Abstract
Background
Substantial heterogeneity exists in the cardiorespiratory fitness (CRF) change in response to exercise training, and its long‐term prognostic implication is not well understood. We evaluated the association between the short‐term supervised training‐related changes in CRF and CRF levels 10 years later.
Methods and Results
STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) trial participants who were originally randomized to exercise training for 8 months and participated in the 10‐year follow‐up visit were included. CRF levels were measured at baseline, after training (8 months), and at 10‐year follow‐up as peak oxygen uptake (vo 2, mL/kg per min) using the maximal treadmill test. Participants were stratified into low, moderate, and high CRF response groups according to the training regimen–specific tertiles of CRF change. The study included 80 participants (age: 52 years; 35% female). At 10‐year follow‐up, the high‐response CRF group had the least decline in CRF compared with the moderate‐ and low‐response CRF groups (−0.35 versus −2.20 and −4.25 mL/kg per minute, respectively; P=0.02). This result was largely related to the differential age‐related changes in peak oxygen pulse across the 3 groups (0.58, −0.23, and −0.86 mL/beat, respectively; P=0.03) with no difference in the peak heart rate change. In adjusted linear regression analysis, high response was significantly associated with greater CRF at follow‐up independent of other baseline characteristics (high versus low [reference] CRF response: standard β=0.25; P=0.004).
Conclusions
Greater CRF improvement in response to short‐term training is associated with higher CRF levels 10 years later. Lack of CRF improvements in response to short‐term training may identify individuals at risk for exaggerated CRF decline with aging.
Keywords: aging, exercise, exercise training
Subject Categories: Aging, Exercise
Clinical Perspective
What Is New?
The prognostic implications of fitness change in response to short‐term exercise training for age‐related fitness decline over 10‐year follow‐up are not known.
What Are the Clinical Implications?
Our study findings suggest that lack of fitness improvement in response to short‐term exercise training may identify individuals at risk for exaggerated fitness decline with aging, associated functional disabilities, and incidence of cardiovascular disease.
Introduction
Low cardiorespiratory fitness (CRF) in midlife is associated with greater risk of adverse cardiovascular events—heart failure, myocardial infarction, stroke, and death—in older age.1, 2, 3, 4, 5, 6, 7, 8 CRF declines with aging in a nonlinear fashion, with prior studies demonstrating a greater rate of decline in older age.9 An accelerated decline in CRF is associated with greater burden of functional disability and comorbidities.10, 11, 12, 13 Thus, low fitness and fitness decline are potentially modifiable targets to prevent disability and lower morbidity burden in older age.
Supervised exercise training is one of the well‐established strategies to improve CRF. Several randomized controlled trials have demonstrated that short‐term supervised exercise training is associated with significant improvement in CRF levels, cardiometabolic risk profile, and quality of life.14, 15, 16, 17, 18 However, there is substantial heterogeneity in CRF response to short‐term training; a proportion of participants experience no improvement in CRF levels with training.19, 20, 21, 22, 23 The prognostic implications of CRF response to short‐term training for CRF decline and cardiometabolic health over long‐term follow‐up are not well established. In this study, we aimed to determine the association of CRF changes in response to short‐term supervised exercise training with CRF and cardiometabolic risk profiles a decade later among participants of the STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) trial.24 We hypothesized that greater CRF response to short‐term training would be associated with greater CRF and more favorable cardiometabolic risk profiles a decade later.
Methods
Patient Population
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to author William E. Kraus at Duke University School of Medicine. STRRIDE was a randomized controlled trial conducted from 1999 to 2003 that examined the effects of short‐term exercise training on various cardiovascular risk factors. The study design and the inclusion and exclusion criteria for STRRIDE were published previously.24 Participants in the trial were middle‐aged (40–65 years old), were overweight to obese (body mass index 25–35), and had low fitness with peak oxygen uptake (peak VO2) <35 mL/kg per min and blood pressure <160/90 mm Hg.24 Men and women were equally represented, and 30% of participants were black. Participants with overt coronary artery disease, metabolic or musculoskeletal disorders, or active engagement in a dieting regimen were excluded. Study participants were randomized to an inactive control arm with no exercise training or to 1 of 3 exercise training arms: (1) low amount/moderate intensity (caloric equivalent of ≈12 miles/week at 40–55% peak VO2); (2) low amount/vigorous intensity (caloric equivalent of ≈12 miles/week at 65–80% peak VO2); and (3) high amount/vigorous intensity (caloric equivalent of ≈20 miles/week at 65% to 80% peak VO2).16, 24 The amount and intensity of exercise training was uptitrated over the first 2 to 3 months, followed by 6 months of supervised training at the appropriate exercise prescription.
A subset of the STRRIDE participants who enrolled at the Duke site and graduated from the intervention program (n=161 [n=145 in exercise training arms], 1999–2003) and were within 10 years (+6 months) of trial completion (n=153) were invited to return for a follow‐up assessment as part of the STRRIDE‐Reunion study.25, 26 Of these, 28 were lost to follow‐up (5 deceased, 1 in another exercise study, 7 relocated, 15 could not be contacted), and 21 refused to participate. A total of 104 participants were enrolled in the STRRIDE‐Reunion study after informed consent at the 10‐year visit. CRF was measured in 89 of these participants. For the present analysis, we included all participants of the STRRIDE trial who had measures of CRF levels at baseline, at the end of the exercise intervention, and at the 10‐year follow‐up visit (N=80). The baseline characteristics of all exercise training graduates from the Duke site of the primary trial (n=145), participants included in the present study (n=80), and participants not included in this study (n=65) are shown in Table S1. Both the primary STRRIDE trial and the STRRIDE‐Reunion study were approved by the Duke University institutional review boards.
CRF Assessment
All CRF testing was performed using graded maximal treadmill exercise with a 12‐lead ECG, and maximal oxygen consumption was measured with a TrueMax Parvomedics metabolic cart.27 CRF was assessed as both relative peak VO2 (mL/kg per min) and absolute peak VO2 (L/min). Peak oxygen pulse, a measure of the cross‐product of stroke volume and peripheral oxygen extraction, was calculated as the ratio of peak VO2 and peak exercise heart rate.
Covariate Assessments
For the baseline STRRIDE study, participant characteristics such as age, sex, ethnicity, and smoking status were self‐reported. Blood pressure was measured in a seated position before the treadmill test. Height and weight were measured for each participant using a standard scale and stadiometer without shoes. Blood samples were obtained following an overnight fast. Plasma HDL (high‐density lipoprotein) cholesterol, and triglyceride concentrations were measured via nuclear magnetic resonance spectroscopy (LipoScience). During the original STRRIDE study, plasma glucose was determined with a YSI analyzer. Plasma insulin levels were determined by immunoassay (Access Immunoassay System; Beckman Coulter).
For the 10‐year follow‐up visit, plasma glucose was analyzed with a Beckman Coulter CxC600 clinical analyzer, and plasma insulin was measured by electrochemiluminescent plate assay (Mesoscale Discovery). Exercise frequency at 10‐year follow‐up was determined based on self‐report.
Statistical Analysis
For this analysis, the primary exposure variable of interest was the categorical change in CRF (relative peak VO2) from baseline to the end of the exercise training intervention in the STRRIDE trial (Δpeak VO2, mL/kg per min). To account for the differences in exercise training dose and intensity across the 3 training arms, we stratified the study participants into 3 ordinal groups based on the training arm–specific Δpeak VO2: low CRF response (lowest tertile of Δpeak VO2 in each training arm), moderate CRF response (middle tertile of Δpeak VO2 in each training arm), and high CRF response (highest tertile of Δpeak VO2 in each training arm). The primary outcome of interest was CRF (peak VO2) measured at the 10‐year follow‐up visit. Other outcomes of interest were systolic blood pressure, minimal waist circumference (measured as the smallest horizontal circumference between the umbilicus and xiphoid process), and insulin resistance at the 10‐year visit.28 Participant characteristics were reported for the 3 CRF response categories as median (interquartile range) for continuous variables and percentages for categorical variables and compared across categories using the Kruskal–Wallis test (for continuous variables) or χ2 tests (for categorical variables). The paired changes in continuous variables from baseline to 10‐year follow‐up were compared using the Wilcoxon rank sum test. The association between CRF response to short‐term training and CRF at 10 years was assessed using multivariable linear regression models including the following covariates measured at the baseline visit: age, sex, ethnicity, exercise training group, baseline CRF, body mass index, mean arterial blood pressure, smoking status, and insulin resistance. The covariates were selected a priori based on the existing literature on the potential biological factors that may be associated with CRF levels and CRF response to training. Separate models were constructed using categorical (low [referent], moderate, high) and continuous (Δpeak VO2) measures of CRF response to short‐term training. Association between continuous measures of CRF change in response to short‐term training and peak oxygen pulse and maximum exercise heart rate at 10‐year follow‐up was evaluated using multivariable adjusted models with same covariates as described. Association between CRF response with short‐term training and other outcomes such as waist circumference, systolic blood pressure, and insulin resistance at 10 years was also assessed using multivariable adjusted models with adjustment for confounders. The statistical analysis was performed using JMP Pro v13.0. All statistical tests performed in the study were 2‐sided; P<0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Participants
This study included 80 participants who underwent supervised exercise training in the STRRIDE trial and had follow‐up CRF assessment 10 years later. The mean changes in CRF from baseline to the end of training in the low, moderate, and high CRF response groups were 0.9 mL/kg per minute (3% increase), 3 mL/kg per minute (10.7% increase), and 5.9 mL/kg per minute (19.7% increase), respectively (Figure). The baseline characteristics of the study participants stratified by CRF response to training are shown in Table 1. The proportions of female and black participants were significantly lower in the high‐response CRF group compared with the moderate‐ and low‐response CRF groups. Baseline CRF and body mass index were not different across the CRF response groups. Similarly, adherence to the exercise training program, as determined based on direct observation or downloadable Polar heart rate monitor data, was not different across the 3 CRF response groups.
Figure 1.
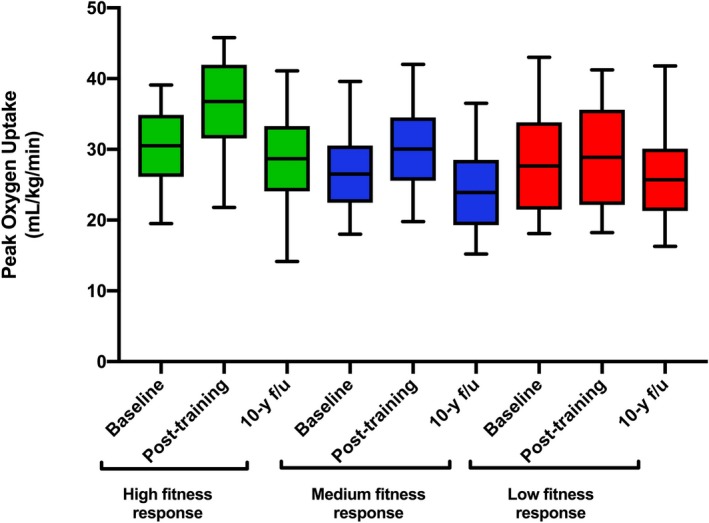
Peak oxygen uptake at baseline, end of training, and 10‐year follow‐up (f/u) across the 3 cardiorespiratory fitness response groups.
Table 1.
Baseline Characteristics of Study Participants Across Categories of CRF Change in Response to Short‐Term Exercise Training
| Low CRF Response (n=24) | Moderate CRF Response (n=28) | High CRF Response (n=28) | P Value | |
|---|---|---|---|---|
| Increase in VO2 from baseline with training | 3% | 10.7% | 19.6% | |
| Age, y | 51.5 (9.0) | 54.0 (7) | 50.0 (10.5) | 0.23 |
| Female, % | 41.7% | 60.7% | 28.5% | 0.02 |
| Black, % | 12.5% | 28.5% | 7.1% | 0.04 |
| Body weight, kg | 84.9 (19.3) | 88.3 (24.6) | 89.4 (21.5) | 0.48 |
| BMI, kg/m2 | 29.0 (3.6) | 29.5 (4.8) | 28.6 (3.4) | 0.83 |
| SBP, mm Hg | 127 (16) | 122 (15) | 123 (13) | 0.26 |
| DBP, mm Hg | 81.2 (6.4) | 78.5 (5.4) | 80.6 (7.4) | 0.004 |
| WC, cm | 95.5 (17.1) | 95.9 (18.7) | 96.6 (12.5) | 0.24 |
| Pretraining VO2peak relative, mL/kg/min | 27.6(12.4) | 26.5 (8.1) | 30.5 (8.8) | 0.43 |
| Pretraining VO2peak absolute, L/min | 2.3 (1.6) | 2.2 (1.2) | 2.8 (1.0) | 0.24 |
| Posttraining VO2peak relative, mL/kg/min | 28.8 (13.4) | 30.0 (8.9) | 36.8 (10.4) | <0.05 |
| Cholesterol, mg/dL | 211 (45) | 196 (26.1) | 195 (44.8) | 0.06 |
| HDL, mg/dL | 47.5 (19.2) | 46.1 (17.8) | 37.0 (10.6) | 0.005 |
| HOMA‐IR insulin resistance | 1.52 (1.27) | 1.77 (1.65) | 1.57 (1.04) | 0.79 |
| Proportional adherence to exercise training program | 0.92 (0.13) | 0.94 (0.14) | 0.91 (0.15) | 0.81 |
| Fasting glucose, mg/dL | 95.4 (13.4) | 90.8 (9.3) | 92.4 (12.0) | 0.13 |
Data presented as percentage or median (interquartile range). BMI indicates body mass index; CRF, cardiorespiratory fitness; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment–insulin resistance; SBP, systolic blood pressure; VO2, peak exercise oxygen uptake; WC, waist circumference.
Participant Characteristics Across CRF Response Groups at 10‐Year Follow‐Up
The characteristics of study participants across the 3 CRF response groups at 10 years are shown in Table 2. No significant differences were noted in the follow‐up body mass index, waist circumference, blood pressure levels, and insulin resistance across the 3 groups. Furthermore, exercise frequency at 10‐year follow‐up was not different across the 3 groups at follow‐up. In contrast, CRF at 10 years was significantly greater among participants who had the greatest CRF response to short‐term training in STRRIDE.
Table 2.
Ten‐Year Follow‐Up Characteristics of Study Participants Across Categories of CRF Change in Response to Short‐Term Exercise Training
| Low CRF Response (n=24) | Moderate CRF Response (n=28) | High CRF Response (n=28) | P Value | |
|---|---|---|---|---|
| Age, y | 62 (8.5) | 65 (7) | 61 (10.5) | 0.17 |
| Body weight, kg | 82.2 (17.8) | 87.6 (27.6) | 86.2 (20.6) | 0.76 |
| BMI, kg/m2 | 28.9 (3.9) | 29.3 (7.1) | 28.7 (6.0) | 0.50 |
| SBP, mm Hg | 122 (16) | 118.5 (12.5) | 122.5 (12.5) | 0.33 |
| DBP, mm Hg | 80.5 (15) | 75.5 (14) | 77 (6.5) | 0.66 |
| WC, cm | 95.4 (14.2) | 96.0 (20.2) | 95.8 (14.1) | 0.62 |
| 10‐year follow‐up VO2peak relative, mL/kg/min | 25.7 (8.8) | 23.9 (9.2) | 28.7 (9.2) | 0.003 |
| 10‐year follow‐up VO2peak absolute, L/min | 1.94 (1.04) | 2.06 (1.18) | 2.64 (0.84) | 0.02 |
| Exercise frequency (sessions/wk) | 1 (3) | 3 (2) | 2 (3) | 0.57 |
| Cholesterol, mg/dL | 211 (64) | 211.5 (47) | 181 (41.5) | 0.12 |
| HDL, mg/dL | 50.8 (17.9) | 45.8 (15.1) | 43.6 (11.2) | 0.13 |
| HOMA‐IR | 1.34 (1.18) | 1.45 (1.26) | 1.56 (2.06) | 0.74 |
| Fasting glucose, mg/dL | 104 (15.5) | 101.5 (11.5) | 103.5 (12.5) | 0.70 |
| Diabetes mellitus, % | 8.3 | 10.7 | 10.7 | 0.14 |
| Antihypertensive use on follow‐up, % | 25 | 32 | 43 | 0.09 |
Data presented as percentage or median (interquartile range). BMI indicates body mass index; CRF, cardiorespiratory fitness; DBP, diastolic blood pressure; HDL, high density lipoprotein; HOMA‐IR, Homeostatic model assessment–insulin resistance; SBP, systolic blood pressure; VO2, peak exercise oxygen uptake; WC, waist circumference.
Table 3 compares the changes in different exercise test parameters from baseline to 10 years follow‐up across the CRF response groups. A significant decline in CRF with aging was noted in the low‐ and moderate‐response CRF groups but not in the high‐response group (respective changes in peak VO2: −4.25 mL/kg per min [interquartile range: 5.29 mL/kg per min], P<0.0001; −2.20 mL/kg per min [interquartile range: 2.45 mL/kg per min], P<0.0005; and −0.35 mL/kg per min [interquartile range: 7.8 mL/kg per min]; P=0.99; Figure, Table 3). Among other exercise test characteristics, there was a significant decrease in the peak oxygen pulse from baseline to 10 years in the low‐response CRF group but not in the other 2 groups (−0.86 mL/beat [interquartile range: 1.6 mL/beat], P=0.01). The peak exercise heart rate declined significantly across all 3 groups with no significant between‐group differences (Table 3).
Table 3.
Comparison of Changes Across in Exercise Test and Cardiometabolic Parameters From Baseline to 10‐Year Follow‐Up Across Categories of CRF Change in Response to Short‐Term Exercise Training
| Median Change | Low Response (n=24) | Moderate Response (n=28) | High Response (n=28) | P Value |
|---|---|---|---|---|
| VO2peak relative, mL/kg/min | −4.25 (5.29)a | −2.20 (2.45)a | −0.35 (7.8) | 0.02 |
| Oxygen pulse | −0.86 (1.6)a | −0.23 (1.8) | 0.58 (2.0) | 0.03 |
| Maximal exercise HR | −14 (13)a | −14 (12.5)a | −11.5 (17.5)a | 0.57 |
| BMI | 0.03 (2.00) | 0.80 (0.66) | −0.25 (2.89) | 0.32 |
| SBP | −4.4 (22.5) | −3.2 (9.8) | −1.6 (14.8) | 0.88 |
| DBP | −4.0 (9.1)a | −2.0 (8.6) | −3.1 (6.9)a | 0.64 |
| WC | 0.30 (7.9) | 3.2 (8.85) | −0.97 (9.1) | 0.16 |
| HOMA‐IR | −0.36 (1.01)a | −0.09 (1.56) | 0.14 (1.25) | 0.13 |
Data presented as median (interquartile range, Q3–Q1) of the change in parameters across all participants. BMI indicates body mass index; CRF, cardiorespiratory fitness; DBP, diastolic blood pressure; HOMA‐IR, homeostatic model assessment–insulin resistance; HR, heart rate; SBP, systolic blood pressure; VO2, peak exercise oxygen uptake; WC, waist circumference.
Significant within‐group change from baseline to 10‐year follow‐up using the Wilcoxon rank sum test.
Association Between CRF Response to Training and CRF at 10 Years of Follow‐Up
In adjusted linear regression analysis, high CRF response to short‐term training was significantly associated with greater CRF levels at 10 years (standard β=0.25, P=0.004; reference group: low CRF response; Table 4). In contrast, moderate CRF response to short‐term training was not associated with CRF at follow‐up (standard β=−0.01, P=0.95; reference group: low CRF response). When CRF change in response to short‐term training was modeled as a continuous variable, 1‐SD increase in CRF was associated with 0.24 SD greater CRF at 10 years. CRF change in response to short‐term training was also significantly associated with peak oxygen pulse (standard β=0.23, P=0.005) but not maximum exercise heart rate (standard β=−0.05, P=0.69) at 10‐year follow‐up in adjusted analysis.
Table 4.
Adjusted Association Between CRF Change in Response to Short‐Term Exercise Training and Fitness Levels at 10‐Year Follow‐Up
| Std. β | P Value | |
|---|---|---|
| Categorical measure of CRF response | ||
| Moderate‐fitness responder (vs low‐fitness responder) | −0.01 | 0.93 |
| High‐fitness responder (vs low‐fitness responder) | 0.25 | 0.004 |
| Continuous measure of CRF response | ||
| Per 1‐SD greater increase in peak VO2 | 0.24 | 0.01 |
Separate models were created for the categorical and continuous measures of the CRF response to short‐term training with adjustment for baseline measures of age, sex, ethnicity, body mass index, baseline peak VO2 (before STRRIDE [Studies of a Targeted Risk Reduction Intervention Through Defined Exercise]), exercise group, mean arterial blood pressure, smoking status, and insulin resistance. CRF indicates cardiorespiratory fitness; VO2, peak exercise oxygen uptake.
Among other cardiometabolic outcomes, there was no consistent dose‐dependent association between CRF response to short‐term training and waist circumference on follow‐up. Participants with moderate CRF response to training had modestly higher waist circumference on follow‐up. However, high CRF response was not associated with waist circumference at 10‐year follow‐up. Furthermore, CRF response to short‐term training was not associated with systolic blood pressure and insulin resistance at 10 years in adjusted analysis (Table S2).
Discussion
In this analysis of participants from the STRRIDE trial who underwent supervised exercise training and had repeated CRF assessment a decade later, we observed that greater improvement in CRF with short‐term training was associated with greater CRF 10 years later. Furthermore, CRF changes with short‐term training were significantly associated with peak oxygen pulse, a measure of exercise stroke volume, but not peak exercise heart rate at 10‐year follow‐up. This suggests that the differences in CRF levels at 10 years across the short‐term training response groups were driven by differences in peak oxygen pulse, a measure of exercise stroke volume, and not peak exercise heart rate. Finally, CRF response to short‐term training was not associated with other cardiometabolic parameters such as blood pressure and insulin resistance at 10 years. Taken together, our study findings highlight the potential legacy effect of CRF improvement in response to short‐term training on long‐term CRF decline with aging.
While short‐term exercise training is a well‐established strategy to improve CRF levels, significant heterogeneity has been reported in the CRF response to exercise training. Recent analysis from the DREW (Dose Response to Exercise in Women) and HART‐D (Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes) trials demonstrated that 30% to 43% of participants undergoing supervised exercise training did not have any improvement in CRF.21, 23 The prognostic value of the CRF response to short‐term training is not well established. Some studies have demonstrated that a blunted improvement in CRF with exercise training among patients with known cardiovascular disease is associated with greater risk of adverse outcomes.29 In contrast, other studies have demonstrated consistent improvements in cardiometabolic parameters such as hemoglobin A1c among all training participants, regardless of the changes in CRF levels.21 Findings from the present study add to the existing literature by evaluating the long‐term associations of CRF response to short‐term training with CRF and other cardiometabolic parameters at 10 years of follow‐up. We observed that greater improvements in CRF with short‐term training were associated with significantly greater CRF at 10 years.
Several factors may underlie the observed associations. First, short‐term training may have a legacy effect on the trajectory of CRF decline.26 Thus, the initial CRF improvement in response to training could reset the baseline such that the subsequent decline over 10 years leaves one with greater CRF levels over that period. Second, it is possible that greater CRF improvement with exercise may identify a subset of participants with more favorable adaptations in cardiac structure and function to training, which also may be associated with attenuated age‐related CRF decline.30, 31 The finding of a significant association between CRF change in response to short‐term training and peak exercise oxygen pulse (stroke volume) but not peak exercise heart rate 10 years later supports this supposition. Recent studies have identified distinct subclinical cardiac phenotypes associated with both blunted fitness response to training and exaggerated decline in CRF with aging.31 Specifically, abnormal left ventricular remodeling patterns are associated with blunted CRF response among exercise training participants and with greater age‐related decline in CRF among young adults.30, 31
In contrast to CRF, we did not observe a consistent association between CRF response to short‐term training and other cardiometabolic parameters, particularly insulin resistance and systolic blood pressure, at 10‐year follow‐up. These findings are consistent with prior observations of no significant associations between CRF change with training and improvements in hemoglobin A1c.21, 32 It is plausible that the cardiometabolic benefits of exercise are related to pleotropic effects of exercise on multiple downstream targets and are not strongly related to CRF improvements.33, 34 These findings also suggest that the potential legacy effect of CRF improvement with short‐term training may be unique to age‐related CRF decline.
Our study findings have important clinical implications for healthy aging. Physical inactivity, low CRF, and greater decline in CRF have been associated with greater functional disability and chronic cardiac and noncardiac comorbidities with older age.12, 28, 35 Although the benefits of short‐term exercise training in improving CRF levels are well known, the findings from the present study highlight the potential long‐term implications of short‐term training. These findings suggest that CRF gains with short‐term exercise regimens may help attenuate CRF decline with aging and lower the burden of low CRF and perhaps associated comorbidities with older age. This is particularly relevant considering the challenges associated with long‐term maintenance of exercise training programs in the population.
Several limitations to our study are noteworthy. First, our study included participants who underwent exercise training during the initial STRRIDE trial and subsequently had a follow‐up CRF assessment 10 years later. A significant proportion of participants who underwent exercise training during the initial STRRIDE trial could not participate in the 10‐year follow‐up visit, limiting the generalizability of our study findings. However, the loss to follow‐up in our study seems random, with no significant difference between baseline characteristics and CRF response to training among the participants with versus without a 10‐year follow‐up visit. Second, we do not have data on exercise and physical activity patterns among study participants over the intervening 10 years that might have influenced the follow‐up CRF assessments. However, the reported exercise frequency 3 months before the 10‐year CRF assessments did not differ across the CRF response groups. Third, we do not have detailed anthropometric assessment such as lean body mass, percentage of body fat, and regional adiposity measurements available at the 10‐year follow‐up visit. Therefore, we cannot assess the contribution of changes in these anthropometric parameters toward long‐term CRF changes. Finally, there is a possibility of residual confounding in the observed associations owing to the observational nature of the analysis.
In conclusion, greater improvements in CRF in response to short‐term supervised exercise training were significantly associated with greater CRF on long‐term follow‐up. Such legacy effects were not related to legacy effects on other cardiometabolic risk factors. Future studies are needed to determine whether this legacy effect of CRF improvement with a short‐term training intervention may be harnessed to modify the trajectory of CRF decline in middle‐age and to lower the burden of functional disability and comorbidities in older age.
Sources of Funding
The original STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) trial was funded by National Heart, Lung, and Blood Institute (NHLBI) grant HL‐057354. Ross is supported by NHLBI fellowship T32HL007101. Pandey is supported by the Texas Health Resources Research Scholarship.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) Exercise Trial Participants Who Were or Were Not Included in the STRRIDE Follow‐Up Study
Table S2. Cardiorespiratory Fitness Change in Response to Short‐Term Exercise Training and Other Measures of Cardiometabolic Parameters at 10‐Year Follow‐Up (Mean Arterial Blood Pressure, Waist Circumference, Insulin Resistance)
Acknowledgments
We would like to thank all of the STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) participants and staff members.
(J Am Heart Assoc. 2019;8:e012876 DOI: 10.1161/JAHA.119.012876.)
References
- 1. Berry JD, Pandey A, Gao A, Leonard D, Farzaneh‐Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. [DOI] [PubMed] [Google Scholar]
- 3. Pandey A, Patel MR, Willis B, Gao A, Leonard D, Das SR, Defina L, Berry JD. Association between midlife cardiorespiratory fitness and risk of stroke: the cooper center longitudinal Study. Stroke. 2016;47:1720–1726. [DOI] [PubMed] [Google Scholar]
- 4. Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72:1622–1639. [DOI] [PubMed] [Google Scholar]
- 6. Kaminsky LA, Arena R, Ellingsen O, Harber MP, Myers J, Ozemek C, Ross R. Cardiorespiratory fitness and cardiovascular disease—the past, present, and future. Prog Cardiovasc Dis. 2019;62:86–93. [DOI] [PubMed] [Google Scholar]
- 7. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124:799–815. [DOI] [PubMed] [Google Scholar]
- 8. Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 9. Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. [DOI] [PubMed] [Google Scholar]
- 10. Pandey A, Patel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L, Berry JD. Changes in mid‐life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–297.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338:1035–1041. [DOI] [PubMed] [Google Scholar]
- 12. Wang BW, Ramey DR, Schettler JD, Hubert HB, Fries JF. Postponed development of disability in elderly runners: a 13‐year longitudinal study. Arch Intern Med. 2002;162:2285–2294. [DOI] [PubMed] [Google Scholar]
- 13. Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL, Fleenor BS, Kaminsky LA. The association between the change in directly measured cardiorespiratory fitness across time and mortality risk. Prog Cardiovasc Dis. 2019;62:157–162. [DOI] [PubMed] [Google Scholar]
- 14. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. [DOI] [PubMed] [Google Scholar]
- 16. Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. [DOI] [PubMed] [Google Scholar]
- 17. Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Myers VH, McVay MA, Brashear MM, Johannsen NM, Swift DL, Kramer K, Harris MN, Johnson WD, Earnest CP, Church TS. Exercise training and quality of life in individuals with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2013;36:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouchard C. Individual differences in the response to regular exercise. Int J Obes Relat Metab Disord. 1995;19(suppl 4):S5–S8. [PubMed] [Google Scholar]
- 20. Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). 1999;87:1003–1008. [DOI] [PubMed] [Google Scholar]
- 21. Pandey A, Swift DL, McGuire DK, Ayers CR, Neeland IJ, Blair SN, Johannsen N, Earnest CP, Berry JD, Church TS. Metabolic effects of exercise training among fitness‐nonresponsive patients with type 2 diabetes: the HART‐D study. Diabetes Care. 2015;38:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, Skinner JS, Castro A, Irving BA, Noland RC, Sparks LM, Spielmann G, Day AG, Pitsch W, Hopkins WG, Bouchard C. Precision exercise medicine: understanding exercise response variability. Br J Sports Med. 2019;53:1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc. 2009;41:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CW, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc. 2001;33:1774–1784. [DOI] [PubMed] [Google Scholar]
- 25. Kraus WE, Slentz CA, Duscha BD, Willis LH, Johnson JL. Abstract 46: legacy effects of STRRIDE exercise training programs on cardiometabolic health observed ten years later. Circulation. 2016;133:A46. [Google Scholar]
- 26. Johnson JL, Slentz CA, Ross LM, Huffman KM, Kraus WE. Ten‐year legacy effects of three eight‐month exercise training programs on cardiometabolic health parameters. Front Physiol. 2019;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and intensity on peak oxygen consumption in middle‐age men and women at risk for cardiovascular disease. Chest. 2005;128:2788–2793. [DOI] [PubMed] [Google Scholar]
- 28. Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, Houmard JA, Kraus WE. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985). 2012;113:1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Schutter A, Kachur S, Lavie CJ, Menezes A, Shum KK, Bangalore S, Arena R, Milani RV. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4:173–179. [DOI] [PubMed] [Google Scholar]
- 30. Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR Jr, Chow LS, de Lemos JA, Carnethon M, Berry JD. Fitness in young adulthood and long‐term cardiac structure and function: the CARDIA study. JACC Heart Fail. 2017;5:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandey A, Ayers C, Blair SN, Swift DL, Earnest CP, Kitzman DW, Khera A, Church TS, Berry JD. Cardiac determinants of heterogeneity in fitness change in response to moderate intensity aerobic exercise training: the DREW study. J Am Coll Cardiol. 2015;65:1057–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan AM, Lam M, Stotz P, Hudson R, Ross R. Exercise‐induced improvement in insulin sensitivity is not mediated by change in cardiorespiratory fitness. Diabetes Care. 2014;37:e95–e97. [DOI] [PubMed] [Google Scholar]
- 33. Vollaard NB, Constantin‐Teodosiu D, Fredriksson K, Rooyackers O, Jansson E, Greenhaff PL, Timmons JA, Sundberg CJ. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J Appl Physiol (1985). 2009;106:1479–1486. [DOI] [PubMed] [Google Scholar]
- 34. Booth FW, Laye MJ. The future: genes, physical activity and health. Acta Physiol (Oxf). 2010;199:549–556. [DOI] [PubMed] [Google Scholar]
- 35. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of STRRIDE (Studies of a Targeted Risk Reduction Intervention Through Defined Exercise) Exercise Trial Participants Who Were or Were Not Included in the STRRIDE Follow‐Up Study
Table S2. Cardiorespiratory Fitness Change in Response to Short‐Term Exercise Training and Other Measures of Cardiometabolic Parameters at 10‐Year Follow‐Up (Mean Arterial Blood Pressure, Waist Circumference, Insulin Resistance)


