention‐to‐treat analysis was preferred: although some patients were switched from ticagrelor to clopidogrel during the initial hospital stay, they remained in the ticagrelor group.
Definition of STEMI
STEMI was defined as persistent angina pectoris for ≥20 minutes in conjunction with an ST‐segment elevation in 2 contiguous leads of ≥0.25 mV in men aged <40 years, ≥0.2 mV in men aged >40 years, or ≥0.15 mV in women in leads V2 to V3 and/or ≥0.1 mV in all other leads or new left bundle branch block.4
Subacute STEMIs were defined as STEMIs with >12 hours between first symptoms and first medical contact and/or signs of a subacute myocardial infarction (MI) in the ECG at admission.
Surrogate Parameters of Severity of STEMI
Peak creatine kinase (CK) was assessed to estimate the severity of MI. CK was routinely measured every 12 hours in patients experiencing STEMI for the first 48 hours, and afterwards every 24 hours until hospital dismissal or until transfer to a local hospital. Left ventricular function was assessed by either ventriculography or echocardiography after STEMI.
Bleeding Criteria
For evaluation of bleeding events, the TIMI bleeding criteria were used to assess in‐hospital bleeding events13 and stratified in TIMI minimal (<3 g/dL decrease in hemoglobin concentration or <9% decrease in hematocrit), TIMI minor (hemoglobin decrease of 3–<5 g/dL or ≥10% decrease), and TIMI major (any intracranial bleeding or decrease in hemoglobin of ≥5 g/dL or a ≥15% absolute decrease in hematocrit). A bleeding event after hospital discharge was defined as a serious bleeding event requiring medical attention within 1 year after STEMI.
Definition of Outcomes
In‐hospital outcomes were evaluated at discharge or at time of patient transfer to a local hospital. The 30‐day and 1‐year follow‐up outcomes were evaluated in a telephone interview. To measure efficacy, the primary outcome, major adverse cardiac and cerebrovascular events (MACCEs), was defined as a combination of death, myocardial reinfarction, or stroke within 1 year after STEMI. The composite safety end point was defined as TIMI minor or major bleeding during hospital stay or any bleeding event from hospital discharge until 1 year of follow‐up.
To estimate benefit/risk ratio of antiplatelet medication, net adverse clinical events, as the combination of MACCEs and bleeding events, were calculated.
Statistical Analysis
All patients admitted with STEMI to the Bremen Heart Center and treated with primary PCI between January 1, 2006, and June 30, 2017, were initially assessed. For the unadjusted comparisons, all patients aged <75 years, patients with initial atrial fibrillation and/or receiving triple therapy, patients without stent implantation, patients who did not receive any DAPT or who were treated with prasugrel were excluded. Baseline characteristics of patients were described by mean values and SDs or SEMs for continuous variables (age, glomerular filtration rate, mean heart rate, systolic blood pressure, coronary vessels diseased, peak CK, and left ventricular ejection fraction). Absolute numbers and percentages were reported for categorical variables (sex, diabetes mellitus, obesity, first coronary artery event, first cardiovascular event, preclinical resuscitation/defibrillation, subacute MI, anterior MI/new left bundle branch block, Killip class 2 to 4, multivessel disease, and TIMI flow <2). Univariate comparison was done with Mann‐Whitney U‐tests for continuous variables (because no normal distribution was found) and χ2 tests for categorical variables. Propensity score (PPS) matching was used to control for possible confounding by indication. The PPS was estimated by a logistic regression model using ticagrelor versus clopidogrel as the dependent variable and the following covariates: sex, subacute MI, heart failure, peak CK, multivessel disease, type of stent implanted (drug‐eluting stent or bare metal stent), TIMI flow after PCI, medical therapy with a glycoprotein IIb/IIIa antagonist, diabetes mellitus, medication with a β blocker, triple therapy, or an angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker at hospital discharge. Ticagrelor users were matched to clopidogrel users using the logit of the PPS by a 1:1 nearest neighbor matching with a caliper of 0.2 times the SD of the logit of the PPS on the entire PCI database for patients aged ≥75 years. The variables included in PPS were selected among available baseline variables, which are known or suspected to be associated with therapy and/or study outcomes. The balance of covariates before and after matching was compared by means of absolute standardized differences between both treatment groups. Hazard ratios (HRs) and 95% CIs were calculated by means of Cox regression model, derived from the PHREG procedure (SAS Institute, Inc, 2018).
Results
Study Population
Between January 1, 2006, and June 30, 2017, 7466 patients admitted with STEMI were treated with emergency primary PCI. The proportion of elderly patients with STEMI in the PCI cohort increased from 19.5% in 2006 to 26.4% in 2017 (Figure 1A). At the same time, treatment strategies changed for elderly patients with STEMI: while in 2006, 72.1% of elderly patients with STEMI were treated with primary PCI, this rate increased to 92.7% in 2017 (Figure 1B). After the introduction of the modern P2Y12 inhibitors, >60% of elderly patients with STEMI received ticagrelor after 2011 (Figure 2).
Figure 1.
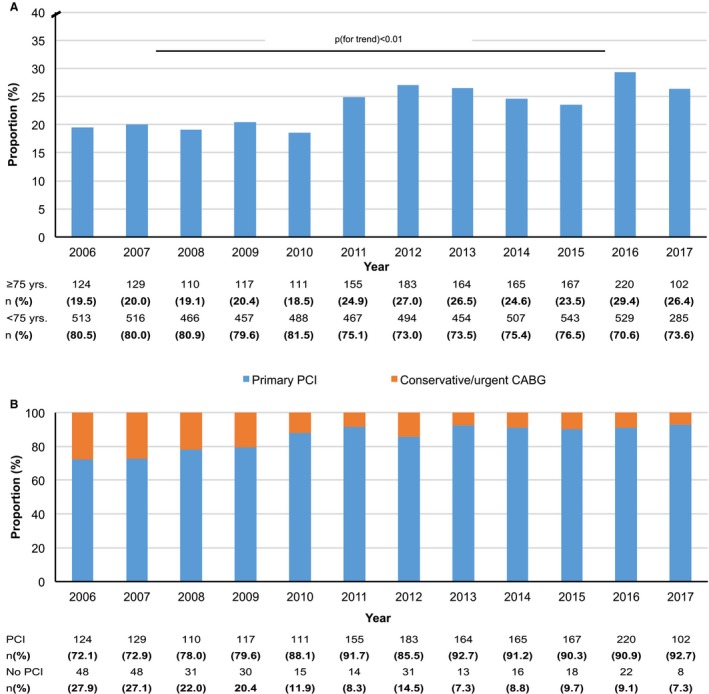
Study population. A, Proportion of patients with ST‐segment–elevation myocardial infarction (STEMI), aged ≥75 years, of the total STEMI cohort undergoing primary percutaneous coronary intervention (PCI). B, Proportion of elderly patients by year treated with PCI vs urgent coronary artery bypass graft (CABG) surgery/medical therapy (no PCI).
Figure 2.
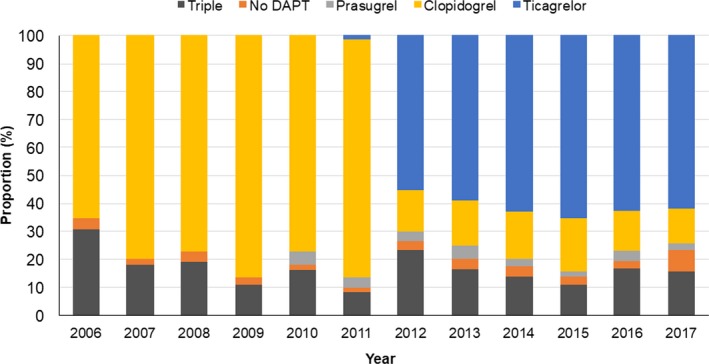
Medical therapy. Proportion of elderly patients treated with either ticagrelor or other medical therapies (clopidogrel with and without triple therapy, no dual‐antiplatelet therapy [DAPT], or DAPT with prasugrel), by year.
For further analysis, all patients aged <75 years (n=5719), patients with initial atrial fibrillation and/or receiving triple therapy (n=483), patients without stent implantation (n=113), patients who did not receive any DAPT (n=26), and patients who were treated with prasugrel (n=38) were excluded. Of the remaining 1087 patients, 552 (51%) were treated with clopidogrel and 535 (49%) were treated with ticagrelor.
Baseline Characteristics
Patients receiving either ticagrelor or clopidogrel showed a similar mean age of 81 years, with more than half of patients in both treatment arms being women (Table 1). For cardiovascular risk profile, no differences in rates of obesity or diabetes mellitus could be detected, whereas rates of active smokers were higher in patients treated with clopidogrel. There were no differences between patients treated with ticagrelor or clopidogrel on a history of coronary artery disease, coronary events, history of stroke/transient ischemic attack, or known peripheral artery disease (Table 1).
Table 1.
Baseline Characteristics and Clinical Status at Admission
| Characteristics | Clopidogrel (n=552) | Ticagrelor (n=535) | P Value |
|---|---|---|---|
| Age, mean±SD, y | 80.9±4.6 | 80.9±4.7 | 0.8 |
| Aged ≥80 y, % | 54.0 | 52.1 | 0.5 |
| Women, % | 51.4 | 49.9 | 0.5 |
| BMI, mean±SD, kg/m2 | 25.9±3.9 | 26.1±4.1 | 0.5 |
| Obesity (BMI >30 kg/m2), % | 13.3 | 15.9 | 0.24 |
| Diabetes mellitus, % | 24.1 | 20.6 | 0.16 |
| Current smokers, % | 14.3 | 9.6 | 0.02 |
| Medical history, % | |||
| Coronary artery disease | 16.4 | 16.0 | 0.8 |
| Percutaneous coronary intervention | 10.2 | 11.7 | 0.5 |
| Acute myocardial infarction | 12.0 | 9.5 | 0.18 |
| Coronary artery bypass graft surgery | 2.9 | 3.2 | 0.8 |
| Stroke/transient ischemic attack | 8.4 | 6.0 | 0.15 |
| Peripheral artery disease | 7.2 | 6.2 | 0.3 |
| Clinical presentation | |||
| Subacute STEMI, % | 12.4 | 15.7 | 0.11 |
| Anterior STEMI or new left bundle branch block, % | 47.8 | 51.8 | 0.2 |
| Initial SBP, mean±SD, mm Hg | 132.4±28 | 135.4±29 | 0.09 |
| SBP <100 mm Hg, % | 13.8 | 10.2 | 0.07 |
| Initial heart rate, mean±SD, /min | 77.9±20 | 79.9±22 | 0.13 |
| Heart rate >100/min, % | 10.9 | 11.5 | 0.3 |
| Killip class, % | |||
| 1 | 80.9 | 85.6 | 0.05 |
| 2 | 2.7 | 1.1 | 0.06 |
| 3 | 2.9 | 1.5 | 0.12 |
| 4 | 13.4 | 11.6 | 0.36 |
| Initial renal function | |||
| GFR, mean±SD, mL/min | 57.4±20 | 63.6±21 | <0.01 |
| GFR <60 mL/min, % | 55.4 | 40.8 | <0.01 |
| GFR <45 mL/min, % | 27.8 | 22.5 | 0.02 |
BMI indicates body mass index; GFR, glomerular filtration rate; SBP, systolic blood pressure; STEMI, ST‐segment–elevation myocardial infarction.
Clinical Presentation and Interventional Details
There were no differences on rates of subacute STEMIs or localization of STEMI between treatment arms. There was a trend toward fewer patients with Killip class I and higher rates of initial hypotension in the clopidogrel group. Furthermore, the mean glomerular filtration rate was lower in patients treated with clopidogrel (Table 1). Although there were no differences for severity of coronary artery disease, patients treated with clopidogrel were more likely to present with initial TIMI 0 flow, less likely to undergo thrombaspiration, and more likely to be medicated with a glycoprotein IIb/IIIa antagonist during or after the coronary intervention (Table 2). Reflecting changes in guideline recommendations, drug‐eluting stents were more likely to be implanted in patients receiving ticagrelor, whereas bare metal stents were implanted in most patients treated with clopidogrel. The immediate results after primary PCI did not differ significantly between patient cohorts treated with ticagrelor or clopidogrel, and neither did size of STEMI (estimated by peak CK) or left ventricular ejection fraction after STEMI (Table 2).
Table 2.
Severity of Coronary Artery Disease and Interventional Details
| Variable | Clopidogrel (n=552) | Ticagrelor (n=535) | P Value |
|---|---|---|---|
| No. of coronary vessels diseased, % | |||
| 1 | 27.5 | 26.4 | 0.6 |
| 2 | 34.1 | 31.4 | 0.3 |
| 3 | 38.3 | 42.2 | 0.18 |
| Initial TIMI 0 flow, % | 69.5 | 62.7 | 0.04 |
| Thrombaspiration, % | 6.1 | 13.3 | <0.01 |
| No. of stents implanted, mean±SD | 1.33±0.6 | 1.49±0.8 | <0.01 |
| Glycoprotein IIb/IIIa antagonists, % | 63.7 | 42.2 | <0.01 |
| Type of stent, % | |||
| Bare metal stent | 89.1 | 21.4 | <0.01 |
| Drug‐eluting stent | 10.8 | 76.7 | <0.01 |
| PCI result (TIMI flow), % | |||
| TIMI flow 0/1 | 2.9 | 3.6 | 0.5 |
| TIMI flow 2 | 8.3 | 7.6 | 0.6 |
| TIMI flow 3 | 88.6 | 88.7 | 0.9 |
| Peak CK, mean±SD, U/mL | 1660±1742 | 1499±1515 | 0.1 |
| Peak CK >2000 U/mL, % | 29.1 | 26.9 | 0.5 |
| LVEF after STEMI, mean±SD, % | 45.9±9 | 47.0±9.5 | 0.16 |
| LVEF <40%, % | 18.1 | 16.1 | 0.2 |
CK indicates creatine kinase; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
In‐Hospital Outcome, Bleeding Rates, and Medication at Discharge
There were no significant differences in rates of in‐hospital resuscitations or in‐hospital strokes between the groups; however, a nonsignificant trend toward a higher in‐hospital mortality in patients with clopidogrel was observed (Table 3). No significant differences were found for in‐hospital TIMI minor or major bleedings (clopidogrel versus ticagrelor, 4.4% versus 3.3%; P=0.4). At hospital discharge, patients treated with clopidogrel were more likely to have a concomitant medication with a β blocker, whereas patients receiving ticagrelor were more likely to have a comedication with an angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker or a mineralocorticoid receptor antagonist (Table 3). A total of 31 patients were switched from ticagrelor to clopidogrel before discharge from the PCI center; however, these patients remained in the ticagrelor group for this study.
Table 3.
In‐Hospital and 1‐Year Event Rates After STEMI and Concomitant Medication at Discharge
| Variable | Clopidogrel (n=552) | Ticagrelor (n=535) | P Value |
|---|---|---|---|
| In‐hospital mortality, % | 13.0 | 9.7 | 0.09 |
| In‐hospital resuscitations, % | 4.3 | 3.6 | 0.5 |
| In‐hospital strokes | 0.9 | 0.8 | 0.7 |
| In‐hospital bleeding events, % | |||
| TIMI minimal | 11.5 | 14.4 | 0.15 |
| TIMI minor | 3.0 | 1.4 | 0.07 |
| TIMI major | 1.4 | 1.9 | 0.4 |
| TIMI minor or major | 4.4 | 3.3 | 0.4 |
| Concomitant medication at discharge, % | |||
| Acetylsalicylic acid | 98.0 | 97.9 | 0.5 |
| β Blocker | 76.0 | 69.8 | 0.02 |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 72.9 | 83.6 | <0.01 |
| Mineralocorticoid receptor antagonist | 9.4 | 17.2 | <0.01 |
| Statin | 85.9 | 86.5 | 0.7 |
| Efficacy end points at 1 y, % | |||
| Reinfarctions | 4.8 | 3.5 | 0.27 |
| Strokes/TIAs | 2.6 | 2.0 | 0.5 |
| All‐cause mortality | 26.8 | 21.1 | 0.035 |
| Major adverse cardiac and cerebrovascular events | 32.4 | 25.5 | 0.015 |
| Safety end points at 1 y, % | |||
| Bleeding events after hospital discharge | 0.4 | 1.8 | 0.03 |
| Cumulative bleeding events (in hospital+after discharge) | 4.9 | 5.1 | 0.6 |
| Net adverse clinical events at 1 y | 37.3 | 30.6 | 0.028 |
STEMI indicates ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack; TIMI, Thrombolysis in Myocardial Infarction.
Long‐Term Outcome and Bleeding Rates After 1 Year
Analysis of follow‐up data revealed a significant reduction of the primary efficacy outcome, MACCE (combination of death, reinfarction, and stroke) after 1 year that occurred in 25.5% of patients treated with ticagrelor and in 32.4% of patients treated with clopidogrel (P=0.015) (Table 3). All‐cause mortality at 1 year was lower in patients with ticagrelor (21.1%) compared with patients with clopidogrel (26.8%) (P=0.035). Bleeding events after hospital discharge were rare; however, they were significantly more frequent in patients treated with ticagrelor (1.8% versus 0.4%; P=0.03). The cumulative bleeding rate of in‐hospital and postdischarge bleedings was not different between the groups: In 4.9% of patients with clopidogrel and 5.1% of patients with ticagrelor, bleeding events occurred (P=0.6). When calculating the net clinical benefit of choice of DAPT (net adverse clinical events=MACCEs and bleedings), net adverse clinical event rates at 1 year for ticagrelor were significantly lower than for clopidogrel (30.6% versus 37.3%; P=0.028).
Results From the Propensity‐Matched Approach
Analyses based on the PPS‐matched cohort (238 matched pairs; Table S1) showed that reduction in MACCEs remained significant within 1 year after STEMI: A 31% reduction in MACCE events (HR, 0.69; 95% CI, 0.49–0.97; P=0.03) could be observed in patients receiving ticagrelor compared with patients receiving clopidogrel (Figure 3). In contrast, the reduction in overall mortality did not remain significant after PPS matching (HR, 0.89; 95% CI, 0.67–1.28; P=0.5) and neither did in‐hospital bleeding rates or rates of bleeding events after hospital discharge (Figure 2). The cumulative rates of bleedings after 1 year were, therefore, indifferent between the groups (HR, 1.08; 95% CI, 0.49–2.37; P=0.84) (Figure 3).
Figure 3.
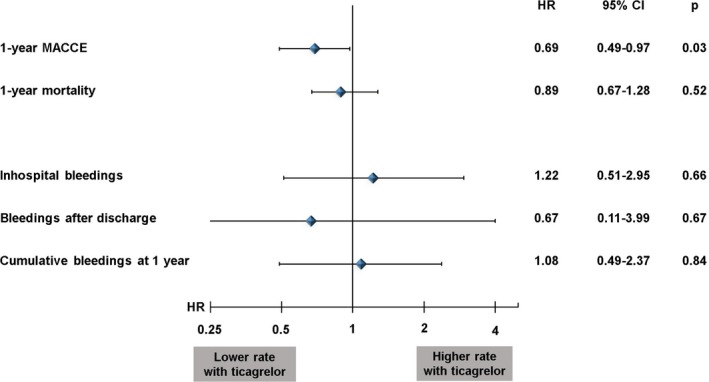
Propensity score–matched analysis of ticagrelor vs clopidogrel; impact on 1‐year major adverse cardiac and cerebrovascular events (MACCEs), 1‐year mortality, and bleeding events. HR indicates hazard ratio.
Additional sensitivity analyses were conducted to evaluate the robustness of our results: First, to account for a possible impact of other medications at discharge, statins and mineralocorticoid receptor antagonists were added to the PPS model. Furthermore, we additionally adjusted for age, body mass index, and year to account for residual confounding. In this extended PPS model, the impact of ticagrelor on MACCEs remained significant (HR, 0.61; 95% CI, 0.39–0.94; P=0.024). Second, to account for confounding by other changes in therapy during the study period, a separate PPS model was calculated, only including patients with STEMI treated after 2011. In this PPS model, the number of pairs was reduced from 238 to 135. The reduction in MACCEs for the ticagrelor cohort remained significant (HR, 0.65; 95% CI, 0.44–0.96; P=0.032), whereas there was again no impact on 1‐year‐mortality (HR, 0.85; 95% CI, 0.56–1.30; P=0.45) and cumulative bleeding after 1 year (HR, 1.00; 95% CI, 0.40–2.51; P=1.0). When calculating the PPS cohorts with an on‐treatment protocol, the impact of ticagrelor for 1‐year MACCEs remained as a trend (HR, 0.76; 95% CI, 0.55–1.05; P=0.09).
Discussion
The major result of the present study is that the beneficial effect of ticagrelor compared with clopidogrel in patients with STEMI, which has been shown in randomized controlled trials, could be confirmed in a real‐world cohort specifically for elderly patients (aged ≥75 years) with a reduced rate of MACCEs and no significant difference in bleeding events, even after PPS‐matched analysis of data. To our knowledge, this is the first study investigating benefits versus risks of ticagrelor compared with clopidogrel with a focus on elderly patients with STEMI from a large all‐comers registry.
Impact of DAPT on MACCEs and Bleeding Events in Elderly Patients
In the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial published in 2001, Yusuf et al showed that the addition of clopidogrel to aspirin in patients with ACS was associated with a significant decrease in the cumulative rate of cardiovascular death, nonfatal MIs, and stroke; however, this occurred with a significant increase in major bleeding events.1 Despite the higher bleeding rates, these results led to a recommendation for DAPT with clopidogrel in patients presenting with ACS.14 For the new‐generation P2Y12 inhibitors, the PLATO2 and TRITON TIMI 383 trials showed that ticagrelor or prasugrel had even lower MACCE rates in patients with ACS when compared with clopidogrel. The advantage was explained by the increased potency of these P2Y12 inhibitors, their faster onset of action, and less interindividual variation of the drug effect.15 However, for both new P2Y12 inhibitors, the lower MACCE rates were accompanied by more bleeding events, with a 32% increase in non–coronary artery bypass graft surgery–related TIMI major bleedings for prasugrel3 and a 25% increase for ticagrelor.2
Elderly patients are regularly underrepresented in randomized trials; elderly patients in the TRITON TIMI 38 trial only constituted 13%3 and in the PLATO trial only 15% of the study population,2 whereas patients aged >75 years were excluded from other trials, like CLARITY (Clopidogrel as Adjunctive Reperfusion Therapy),16 which compared the effect of clopidogrel versus placebo in patients with STEMI.
Elderly patients have a high rate of comorbidity and are at highest risk not only for bleeding events, but also for coronary events. Despite their higher natural risk, recent observational studies show a decline in cardiac mortality for elderly patients with coronary artery disease: Spoon et al reported in 2014 that at the Mayo Clinic (Rochester, MN), patients aged ≥80 years undergoing PCI had the steepest relative change in cardiac mortality between 1991 and 2008, whereas non–cardiac mortality rates were increasing for this age group.17 It is, therefore, crucial to analyze the effects of the new P2Y12 inhibitors in elderly patients in an all‐comer study, which reflects “real‐world” management of patients with ACS.
The increased importance of elderly patients in STEMI registries can be observed in our data because an absolute and relative increase in numbers of STEMIs for elderly patients was evident between 2006 and 2017 in the BSR. Furthermore, during the study period, elderly patients with STEMI were more likely to undergo emergency PCI instead of a conservative treatment strategy or being transferred to urgent coronary artery bypass graft surgery. Combining these findings with a recent publication, which showed that in particular elderly patients benefitted from an optimal interventional revascularization result,18 elderly patients represent a patient cohort of growing proportion and importance, which the interventional cardiologist can effectively help through emergency PCI.
The results of the present study underline the safety of ticagrelor in elderly patients with relatively low bleeding rates and a significant benefit for MACCEs. These findings are in good accordance with the PLATO trial2; however, they are observed in a much older (age, 81 versus 62 years) patient cohort than in the PLATO trial, without the inclusion and exclusion criteria, which limit randomized trials.
The recently published GLOBAL LEADERS (A Clinical Study Comparing Two Forms of Anti‐platelet Therapy After Stent Implantation) trial, which tested a prolonged DAPT with aspirin and ticagrelor for 24 versus 12 months in patients undergoing PCI with ACS or stable coronary artery disease, seems to corroborate a potential long‐term benefit of modern P2Y12 inhibitors, especially for elderly patients: although the overall efficacy end point was not met for the entire study population, the elderly subgroup (aged >75 years) did show a significant reduction in the primary study endpoint (HR, 0.75; 95% CI, 0.58–0.99; P=0.04) when the dual therapy with ticagrelor was extended to 24 months compared with 12 months.19
Concurrent Changes in Preferred Stent Type and Medications During the Study Period
Almost simultaneously to the introduction of ticagrelor and prasugrel in STEMI, the recommendations for the preferred stent type were changed in the guidelines.4, 5, 6 Thus, bare metal stents were preferably implemented before 2012 and drug‐eluting stents were used in most elderly patients with STEMI after 2011 in the present study. It can be debated what impact new‐generation stents had on outcome for elderly patients with STEMI undergoing emergency PCI. A meta‐analysis showed that the advantage of drug‐eluting stents over bare metal stents in randomized controlled trials was often mainly generated by including target‐lesion, target‐vessel‐revascularization, or in‐stent‐restenosis events into the composite end points, with no benefit for mortality or reinfarction rates.20 These findings were confirmed in the NORSTENT (Norwegian Coronary Stent Trial).21 Because the MACCE definition in the present study did not include target‐lesion/target‐vessel‐revascularization events, confounding by type of stent may not play a decisive role.
There were some differences on comedication between the clopidogrel versus ticagrelor group. β Blockers were more likely to be given in combination with clopidogrel, probably to avoid bradycardias, a known adverse effect of ticagrelor. In contrast, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers as well as mineralocorticoid receptor antagonists were more likely to be given in combination with ticagrelor.
The role of β‐blocker therapy in survivors of acute MIs is not established, at least not for patients with a preserved ejection fraction. One observational study found a benefit for β‐blocker medication in patients with STEMI at higher risk (eg, advanced age)22; however, a recently published study by Puymirat et al23 failed to show a prognostic impact of β‐blocker medication with respect to mortality within 1 year after infarction. Similarly, mineralocorticoid receptor antagonists, despite their proven prognostic effect in patients with reduced ejection fraction, have failed to have a significant positive impact, if medication is started directly after acute MI.24
Limitations
Because of the design of this long‐term observational study, selection effects over time are likely; and it is a limitation of the study that changes in treatment of STEMI took place during the study period (eg, the introduction of new‐generation stents or modifications in drug therapy). The study design aimed to minimize the impact of this bias through PPS matching and by adding a separate PPS‐matched analysis, which only included patients treated after 2011. However, residual confounding effects cannot be excluded, and sample sizes of the PPS‐matched subcohorts were small. Furthermore, our study is limited in scope because its results were limited to one country and one PCI center. Extrapolation of the present analysis to multicenter or multinational registries might allow to reexamine these results and possibly help to generalize the findings.
Conclusions
In this study with data from an all‐comers STEMI registry, it could be shown that ticagrelor was preferred in most elderly patients with STEMI, treated with primary PCI after 2011. In propensity‐matched treatment cohorts, the elderly patients, who received ticagrelor, did not show an excess of bleeding events, whereas a significant reduction in 1‐year MACCE rates could observed in the ticagrelor cohort compared with a treatment with clopidogrel. Therefore, the safety and efficacy of treatment with ticagrelor, which has been shown in randomized controlled trials, could be confirmed in this real‐world cohort of elderly patients with STEMI.
Disclosures
None.
Supporting information
Table S1. Characteristics of Matched Sample (238 pairs)
(J Am Heart Assoc. 2019;8:e012530 DOI: 10.1161/JAHA.119.012530.)
References
- 1. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 2. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 4. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 5. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 6. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 7. Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, Cannon CP, Heras M, Lopes RD, Morais J, Mahaffey KW, Bach RG, Wojdyla D, Wallentin L. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5:680–688. [DOI] [PubMed] [Google Scholar]
- 8. Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part I: non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. [DOI] [PubMed] [Google Scholar]
- 9. Schmucker J, Wienbergen H, Seide S, Fiehn E, Fach A, Würmann‐Busch B, Gohlke H, Günther K, Ahrens W, Hambrecht R. Smoking ban in public areas is associated with a reduced incidence of hospital admissions due to ST‐elevation myocardial infarctions in non‐smokers: results from the Bremen STEMI Registry. Eur J Prev Cardiol. 2014;21:1180–1186. [DOI] [PubMed] [Google Scholar]
- 10. Schmucker J, Fach A, Becker M, Seide S, Bünger S, Zabrocki R, Ahrens W, Hambrecht R, Wienbergen H. Predictors of acute kidney injury in patients admitted with ST‐elevation myocardial infarction: results from the Bremen STEMI‐Registry. Eur Heart J Acute Cardiovasc Care. 2018;7:710–722. [DOI] [PubMed] [Google Scholar]
- 11. Schmucker J, Seide S, Wienbergen H, Fiehn E, Stehmeier J, Günther K, Ahrens W, Hambrecht R, Pohlabeln H, Fach A. Socially disadvantaged city districts show a higher incidence of acute ST‐elevation myocardial infarctions with elevated cardiovascular risk factors and worse prognosis. BMC Cardiovasc Disord. 2017;17:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fach A, Backhaus T, Schmucker J, Zabrocki R, Garstka D, Stehmeier J, Fiehn E, Hambrecht R, Wienbergen H. Bivalirudin versus heparin and provisional GP IIb/IIIa inhibitors in patients treated for ST‐segment elevation myocardial infarctions: comparison of outcomes in a “real‐world” setting. J Interv Cardiol. 2017;30:301–308. [DOI] [PubMed] [Google Scholar]
- 13. Roxana M, Rao SV, Deepak L, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials; a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 14. Antman EM, Anbe DT, Jacobs AK, Ornato JP. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary. Circulation. 2004;110:588–636. [DOI] [PubMed] [Google Scholar]
- 15. Qaderdan K, Ishak M, Heestermans AA, de Vrey E, Jukema JW, Voskuil M, de Boer MJ, van't Hof AW, Groenemeijer BE, Vos GJ, Janssen PW, Bergmeijer TO, Kelder JC, Deneer VH, ten Berg JM. Ticagrelor or prasugrel versus clopidogrel in elderly patients with an acute coronary syndrome: optimization of antiplatelet treatment in patients 70 years and older–rationale and design of the POPular AGE study. Am Heart J. 2015;170:981–985. [DOI] [PubMed] [Google Scholar]
- 16. Sabatine MS, Cannon CP, Gibson CM, López‐Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E; CLARITY‐TIMI 28 Investigators . Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med. 2005;352:1179–1189. [DOI] [PubMed] [Google Scholar]
- 17. Spoon DB, Psaltis PJ, Singh M, Holmes DR Jr, Gersh BJ, Rihal CS, Lennon RJ, Moussa ID, Simari RD, Gulati R. Trends in cause of death after percutaneous coronary intervention. Circulation. 2014;129:1286–1294. [DOI] [PubMed] [Google Scholar]
- 18. Fach A, Bünger S, Zabrocki R, Schmucker J, Conradi P, Garstka D, Fiehn E, Hambrecht R, Wienbergen H. Comparison of outcomes of patients with ST‐segment elevation myocardial infarction treated by primary percutaneous coronary intervention analyzed by age groups (<75, 75 to 85, and >85 years); (results from the Bremen STEMI Registry). Am J Cardiol. 2015;116:1802–1809. [DOI] [PubMed] [Google Scholar]
- 19. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Möllmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug‐eluting stent: a multicentre, open‐label, randomised superiority trial. Lancet. 2018;392:940–949. [DOI] [PubMed] [Google Scholar]
- 20. Hao PP, Chen YG, Wang XL, Zhang Y. Efficacy and safety of drug‐eluting stents in patients with acute ST‐segment–elevation myocardial infarction: a meta‐analysis of randomized controlled trials. Tex Heart Inst J. 2010;37:516–524. [PMC free article] [PubMed] [Google Scholar]
- 21. Bønaa K, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen D, Kløw N, Uchto M, Trovik T, Bendz B, Stavnes S, Bjørnerheim R, Larsen A, Slette M, Steigen T, Jakobsen OJ, Bleie Ø, Fossum E, Hanssen T, Dahl‐Eriksen Ø, Njølstad I, Rasmussen K, Wilsgaard T, Nordrehaug J. Drug‐eluting or bare‐metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. [DOI] [PubMed] [Google Scholar]
- 22. Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, Hara M, Uematsu M, Fukunami M, Hamasaki T, Sato H, Hori M, Komuro I; Osaka Acute Coronary Insufficiency Study (OACIS) Investigators . Impact of beta blockade therapy on long‐term mortality after ST‐segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol. 2013;111:457–464. [DOI] [PubMed] [Google Scholar]
- 23. Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, Cattan S, Steg G, Schiele F, Ferrières J, Juillière Y, Simon T, Danchin N. β Blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montalescot G, Pitt B, Lopez de Sa E, Hamm CW, Flather M, Verheugt F, Shi H, Turgonyi E, Orri M, Vincent J, Zannad F. Early eplerenone treatment in patients with acute ST‐elevation myocardial infarction without heart failure: the Randomized Double‐Blind Reminder Study. Eur Heart J. 2014;35:2295–2302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Matched Sample (238 pairs)


