Abstract
Background
The incidence and predictors of atrial fibrillation (AF) progression are currently not well defined, and clinical AF progression partly overlaps with rhythm control interventions (RCIs).
Methods and Results
We assessed AF type and intercurrent RCIs during yearly follow‐ups in 2869 prospectively followed patients with paroxysmal or persistent AF. Clinical AF progression was defined as progression from paroxysmal to nonparoxysmal or from persistent to permanent AF. An RCI was defined as pulmonary vein isolation, electrical cardioversion, or new treatment with amiodarone. During a median follow‐up of 3 years, the incidence of clinical AF progression was 5.2 per 100 patient‐years, and 10.9 per 100 patient‐years for any RCI. Significant predictors for AF progression were body mass index (hazard ratio [HR], 1.03; 95% CI, 1.01–1.05), heart rate (HR per 5 beats/min increase, 1.05; 95% CI, 1.02–1.08), age (HR per 5‐year increase 1.19; 95% CI, 1.13–1.27), systolic blood pressure (HR per 5 mm Hg increase, 1.03; 95% CI, 1.00–1.05), history of hyperthyroidism (HR, 1.71; 95% CI, 1.16–2.52), stroke (HR, 1.50; 95% CI, 1.19–1.88), and heart failure (HR, 1.69; 95% CI, 1.34–2.13). Regular physical activity (HR, 0.80; 95% CI, 0.66–0.98) and previous pulmonary vein isolation (HR, 0.69; 95% CI, 0.53–0.90) showed an inverse association. Significant predictive factors for RCIs were physical activity (HR, 1.42; 95% CI, 1.20–1.68), AF‐related symptoms (HR, 1.84; 95% CI, 1.47–2.30), age (HR per 5‐year increase, 0.88; 95% CI, 0.85–0.92), and paroxysmal AF (HR, 0.61; 95% CI, 0.51–0.73).
Conclusions
Cardiovascular risk factors and comorbidities were key predictors of clinical AF progression. A healthy lifestyle may therefore reduce the risk of AF progression.
Keywords: atrial fibrillation, epidemiology, predictors, progression, rhythm control
Subject Categories: Atrial Fibrillation, Epidemiology
Clinical Perspective
What Is New?
The incidence for clinical atrial fibrillation (AF) progression was relatively low (5.2 cases per 100 patient‐years of follow‐up).
Several potentially modifiable risk factors and comorbidities were key predictors of clinical AF progression.
Patients with and without rhythm control interventions had a similar AF progression rate, although pulmonary vein isolation was associated with a lower AF progression rate.
What Are the Clinical Implications?
A healthy lifestyle may help to reduce the risk of AF progression.
The role of rhythm control interventions in the prevention of AF progression is less clear.
Introduction
Current thinking indicates that atrial fibrillation (AF) usually progresses from short, rare episodes to longer and more frequent attacks.1 Patients who develop more sustained forms of the disease are less amenable to treatment and are thought to have a worse outcome.2, 3 A recent meta‐analysis suggested a higher risk of thromboembolism and death among patients with sustained compared with intermittent forms of AF.4
In clinical practice, AF is classified into paroxysmal, persistent, or permanent AF.1 Even though the classification poorly reflects temporal persistence of the arrhythmia,5 it is commonly used in daily clinical practice. In a recent meta‐analysis, the cumulative incidence of AF progression was 8.1 per 100 patient‐years. Main predictors explaining between‐study heterogeneity were age, hypertension, follow‐up duration, and baseline AF type.6
However, previous studies did not take into account at least 2 important issues. First, the change in AF type is not exclusively unidirectional, as AF may also regress to less sustained forms, and prior studies have not acknowledged this. Second, AF progression may be masked to some extent by the use of rhythm control interventions (RCIs). The most effective RCIs currently available are antiarrhythmic treatment with amiodarone, direct electrical cardioversion (ECV) and pulmonary vein isolation (PVI).7 While RCIs partly overlap with the definition of the clinical AF type, they constitute an independent entity that needs to be taken into account separately.
A better understanding of clinical AF progression and its associated risk factors will improve risk prediction and help to plan specific intervention studies to prevent AF progression. In the current study, we aimed to assess the incidence and associated predictors of clinical AF progression and RCIs in a large cohort of prospectively followed patients with paroxysmal and persistent AF.
Methods
The consent forms, as approved by the local ethics committee (Ethikkommission Nordwest‐ und Zentralschweiz), do not allow the data to be made publicly available. Researchers may contact the authors for the potential submission of research proposals for future analyses.
Study Design and Participants
To increase sample size and power, we combined data from 2 ongoing prospective, observational, multicenter cohort studies of AF patients in Switzerland. Both cohorts used similar enrollment criteria, outcome definitions and case report forms.
Between 2010 and 2014, the BEAT‐AF (Basel Atrial Fibrillation) cohort study recruited 1553 patients with documented AF across 7 centers in Switzerland. The Swiss‐AF (Swiss Atrial Fibrillation) study enrolled 2415 AF patients between 2014 and 2017 across 14 centers in Switzerland. The detailed methodology for Swiss‐AF was described earlier.8 In both cohorts, all patients were required to have previously documented AF. The main exclusion criteria for both cohorts were the inability to sign informed consent and secondary forms of AF (eg, AF after cardiac surgery). Patients with an acute illness within the past 4 weeks could only be enrolled once the acute episode had resolved. Patients enrolled in BEAT‐AF were not eligible for participation in Swiss‐AF and vice versa.
From the combined data set of BEAT‐AF and Swiss‐AF, we excluded 942 (23.7%) patients with permanent AF at baseline. From the remaining 3026 patients, we excluded 144 (4.8%) patients without follow‐up information on AF type, 8 (0.3%) patients without follow‐up information on RCIs, and 5 (0.1%) patients with double inclusion, such that 2869 patients remained in the final analyses. For the present analysis, we used available data up to October 12, 2018.
The study protocols of both studies were approved by the local ethics committees, and informed written consent was obtained from each participant.
Assessments
In both cohorts, patients completed similar questionnaires about personal, medical, nutritional, and lifestyle factors on a yearly basis. Smoking status was categorized into current smokers and noncurrent smokers (past or never smokers). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Local investigators classified the current AF type according to the 2010 guidelines of the European Society of Cardiology into paroxysmal AF (self‐terminating, usually within 48 hours), persistent AF (episodes lasting >7 days or requiring termination by electrical or medical cardioversion) or permanent AF (AF is accepted by the patient and the physician, and no further attempts to restore sinus rhythm are performed).1 For consistency reasons we did not apply the updated 2016 guidelines in which AF episodes cardioverted within 7 days are considered paroxysmal AF.7 The current AF type was determined by the local study investigator during the baseline or follow‐up visits on the basis of all available clinical patient data. We did not distinguish between long‐standing persistent and permanent AF. Coronary artery disease was defined as either a history of myocardial infarction and/or percutaneous transluminal coronary angioplasty and/or coronary bypass graft. Physical activity was assessed using a question about whether participants perform physical activity on a regular basis or not.
After a face‐to‐face examination at baseline, all yearly follow‐up examinations in BEAT‐AF were performed by paper‐based mailed questionnaires and subsequent telephone interviews. In Swiss‐AF, all patients were assessed yearly by clinical follow‐up visits. In both cohorts, patients completed information about personal factors, and trained study personnel subsequently updated AF type, comorbidities, medication, medical interventions, and intercurrent adverse events during the clinical visits (Swiss‐AF) or telephone interviews (BEAT‐AF), respectively. The current AF type was assessed in both cohorts during each baseline and follow‐up visit, into paroxysmal, persistent, or permanent on the basis of clinical information and medical reports.
Definitions and Outcomes
Clinical AF progression was defined as AF progression from paroxysmal AF at baseline to nonparoxysmal AF (persistent or permanent AF) at the latest follow‐up or as AF progression from persistent AF at baseline to permanent AF at the latest follow‐up. To take into account AF regression, intermittent classification into higher clinical AF categories with subsequent regression to the same or a lower clinical category by the latest follow‐up was not counted as AF progression.
Intercurrent RCI was defined as undergoing either ECV, PVI, or treatment with amiodarone during prospective follow‐up. For the RCI analyses we excluded all patients (n=597; 20.8%) who were receiving amiodarone at baseline.
Statistical Analysis
Baseline characteristics were stratified by baseline AF type (paroxysmal versus persistent). Categorical variables were presented as numbers (percentages) and compared using χ2 tests. The distribution of continuous variables was checked using kurtosis, skewness, and visual inspection of the histogram (not presented). They were presented as means± SDs or median (interquartile range) and compared using Student t tests or Wilcoxon rank‐sum tests, as appropriate.
We constructed Kaplan–Meier Cumulative Incidence Curves for clinical AF progression and RCIs. Differences in incidence rates for AF progression from paroxysmal AF to nonparoxysmal AF versus AF progression from persistent AF to permanent AF were compared using a log‐rank test.
To identify independent predictors of clinical AF progression or RCI, we constructed Cox regression models to calculate hazard ratios (HRs) and 95% CIs. The proportional hazard assumption for the Cox models has been assessed by creating interactions of the predictors and a function of the survival time (not presented). All models included a predefined set of covariates: age, sex, BMI, heart rate, systolic blood pressure, history of diabetes mellitus, history of coronary artery disease, history of hypertension, history of heart failure, history of stroke and/or transient ischemic attack, history of hyperthyroidism, history of renal failure, regular physical activity, smoking (current versus history/never smoker), AF type at baseline (paroxysmal versus persistent), history of PVI, and presence of AF‐related symptoms. Regression models on clinical AF progression were additionally adjusted for amiodarone treatment at baseline. P values for interaction were calculated by adding a multiplicative interaction term.
We also performed sensitivity analyses for RCI and clinical AF progression among patients without a history of PVI or ECV at baseline.
Statistical analyses were performed using SAS 9.4 (SAS Corporation, Cary, NC) or STATA software version 12.0 (StataCorp, College Station, TX). A 2‐sided P<0.05 was considered to indicate statistical significance.
Results
We included 1854 (65%) patients with paroxysmal and 1015 (35%) patients with persistent AF in the analysis. Baseline characteristics stratified by baseline AF type are shown in Table 1. Age was similar in patients with paroxysmal and persistent AF (70±11 versus 70±9 years; P=0.211). Patients with paroxysmal AF had a lower BMI (27.0±4.8 versus 27.8±4.7; P<0.001), engaged more often in regular physical activity (995 [54%] versus 490 [48%]; P=0.005) and reported more AF‐related symptoms (1371 [75%] versus 664 [66%]; P<0.001), while they had a lower prevalence of heart failure (285 [15%] versus 278 [27%]; P<0.001) and hypertension (1182 [64%] versus 709 [70%]; P=0.001) (Table 1).
Table 1.
Baseline Characteristics Stratified by Baseline AF Type
| Characteristic | Paroxysmal (n=1854) | Persistent (n=1015) | P Value |
|---|---|---|---|
| Age, y | 70±11 | 70±9 | 0.211 |
| Female sex, N (%) | 598 (32.3) | 254 (25.0) | <0.001 |
| White race, N (%) | 1825 (98.4) | 1008 (99.3) | 0.156 |
| Body mass index, kg/m2 | 27.0±4.8 | 27.8±4.7 | <0.001 |
| Heart rate, beats/min | 63 (56–72) | 68 (59–80 | <0.001 |
| Systolic blood pressure, mm Hg | 135±19 | 134±19 | 0.025 |
| History of coronary heart disease, N (%) | 430 (23.2) | 251 (24.7) | 0.355 |
| History of stroke/TIA, N (%) | 327 (17.6) | 144 (14.2) | 0.018 |
| History of hypertension, N (%) | 1182 (63.8) | 709 (69.9) | 0.001 |
| History of heart failure, N (%) | 285 (15.4) | 278 (27.4) | <0.001 |
| History of diabetes mellitus, N (%) | 233 (12.6) | 148 (14.6) | 0.129 |
| History of renal failure, N (%) | 263 (14.2) | 181 (17.8) | 0.010 |
| History of hyperthyroidism, N (%) | 55 (3.0) | 57 (5.6) | <0.001 |
| Current smoker, N (%) | 152 (8.2) | 86 (8.5) | 0.809 |
| Regular physical activity, N (%) | 995 (53.9) | 490 (48.4) | 0.005 |
| History of pulmonary vein isolation, N (%) | 476 (25.7) | 269 (26.5) | 0.640 |
| History of electrical cardioversion, N (%) | 336 (18.2) | 676 (66.7) | <0.001 |
| AF‐related symptoms, N (%) | 1371 (75.4) | 664 (66.1) | <0.001 |
P values are based on χ2 tests, Student t tests or Wilcoxon rank‐sum tests as appropriate. AF indicates atrial fibrillation; TIA, transient ischemic attack.
Clinical Atrial Fibrillation Progression
During a median (interquartile range) follow‐up of 3.0 (2.0; 5.0) years, 458 of 2869 (16.0%) patients had clinical AF progression (incidence 5.2 per 100 patient‐years of follow‐up (Table S1). The corresponding Kaplan–Meier estimates are presented in Figure 1. The incidence per 100 patient‐years was 4.9 for AF progression from paroxysmal to nonparoxysmal versus 5.8 for AF progression from persistent to permanent AF (P for difference=0.082; Table S1). When excluding patients with a history of ECV or PVI at baseline and those with any RCI during follow‐up, 151 of the remaining 963 patients (15.7%) had clinical AF progression, corresponding to an incidence of 5.4 per 100 patient‐years (Table S1).
Figure 1.
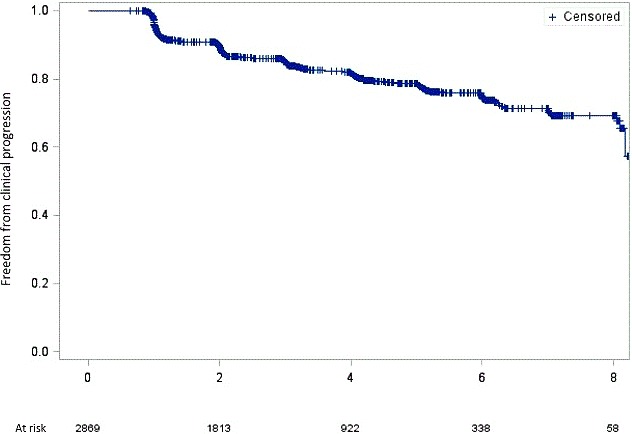
Kaplan–Meier estimates for clinical atrial fibrillation progression. The x axis represents the time of follow‐up in years. The y axis represents freedom from clinical atrial fibrillation progression.
Predictors for clinical AF progression are presented in Table 2. BMI (HR, 1.03; 95% CI, 1.01‐1.05; P=0.016), heart rate (HR per 5 beats/min increase, 1.05; 95% CI, 1.02–1.08; P<0.001), age (HR per 5‐year increase, 1.19; 95% CI, 1.13–1.27; P<0.001), systolic blood pressure (HR per 5 mm Hg, 1.03; 95% CI, 1.00–1.05; P=0.050), history of hyperthyroidism (HR, 1.71; 95% CI, 1.16–2.52; P=0.007), history of stroke/transient ischemic attack (HR, 1.50; 95% CI, 1.20; 1.88; P<0.001) and history of heart failure (HR, 1.69; 95% CI, 1.34–2.13; P<0.001) were associated with a higher incidence of AF progression. Regular physical activity (HR, 0.80; 95% CI, 0.66–0.98; P=0.028) and previous PVI (HR, 0.69; 95% CI, 0.53–0.90; P=0.006) were protective for AF progression. The associations were widely comparable, both in direction and magnitude, in models that were stratified for either baseline AF type or study cohort (Tables S2 and S3).
Table 2.
Risk Factors for Clinical AF Progression
| Characteristic (n=2869) | Age/Sex Adjusted | P Value | Multivariable Adjusted | P Value |
|---|---|---|---|---|
| Age | 1.25 (1.19–1.32) | <0.001 | 1.19 (1.13–1.27) | <0.001 |
| Female sex | 0.85 (0.70–1.04) | 0.119 | 0.87 (0.70–1.08) | 0.220 |
| BMI | 1.04 (1.02–1.06) | <0.001 | 1.03 (1.01–1.05) | 0.016 |
| Heart rate | 1.06 (1.03–1.08) | <0.001 | 1.05 (1.02–1.08) | <0.001 |
| Systolic blood pressure | 1.01 (0.99–1.04) | 0.436 | 1.03 (1.00–1.05) | 0.050 |
| History of diabetes mellitus | 1.14 (0.88–1.48) | 0.328 | 0.92 (0.69–1.21) | 0.549 |
| History of coronary artery disease | 1.14 (0.91–1.41) | 0.250 | 0.98 (0.77–1.23) | 0.833 |
| History of hypertension | 1.14 (0.93–1.41) | 0.207 | 0.94 (0.75–1.18) | 0.611 |
| History of stroke and/or TIA | 1.51 (1.21–1.88) | <0.001 | 1.50 (1.19–1.88) | <0.001 |
| History of heart failure | 1.82 (1.48–2.24) | <0.001 | 1.69 (1.34–2.13) | <0.001 |
| History of hyperthyroidism | 1.72 (1.17–2.51) | 0.006 | 1.71 (1.16–2.52) | 0.007 |
| History of renal failure | 1.31 (1.03–1.66) | 0.029 | 1.09 (0.84–1.42) | 0.514 |
| Regular physical activity | 0.72 (0.60–0.87) | <0.001 | 0.80 (0.66–0.98) | 0.028 |
| Current smoking | 1.19 (0.84–1.68) | 0.339 | 1.04 (0.72–1.49) | 0.844 |
| Paroxysmal AF | 0.84 (0.69–1.02) | 0.072 | 0.99 (0.80–1.21) | 0.903 |
| History of pulmonary vein isolation | 0.62 (0.48–0.80) | <0.001 | 0.69 (0.53–0.90) | 0.006 |
| AF‐related symptoms at baseline | 0.82 (0.66–1.01) | 0.058 | 0.86 (0.69–1.06) | 0.164 |
| Amiodarone use at baseline | 0.97 (0.77–1.22) | 0.787 | 0.89 (0.70–1.13) | 0.343 |
Data are hazard ratios (95% CI) based on Cox regression models. Age per 5‐year increase; heart rate per 5 beats/min increase, systolic blood pressure per 5 mm Hg increase; multivariable models included all variables from the table (age, sex, BMI, heart rate, systolic blood pressure, history of diabetes mellitus, history of coronary artery disease, history of hypertension, history of stroke/TIA, history of heart failure, history of hyperthyroidism, history of renal failure, regular physical activity, current smoking, history of pulmonary vein isolation, AF‐related symptoms, amiodarone). A maximum of 85 (3.0%) observations were deleted because of missing variables. AF indicates atrial fibrillation; BMI, body mass index; TIA, transient ischemic attack.
Rhythm Control Interventions
During follow‐up, 617 of 2272 patients (27.2%) not on amiodarone treatment at baseline were treated with an RCI, defined as either a PVI and/or ECV and/or new treatment with amiodarone (incidence, 10.9 per 100 patient‐years of follow‐up; Figure 2 and Table S1). When separating the combined RCI end point into its components, there were 199 (8.8%) patients receiving newly prescribed amiodarone (incidence, 2.8 per 100 patient‐years), 282 (12.4%) having ≥1 ECVs (incidence, 4.2 per 100 patient‐years), and 358 (15.8%) having ≥1 PVI (incidence, 5.7 per 100 patient‐years; Table S1). Among 1221 patients without a history of PVI or ECV at baseline, 258 (21.1%) had any RCI during follow‐up. The corresponding incidence was 8.0 per 100 patient‐years of follow‐up (Table S1). Patients who were receiving a PVI during follow‐up were younger (62±10 versus 68±10 versus 73±6; P<0.001), had a lower BMI (26±4 versus 28±4 versus 28±5; P<0.001) and reported more often to perform regular physical activity (144 [67%] versus 64 [60%] versus 56 [57%]; P<0.001) than patients treated with ECV or amiodarone. On the other hand, most of the comorbidities were more prevalent among patients receiving an ECV or amiodarone during follow‐up compared with those treated with a PVI (Table S4).
Figure 2.
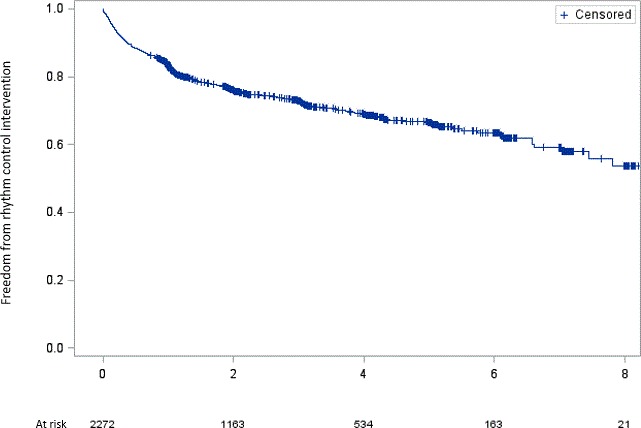
Kaplan–Meier estimates for rhythm control intervention. The x axis represents the time of follow‐up in years. The y axis represents freedom from rhythm control intervention.
Table 3 shows the association of covariates with any RCI during follow‐up. Variables independently associated with RCI were regular physical activity (HR, 1.42; 95% CI, 1.20–1.68; P<0.001), AF‐related symptoms at baseline (HR, 1.84; 95% CI, 1.47–2.30; P<0.001), age (HR per 5‐year increase, 0.88; 95% CI, 0.85–0.92; P<0.001), and paroxysmal AF at baseline (HR, 0.61; 95% CI, 0.51–0.73; P<0.001). Exclusion of patients with a history of PVI and/or ECV at baseline did not influence the results (Table S5).
Table 3.
Factors Associated With Rhythm Control Interventions
| Characteristic (n=2272) | Age/Sex Adjusted | P Value | Multivariable Adjusted | P Value |
|---|---|---|---|---|
| Age | 0.86 (0.84–0.89) | <0.001 | 0.88 (0.85–0.92) | <0.001 |
| Female sex | 1.12 (0.95–1.33) | 0.191 | 1.05 (0.88–1.26) | 0.567 |
| BMI | 1.01 (1.00–1.03) | 0.180 | 1.01 (1.00–1.03) | 0.111 |
| Heart rate | 1.03 (1.01–1.05) | 0.013 | 1.02 (1.00–1.05) | 0.079 |
| Systolic blood pressure | 1.02 (0.99–1.04) | 0.120 | 1.02 (1.00–1.04) | 0.118 |
| History of diabetes mellitus | 0.95 (0.73–1.23) | 0.692 | 1.02 (0.77–1.34) | 0.903 |
| History of coronary artery disease | 0.75 (0.60–0.94) | 0.014 | 0.79 (0.62–1.01) | 0.058 |
| History of hypertension | 1.05 (0.88–1.24) | 0.598 | 1.03 (0.86–1.23) | 0.775 |
| History of stroke/TIA | 0.85 (0.67–1.09) | 0.197 | 0.91 (0.72–1.17) | 0.466 |
| History of heart failure | 1.06 (0.84–1.33) | 0.650 | 1.14 (0.89–1.47) | 0.285 |
| History of hyperthyroidism | 1.24 (0.86–1.78) | 0.260 | 1.08 (0.75–1.57) | 0.678 |
| History of renal failure | 0.80 (0.60–1.05) | 0.109 | 0.80 (0.59–1.07) | 0.129 |
| Regular physical activity | 1.32 (1.12–1.56) | <0.001 | 1.42 (1.20–1.68) | <0.001 |
| Current smoking | 0.98 (0.74–1.28) | 0.858 | 1.04 (0.79–1.37) | 0.778 |
| Paroxysmal AF | 0.62 (0.52–0.73) | <0.001 | 0.61 (0.51–0.73) | <0.001 |
| AF‐related symptoms at baseline | 1.70 (1.37–2.11) | <0.001 | 1.84 (1.47–2.30) | <0.001 |
Data are hazard ratios (95% CI) based on Cox regression models. Rhythm control intervention was defined as either pulmonary vein isolation, electrical cardioversion, and/or new amiodarone. Age per 5‐year increase; heart rate per 5 beats/min increase, systolic blood pressure per 5 mm Hg increase; multivariable models included all variables from the table (age, sex, BMI, heart rate, systolic blood pressure, history of diabetes mellitus, history of coronary artery disease, history of hypertension, history of stroke/TIA, history of heart failure, history of hyperthyroidism, history of renal failure, regular physical activity, current smoking, AF type [paroxysmal AF vs nonparoxysmal AF], AF‐related symptoms). A maximum of 60 (2.6%) observations were deleted because of missing variables. AF indicates atrial fibrillation; BMI, body mass index; TIA, transient ischemic attack.
Discussion
In this large prospective cohort of patients with paroxysmal or persistent AF, we found that the incidence of clinical AF progression was relatively low and independent of baseline AF type. Several risk factors and comorbidities potentially amenable to therapeutic or lifestyle interventions were significantly associated with AF progression. The incidence of RCI was twice as high as the incidence of clinical AF progression, and main determinants for RCI were symptoms, younger age, and physical activity. RCI did not seem to have a major impact on the clinical progression rate.
The incidence of clinical AF progression was 5.2 progression cases per 100 patient‐years of follow‐up. In a recent meta‐analysis we found a cumulative incidence of clinical AF progression of 8.1 per 100 patient‐years of follow‐up.6 Our slightly revised definition of AF progression might partly explain the lower incidence in the current study. The relatively low incidence of clinical AF progression seems to be in contrast with the high overall prevalence of nonparoxysmal AF. For example, in the GARFIELD (Global Anticoagulant Registry in the FIELD) registry, which enrolled >17 000 patients with newly diagnosed AF almost 30% of the study participants had nonparoxysmal AF at baseline.9 We observed a comparable prevalence of 38% nonparoxysmal AF among patients with recent‐onset AF.10 This raises the question of whether there is a specific population of AF patients in which persistent or permanent AF might be the first clinical manifestation of the arrhythmia.
Interestingly, patients without a history of RCI at baseline and not receiving any RCI during follow‐up had a similar incidence of AF progression as the overall cohort. While a history of ECV has been incorporated in the definition of AF type, the other 2 interventions are independent of it. In our study, PVI was associated with a lower AF progression rate. These data are in line with previous studies showing that PVI is more effective than antiarrhythmic medication in restoring and maintaining sinus rhythm in symptomatic AF patients.11, 12, 13 Moreover, a meta‐analysis assessing the rate of AF progression showed a significantly lower AF progression rate among patients with catheter ablation compared with patients without an intervention.14 Underlying mechanisms might include an inverse remodeling of the left atrium after PVI,15, 16, 17 even though the positive effect is still debated.18, 19 While these data suggest that PVI may slow the natural history of AF progression, causality cannot be proven in observational studies, residual confounding might persist even after comprehensive multivariable adjustment, and patient selection may play an important role in this association. Our multivariable models suggest that younger and active individuals with fewer comorbidities are more likely to receive an RCI, probably reflecting this selection process. Overall, the impact of RCI on the incidence of AF progression is unclear and needs to be assessed in future studies.
Several factors reflecting a healthy lifestyle were inversely and independently associated with clinical AF progression, including regular physical activity, a lower BMI, and a lower systolic blood pressure. Although the relationship between physical activity and new‐onset AF is not clear and may not be linear,20, 21, 22 our study suggests that regular physical activity might prevent AF progression. Overweight and obesity have been described as risk factors for new‐onset AF in different cohorts,23, 24, 25 possibly mediated by left atrial dilation.26 BMI was also associated with AF progression from paroxysmal to permanent, and the hazard ratio was similar to the one we observed in our study.27 Blood pressure is closely related to physical activity and BMI. Hypertension explained 18% of the heterogeneity in a recently published meta‐analysis on risk factors for AF progression.6 The association of hypertension with new‐onset AF was described earlier, and hypertension is thought to account for 22% of incident AF cases.28, 29 These data show the importance of blood pressure control for both new‐onset AF and AF progression, and also suggest that a healthy lifestyle not only plays a key role for primary AF prevention but may also lower the risk of AF progression.
We are in line with previous studies that history of heart failure,30, 31, 32 history of stroke,2 and increasing age2, 31, 32, 33, 34, 35, 36, 37 are associated with clinical AF progression. It is well known that heart failure is an important comorbidity associated with AF, and both entities frequently coexist.38 Moreover, older age is strongly and independently associated with AF.39, 40 There seems to be an overlap of risk factors for AF progression with predisposing factors for new‐onset AF. The factors might lead to structural and electrical changes in the atria that may explain the increased risk for AF progression. Possible changes could include atrial dilation, stiffness of the left atrium, and increased myocardial fibrosis, but also electro‐anatomical changes and conduction disturbances.41, 42, 43, 44 An additional independent predictor in our analysis was a history of hyperthyroidism. Subclinical and clinical hyperthyroidism have been associated with incident AF.45, 46 The underlying causes are currently not completely understood. Possible mechanisms that may explain the risk for incident AF and AF progression include elevated left atrial pressure secondary to impaired left ventricular relaxation,47 ectopic atrial activity,47 and shortening of action potential duration.48 Another pathophysiological concept suggests an autoimmune‐endocrine disorder. In animal studies, activating autoantibodies to the β1‐adrenergic and M2 muscarinic receptors has shown to induce AF.49
Strengths and Limitations
Yearly standardized assessments of AF type in a large number of patients with AF is one of the key strengths of this analysis. On the other hand, some potential limitations need to be taken into account in the interpretation of our findings. Because of the observational study design, we are unable to prove causality, and residual confounding may persist despite multivariable adjustment. We did not obtain an ECG recording during BEAT‐AF follow‐up visits. However, we collected all available clinical data (including changes in medication, available ECGs, and RCIs) to classify the clinical AF type as accurately as possible. Stratified analyses showed consistent results without meaningful differences between the 2 cohorts. As we did not have continuous ECG monitoring, our analysis cannot provide an assessment of AF burden but is a reflection of the clinical AF type that is currently used in daily clinical practice to classify AF patients.
Conclusions
In this large, prospective study of patients with nonpermanent AF, the incidence of clinical AF progression was relatively low. A healthy lifestyle may help to prevent a significant proportion of AF progression. The role of RCIs in the prevention of AF progression is less clear, as patients without RCIs have a similar progression rate, and determinants for RCIs significantly differed from predictors of AF progression.
Sources of Funding
BEAT‐AF has been supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Boehringer Ingelheim, Sanofi‐Aventis, Merck Sharp & Dome, Bayer, Daiichi‐Sankyo, and Pfizer/Bristol‐Myers Squibb. The Swiss‐AF cohort study is supported by grants of the Swiss National Science Foundation (grant numbers 33CS30_1148474 and 33CS30_177520), the Foundation for Cardiovascular Research Basel, and the University of Basel. Dr Conen holds a McMaster University Department of Medicine Mid‐Career Research Award. His work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program.
Disclosures
Dr Auricchio is a consultant to Boston Scientific, Backbeat, Biosense Webster, Cairdac, Corvia, Daiichi‐Sankyo, Medtronic, Merit, Microport CRM, Philips, and V‐Wave. He received speaker fees from Daiichi‐Sankyo, Boston Scientific, Biosense Webster, Medtronic, Microport CRM, and Philips. Dr. Auricchio also participates in clinical trials for Boston Scientific, Medtronic, Microport CRM, and Zoll Medical. He also holds intellectual properties with the following: Boston Scientific, Biosense Webster, and Microport CRM. Dr Kobza has received institutional grant support from Abbott, Biotronik, Biosense Webster, Boston, and Medtronic. He has served on the speakers’ bureau for Biosense Webster. Dr Shah received honoria from Daiichi‐Sankyo and Pfizer; He received speakers’ fees from Biosense Webster, Daiichi Sankyo, Boehringer Ingelheim, Bristol Myers Squibb, and Bayer, and consultancy honoraria from Biosense Webster; Dr Schläpfer served on the advisory boards for Daiichi‐Sankyo, Bayer, and Boehringer‐Ingelheim. Dr Sticherling has received speaker honoraria from Biosense Webster and Medtronic and research grants from Biosense Webster, Daiichi‐Sankyo, and Medtronic; Dr Kühne has served on the speakers’ bureau for Boston Scientific, St. Jude Medical, and Biotronik. He has received lecture/consulting fees from Sorin, Boehringer Ingelheim, Bayer, Sanofi Aventis, Novartis, Medtronic, and Pfizer‐BMS and has received unrestricted grants from Bayer and Pfizer‐BMS. He is a proctor for Medtronic (Cryoballoon). Dr Conen has received consultant/speaker fees from Servier Canada. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. The members of the Swiss‐AF Investigators and Contributors.
Table S1. Incidence Models
Table S2. Risk Factors for Clinical Atrial Fibrillation Progression in Paroxysmal vs Persistent Atrial Fibrillation
Table S3. Risk Factors for Clinical Atrial Fibrillation Progression in BEAT‐AF vs Swiss‐AF
Table S4. Baseline Characteristics Stratified by Rhythm Control Intervention
Table S5. Factors Associated With Rhythm Control Interventions Among Patients Without a History of Pulmonary Vein Isolation and/or Electrical Cardioversion
(J Am Heart Assoc. 2019;8:e012554 DOI: 10.1161/JAHA.119.012554.)
References
- 1. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, Ezekowitz J, Alings M, Yang H, Alexander JH, Flaker G, Hanna M, Granger CB. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 4. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, McGavigan AD. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta‐analysis. Eur Heart J. 2016;37:1591–1602. [DOI] [PubMed] [Google Scholar]
- 5. Charitos EI, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol. 2014;63:2840–2848. [DOI] [PubMed] [Google Scholar]
- 6. Blum S, Meyre P, Aeschbacher S, Berger S, Auberson C, Briel M, Osswald S, Conen D. Incidence and predictors of atrial fibrillation progression: a systematic review and meta‐analysis. Heart Rhythm. 2019;16:502–510. [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 8. Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, Hayoz D, Kobza R, Moschovitis G, Shah D, Schlaepfer J, Novak J, di Valentino M, Erne P, Sticherling C, Bonati L, Ehret G, Roten L, Fischer U, Monsch A, Stippich C, Wuerfel J, Schwenkglenks M, Kuehne M, Osswald S. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss‐AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467. [DOI] [PubMed] [Google Scholar]
- 9. Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, Goldhaber SZ, Goto S, Haas S, Hacke W, Kayani G, Mantovani LG, Misselwitz F, Ten Cate H, Turpie AG, Verheugt FW, Kakkar AK. Two‐year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD‐AF. Eur Heart J. 2016;37:2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruperti Repilado FJ, Doerig L, Blum S, Aeschbacher S, Krisai P, Ammann P, Erne P, Moschovitis G, di Valentino M, Shah D, Schlapfer J, Stempfel S, Kuhne M, Sticherling C, Osswald S, Conen D. Prevalence and predictors of atrial fibrillation type among individuals with recent onset of atrial fibrillation. Swiss Med Wkly. 2018;148:w14652. [DOI] [PubMed] [Google Scholar]
- 11. Al Halabi S, Qintar M, Hussein A, Alraies MC, Jones DG, Wong T, MacDonald MR, Petrie MC, Cantillon D, Tarakji KG, Kanj M, Bhargava M, Varma N, Baranowski B, Wilkoff BL, Wazni O, Callahan T, Saliba W, Chung MK. Catheter ablation for atrial fibrillation in heart failure patients: a meta‐analysis of randomized controlled trials. JACC Clin Electrophysiol. 2015;1:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- 13. Scherr D, Khairy P, Miyazaki S, Aurillac‐Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O'Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haissaguerre M, Jais P. Five‐year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol. 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 14. Proietti R, Hadjis A, AlTurki A, Thanassoulis G, Roux JF, Verma A, Healey JS, Bernier ML, Birnie D, Nattel S, Essebag V. A systematic review on the progression of paroxysmal to persistent atrial fibrillation: shedding new light on the effects of catheter ablation. JACC Clin Electrophysiol. 2015;1:105–115. [DOI] [PubMed] [Google Scholar]
- 15. Xiong B, Li D, Wang J, Gyawali L, Jing J, Su L. The effect of catheter ablation on left atrial size and function for patients with atrial fibrillation: an updated meta‐analysis. PLoS One. 2015;10:e0129274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsan NA, Tops LF, Holman ER, Van de Veire NR, Zeppenfeld K, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Comparison of left atrial volumes and function by real‐time three‐dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. 2008;102:847–853. [DOI] [PubMed] [Google Scholar]
- 17. Hof IE, Velthuis BK, Chaldoupi SM, Wittkampf FH, van Driel VJ, van der Heijden JF, Cramer MJ, Meine M, Hauer RN, Loh P. Pulmonary vein antrum isolation leads to a significant decrease of left atrial size. Europace. 2011;13:371–375. [DOI] [PubMed] [Google Scholar]
- 18. Wylie JV Jr, Peters DC, Essebag V, Manning WJ, Josephson ME, Hauser TH. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:656–662. [DOI] [PubMed] [Google Scholar]
- 19. Kim YG, Shim J, Oh S‐K, Park H‐S, Lee K‐N, Hwang SH, Choi J‐I, Kim Y‐H. Different responses of left atrium and left atrial appendage to radiofrequency catheter ablation of atrial fibrillation: a follow up MRI study. Sci Rep. 2018;8:7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proietti M, Boriani G, Laroche C, Diemberger I, Popescu MI, Rasmussen LH, Sinagra G, Dan GA, Maggioni AP, Tavazzi L, Lane DA, Lip GYH. Self‐reported physical activity and major adverse events in patients with atrial fibrillation: a report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP‐AF) General Registry. Europace. 2017;19:535–543. [DOI] [PubMed] [Google Scholar]
- 21. Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, Wisloff U, Loennechen JP. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473. [DOI] [PubMed] [Google Scholar]
- 22. Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes. 2011;4:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berkovitch A, Kivity S, Klempfner R, Segev S, Milwidsky A, Erez A, Sabbag A, Goldenberg I, Sidi Y, Maor E. Body mass index and the risk of new‐onset atrial fibrillation in middle‐aged adults. Am Heart J. 2016;173:41–48. [DOI] [PubMed] [Google Scholar]
- 24. Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 27. Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Vos CB, Breithardt G, Camm AJ, Dorian P, Kowey PR, Le Heuzey JY, Naditch‐Brule L, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub WS, Crijns HJ. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm‐control therapy. Am Heart J. 2012;163:887–893. [DOI] [PubMed] [Google Scholar]
- 31. Kato T, Yamashita T, Sagara K, Iinuma H, Fu LT. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14‐year follow‐up study. Circ J. 2004;68:568–572. [DOI] [PubMed] [Google Scholar]
- 32. Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. [DOI] [PubMed] [Google Scholar]
- 33. Zhang YY, Qiu C, Davis PJ, Jhaveri M, Prystowsky EN, Kowey P, Weintraub WS. Predictors of progression of recently diagnosed atrial fibrillation in REgistry on Cardiac Rhythm DisORDers Assessing the Control of Atrial Fibrillation (RecordAF)‐United States cohort. Am J Cardiol. 2013;112:79–84. [DOI] [PubMed] [Google Scholar]
- 34. Takigawa M, Takahashi A, Kuwahara T, Okubo K, Takahashi Y, Watari Y, Takagi K, Fujino T, Kimura S, Hikita H, Tomita M, Hirao K, Isobe M. Long‐term follow‐up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:267–273. [DOI] [PubMed] [Google Scholar]
- 35. Potpara TS, Stankovic GR, Beleslin BD, Polovina MM, Marinkovic JM, Ostojic MC, Lip GY. A 12‐year follow‐up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade Atrial Fibrillation study. Chest. 2012;141:339–347. [DOI] [PubMed] [Google Scholar]
- 36. Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S, Mazzone P, Paglino G, Gulletta S, Sala S, Santinelli V. Atrial fibrillation progression and management: a 5‐year prospective follow‐up study. Heart Rhythm. 2008;5:1501–1507. [DOI] [PubMed] [Google Scholar]
- 37. Holmqvist F, Kim S, Steinberg BA, Reiffel JA, Mahaffey KW, Gersh BJ, Fonarow GC, Naccarelli GV, Chang P, Freeman JV, Kowey PR, Thomas L, Peterson ED, Piccini JP. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart. 2015;101:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. [DOI] [PubMed] [Google Scholar]
- 42. Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–1635. [DOI] [PubMed] [Google Scholar]
- 43. Boyd AC, Schiller NB, Leung D, Ross DL, Thomas L. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc Imaging. 2011;4:234–242. [DOI] [PubMed] [Google Scholar]
- 44. Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study. Heart Rhythm. 2007;4:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, Faber J, Hansen PR, Pedersen OD, Torp‐Pedersen C, Gislason GH. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WC, den Heijer M, Visser TJ, Witteman JC. High‐normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219–2224. [DOI] [PubMed] [Google Scholar]
- 47. Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50. [DOI] [PubMed] [Google Scholar]
- 48. Tse HF, Lau CP. Electrophysiological properties of the fibrillating atrium: implications for therapy. Clin Exp Pharmacol Physiol. 1998;25:293–302. [DOI] [PubMed] [Google Scholar]
- 49. Li H, Murphy T, Zhang L, Huang B, Veitla V, Scherlag BJ, Kem DC, Yu X. Beta1‐adrenergic and M2 muscarinic autoantibodies and thyroid hormone facilitate induction of atrial fibrillation in male rabbits. Endocrinology. 2016;157:16–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The members of the Swiss‐AF Investigators and Contributors.
Table S1. Incidence Models
Table S2. Risk Factors for Clinical Atrial Fibrillation Progression in Paroxysmal vs Persistent Atrial Fibrillation
Table S3. Risk Factors for Clinical Atrial Fibrillation Progression in BEAT‐AF vs Swiss‐AF
Table S4. Baseline Characteristics Stratified by Rhythm Control Intervention
Table S5. Factors Associated With Rhythm Control Interventions Among Patients Without a History of Pulmonary Vein Isolation and/or Electrical Cardioversion


