Abstract
Background
High levels of physical activity have been associated with longer life expectancy free of cardiovascular disease (CVD), but specific types of CVD and sedentary behavior have not been examined. We examined associations of leisure‐time moderate‐to‐vigorous physical activity (LTPA) and television viewing with life expectancy free of 3 types of CVD.
Methods and Results
We included 13 534 participants from the ARIC (Atherosclerosis Risk in Communities) cohort. We used multistate survival models to estimate associations of LTPA in the past year (no LTPA, less than the median, equal to or greater than the median) and television viewing (often or very often, sometimes, seldom or rarely) with life expectancy at age 50 free of nonfatal coronary heart disease (CHD), stroke, and heart failure (HF). Over 27 years of follow‐up, 4519 participants developed one of the 3 nonfatal CVDs and 5475 deaths occurred. Compared with participants who engaged in no LTPA, participants who engaged in LTPA equal to or greater than the median had longer life expectancy free of nonfatal CHD (men: 1.5 years [95% CI, 1.0–2.0]; women: 1.6 years [95% CI, 1.1–2.2]), stroke (men: 1.8 years [95% CI, 1.2–2.3]; women: 1.8 years [95% CI, 1.3–2.3]), and HF (men: 1.6 years [95% CI, 1.1–2.1]; women: 1.7 years [95% CI, 1.2–2.2]). Compared with viewing more television, watching less television was associated with longer life expectancy free of CHD, stroke, and HF (≈0.8 year).
Conclusions
Higher levels of LTPA and less television viewing were associated with longer life expectancy free of CHD, stroke, and HF. Engaging in LTPA and watching less television may increase the number of years lived free of CHD, stroke, and HF.
Keywords: coronary heart disease, epidemiology, heart failure, physical exercise, sedentary behavior, stroke
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Primary Prevention
Clinical Perspective
What Is New?
Men and women who engaged in any leisure‐time physical activity and viewed less television lived more years free of coronary heart disease, stroke, and heart failure.
To our knowledge, this study is the first to provide estimates of years expected to live free of coronary heart disease, stroke, and heart failure (rather than using a composite measure of cardiovascular disease) and to demonstrate that less sedentary behavior, measured as television viewing, was linked to living more years free of 3 types of cardiovascular disease.
What Are the Clinical Implications?
Increased participation in leisure‐time moderate‐to‐vigorous physical activity and reduction of time spent viewing television should be promoted to increase the number of years lived free of coronary heart disease, stroke, and heart failure.
Cardiovascular disease (CVD) is the leading cause of death, premature mortality, and years lived with disability.1 In the United States, >92.1 million people have CVD (36.6%).2 The major contributors to the CVD burden are coronary heart disease (CHD), stroke, and heart failure (HF).2 This high burden of CVD suggests that many years of life may be spent living with CVD. Consequently, it is important to identify opportunities to extend the number of years lived free of CVD. Increasingly, studies have begun using health expectancy metrics that combine incidence and mortality to estimate years lived with and without disease.3
Greater amounts of physical activity have been associated with a reduced risk of CVD,4, 5 all‐cause mortality,4, 6 longer life expectancy,6, 7 and, most recently, longer CVD‐free survival.8, 9, 10 Results from observational studies suggest a positive association of physical activity with life expectancy free of CVD.8, 9, 10 In the Framingham Heart Study cohort,8 for example, men who self‐reported high levels of physical activity based on a weighted daily sum of activities could expect to live 22.8 years (95% CI, 21.6–23.9) CVD free, which was 3.2 years (95% CI, 1.9–4.3) longer than men who self‐reported low levels of physical activity (19.7 years CVD free [95% CI, 18.7–20.6]). Research in this area has been limited to analysis of 1 population‐based cohort in the United States and 3 cohorts in Europe. Furthermore, existing studies used various methods of physical activity ascertainment, making it difficult to generalize findings to other populations.
Greater amounts of sedentary behavior are linked to an increased risk of incident CVD11 and all‐cause mortality.12 Sedentary behavior is any waking behavior that expends little energy expenditure (≤1.5 metabolic equivalent of task [MET]) while in a sitting or reclining posture.13 On average, Americans spend about 8 hours per day in sedentary behaviors.14 Little information exists about how sedentary behaviors are associated with years lived with and without CVD.
Our objective was to examine the associations of physical activity and sedentary behavior with life expectancy free of disease for 3 types of CVD: CHD, stroke, and HF. Gaps in the existing literature addressed by this study include assessment of physical activity as MET hours/week, to account for intensity of activity and to enhance translation of results, and, for the first time, examination of sedentary behavior.
Methods
Study Population and Study Design
We used data from the ARIC (Atherosclerosis Risk in Communities) study. ARIC is a population‐based prospective cohort of 15 792 mostly white and black adults aged 45 to 64 years at baseline (white, n=11 478; black, n=4266; Asian, n=34; American Indian/Alaskan Indian, n=14). Participants were recruited from 4 geographic areas in the United States (Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and Minneapolis, Minnesota).15 The study has conducted 6 examination visits (visit 1, 1987–1989; visit 2, 1990–1992; visit 3, 1993–1995; visit 4, 1996–1998; visit 5, 2011–2013; and visit 6, 2016–2017). Cohort members were asked to participate in interviews, clinical examinations, and annual (semiannual since 2012) telephone interviews.15 Participants provided written consent at each examination. The ARIC study was approved by the institutional review boards of the 4 participating ARIC study centers. The data and analytic methods will not be made available for the purposes of reproducing the results because of human subject confidentiality. However, ARIC study data can be accessed by contacting the ARIC Coordinating Center at the University of North Carolina at Chapel Hill.
For our analysis, we used the prospective cohort design of the ARIC study. Exposures included physical activity, which was assessed at visits 1 and 3, and television viewing assessed at visit 1. Outcomes included all‐cause mortality and nonfatal CHD, stroke, and HF, which were all ascertained from baseline to December 31, 2016. Covariates assessed at visit 1 and included in analysis were age, sex, race by ARIC center, and education. Covariates assessed at visits 1, 2, 3, and 4 included alcohol intake, smoking status, body mass index (BMI), hypertension, diabetes mellitus status, and HDL (high‐density lipoprotein) cholesterol (HDL‐C; Table S1).
Physical Activity and Sedentary Behavior
Physical activity was ascertained at visits 1 and 3 with the Baecke questionnaire.16, 17 Participants were asked to list up to 4 leisure‐time physical activities they participated in the past year and to provide the number of hours per week (duration) and months per year (frequency) for each activity. Activities were assigned a MET value based on the Compendium of Physical Activities.18 Activities with a MET value of ≥3 were classified as moderate‐to‐vigorous intensity.19 The MET, frequency, and duration of each activity were used to calculate MET hours/week spent in leisure‐time moderate‐to‐vigorous physical activity (LTPA). MET hours/week spent in LTPA at both visits was categorized as none, less than the median (0.1 to <13.2 MET hours/week) and equal to or greater than the median (≥13.2 MET hours/week). The cut point was based on the median value of MET hours/week at visit 1 among those reporting any LTPA.
Television viewing, an indicator of sedentary behavior, was ascertained at visit 1 with a question on how often participants viewed television (response options included never, seldom, sometimes, often, and very often), which was further classified as never/seldom, sometimes, and often/very often because of small numbers in some categories.
All‐Cause Mortality
Deaths were ascertained from cohort entry until December 31, 2016, and were identified by annual phone calls, active surveillance of hospitalizations in ARIC communities, and linkage with the National Death Index. Death certificates were reviewed by medical abstractors to determine underlying causes of death, and the circumstances of death were confirmed with family members.15, 20
CVD Outcomes
We separately examined incident nonfatal CHD, stroke, and HF. Prevalent CHD at baseline was defined as a myocardial infarction (MI) from visit 1 ECG data or a self‐reported history of MI or coronary revascularization. Incident nonfatal CHD was defined as definite or probable MI, silent MI between examinations detected by ECG, or cardiac revascularization procedures. MI events were identified from active surveillance of hospitalizations occurring among ARIC cohort members.20, 21 Trained study personnel abstracted clinical information from the medical records of eligible hospitalizations. Definite or probable MI was defined from a diagnostic algorithm based on chest pain, ECG, and cardiac enzyme data.20 A committee reviewed all CHD events, and disagreements were adjudicated.
Prevalent stroke at baseline was defined as self‐reported history of a stroke or transient ischemic attack. Incident nonfatal stroke events were identified by self‐report from ARIC visit examinations, annual follow‐up telephone calls, and active surveillance of hospitalizations in the ARIC communities.22 A trained nurse abstracted the medical record if a hospitalization had stroke diagnostic codes, if discharge summaries contained stroke‐related keywords, if neuroimaging had cerebrovascular findings, or if the patient had been admitted to the neurological intensive care unit.22 Stroke events were classified as definite or probable by computer algorithm and by a physician reviewer according to criteria adapted from the National Survey of Stroke.22, 23 A second physician adjudicated disagreements.
Prevalent HF at baseline was defined as self‐report of taking a medication for HF in the past 2 weeks or a score of 3 on the Gothenburg criteria.24 Incident HF was identified by active surveillance of hospitalizations with International Classification of Diseases, Ninth Revision (ICD‐9) code 428.xx in any position and relevant ICD‐10 codes. Medical records of eligible hospitalizations that occurred after 2004 were abstracted and independently reviewed by 2 trained and certified physicians on the ARIC HF classification committee.25
Covariates
Sociodemographic factors ascertained at visit 1 and included in analyses were age, race by ARIC study field center (white, Forsyth County; black, Forsyth County; white, Washington County; white Minneapolis; black, Jackson), sex, and education (high school or less, vocational school, some college or college degree, higher than college degree).
Smoking status, alcohol intake, BMI, blood pressure, diabetes mellitus, and HDL‐C were assessed following a standardized protocol at visits 1 to 4. Smoking status was based on self‐report and categorized as never, former, and current smokers. Alcohol intake was measured by asking participants to report their usual intake of alcoholic beverages of a standard serving of wine, beer, and hard liquor per week. The alcohol amount of each type of drink (4‐oz glass of wine=10.8 g, 12 oz of beer=13.2 g, 1.5 oz of hard liquor=15.1 g) was multiplied by number of drinks and summed for a weekly intake. Alcohol intake was further categorized as no current intake, ≤100 g, and >100 g.26
Weight and height were measured and used to calculate BMI as weight in kilograms divided by square of height in meters (categorized as underweight/normal <25.0, overweight 25.0 to <30, and obese ≥30.0).27 Seated blood pressure after 5 minutes of rest was measured at all visits by a trained technician. The average of the second and third measurements was used. Participants were classified as hypertensive if systolic blood pressure was ≥140 mm Hg, if diastolic blood pressure was ≥90 mm Hg, or if use of antihypertensive medication was reported in the past 2 weeks. Diabetes mellitus status was considered positive if study participants had been told by a physician they had diabetes mellitus or if they had fasting plasma glucose ≥126 mg/dL, had nonfasting glucose ≥200 mg/dL, or reported use of hypoglycemic medication in the past 2 weeks. HDL‐C was collected from 12‐hour fasting blood samples drawn by venipuncture from an antecubital vein and specified as a continuous variable.
Statistical Analysis
Of the 15 792 ARIC participants examined at baseline, we excluded participants with prevalent CVD (having prevalent CHD, stroke, or HF; n=2023) and participants who experienced a CVD event (CHD, stroke, or HF) or death within the first year of follow‐up (n=132). Asian or American Indian/Alaskan Indian participants (n=48) and black participants in Minnesota and Washington County (n=55) were excluded given small numbers. After exclusions, the analytic set comprised 13 534 (86%) participants.
A nonrecoverable illness‐death multistate survival model was used to estimate how participants moved between states of health (state 1), disease (state 2), and all‐cause mortality (state 3; further explained in Data S1, Figure S1A–S1C). In this model, participants started free of disease and moved (1) from health to developing disease or (2) from health to death from any cause. Once a participant developed disease, he or she could move from the disease state to all‐cause mortality. This model had 3 types of transitions: health to disease (T1), health to all‐cause mortality (T2), and disease to all‐cause mortality (T3; number of events for each transition and model is shown in Table S2, and the probability of being in each state given a participant's starting health state is shown in Figure S2A through S2C). Separate models were specified with different nonfatal disease states: CHD, stroke, and HF. No backward transitions were allowed between states. If a participant experienced a fatal disease event (date of CVD event and death were on the same day), he or she moved directly to the all‐cause mortality state. In our analytic sample there were 282 fatal CHD events, 7 fatal stroke events, and 132 fatal HF events; these participants moved directly to the all‐cause mortality state.
The multistate model was estimated as a Markov log‐linear parametric model with an exponential distribution and time‐constant hazard using the msm R package (further explained in Data S1).28, 29 Transition‐specific hazard ratios (HRs) and 95% CIs were estimated for each type of transition (T1, T2, T3). Time‐varying covariates closest in time preceding each transition were used for the specific transition. The exposures and covariates were used on all transitions and did not vary by type of transition. Age was used as the time scale for all models; participants started contributing time to the study at the age they entered at visit 1. Follow‐up continued until the date of last known contact with each participant, death, or the end of the study (December 31, 2016).
Life expectancy at age 50 (upper limit: 95 years), the expected average number of remaining years of life in health and disease states conditional on reaching age 50, was calculated using the Estimating Life Expectancies in Continuous Time (ELECT) R package (described in Data S1).30 We estimated life expectancy at age 50 for comparison to existing studies on physical activity and CVD life expectancy outcomes that used age 50 for their life expectancy estimates.8, 9, 10
We estimated 3 sets of analytic models for each of the multistate models represented in Figure S1A through S1C. First, we specified models that included LTPA and covariates. The covariates included age, sex, race by ARIC center, education, alcohol, and smoking. Second, we specified models that included television and the same covariates used in the LTPA models. Third, we specified models that included LTPA, television, and the same covariates used in both the LTPA and television models.
In LTPA models that did not include television, we also evaluated the impact of additional adjustment for BMI, diabetes mellitus status (yes, no), hypertension (yes, no), and HDL‐C (continuous) in addition to previously mentioned confounders. The additional covariates were limited to sensitivity analyses because they could mediate transitions between LTPA from health to disease, health to all‐cause mortality, and disease to all‐cause mortality.
We used multiple imputation by chained equations to impute missing exposure and covariate data that occurred at each visit (further explained in Data S1).31, 32 We imputed 10 data sets, and the multistate models and life expectancies were estimated in each imputed data set. The HRs, standard errors, and life expectancy estimates were averaged using the Rubin rule.33 All analyses were carried out with SAS v9.4 (SAS Institute) and R v3.3.2 (R Foundation for Statistical Computing) and approved by the institutional review board of the University of North Carolina at Chapel Hill.
Results
The average age at visit 1 was 54 years (SD: 5.7), 56% of participants were female, 26% were black, and 55% had received a high school education or less (Table 1). At visit 1, almost 40% reported no LTPA; of those who reported LTPA, the median was 13.2 MET hours/week. Close to half of all participants viewed television sometimes (47%).
Table 1.
Visit 1 Characteristics of ARIC Study Participants by LTPA and Television Viewing (n=13 534)
| LTPA | Television Viewing | Overall | |||||
|---|---|---|---|---|---|---|---|
| None | < Median | ≥ Median | Seldom/Never | Sometimes | Often/Very Often | ||
| n=5114 | n=4207 | n=4207 | n=2601 | n=6393 | n=4528 | n=13 534 | |
| Age at visit 1, mean (SD) | 54.3 (5.7) | 54.4 (5.7) | 54.5 (5.8) | 53.9 (5.7) | 54.4 (5.7) | 54.7 (5.8) | 54.4 (5.7) |
| Male, % | 2045 (40.0) | 1643 (39.1) | 2222 (52.8) | 1027 (39.5) | 2650 (41.5) | 2231 (49.3) | 5915 (43.7) |
| ARIC center, n (%) | |||||||
| Forsyth County | 1111 (21.7) | 1148 (27.3) | 1225 (29.1) | 694 (26.7) | 1681 (26.3) | 1107 (24.5) | 3485 (25.8) |
| Jackson | 1893 (37.0) | 722 (17.2) | 552 (13.1) | 370 (14.2) | 1484 (23.2) | 1313 (29.0) | 3171 (23.4) |
| Minneapolis | 915 (17.9) | 1255 (29.8) | 1406 (33.4) | 866 (33.3) | 1636 (25.6) | 1074 (23.7) | 3576 (26.4) |
| Washington County | 1195 (23.4) | 1082 (25.7) | 1024 (24.3) | 671 (25.8) | 1592 (24.9) | 1034 (22.8) | 3302 (24.4) |
| Race/ethnicity, n (%) | |||||||
| White | 3076 (60.2) | 3333 (79.2) | 3566 (84.8) | 2190 (84.2) | 4773 (74.7) | 3006 (66.4) | 9977 (73.7) |
| Black | 2038 (39.9) | 874 (20.8) | 641 (15.2) | 411 (15.8) | 1620 (25.3) | 1522 (33.6) | 3557 (26.3) |
| Education, n (%) | |||||||
| High school or less | 3355 (65.7) | 2234 (53.2) | 1808 (43.0) | 1230 (47.3) | 3436 (53.8) | 2726 (60.3) | 7403 (54.8) |
| Vocational | 386 (7.6) | 387 (9.2) | 365 (8.7) | 208 (8.0) | 551 (8.6) | 378 (8.4) | 1138 (8.4) |
| College | 987 (19.3) | 1168 (27.8) | 1398 (33.3) | 821 (31.6) | 1702 (26.7) | 1030 (22.8) | 3553 (26.3) |
| Graduate/professional | 376 (7.4) | 414 (9.9) | 632 (15.0) | 339 (13.1) | 695 (10.9) | 388 (8.6) | 1422 (10.5) |
| Missing | 10 | 4 | 4 | 3 | 9 | 6 | 18 |
| LTPA, n (%) | |||||||
| No LTPA | 807 (31.0) | 2408 (37.7) | 1897 (41.9) | 5114 (37.8) | |||
| <13.2 MET h/wk | 791 (30.4) | 1941 (30.4) | 1473 (32.5) | 4207 (31.1) | |||
| ≥13.2 MET h/wk | 1003 (38.6) | 2044 (32.0) | 1158 (25.6) | 4207 (31.1) | |||
| Missing | 0 | 0 | 0 | 6 | |||
| Television viewing, n (%) | |||||||
| Seldom/never | 807 (15.8) | 791 (18.8) | 1003 (23.9) | 2601 (19.2) | |||
| Sometimes | 2408 (47.1) | 1941 (46.2) | 2044 (48.6) | 6393 (47.3) | |||
| Often/very often | 1897 (37.1) | 1473 (35.0) | 1158 (27.5) | 4528 (33.5) | |||
| Missing | 2 | 2 | 2 | 12 | |||
| Smoking, n (%) | |||||||
| Current smoker | 1616 (31.6) | 994 (23.6) | 833 (19.8) | 521 (20.1) | 1540 (24.1) | 1381 (30.5) | 3446 (25.5) |
| Past smoker | 1347 (26.4) | 1311 (31.2) | 1584 (37.7) | 836 (32.2) | 1971 (30.8) | 1435 (31.7) | 4244 (31.4) |
| Never smoker | 2147 (42.0) | 1900 (45.2) | 1788 (42.5) | 1242 (47.8) | 2880 (45.1) | 1708 (37.8) | 5836 (43.2) |
| Missing | 4 | 2 | 2 | 2 | 2 | 4 | 8 |
| Alcohol intake, n (%) | |||||||
| Not current drinker | 3419 (67.4) | 2543 (60.7) | 2185 (52.2) | 1586 (61.2) | 3886 (61.1) | 2669 (59.3) | 8150 (60.5) |
| ≤100 g | 1020 (20.1) | 1114 (26.6) | 1303 (31.1) | 671 (25.9) | 1635 (25.7) | 1131 (25.1) | 3437 (25.5) |
| >100 g | 637 (12.6) | 535 (12.8) | 701 (16.7) | 333 (12.9) | 842 (13.2) | 698 (15.5) | 1875 (13.9) |
| Missing | 38 | 15 | 18 | 11 | 30 | 30 | 72 |
| BMI, mean (SD) | 28.5 (5.8) | 27.2 (5.0) | 26.7 (4.5) | 26.7 (4.9) | 27.5 (5.2) | 28.0 (5.4) | 27.5 (5.2) |
| Missing | 5 | 2 | 1 | 2 | 1 | 5 | 8 |
| Have hypertension, n (%) | 1917 (37.5) | 1230 (29.3) | 1087 (25.9) | 652 (25.1) | 1971 (30.9) | 1611 (35.6) | 4237 (31.3) |
| Missing | 7 | 5 | 2 | 0 | 9 | 4 | 14 |
| Have diabetes mellitus, n (%) | 649 (12.9) | 395 (9.4) | 332 (7.9) | 202 (7.8) | 631 (10.0) | 542 (12.1) | 1377 (10.3) |
| Missing | 64 | 20 | 24 | 14 | 52 | 42 | 108 |
| HDL‐C, mean (SD) | 51.8 (17.0) | 52.6 (16.7) | 52.6 (17.3) | 54.4 (17.6) | 52.5 (16.7) | 50.9 (17.0) | 52.3 (17.0) |
| Missing | 119 | 39 | 31 | 23 | 94 | 72 | 189 |
ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LTPA, leisure‐time moderate‐to‐vigorous physical activity; MET, metabolic equivalent of task.
Over a median of 27.2 years of follow‐up, 4519 (34%) participants experienced at least 1 nonfatal CVD event and 5475 (40%) died. The average age at incident nonfatal CHD was 69 years (SD: 8.5), 72 years (SD: 8.6) at incident nonfatal stroke, and 74 years (SD: 8.5) at incident nonfatal HF.
LTPA and Television Viewing With Nonfatal Disease and Death
Engagement in any level of LTPA compared with none was associated with a reduced risk of developing nonfatal CHD, stroke, and HF (Table 2). Participation in any LTPA compared with none was also associated with a lower risk of all‐cause mortality in the absence of having each type of CVD. Participants who engaged in LTPA equal to or greater than the median compared with none had a lower risk of all‐cause mortality after developing nonfatal CHD. Similarly, for nonfatal HF, engagement in any level of LTPA compared with none was associated with lower risk of all‐cause mortality after developing nonfatal HF. Results were not sensitive to controlling for additional factors (BMI, hypertension, diabetes mellitus, HDL‐C) that may act as mediators or confounders.
Table 2.
Associations (HR [95% CI]) of LTPA With Nonfatal CHD, Stroke, HF, and All‐Cause Mortality, ARIC Participants (n=13 534)
| T1: Health to Disease | T2: Health to Death | T3: Disease to Death | |
|---|---|---|---|
| Nonfatal CHD | |||
| Events, n | 2426 | 4241 | 1234 |
| Sum of person‐years | 292 957 | 292 957 | 23 887 |
| LTPA | |||
| No LTPA | Ref | Ref | Ref |
| <13.2 MET h/wk | 0.92 (0.82–1.02) | 0.91 (0.84–0.99) | 0.98 (0.84–1.13) |
| ≥13.2 MET h/wk | 0.88 (0.79–0.97) | 0.80 (0.74–0.87) | 0.83 (0.72–0.96) |
| Nonfatal stroke | |||
| Events, n | 1144 | 4741 | 734 |
| Sum of person‐years | 309 746 | 309 746 | 7099 |
| LTPA | |||
| No LTPA | Ref | Ref | Ref |
| <13.2 MET h/wk | 0.86 (0.74–1.00) | 0.92 (0.85–0.99) | 1.06 (0.88–1.28) |
| ≥13.2 MET h/wk | 0.80 (0.68–0.92) | 0.80 (0.74–0.86) | 1.01 (0.83–1.23) |
| Nonfatal HF | |||
| Events, n | 2602 | 3655 | 1820 |
| Sum of person‐years | 304 313 | 304 313 | 12 531 |
| LTPA | |||
| No LTPA | Ref | Ref | Ref |
| <13.2 MET h/wk | 0.87 (0.79–0.95) | 0.94 (0.87–1.02) | 0.88 (0.79–0.99) |
| ≥13.2 MET h/wk | 0.80 (0.72–0.88) | 0.82 (0.76–0.89) | 0.82 (0.73–0.92) |
Models specified separately for each type of disease state. Models adjusted for age, sex, race by ARIC center, education, smoking, and alcohol intake. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; HF, heart failure; HR, hazard ratio; LTPA, leisure‐time moderate‐to‐vigorous physical activity; MET, metabolic equivalent of task; Ref, referent; T, transition.
Watching television seldom/never compared with viewing television often/very often was associated with a reduced risk of nonfatal CHD and HF but was not associated with nonfatal stroke (Table 3). Across all diseases, watching television sometimes or seldom/never was associated with a lower risk of all‐cause mortality in the absence of having each type of CVD. The associations of television viewing with all‐cause mortality after developing each disease were close to the null.
Table 3.
Associations (HR [95% CI]) of Television Viewing With Nonfatal CHD, Stroke, HF, and All‐Cause Mortality, ARIC Participants (n=13 534)
| T1: Health to Disease | T2: Health to Death | T3: Disease to Death | |
|---|---|---|---|
| Nonfatal CHD | |||
| Events, n | 2426 | 4241 | 1234 |
| Sum of person‐years | 292 957 | 292 957 | 23 887 |
| Television viewing | |||
| Often/very often | Ref | Ref | Ref |
| Sometimes | 0.98 (0.89–1.07) | 0.92 (0.86–0.99) | 0.89 (0.79–1.01) |
| Seldom/never | 0.83 (0.74–0.94) | 0.91 (0.84–1.00) | 0.93 (0.79–1.11) |
| Nonfatal stroke | |||
| Events, n | 1144 | 4741 | 734 |
| Sum of person‐years | 309 746 | 309 746 | 7099 |
| Television viewing | |||
| Often/very often | Ref | Ref | Ref |
| Sometimes | 0.98 (0.86–1.12) | 0.90 (0.85–0.96) | 1.04 (0.89–1.22) |
| Seldom/never | 0.98 (0.82–1.17) | 0.89 (0.82–0.97) | 0.95 (0.76–1.19) |
| Nonfatal HF | |||
| Events, n | 2602 | 3655 | 1820 |
| Sum of person‐years | 304 313 | 304 313 | 12 531 |
| Television viewing | |||
| Often/very often | Ref | Ref | Ref |
| Sometimes | 0.81 (0.74–0.88) | 0.97 (0.90–1.04) | 0.97 (0.87–1.07) |
| Seldom/never | 0.87 (0.78–0.97) | 0.91 (0.82–1.00) | 1.02 (0.89–1.17) |
Models are specified separately for each type of disease state. Models are adjusted for age, sex, race by ARIC center, education, smoking, and alcohol intake. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; HF, heart failure; HR, hazard ratio; Ref, referent; T, transition.
The HRs from models that included both LTPA and television were similar to the models that examined these factors separately.
LTPA and Nonfatal CHD, Stroke, and HF Life Expectancy
Engagement in any LTPA compared with none was associated in a positive dose‐response fashion with longer nonfatal disease‐free life expectancy for each type of CVD (Figure 1). At LTPA levels less than the median, disease‐free life expectancy was ≈0.8 year longer compared with no LTPA for each type of nonfatal CVD for men and women. At LTPA equal to or greater than the median, compared with no LTPA, disease‐free life expectancy was ≈1.5 years longer (CHD, men: 1.5 years [95% CI, 1.0–2.0]; women: 1.6 years [95% CI, 1.1–2.2]; stroke, men: 1.8 years [95% CI, 1.2–2.3]; women: 1.8 years [95% CI, 1.3–2.3]; HF, men: 1.6 years [95% CI, 1.1–2.1]; women 1.7 years [95% CI, 1.2–2.2]). For each type of CVD, life expectancy with disease was similar for the 3 levels of LTPA.
Figure 1.
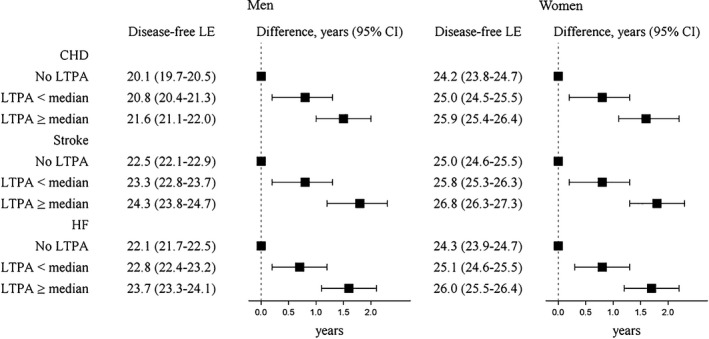
Life expectancy (LE; years) free of nonfatal disease and LE differences at age 50 by LTPA, ARIC (Atherosclerosis Risk in Communities) participants (n=13 534). LTPA categories: less than the median (0.1 to <13.2 MET hours/week), equal to or greater than the median (≥13.2 MET hours/week). All disease states are nonfatal. Models are specified separately for each type of disease state. Models are adjusted for age, sex, race by ARIC center, education, smoking status, and alcohol intake. CHD indicates coronary heart disease; HF, heart failure; LTPA, leisure‐time moderate‐to‐vigorous physical activity; MET, metabolic equivalent of task.
Television and Nonfatal CHD, Stroke, and HF Life Expectancy
Watching television sometimes or seldom/never was associated with longer disease‐free life expectancy compared with viewing often/very often (Figure 2). Compared, for example, with participants who often/very often viewed television, participants who seldom/never watched had longer nonfatal CHD‐free life expectancy (men: 1.1 years [95% CI, 0.5–1.7]; women: 0.9 year [95% CI, 0.3–1.6]), longer nonfatal stroke‐free life expectancy (men: 0.8 year [95% CI, 0.2–1.4]; women: 0.8 year [95% CI, 0.2–1.4]), and longer nonfatal HF‐free life expectancy (men: 0.9 year [95% CI, 0.3–1.5]; women: 1.0 years [95% CI, 0.4–1.6]). Across all diseases, disease‐free life expectancy was similar for the sometimes and seldom/never levels of viewing television. For each type of CVD, life expectancy with nonfatal disease was similar by level of television viewing.
Figure 2.
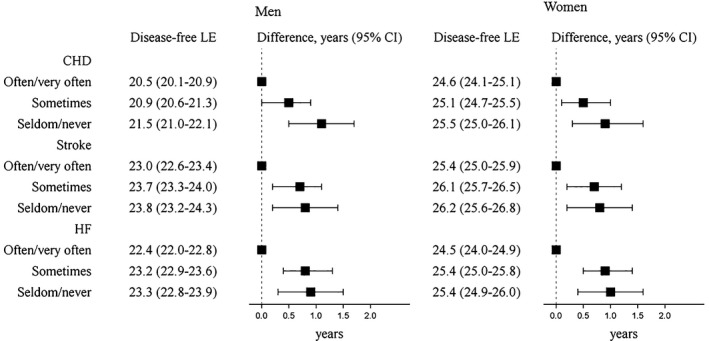
Life expectancy (LE; years) free of nonfatal disease and LE differences at age 50 by television viewing, ARIC (Atherosclerosis Risk in Communities) participants (n=13 534). All disease states are nonfatal. Models are specified separately for each type of disease state. Models are adjusted for age, sex, race by ARIC center, education, smoking status, and alcohol intake. CHD indicates coronary heart disease; HF, heart failure.
Models With Both LTPA and Television With Nonfatal CHD, Stroke, and HF Life Expectancy
Across all diseases, participants who engaged in LTPA equal to or greater than the median and seldom/never viewed television had nonfatal disease‐free life expectancy ≈2.5 years longer compared with participants who reported no LTPA and often/very often watched television (CHD, men: 2.4 years [95% CI, 1.7–3.2]; women: 2.4 years [95% CI, 1.7–3.2]; stroke, men: 2.4 years [95% CI, 1.6–3.1]; women: 2.4 years [95% CI, 1.7–3.2]; HF, men: 2.4 years [95% CI, 1.7–3.1]; women: 2.5 years [95% CI, 1.8–3.2]; Figure 3).
Figure 3.

Life expectancy (LE; years) free of nonfatal disease and LE differences at age 50 by LTPA and television viewing, ARIC (Atherosclerosis Risk in Communities) participants (n=13 534). A, Nonfatal coronary heart disease. B, Nonfatal stroke. C, Nonfatal heart failure. LTPA categories: < median (0.1 to <13.2 MET hours/week), ≥ median (≥13.2 MET hours/week) television categories: high television is viewing often/very often, some television is sometimes viewing, and low television is seldom/never viewing television. Models are specified separately for each type of disease state. Models are adjusted for age, sex, race by ARIC center, education, smoking status, and alcohol intake. LTPA indicates leisure‐time moderate‐to‐vigorous physical activity; MET, metabolic equivalent of task; TV, television.
Discussion
In this large prospective cohort of adults, engagement in any level of LTPA was associated with longer nonfatal CVD‐free life expectancy compared with engaging in no LTPA. Such associations were observed for nonfatal CHD, stroke, and HF. The inverse association of television viewing with CVD‐free life expectancy outcomes was of modest magnitude, with a small gain in CVD‐free life expectancy for viewing television sometimes and seldom/never compared with often/very often.
The observed pattern of extended disease‐free life expectancy with higher LTPA levels is consistent with published reports.8, 9, 10 In reports from the Framingham Heart Study, participants who self‐reported high compared with low levels of physical activity had CVD‐free life expectancy that was longer by about 3 years.8, 9 Nevertheless, it is difficult to compare our MET hours/week of LTPA with the physical activity measurement used in the Framingham cohort, which was estimated as a weighted sum of daily activities based on sleeping, resting, and engaging in light, moderate, or heavy activity.8 O'Doherty et al10 separately analyzed data from 3 European‐based cohorts and observed that participation in vigorous activity compared with no vigorous physical activity was associated with longer CVD‐free life expectancy (estimates ranged from 3 to 5 years). Although we found similar trends in LTPA extending years spent free of CVD, the magnitude of our findings was smaller compared with these other studies. These differences may be due to characteristics of each cohort, the various methods of physical activity measurement, and different analytic methods and covariates used.
Our report extends the understanding of how physical activity is associated with CVD life expectancy outcomes by measuring LTPA as MET hours/week to account for intensity of activity and separately examining 3 types of CVD. We observed a positive dose‐response relationship between LTPA and nonfatal disease‐free life expectancy. At LTPA less than the median, nonfatal disease‐free life expectancy was longer by 0.8 years for each type of CVD compared with no LTPA. At LTPA equal to or greater than the median, we observed nonfatal disease‐free life expectancy close to 2 years longer compared with no LTPA. For reference, a person who engages in a walk at 3 miles/hour, estimated to be 3.3 METs, for 5 days/week at 48 min/day can expend 13.2 MET hours/week (the cut point for our highest LTPA category).
We separately examined 3 types of nonfatal CVD and observed homogeneity of associations of LTPA with nonfatal CHD, stroke, and HF. The HRs we estimated for how LTPA was associated with nonfatal incident CHD, stroke, and HF are consistent with existing research regarding the direction and magnitude of estimates when physical activity has been specified as MET hours/week.5 The similarities of associations of LTPA with these 3 CVDs may be because physical activity has been linked to reduced adiposity, improved blood pressure, prevention of diabetes mellitus, and improvement in lipid profiles—factors that are all risk factors for CHD, stroke, and HF.34
Our study is the first, to our knowledge, to examine how sedentary behavior is associated with nonfatal CVD life expectancy outcomes. We observed a gain in nonfatal disease‐free life expectancy of ≈1 year for sometimes and seldom/never viewing television compared with viewing often/very often, but the estimates for the sometimes and seldom/never viewers were similar, with overlapping CIs. Our associations of HRs of television viewing with incident nonfatal CVD and all‐cause mortality are in the same direction as existing literature but are somewhat weaker.35, 36 Often television viewing is measured as hours/week spent viewing, and studies using this type of measure have estimated a higher risk of incident CVD at ≥2 hours of viewing per day and a higher risk of all‐cause mortality at 3 and 4 hours per day of viewing.35, 36 A limitation of our study is that it did not assess the number of hours spent watching television.
We analyzed each type of nonfatal CVD separately but observed that 32% participants who developed CVD experienced multiple CVD events. In additional analysis, we estimated life expectancy at age 50 for a composite CVD outcome that was the first occurrence of nonfatal CHD, stroke, or HF. The life expectancy differences by LTPA and television with the composite CVD outcome were similar to associations observed when examining each disease individually. However, life expectancy free of nonfatal CVD was lower and life expectancy with nonfatal CVD was higher than the respective life expectancy estimates when separately examining each disease.
To estimate CVD‐free life expectancy, we used a health expectancy outcome, a summary measure that estimates expected years to live in good and poor health. Improving and maintaining the health of older adults is a priority because this population is projected to grow and chronic diseases often develop at older ages.37 Other strengths of this study include use of time‐varying covariates, well‐measured outcomes from active surveillance and adjudication of outcomes, long‐term follow‐up of >30 years, and multiple imputation of missing exposure and covariate data. Although we included time‐varying measurements of physical activity, we did not have a physical activity measurement after visit 3 and we used a baseline measure of television viewing. It is possible that participant levels for both types of behaviors changed after the last measurement of each. In addition, many participants transitioned into retirement over follow‐up. For many participants, we were not able to capture changes that may have occurred with LTPA and television behaviors after this life transition.
Other limitations of this report include the self‐reported nature of LTPA and extent of television viewing and the single domains of physical activity and sedentary behavior. Levels of physical activity tend to be overestimated when collected by self‐report; however, the Baecke questionnaire is similar in reliability and validity to other physical activity questionnaires.38, 39 We included physical activity during leisure‐time and did not include activity that takes place during occupation, transport, or housework activities. It is possible our associations could be stronger with the inclusion of physical activity that occurs from multiple domains. Television viewing represents only one domain of sedentary behavior and does not include time spent sitting for work or transport.
Conclusion
Engagement in any level of LTPA and viewing television sometimes or seldom/never were associated with longer life expectancy free of nonfatal CHD, stroke, and HF. Our findings suggest that engaging in LTPA and watching less television could increase the number of years lived free of nonfatal CHD, stroke, and HF.
Sources of Funding
Cuthbertson was supported by a National Heart, Lung, and Blood Institute National Research Service Award (T32‐HL007055‐41). The ARIC (Atherosclerosis Risk in Communities) study was funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. ARIC (Atherosclerosis Risk in Communities) Visits When Exposures and Covariates Were Assessed
Table S2. Number of Participants Who Experienced Each State*
Figure S1. Three‐state multistate survival models used for coronary heart disease, stroke, and heart failure analysis.
Figure S2. Stacked transition probabilities to all states from the health state.
Acknowledgments
We would like to thank Dr Chirayath M. Suchindran for his guidance on multistate models and multistate life expectancies. The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions.
(J Am Heart Assoc. 2019;8:e012657 DOI: 10.1161/JAHA.119.012657.)
References
- 1. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, Ferrari AJ, Kasaeian A, Werdecker A, Carter A, Zipkin B, Sartorius B, Serdar B, Sykes BL, Troeger C, Fitzmaurice C, Rehm CD, Santomauro D, Kim D, Colombara D, Schwebel DC, Tsoi D, Kolte D, Nsoesie E, Nichols E, Oren E, Charlson FJ, Patton GC, Roth GA, Hosgood HD, Whiteford HA, Kyu H, Erskine HE, Huang H, Martopullo I, Singh JA, Nachega JB, Sanabria JR, Abbas K, Ong K, Tabb K, Krohn KJ, Cornaby L, Degenhardt L, Moses M, Farvid M, Griswold M, Criqui M, Bell M, Nguyen M, Wallin M, Mirarefin M, Qorbani M, Younis M, Fullman N, Liu P, Briant P, Gona P, Havmoller R, Leung R, Kimokoti R, Bazargan‐Hejazi S, Hay SI, Yadgir S, Biryukov S, Vollset SE, Alam T, Frank T, Farid T, Miller T, Vos T, Barnighausen T, Gebrehiwot TT, Yano Y, Al‐Aly Z, Mehari A, Handal A, Kandel A, Anderson B, Biroscak B, Mozaffarian D, Dorsey ER, Ding EL, Park EK, Wagner G, Hu G, Chen H, Sunshine JE, Khubchandani J, Leasher J, Leung J, Salomon J, Unutzer J, Cahill L, Cooper L, Horino M, Brauer M, Breitborde N, Hotez P, Topor‐Madry R, Soneji S, Stranges S, James S, Amrock S, Jayaraman S, Patel T, Akinyemiju T, Skirbekk V, Kinfu Y, Bhutta Z, Jonas JB, Murray CJL. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3. Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, Murray CJ. Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet. 2012;380:2144–2162. [DOI] [PubMed] [Google Scholar]
- 4. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018. Available at: https://health.gov/paguidelines/second-edition/report.aspx. Accessed July 24, 2019.
- 5. Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Friedemann Smith C, Wilkins E, Rayner M, Roberts N, Scarborough P. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e002495 DOI: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9:e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. [DOI] [PubMed] [Google Scholar]
- 8. Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165:2355–2360. [DOI] [PubMed] [Google Scholar]
- 9. Nusselder WJ, Franco OH, Peeters A, Mackenbach JP. Living healthier for longer: comparative effects of three heart‐healthy behaviors on life expectancy with and without cardiovascular disease. BMC Public Health. 2009;9:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Doherty MG, Cairns K, O'Neill V, Lamrock F, Jorgensen T, Brenner H, Schottker B, Wilsgaard T, Siganos G, Kuulasmaa K, Boffetta P, Trichopoulou A, Kee F. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur J Epidemiol. 2016;31:455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey A, Salahuddin U, Garg S, Ayers C, Kulinski J, Anand V, Mayo H, Kumbhani DJ, de Lemos J, Berry JD. Continuous dose‐response association between sedentary time and risk for cardiovascular disease: a meta‐analysis. JAMA Cardiol. 2016;1:575–583. [DOI] [PubMed] [Google Scholar]
- 12. Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, Bauman AE, van der Ploeg HP. Daily sitting time and all‐cause mortality: a meta‐analysis. PLoS One. 2013;8:e80000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–542. [DOI] [PubMed] [Google Scholar]
- 14. Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 17. Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle‐aged women and men. Med Sci Sports Exerc. 1997;29:901–909. [DOI] [PubMed] [Google Scholar]
- 18. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services . Physical Activity Guidelines Advisory Committee report. 2008. Available at: https://health.gov/paguidelines/guidelines/report.aspx. Accessed March 6, 2017.
- 20. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 21. Atherosclerosis Risk in Communities Study . Manual 3 Surveillance Component Procedures Manual of Operations Version 6.6. 2015. Available at: https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Manual3_Ver%206.6_20151112.pdf. Accessed July 24, 2019.
- 22. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 23. Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2014;45:3219–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atherosclerosis Risk in Communities Study . Manual 3A Surveillance of Heart Failure Manual of Operations Version 2.1. 2011. Available at: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/GetPdf.cgi?id=phd004516.1. Accessed July 24, 2019.
- 25. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) Study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njolstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Camara AG, Volzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno‐Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG II, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca‐Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundstrom J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kuhn T, Grobbee DE, Barrett‐Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Despres JP, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjonneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J. Risk thresholds for alcohol consumption: combined analysis of individual‐participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 28. Jackson CH. Multi‐state models for panel data: the msm package for R. J Stat Softw. 2011;38:1–28. [Google Scholar]
- 29. Jackson CH. Multi‐state modelling with R: the msm package. 2016. Available at: https://cran.r-project.org/web/packages/msm/vignettes/msm-manual.pdf. Accessed July 24, 2019.
- 30. van den Hout A. ELECT: estimation of life expectancies using continuous‐time multi‐state survival models. 2014. Available at: http://www.ucl.ac.uk/~ucakadl/ELECT/ELECTManual_version0_1_2.pdf. Accessed February 20, 2017. [DOI] [PubMed] [Google Scholar]
- 31. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 33. Rubin DR. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 34. Labarthe DR. Epidemiology and Prevention of Cardiovascular Diseases: A Global Challenge. Sudbury, MA: Jones and Bartlett Publishers; 2011. [Google Scholar]
- 35. Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all‐cause mortality: a meta‐analysis. JAMA. 2011;305:2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Association between television viewing time and all‐cause mortality: a meta‐analysis of cohort studies. Am J Epidemiol. 2015;182:908–916. [DOI] [PubMed] [Google Scholar]
- 37. He W, Goodkind D, Kowal P. An aging world: 2015. International Population Reports, P95/16‐1 2016. Available at: https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf. Accessed July 24, 2019.
- 38. Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure‐time physical activity. Int J Epidemiol. 1995;24:685–693. [DOI] [PubMed] [Google Scholar]
- 39. Jacobs DR Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. ARIC (Atherosclerosis Risk in Communities) Visits When Exposures and Covariates Were Assessed
Table S2. Number of Participants Who Experienced Each State*
Figure S1. Three‐state multistate survival models used for coronary heart disease, stroke, and heart failure analysis.
Figure S2. Stacked transition probabilities to all states from the health state.


