Abstract
Background
Endothelial dysfunction is a consequence of type 2 diabetes mellitus, but it is unclear whether endothelial dysfunction of conductance versus resistance vessels may also precede type 2 diabetes mellitus development.
Methods and Results
In a population‐based cohort of 15 010 individuals from the GHS (Gutenberg Health Study) (aged 35–74 years at enrollment in 2007–2012), we identified 1610 cases of incident pre–diabetes mellitus and 386 cases of incident type 2 diabetes mellitus by hemoglobin A1c (HbA1c) and/or medical history between 2012 and 2017. Endothelial function of conductance and resistance vessels was measured by flow‐mediated dilation and digital volume plethysmography–derived reactive hyperemia index, respectively. Multivariable regression modeling was used to estimate β coefficients of HbA1c levels at follow‐up and relative risks of incident (pre–)diabetes mellitus. Reactive hyperemia index was independently associated with HbA1c after multivariable adjustment for baseline HbA1c, sex, age, socioeconomic status, arterial hypertension, waist/height ratio, pack‐years of smoking, non–high‐density lipoprotein/high‐density lipoprotein ratio, physical activity, family history of myocardial infarction/stroke, prevalent cardiovascular disease, medication use, and C‐reactive protein (β=−0.020; P=0.0029). The adjusted relative risk per SD decline in reactive hyperemia index was 1.08 (95% CI, 1.02–1.15; P=0.012) for incident pre–diabetes mellitus and 1.16 (95% CI, 1.01–1.34; P=0.041) for incident type 2 diabetes mellitus. Flow‐mediated dilation independently increased the relative risk for developing pre–diabetes mellitus by 8% (95% CI, 1.02–1.14; P=0.012), but it was not independently associated with incident type 2 diabetes mellitus (relative risk, 1.01; 95% CI, 0.86–1.19; P=0.92) and with HbA1c (β=−0.003; P=0.59).
Conclusions
Endothelial dysfunction of resistance rather than conductance vessels may precede the development of (pre–)diabetes mellitus. Assessment of endothelial function by digital volume plethysmography may help to identify subjects at risk for development of type 2 diabetes mellitus.
Keywords: digital volume plethysmography, endothelial dysfunction, flow‐mediated dilation, population based, risk of type 2 diabetes mellitus
Subject Categories: Diabetes, Type 2; Epidemiology
Clinical Perspective
What Is New?
Endothelial dysfunction of resistance rather than conductance vessels predicts incident pre–diabetes mellitus and type 2 diabetes mellitus in a large population‐based cohort.
The analysis demonstrated that a lower reactive hyperemia index, derived from digital volume plethysmography, was a predictor for the development of type 2 diabetes mellitus and its precursor pre–diabetes mellitus independently of other diabetes mellitus risk factors, whereas this association was weaker for flow‐mediated dilation of the brachial artery.
What Are the Clinical Implications?
These findings implicate that microvascular damage may precede the manifestation of diabetes mellitus.
Assessment of microvascular endothelial dysfunction may be useful in the risk stratification for type 2 diabetes mellitus.
Introduction
Type 2 diabetes mellitus is a major cause of death and disability worldwide.1 The International Diabetes Federation estimated that by 2045, the prevalence of diabetes mellitus will increase to 693 million people worldwide.2 Disability resulting from diabetes mellitus has grown substantially over the past decades. In particular, type 2 diabetes mellitus markedly increases the risk of acute and chronic atherosclerotic cardiovascular disease (CVD), even in the presence of adequate glycemic control.3
Endothelial dysfunction of conductance and resistance arteries represents an early subclinical vascular consequence in the early preclinical development of type 2 diabetes mellitus.4, 5 This finding is most commonly explained by the effects of hyperglycemia and insulin resistance, which lead to a decreased bioavailability of vascular NO and inappropriate production of oxygen‐free radicals.6 Moreover, endothelial dysfunction has been shown to be predictive of future cardiovascular events in several disease phenotypes, including patients with coronary and peripheral artery disease, arterial hypertension, and chronic congestive heart failure.7, 8 More important, these alterations have been reported to be already detectable in the prediabetic phase, an intermediate stage along the continuum from normal glucose levels to the clinical entity of type 2 diabetes mellitus.9
In addition, evidence is available that endothelial dysfunction may not only be the consequence but rather precede or even predict the onset of development of type 2 diabetes mellitus. However, most of these studies used solely plasma biomarkers of endothelial activation or used flow‐mediated dilation (FMD) of the brachial artery in small study samples10, 11, 12, 13, 14, 15 without sufficient control for confounding factors. To the best of our knowledge, no study has to date investigated whether endothelial dysfunction of conductance and/or resistance vessels is associated with an increased incidence of future development of pre–diabetes mellitus and type 2 diabetes mellitus independently of the cardiovascular risk profile.
To address this topic, we measured endothelial function of large conductance arteries and small arterioles in the GHS (Gutenberg Health Study).16 We investigated whether endothelial dysfunction predicts incident pre–diabetes mellitus and type 2 diabetes mellitus and how this association is influenced by other cardiovascular risk factors.
Methods
The analysis presents clinical data of a large‐scale population‐based cohort with ongoing follow‐up examinations. This project constitutes a major scientific effort with high methodological standards and detailed guidelines for analysis and publication to ensure scientific analyses on the highest level. Therefore, data are not made available for the scientific community outside the established and controlled workflows and algorithms. To meet the general idea of verification and reproducibility of scientific findings, we offer access to data at the local database in accordance with the ethics vote on request at any time. The GHS steering committee, which comprises a member of each involved department and the head of the GHS, convenes once a month. The steering committee decides on internal and external access of researchers and use of the data and biomaterials based on a research proposal to be supplied by the researcher. Interested researchers make their requests to the head of the GHS (Philipp S. Wild, philipp.wild@unimedizin-mainz.de).
Study Design and Sample
The data presented were derived from the GHS, an age‐, sex‐, and residence‐stratified, population‐based, prospective single‐center cohort study in midwestern Germany. The study design of the GHS has been published previously.16 The multidisciplinary GHS aims at improving individual risk stratification with focus on CVD, metabolic, ophthalmological, cancer, immune system, and mental diseases. The study sample comprises 15 010 individuals (age range, 35–74 years), who underwent a baseline examination between 2007 and 2012 at the University Medical Center Mainz. During the visit at the study center, individuals underwent a standardized 5‐hour baseline examination with deep clinical phenotyping and biobanking, according to standard operating procedures. Sequential phenotyping has been conducted 5 years after enrollment (ie, 2012–2017) at the study platform. The study protocol was approved by the ethics committee of the Statutory Physician Board of the State Rhineland‐Palatinate and the local data safety commissioners. The study design is in accordance with the revised Helsinki protocol and principles outlined in recommendations for Good Clinical and Epidemiological Practice. Written informed consent was collected from all study participants before participation.
Assessment of Endothelial Function by FMD and Digital Volume Plethysmography
Briefly, endothelial function measurements were performed in dark, air‐conditioned rooms (23°C–25°C) after at least 5 minutes of rest and the subjects in a fasted state (8 hours) and supine position. Subjects were particularly advised to refrain from nicotine, caffeine, alcohol, vitamins, and physical activity before measurement.
Brachial artery diameter was measured in resting conditions, and FMD was determined after a 5‐minute upper‐arm occlusion under standardized conditions as percentage increase of baseline artery diameter. Two‐dimensional high‐resolution ultrasound images of the right brachial artery were acquired with a Philips HD11XE CV ultrasound machine (Best, the Netherlands) using a linear array broadband probe, L12–5 (38 mm). Artery diameters were analyzed offline with the Brachial Analyzer software tool, version 5.0 (Medical Imaging Applications LLC, Iowa City, IA).
For digital volume plethysmography, pneumatic pulse amplitude was recorded in both index fingers (left index finger served as control finger) using the Endo‐PAT 2000 device (Itamar Medical, Caesarea, Israel). Digital reactive hyperemia index (RHI) was calculated automatically by the Endo‐PAT software as logarithmic ratio between rest and postocclusion in digital pulse amplitude, normalized to the control finger.
Measurements of FMD and digital volume plethysmography were conducted simultaneously in a single examination by trained technicians who had performed at least 250 vascular function studies before study enrollment and with continuing quality assessment. Reproducibility of the measurements was evaluated and provided good intraclass and interclass variability for all measures of endothelial function (eg, FMD: 0.87–0.93 and 0.90–0.93, respectively). Further details and quality control data about the measurements in the GHS have been described elsewhere.17, 18
Determination of Cardiovascular Risk Factors and CVD
Computer‐assisted interviews, anthropometric measures, and routine laboratory assessments were conducted in a standardized manner to assess cardiovascular risk factors, cardiovascular comorbidities, and humoral biomarkers of glucose metabolism. Medication history was derived from medical records and personal reports and categorized according to the Anatomical Therapeutic Chemical Classification System.19 Variables were defined as follows: waist/height ratio was calculated by dividing the waist circumference by the body height in centimeters. Pack‐years of smoking was calculated as number of cigarettes smoked per day divided by 20 (a pack) and multiplied by the number of years smoked. Arterial hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at rest (mean of second and third standardized measurement after 8 and 11 minutes of rest respectively) or by intake of any antihypertensive drugs within the past 2 weeks or arterial hypertension diagnosed by a physician. Non–high‐density lipoprotein/high‐density lipoprotein ratio was calculated by subtracting high‐density lipoprotein cholesterol from total cholesterol divided by high‐density lipoprotein cholesterol. Physical activity was measured using the validated Short Questionnaire to Assess Health‐Enhancing Physical Activity,20 which contains questions on multiple activities (commuting, leisure time, household, and activities at work and school), referring to a normal week in recent months (days per week, average time per day, and intensity) for the calculation of an index score (total minutes of activity multiplied by intensity score). Prevalent CVD was assessed on the basis of medical history or diagnosis during a standardized computer‐assisted personal interview as any of the following: coronary artery disease, peripheral artery disease, myocardial infarction, congestive heart failure, stroke, or atrial fibrillation. Family history of myocardial infarction or stroke was self‐reported. High‐sensitivity C‐reactive protein was obtained by a standardized laboratory method. Socioeconomic status was assessed via a validated index score combining information about educational background, current occupation, and income (range, 3–21), with a higher score indicating higher socioeconomic status.21 Detailed information on clinical and laboratory examinations has been published previously.17, 22 All data used for the present study underwent detailed quality control by a central data management unit and were screened for completeness according to predefined algorithms and plausibility criteria.
Definition and Ascertainment of Type 2 Diabetes Mellitus
Baseline and incident pre–diabetes mellitus were defined by hemoglobin A1c (HbA1c) levels, whereas baseline and incident type 2 diabetes mellitus were defined by HbA1c levels and medical history.23 Individuals with an intake of antidiabetic medication and/or previous diagnosis of type 2 diabetes mellitus were classified as diabetic. According to HbA1c levels, subjects were classified according to guidelines of the American Diabetes Association23 (ie, <5.7% [<39 mmol/mol] as normoglycemic, 5.7%–6.4% [39–46 mmol/mol] as having increased risk for diabetes mellitus or pre–diabetes mellitus, and ≥6.5% [≥48 mmol/mol] as having diabetes mellitus). Individuals with type 1 diabetes mellitus and other types of diabetes mellitus (n=134) were excluded from the analysis.
Statistical Analysis
Baseline characteristics of the study sample are shown according to glucose status (ie, normoglycemia, pre–diabetes mellitus, and type 2 diabetes mellitus) as absolute and relative frequency for categorical variables and as mean value and SD or median with 25th and 75th percentiles for continuous variables. Statistical comparisons for categorical variables were made by Fisher exact or χ2 tests; and for continuous variables, Mann‐Whitney U or Student t tests were used, respectively. For the evaluation of statistical trends among groups, Jonckheere‐Terpstra trend test was used. Binomial plots were generated for incident cases of (pre–)diabetes mellitus to show their relative frequencies in dependence on endothelial function. To prospectively assess the relationship between endothelial function and levels of HbA1c, linear regression models with β estimates were used. Scatterplots were used for the visual examination of the relationship between the independent and dependent variables, showing a linear approximation to be adequate. Moreover, histograms were used to check if the dependent variable was symmetric and if the residuals were normally distributed. These assumptions were met. We further investigated the variance inflation factor for all included variables, indicating no multicollinearity issues. Relative risks (RRs) from a Poisson regression model with robust variance estimation as a more stable alternative to log‐binomial regression were used to estimate the effect of endothelial function on incident (pre–)diabetes mellitus. FMD and RHI were modeled as inverse term (multiplied by −1), to provide estimates reflecting increased risk. Separate analyses for each endothelial function variable were performed. Models were sequentially adjusted for baseline HbA1c (continuous), sex (male/female), age (continuous), socioeconomic status (continuous), arterial hypertension (categorical), waist/height ratio (continuous), pack‐years of smoking (continuous), non–high‐density lipoprotein/high‐density lipoprotein ratio (continuous), physical activity (continuous), family history of myocardial infarction or stroke (categorical), prevalent CVD (categorical; composite variable combining congestive heart failure, coronary artery disease, myocardial infarction, stroke, atrial fibrillation, and peripheral artery disease), medication use (antithrombotic agents, antihypertensives, diuretics, β blockers, calcium channel blocker, agents acting on the renin‐angiotensin‐aldosterone system, and lipid‐modifying agents), and inflammation by levels of C‐reactive protein (continuous). All effect estimates are given with 95% CIs with corresponding P values. In this explorative analysis, P values should be treated as a continuous measure of statistical strength of an association, and they are therefore reported exactly. All tests were 2 sided, and P<0.05 was seen as significant. The statistical data analyses were performed using the software R, version 3.3.1 (http://www.r-project.org/).
Results
Baseline Characteristics
After exclusion of individuals with type 1 diabetes mellitus (n=79) or unspecified diabetes mellitus (n=55), a total of 14 876 individuals (7522 men and 7354 women) were analyzed (Figure 1). Baseline characteristics of the study sample, according to glucose status, are presented in Table 1. At baseline, 63.4% of individuals were normoglycemic, 27.7% had pre–diabetes mellitus, and 8.8% had type 2 diabetes mellitus (of which, n=913 [69.4%] were classified by HbA1c, n=386 [29.3%] were classified by medical history, and n=17 [1.3%] were classified by history of antidiabetic medications). In comparison to normoglycemic individuals, subjects with pre–diabetes mellitus and diabetes mellitus were more likely to be men, were older, and had a higher waist/height ratio. Moreover, they were less physically active and showed a more pronounced cardiovascular risk profile. In general, the use of antithrombotic and cardiovascular medication increased from subjects with normoglycemia to those with pre–diabetes mellitus and diabetes mellitus. Among individuals with type 2 diabetes mellitus, approximately one third were therapy naïve with regard to antidiabetic medication. With regard to endothelial dysfunction, FMD and RHI decreased from normoglycemic to prediabetic and diabetic subjects, whereas baseline brachial artery diameter and pulse amplitude increased concordantly.
Figure 1.
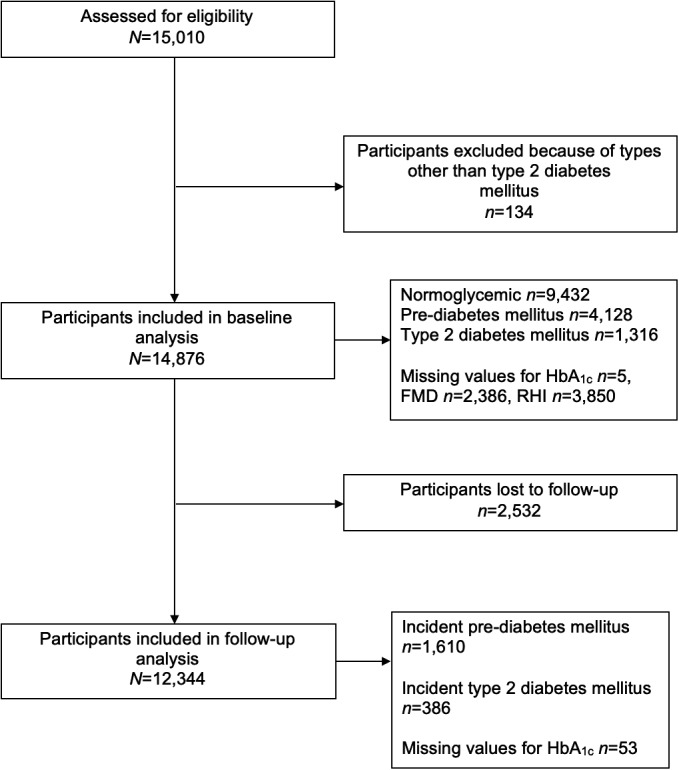
Flow diagram for the study sample in the present analysis. Of 15 010 participants initially enrolled in the GHS (Gutenberg Health Study) at baseline between 2007 and 2012, 134 were excluded from analysis because of having types other than type 2 diabetes mellitus. Of the remaining 14 876 participants, 12 344 attended follow‐up between 2012 and 2017. FMD indicates flow‐mediated dilation; HbA1c, hemoglobin A1c; RHI, reactive hyperemia index.
Table 1.
Characteristics of the Study Sample by Glucose Status at Baseline
| Variable | Normoglycemic (n=9432) | Pre–Diabetes Mellitus (n=4128) | Diabetes Mellitus (n=1316) | P Value for Trend |
|---|---|---|---|---|
| Characteristic | ||||
| Female sex, n (%) | 4786 (50.7) | 2065 (50.0) | 503 (38.2) | <0.0001 |
| Age, y | 52.0±10.8 | 59.3±9.7 | 63.0±8.3 | <0.0001 |
| Socioeconomic statusa | 13.48±4.42 | 12.16±4.39 | 10.95±4.28 | <0.0001 |
| Cardiovascular risk factors | ||||
| Arterial hypertension, n (%) | 3888 (41.2) | 2451 (59.4) | 1056 (80.2) | <0.0001 |
| Pack‐years of smokingb | 0.02 (0/2.43) | 0.26 (0/4.75) | 0.57 (0/4.87) | <0.0001 |
| Waist/height ratioc | 0.54±0.07 | 0.57±0.08 | 0.63±0.08 | <0.0001 |
| Non–high‐density lipoprotein/high‐density lipoprotein ratiod | 2.97±1.17 | 3.27±1.20 | 3.37±1.20 | <0.0001 |
| Physical activitye | 7.74±3.88 | 6.94±4.02 | 5.94±4.22 | <0.0001 |
| Family history of myocardial infarction or stroke, n (%) | 1949 (20.7) | 975 (23.6) | 362 (27.5) | <0.0001 |
| Inflammation | ||||
| C‐reactive protein, mg/L | 1.30 (0.50/2.70) | 2.00 (1.00/3.80) | 2.40 (1.30/5.10) | <0.0001 |
| Cardiovascular comorbidities, n (%) | ||||
| Congestive heart failure | 620 (6.6) | 352 (8.5) | 166 (12.6) | <0.0001 |
| Coronary artery disease | 210 (2.2) | 247 (6.1) | 177 (14.1) | <0.0001 |
| Myocardial infarction | 140 (1.5) | 173 (4.2) | 125 (9.6) | <0.0001 |
| Stroke | 113 (1.2) | 96 (2.3) | 64 (4.9) | <0.0001 |
| Atrial fibrillation | 181 (1.9) | 148 (3.6) | 76 (5.8) | <0.0001 |
| Peripheral artery disease | 204 (2.2) | 184 (4.5) | 107 (8.3) | <0.0001 |
| Any cardiovascular disease | 1190 (12.7) | 842 (20.6) | 445 (34.6) | <0.0001 |
| Measurements of endothelial function | ||||
| Flow‐mediated dilation, % | 8.60±5.41 | 7.61±4.85 | 6.45±4.62 | 0.10 |
| Baseline brachial artery diameter, mm | 4.23±0.86 | 4.42±0.81 | 4.70±0.81 | <0.0001 |
| Reactive hyperemia indexf | 0.69±0.41 | 0.62±0.41 | 0.45±0.40 | <0.0001 |
| Baseline pulse amplitude, mm | 0.38 (0.19/0.74) | 0.52 (0.26/0.92) | 0.69 (0.43/1.03) | <0.0001 |
| Humoral biomarkers of glucose metabolism | ||||
| HbA1c, % | 5.30 (5.10/5.50) | 5.90 (5.70/6.00) | 6.70 (6.30/7.20) | <0.0001 |
| HbA1c, mmol/mol | 34.43 (32.24/36.61) | 40.98 (38.80/42.08) | 49.73 (45.36/55.19) | <0.0001 |
| Fasting glucose, mg/dL | 90.0 (84.0/95.0) | 96.0 (90.0/102.0) | 117.2 (103.0/139.0) | <0.0001 |
| Fasting glucose, mmol/L | 5.00 (4.66/5.27) | 5.33 (5.00/5.66) | 6.51 (5.72/7.71) | <0.0001 |
| Medication, n (%)g | ||||
| Antidiabetic medication (A10) | … | … | 856 (65.2) | … |
| Antithrombotic agents (B01) | 689 (7.4) | 678 (16.5) | 450 (34.3) | <0.0001 |
| Antihypertensives (C02) | 57 (0.6) | 41 (1.0) | 56 (4.3) | <0.0001 |
| Diuretics (C03) | 261 (2.8) | 257 (6.3) | 263 (20.0) | <0.0001 |
| β Blockers (C07) | 1085 (11.7) | 902 (22.0) | 519 (39.5) | <0.0001 |
| Calcium channel blocker (C08) | 432 (4.6) | 356 (8.7) | 290 (22.1) | <0.0001 |
| Agents acting on the renin‐angiotensin‐aldosterone system (C09) | 1464 (15.7) | 1247 (30.4) | 781 (59.5) | <0.0001 |
| Lipid‐modifying agents (C10) | 704 (7.6) | 744 (18.2) | 508 (38.7) | <0.0001 |
Individuals were grouped according to medical history and HbA1c levels as normoglycemic (<5.7% [<39 mmol/mol]), as having increased risk for diabetes mellitus or pre–diabetes mellitus (5.7%–6.4% [39–46 mmol/mol]), and as having diabetes mellitus (≥6.5% [≥48 mmol/mol]). Plus‐minus values are means ± SDs, and 2 values in parentheses are medians with 25th and 75th percentiles. The number (percentage) denotes absolute and relative frequency. P values were derived by Jonckheere‐Terpstra trend test. HbA1c indicates hemoglobin A1c.
Socioeconomic status score ranges from 3 to 21, with higher values indicating higher status.
Pack‐years was calculated as number of cigarettes smoked per day, divided by 20 and multiplied by duration of smoking in years.
Waist/height ratio is the waist circumference divided by the body height in centimeters.
Non–high‐density lipoprotein/high‐density lipoprotein ratio was calculated by subtracting high‐density lipoprotein cholesterol from total cholesterol, divided by high‐density lipoprotein cholesterol.
Physical activity score was calculated by multiplying total minutes of activity by the intensity score, displayed per 1000 units.
Reactive hyperemia index is the logarithmic ratio of digital pulse amplitude at baseline and after cuff release, normalized to the control finger.
Medication is labeled with the anatomical therapeutic chemical code.
Baseline Characteristics of Individuals Without and With Incident Pre–Diabetes Mellitus and Diabetes Mellitus at Follow‐Up
Baseline characteristics of individuals who developed incident (pre–)diabetes mellitus during follow‐up and those who remained free of (pre–)diabetes mellitus are displayed in Table 2. During the 5‐year follow‐up period, incident pre–diabetes mellitus was detected in n=1610 and incident type 2 diabetes mellitus was detected in n=386 individuals (of which, n=267 [69.2%] were classified by HbA1c, n=118 [30.6%] were classified by medical history, and n=1 [0.2%] were classified by history of antidiabetic medications). Distributions of characteristics of subjects with incident (pre–)diabetes mellitus were similar to those with baseline (pre–)diabetes mellitus, showing increased prevalence or alterations of cardiovascular risk factors, CVD, and endothelial function variables from normoglycemic to subjects with (pre–)diabetes mellitus. For visualization of the relation between endothelial function at baseline and incident (pre–)diabetes mellitus, binomial plots were generated for the incidence of (pre–)diabetes mellitus over the range of endothelial function (Figure 2). RHI (Figure 2A) and FMD (Figure 2C) showed a negative, graded relationship for the incidence of (pre–)diabetes mellitus across increasing values, whereas there was a steady increase in incidence of (pre–)diabetes mellitus with increasing baseline pulse amplitude (Figure 2B) and brachial artery diameter (Figure 2D).
Table 2.
Baseline Characteristics of Individuals Without and With Incident (Pre‐) Diabetes Mellitus at Follow‐Up
| Characteristic | Incident Pre–Diabetes Mellitus | Incident Diabetes Mellitus | ||||
|---|---|---|---|---|---|---|
| No (n=6316) | Yes (n=1610) | P Value | No (n=10 990) | Yes (n=386) | P Value | |
| Female sex, n (%) | 3134 (49.6) | 834 (51.8) | 0.12 | 5506 (50.1) | 156 (40.4) | 0.00019 |
| Age, y | 50.7±10.5 | 55.5±10.0 | <0.0001 | 53.7±10.8 | 58.4±9.2 | <0.0001 |
| Socioeconomic statusa | 13.95±4.32 | 13.02±4.38 | <0.0001 | 13.42±4.39 | 11.83±4.23 | <0.0001 |
| Cardiovascular risk factors | ||||||
| Arterial hypertension, n (%) | 2366 (37.5) | 784 (48.7) | <0.0001 | 4878 (44.4) | 286 (74.1) | <0.0001 |
| Pack‐years of smokingb | 0 (0/1.89) | 0.13 (0/3.14) | <0.0001 | 0 (0/2.50) | 1.09 (0/6.80) | <0.0001 |
| Waist/height ratioc | 0.53±0.07 | 0.55±0.07 | <0.0001 | 0.54±0.07 | 0.62±0.08 | <0.0001 |
| Non–high‐density lipoprotein/high‐density lipoprotein ratiod | 2.90±1.13 | 3.13±1.16 | <0.0001 | 3.02±1.15 | 3.72±1.24 | <0.0001 |
| Physical activityd | 7.92±3.76 | 7.62±3.87 | 0.011 | 7.32 (5.30/9.42) | 6.31 (4.00/8.43) | <0.0001 |
| Family history of myocardial infarction or stroke, n (%) | 1270 (20.1) | 362 (22.5) | 0.038 | 2341 (21.3) | 91 (23.6) | 0.28 |
| Inflammation | ||||||
| C‐reactive protein, mg/L | 1.20 (0.50/2.40) | 1.60 (0.57/3.20) | <0.0001 | 1.40 (0.50/2.80) | 2.30 (1.20/4.91) | <0.0001 |
| Cardiovascular comorbidities, n (%) | ||||||
| Congestive heart failure | 390 (6.2) | 101 (6.3) | 0.86 | 731 (6.7) | 30 (7.8) | 0.41 |
| Coronary artery disease | 107 (1.7) | 47 (2.9) | 0.0023 | 318 (2.9) | 27 (7.1) | <0.0001 |
| Myocardial infarction | 63 (1.0) | 33 (2.1) | 0.0012 | 201 (1.8) | 18 (4.7) | 0.00046 |
| Stroke | 63 (1.0) | 20 (1.2) | 0.41 | 140 (1.3) | 13 (3.4) | 0.0020 |
| Atrial fibrillation | 96 (1.5) | 35 (2.2) | 0.079 | 228 (2.1) | 11 (2.9) | 0.28 |
| Peripheral artery disease | 105 (1.7) | 48 (3.0) | 0.0011 | 272 (2.5) | 22 (5.7) | 0.00051 |
| Any cardiovascular disease | 702 (11.2) | 228 (14.2) | 0.00083 | 1498 (13.7) | 83 (21.8) | <0.0001 |
| Measurements of endothelial function | ||||||
| Flow‐mediated dilation, % | 8.76±5.46 | 7.90±4.88 | <0.0001 | 8.34±5.25 | 6.93±4.62 | <0.0001 |
| Baseline brachial artery diameter, mm | 4.20±0.86 | 4.34±0.85 | <0.0001 | 4.28±0.85 | 4.59±0.79 | <0.0001 |
| Reactive hyperemia indexe | 0.70±0.41 | 0.63±0.41 | <0.0001 | 0.68±0.41 | 0.49±0.36 | <0.0001 |
| Baseline pulse amplitude, mm | 0.37 (0.18/0.71) | 0.46 (0.24/0.85) | <0.0001 | 0.41 (0.20/0.78) | 0.67 (0.37/1.07) | <0.0001 |
| Humoral biomarkers of glucose metabolism | ||||||
| HbA1c, % | 5.20 (5.00/5.40) | 5.50 (5.30/5.60) | <0.0001 | 5.41±0.42 | 5.96±0.37 | <0.0001 |
| HbA1c, mmol/mol | 33.33 (31.15/35.52) | 36.61 (34.43/37.71) | <0.0001 | 35.58±4.58 | 41.62±4.08 | <0.0001 |
| Fasting glucose, mg/dL | 89.5±8.0 | 92.4±8.7 | <0.0001 | 91.6±9.0 | 103.4±12.7 | <0.0001 |
| Fasting glucose, mmol/L | 4.97±0.45 | 5.13±0.48 | <0.0001 | 5.08 (0.50) | 5.74±0.70 | <0.0001 |
| Medication, n (%)f | ||||||
| Antidiabetic medication (A10) | … | … | … | … | … | … |
| Antithrombotic agents (B01) | 358 (5.8) | 164 (10.3) | <0.0001 | 958 (8.8) | 65 (16.9) | <0.0001 |
| Antihypertensives (C02) | 31 (0.5) | 14 (0.9) | 0.093 | 67 (0.6) | 7 (1.8) | 0.013 |
| Diuretics (C03) | 126 (2.0) | 60 (3.8) | 0.00014 | 330 (3.0) | 32 (8.3) | <0.0001 |
| β Blockers (C07) | 607 (9.8) | 244 (15.3) | <0.0001 | 1465 (13.5) | 101 (26.3) | <0.0001 |
| Calcium channel blocker (C08) | 220 (3.5) | 103 (6.5) | <0.0001 | 557 (5.1) | 43 (11.2) | <0.0001 |
| Agents acting on the renin‐angiotensin‐aldosterone system (C09) | 810 (13.0) | 345 (21.6) | <0.0001 | 2009 (18.5) | 150 (39.1) | <0.0001 |
| Lipid‐modifying agents (C10) | 357 (5.7) | 202 (12.7) | <0.0001 | 1087 (10.0) | 82 (21.4) | <0.0001 |
Baseline characteristics of individuals who developed incident (pre–)diabetes mellitus at follow‐up and those who remained free of (pre–)diabetes mellitus. Individuals were grouped according to medical history and HbA1c levels as normoglycemic (<5.7% [<39 mmol/mol]), as having increased risk for diabetes mellitus or pre–diabetes mellitus (5.7%–6.4% [39–46 mmol/mol]), and as having diabetes mellitus (≥6.5% [≥48 mmol/mol]). Plus‐minus values are means ± SDs, and 2 values in parentheses are medians with 25th and 75th percentiles. Number (percentage) denotes absolute and relative frequency. P values were derived by Fisher exact or χ2 tests for categorial variables; and for continuous variables, Mann‐Whitney U or Student t tests were used. HbA1c indicates hemoglobin A1c.
Socioeconomic status score ranges from 3 to 21, with higher values indicating higher status.
Pack‐years was calculated as number of cigarettes smoked per day, divided by 20 and multiplied by duration of smoking in years.
Waist/height ratio is the waist circumference divided by the body height in centimeters.
Non–high‐density lipoprotein/high‐density lipoprotein ratio was calculated by subtracting high‐density lipoprotein cholesterol from total cholesterol, divided by high‐density lipoprotein cholesterol.
Physical activity score was calculated by multiplying total minutes of activity by the intensity score, displayed per 1000 units.
Reactive hyperemia index is the logarithmic ratio of digital pulse amplitude at baseline and after cuff release, normalized to the control finger.
Medication is labeled with the anatomical therapeutic chemical code.
Figure 2.
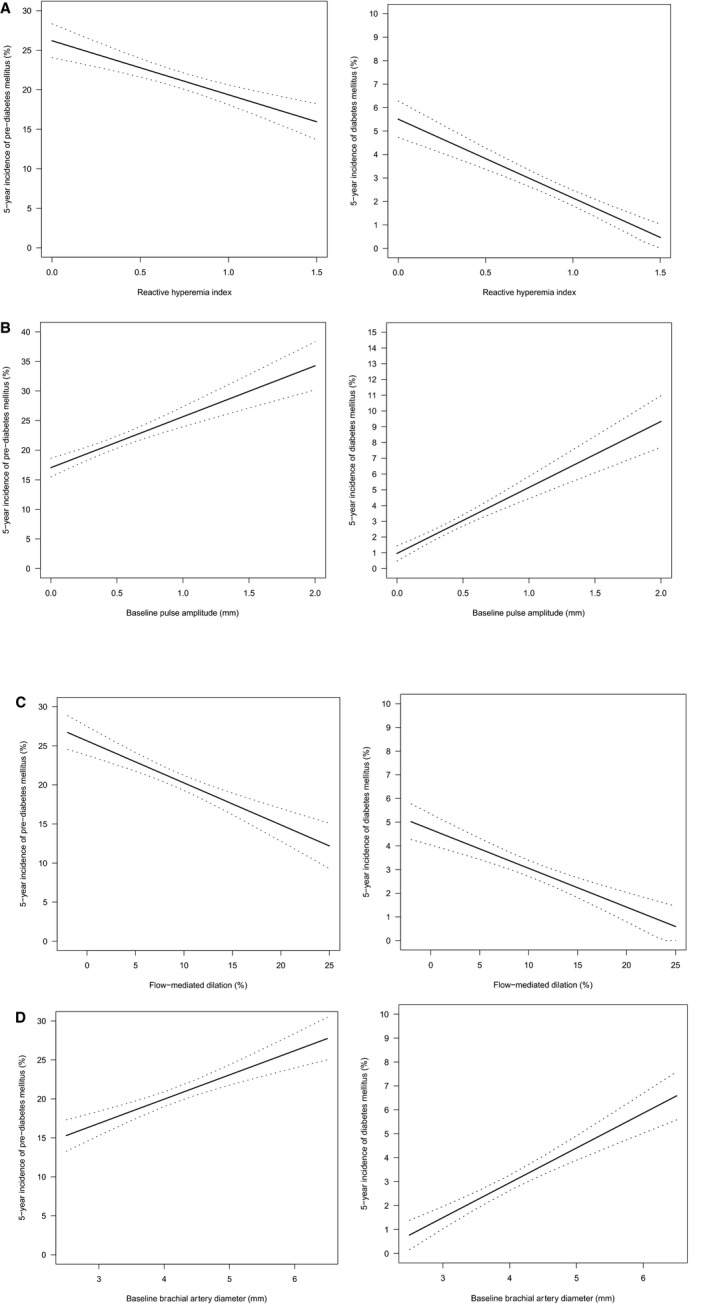
Endothelial function and incidence of (pre–)diabetes mellitus. A, Reactive hyperemia index, B, Baseline pulse amplitude, C, Flow‐mediated dilation, D, Baseline brachial artery diameter. Binomial plots presenting the incidence of (pre–)diabetes mellitus over the range of endothelial function variables at baseline, as indicated by a regression line with 95% CI.
Relation Between Endothelial Function at Baseline and HbA1c Levels at Follow‐Up
In the next step, we evaluated how endothelial function at baseline might impact HbA1c levels after 5‐year follow‐up in all subjects of the study sample without intake of blood glucose influencing drugs at baseline and follow‐up by the use of linear regression models for each measure (Table 3). Decreased RHI (β=−0.020; P=0.0029) and increased baseline pulse amplitude (β=0.015; P=0.033) were strong predictors of increased levels of HbA1c at follow‐up in multivariable regression analysis, with adjustment for baseline HbA1c, sex, age, socioeconomic status, arterial hypertension, waist/height ratio, pack‐years of smoking, non–high‐density lipoprotein/high‐density lipoprotein ratio, physical activity, family history of myocardial infarction or stroke, prevalent CVD, medication use, and inflammation by levels of C‐reactive protein. In contrast, the interrelation between FMD (β=−0.003; P=0.62) as well as baseline brachial artery diameter (β=0.015; P=0.068) and HbA1c was attenuated and did no longer pass the threshold of statistical significance after additional adjustment for cardiovascular risk factors, prevalent CVD, and medication use.
Table 3.
Association of Baseline Endothelial Function on HbA1c Levels After 5‐Year Follow‐Up
| Measurements of Endothelial Function | No. | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|
| β Estimate per SD (95% CI) | P Value | β Estimate per SD (95% CI) | P Value | β Estimate per SD (95% CI) | P Value | ||
| Reactive hyperemia index | 8435 | −0.028 (−0.039 to −0.017) | <0.0001 | −0.020 (−0.034 to −0.007) | 0.0025 | −0.020 (−0.033 to −0.007) | 0.0029 |
| Baseline pulse amplitude | 8435 | 0.027 (0.016 to 0.038) | <0.0001 | 0.015 (0.001 to 0.029) | 0.035 | 0.015 (0.001 to 0.029) | 0.033 |
| Flow‐mediated dilation | 9522 | −0.016 (−0.026 to −0.006) | 0.0016 | −0.003 (−0.015 to 0.008) | 0.62 | −0.003 (−0.015 to 0.009) | 0.59 |
| Baseline brachial artery diameter | 10 261 | 0.032 (0.019 to 0.046) | <0.0001 | 0.015 (−0.001 to 0.030) | 0.068 | 0.015 (−0.001 to 0.030) | 0.066 |
β Estimates and 95% CIs are derived from a linear regression model, modeling for HbA1c levels (dependent variable) per 1‐SD increase in endothelial function (independent variable). Patients with intake of drugs affecting blood glucose at baseline and follow‐up were excluded from analyses. HbA1c indicates hemoglobin A1c.
Model 1 was adjusted for baseline HbA1c, sex, age, and socioeconomic status.
Model 2 was additionally adjusted for arterial hypertension, waist/height ratio, pack‐years of smoking, non–high‐density lipoprotein/high‐density lipoprotein ratio, physical activity, family history of myocardial infarction or stroke, cardiovascular disease (comprising congestive heart failure, coronary artery disease, myocardial infarction, stroke, atrial fibrillation, and peripheral artery disease), and medication use (antithrombotic agents, antihypertensives, diuretics, β blockers, calcium channel blocker, agents acting on the renin‐angiotensin‐aldosterone system, and lipid‐modifying agents).
Model 3 was additionally adjusted for C‐reactive protein.
Baseline Endothelial Function and Incident Pre–Diabetes Mellitus and Diabetes Mellitus
The results of Poisson regression modeling with robust variance estimates for subjects who were free of pre–diabetes mellitus and type 2 diabetes mellitus at baseline are displayed in Table 4. RHI was independently associated with an increased risk of incident pre–diabetes mellitus by 8% (95% CI, 1.02–1.15; P=0.012) and type 2 diabetes mellitus by 16% (95% CI, 1.01–1.34; P=0.041) per 1 SD. Furthermore, baseline pulse amplitude was independently predictive of future development of both pre–diabetes mellitus (RR, 1.12; 95% CI, 1.06–1.19; P=0.00019) and diabetes mellitus (RR, 1.17; 95% CI, 1.02–1.33; P=0.022). FMD and baseline brachial artery diameter were both independently associated with an increased risk of pre–diabetes mellitus (RR, 1.08 [95% CI, 1.02–1.14; P=0.012] for FMD and RR, 1.14 [95% CI, 1.06–1.23; P=0.00080] for baseline brachial artery diameter). However, their impact on development of type 2 diabetes mellitus risk was present in the age‐, sex‐, and socioeconomic status–adjusted model (RR, 1.19 [95% CI, 1.04–1.37; P=0.014] for FMD and RR, 1.20 [95% CI, 1.02–1.41; P=0.027] for baseline brachial artery diameter), but was no longer observed after adjustment for other cardiovascular risk factors, prevalent CVD, and medication use (RR, 1.01 [95% CI, 0.86–1.18; P=0.94] for FMD and RR, 0.96 [95% CI, 0.79–1.16; P=0.65] for baseline brachial artery diameter).
Table 4.
Impact of Endothelial Function on Incident (Pre‐)Diabetes Mellitus After 5‐Year Follow‐Up
| Measurements of Endothelial Function | No. | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value | ||
| Estimates (per 1‐SD decline of endothelial function marker) for incident pre–diabetes mellitus | |||||||
| Reactive hyperemia index | 6125 | 1.15 (1.09–1.21) | <0.0001 | 1.08 (1.02–1.15) | 0.010 | 1.08 (1.02–1.15) | 0.012 |
| Baseline pulse amplitude | 6125 | 1.15 (1.10–1.21) | <0.0001 | 1.12 (1.05–1.18) | 0.00023 | 1.12 (1.06–1.19) | 0.00019 |
| Flow‐mediated dilation | 6849 | 1.08 (1.02–1.14) | 0.00015 | 1.08 (1.02–1.14) | 0.014 | 1.08 (1.02–1.14) | 0.012 |
| Baseline brachial artery diameter | 7271 | 1.18 (1.10–1.26) | <0.0001 | 1.14 (1.06–1.23) | 0.00080 | 1.14 (1.06–1.23) | 0.00080 |
| Estimates (per 1‐SD decline of endothelial function marker) for incident diabetes mellitus | |||||||
| Reactive hyperemia index | 8536 | 1.42 (1.25–1.60) | <0.0001 | 1.16 (1.01–1.34) | 0.040 | 1.16 (1.01–1.34) | 0.041 |
| Baseline pulse amplitude | 8536 | 1.33 (1.20–1.46) | <0.0001 | 1.17 (1.02–1.33) | 0.023 | 1.17 (1.02–1.33) | 0.022 |
| Flow‐mediated dilation | 9633 | 1.19 (1.04–1.37) | 0.014 | 1.01 (0.86–1.18) | 0.94 | 1.01 (0.86–1.19) | 0.92 |
| Baseline brachial artery diameter | 10 371 | 1.20 (1.02–1.41) | 0.027 | 0.96 (0.79–1.16) | 0.65 | 0.95 (0.79–1.16) | 0.63 |
Relative risks and 95% CIs are derived from a Poisson regression model with robust variance estimation, modeling for incident (pre–)diabetes mellitus (dependent variable) per 1‐SD decline in endothelial function (independent variable). Flow‐mediated dilation and reactive hyperemia index were modeled as inverse term (multiplied by −1), to provide estimates reflecting increased risk.
Model 1 was adjusted for sex, age, and socioeconomic status.
Model 2 was additionally adjusted for arterial hypertension, waist/height ratio, pack‐years of smoking, non–high‐density lipoprotein/high‐density lipoprotein ratio, physical activity, family history of myocardial infarction or stroke, cardiovascular disease (comprising congestive heart failure, coronary artery disease, myocardial infarction, stroke, atrial fibrillation, and peripheral artery disease), and medication use (antithrombotic agents, antihypertensives, diuretics, β blockers, calcium channel blocker, agents acting on the renin‐angiotensin‐aldosterone system, and lipid‐modifying agents).
Model 3 was additionally adjusted for C‐reactive protein.
Discussion
To our knowledge, this is the first study investigating the association of different endothelial function measurement methods, in particular digital volume plethysmography, with the incidence of pre–diabetes mellitus and type 2 diabetes mellitus in the general population. The results of the present study demonstrate, for the first time, that a lower RHI, derived from digital volume plethysmography, as well as the baseline pulse amplitude were strong predictors for the development of type 2 diabetes mellitus and its precursor pre–diabetes mellitus independently of other well‐established diabetes mellitus risk factors. This implies that microvascular endothelial dysfunction may be detectable before the development of (pre–)diabetes mellitus and hence rather precede than being solely the consequence of (pre–)diabetes mellitus. In contrast, this association was weaker for FMD, a measure of endothelial function of large conductance vessels, thus enabling differentiation of microvascular and macrovascular endothelial dysfunction for the development and progression of (pre–)diabetes mellitus.
Endothelial Dysfunction Is a Consequence of Type 2 Diabetes Mellitus
In the setting of type 2 diabetes mellitus, endothelial dysfunction is a key component of atherosclerosis and initiates the development of clinical CVD.6 Pathophysiologically, endothelial dysfunction is, besides others, the consequence of recurrent hyperglycemia; elevated free fatty acids; impairment of insulin signaling, leading to systemic insulin resistance; decreased vascular bioavailability of NO caused by reduced production and/or increased inactivation of NO24; and increased oxidative stress with the consequence of enhanced vasoconstriction, inflammation, and thrombosis.25
Several methods are available for the in vivo assessment of endothelial dysfunction in humans. Among these, FMD of the brachial artery and digital volume plethysmography to determine endothelial function of arterioles are validated methods to assess conduit artery and resistance artery endothelial dysfunction, respectively.8 Both methods are partly dependent on NO26 and correlates of coronary endothelial function.27 More important, endothelial dysfunction of large and/or small arteries has consistently shown to be associated with future cardiovascular events in patients with arterial hypertension, coronary and peripheral artery disease, and heart failure.8, 28 Differences in the pathophysiological characteristics of endothelial dysfunction between circulatory beds remain poorly investigated. In the present study, it was clearly demonstrated that in the setting of type 2 diabetes mellitus, the assessment of endothelial dysfunction in different circulatory beds shows a differential picture, highlighting the interrelation between early microvascular deterioration and disease progression.
Endothelial Dysfunction Precedes the Development of Type 2 Diabetes Mellitus
The concept that endothelial dysfunction may precede the development of future type 2 diabetes mellitus has been proposed earlier. Several studies indicated that elevated biomarkers suggestive of endothelial dysfunction/activation, including adhesion molecules intercellular adhesion molecule 1, E‐selectin, and vascular cell adhesion molecule 1, as well as hemostatic markers, such as von Willebrand factor and PAI‐1 (plasminogen activator inhibitor‐1), predicted an increased risk of type 2 diabetes mellitus independent of other diabetes mellitus risk factors, such as obesity, inflammation, and insulin resistance.10, 12, 13, 14 In addition, Rossi et al established that, in postmenopausal women, endothelial dysfunction assessed via FMD of the brachial artery may precede the onset of type 2 diabetes mellitus.11 This study was, however, too small to adjust for other risk factors.
More recently, on the basis of a systematic review and meta‐analysis, Muris et al postulated that diseased microvessels might concur to the onset of type 2 diabetes mellitus.29 However, in none of these studies, methods that allow differentiating between endothelial dysfunction of large‐conductance versus small‐resistance vessels were neither comprehensively investigated nor assessed simultaneously. Furthermore, sample size and the selection of confounders used for adjustments were limited. From a pathophysiological perspective, it has been hypothesized that, in particular, 2 mechanisms may explain the association between microvascular alterations and risk of type 2 diabetes mellitus.29, 30 First, in the arteriolar microcirculation, endothelial dysfunction may impair the function of insulin to redirect blood flow in skeletal muscle from nonnutritive to nutritive capillaries and thereby may reduce insulin‐meditated glucose uptake.31, 32 Second, (pancreatic) microvascular endothelial dysfunction may cause apoptosis of β cells in the pancreas, reducing insulin secretion by the pancreas and thus leading to hyperglycemia, which, in turn, can further impair microvascular endothelial function.33, 34, 35 Mechanistically, insulin stimulates the endothelial NO synthase, leading to production of the vasodilator NO, whereas disrupted endothelial insulin signaling impairs endothelial NO synthase pathways and the balance between production of vasodilator and vasoconstrictor substances.36 In this context, endothelial glucotoxicity, lipotoxicity, and inflammation associated with altered insulin signaling and insulin resistance enhance the production of reactive oxygen species and oxidative stress, thus limiting NO bioavailability and resulting in hypertension, reduced blood flow, and disturbed transport of glucose and insulin to target tissues.36
The present results demonstrate that endothelial dysfunction of arterioles/microvessels, assessed by RHI, independently predicted the development of (pre–)diabetes mellitus, whereas this association was weaker for endothelial dysfunction of conductance vessels, as indicated by FMD. Because the association of large‐conductance artery endothelial dysfunction and risk of type 2 diabetes mellitus was attenuated after adjustment for the cardiovascular risk profile in the case of FMD, this may reflect, at least in part, the stronger dependence of FMD on CVD risk,8 emphasizing the differing role of endothelial dysfunction depending on the arterial bed. This would be consistent with the notion that endothelial dysfunction in large arterial beds is a key step in the pathogenesis of atherosclerotic CVD, whereas endothelial dysfunction at the level of arteriolar microcirculation with a vast surface area in intimate contact with metabolically active, insulin‐sensitive tissues may be associated with the risk of type 2 diabetes mellitus.10, 37 The present study allows us, for the first time, to directly compare the role of microvascular and macrovascular endothelial dysfunction for the development of pre–diabetes mellitus and type 2 diabetes mellitus and supports the concept of microvascular endothelial dysfunction representing an independent precursor to both diabetic phenotypes. In this context, endothelial dysfunction appears to be a shared antecedent of CVD and type 2 diabetes mellitus in a causative framework in which both are consequences of preceding endothelial dysfunction.38, 39 However, the interplay of metabolism, microcirculation, and macrocirculation is complex and still not fully understood. Previous research suggested that microvascular alterations interact within the vascular continuum with larger arteries, subsequently leading to upstream endothelial dysfunction (over time) and atherogenesis (micro‐macro interaction).40, 41, 42, 43 These microvascular alterations in the earlier course of disease development may be reversible through adaption, whereas macrovascular alterations may be more irreversible through maladaptation, which underlines the independent predictive role of RHI in (pre–)diabetes mellitus, whereas FMD may reflect the initiation of atherosclerotic changes over time caused by the preceding microvascular dysfunction.39
In the present analysis, binomial plots revealed that worsening of endothelial function, measured by RHI and FMD, was accompanied by a steady increase in incidence of pre–diabetes mellitus and diabetes mellitus. In addition, structural components of endothelial function variables, such as baseline pulse amplitude and brachial artery diameter, appeared to also influence (pre–)diabetes mellitus risk, partly even to a greater extent than the functional markers, reflecting the arterial state on which functional markers are based on. In contrast to the results for RHI derived from digital volume plethysmography, there was no significant relation between FMD and HbA1c levels at follow‐up as well as risk of type 2 diabetes mellitus, but between FMD and risk of pre–diabetes mellitus, after adjustment for the cardiovascular risk profile. However, as FMD predicted incident pre–diabetes mellitus but not diabetes mellitus, this is likely because of the fact that risk prediction of incident diabetes mellitus includes normoglycemic subjects as well as subjects in the prediabetic stage in which damages may be already present and, thus, FMD may provide less predictive power in case of progression to overt disease.
A lower RHI, measured via digital volume plethysmography, was closely associated with higher HbA1c levels and predicted an increased risk for future development of incident pre–diabetes mellitus and type 2 diabetes mellitus. In a dose‐dependent manner, RR for incident disease increased from pre–diabetes mellitus to diabetes mellitus. More important, RHI appeared to be an independent predictor of increased HbA1c levels, even after adjustment for baseline HbA1c, showing that RHI might be predictive for (pre–)diabetes mellitus development, even when HbA1c levels are below a critical threshold or normal. Because endothelial dysfunction is an indicator of early vascular damage, it might provide incremental value for the detection of metabolic abnormalities that are not completely displayed by blood markers, such as HbA1c, in early stages of disease development. Of interest, regression analysis demonstrated that these relationships were independent of age, sex, socioeconomic status, cardiovascular risk factors, prevalent CVD, medication use, and levels of C‐reactive protein (as an indicator of systemic inflammation). Therefore, it is likely that dysregulated arteriolar endothelial function constitutes an independent risk marker for the development of pre–diabetes mellitus and type 2 diabetes mellitus by displaying clinically relevant, early vascular damage. Given the above, these findings may contribute to identify high‐risk individuals in the setting of primary prevention.
Strengths and Limitations of the Present Study
Strengths of the present study include the novelty of prospectively examining the predictive power of RHI, derived from digital volume plethysmography and FMD, with respect to pre–diabetes mellitus and diabetes mellitus in a population‐based cohort. Furthermore, the large sample size of the population‐representative GHS across a broad age spectrum as well as the comprehensive and standardized assessment of endothelial function in multiple circulatory beds, glucose status, and further included variables are notable. Some limitations, however, need to be considered. More important, this is the first study to investigate a variety of measurements of endothelial dysfunction simultaneously in a population‐based setting. As the investigated sample represents a mid‐European predominantly white population, the findings have limited generalizability to other ethnicities. Although the analyses corrected for a broad spectrum of known confounders, residual confounding cannot be fully excluded. Furthermore, we could not account for the genetic susceptibility to type 2 diabetes mellitus, which is known to play an important role in disease development.44 Because changes in HbA1c were assessed on the basis of 2 measurements (ie, baseline and 5‐year follow‐up), we cannot evaluate the course of HbA1c in between measurements. However, it may be reasonable to assume that HbA1c increased more or less steadily over time, as previously demonstrated.45 Also, we cannot rule out that methodological aspects of the determination of endothelial function may have affected our results because upper arm/brachial occlusion is known to produce a larger dilatory effect (and less NO mediated) compared with a lower arm/wrist occlusion, which could be relevant to the different response to FMD and RHI.46 Nevertheless, rigorous efforts were undertaken for standardized phenotyping and to minimize potential confounding during assessment of endothelial dysfunction.
Conclusions
Our data support the concept that endothelial dysfunction of the microvasculature, in particular resistance (but not conductance) vessels, precedes the development of type 2 diabetes mellitus. This implicates that microvascular damage might occur before the manifestation of diabetes mellitus. Future studies are needed to evaluate whether assessment of microvascular endothelial dysfunction may be useful in the risk stratification in primary prevention given the epidemic of diabetes mellitus development and the limitations of current risk stratification tools for type 2 diabetes mellitus.
Sources of Funding
The GHS (Gutenberg Health Study) is funded through the government of Rhineland‐Palatinate (“Stiftung Rheinland‐Pfalz für Innovation,” contract AZ 961‐386261/733), the research programs “Wissen schafft Zukunft” and “Center for Translational Vascular Biology” of the Johannes Gutenberg–University of Mainz, and its contract with Boehringer Ingelheim and PHILIPS Medical Systems, including an unrestricted grant for the GHS and by the Foundation Heart of Mainz. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
Drs Münzel, Gori, and Wild are principal investigators of the German Center for Cardiovascular Research, partner site Rhine‐Main, Mainz, Germany. Drs Wild and Prochaska are funded by the Federal Ministry of Education and Research (BMBF 01EO1503). The remaining authors have no disclosures to report.
Acknowledgments
We appreciate the contribution of the participants of the GHS (Gutenberg Health Study) as well as the excellent assistance of all technicians, study nurses, and coworkers involved in the GHS. Drs Hahad and Münzel are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2019;8:e012509 DOI: 10.1161/JAHA.119.012509.)
References
- 1. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. Idf diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. [DOI] [PubMed] [Google Scholar]
- 4. Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide‐mediated vasodilation in patients with non‐insulin‐dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. [DOI] [PubMed] [Google Scholar]
- 5. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. [DOI] [PubMed] [Google Scholar]
- 6. Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. [DOI] [PubMed] [Google Scholar]
- 7. Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. [DOI] [PubMed] [Google Scholar]
- 8. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I, Gentile F, Gesualdo M, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Saba PS, Zito A, Mattioli AV, Pedrinelli R. Endothelial function in pre‐diabetes, diabetes and diabetic cardiomyopathy: a review. J Diabetes Metab. 2014;5:1–10. [Google Scholar]
- 10. Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. [DOI] [PubMed] [Google Scholar]
- 11. Rossi R, Cioni E, Nuzzo A, Origliani G, Modena MG. Endothelial‐dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes Care. 2005;28:702–707. [DOI] [PubMed] [Google Scholar]
- 12. Thorand B, Baumert J, Chambless L, Meisinger C, Kolb H, Doring A, Lowel H, Koenig W; MONICA/KORA Study Group . Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle‐aged men and women from the general population. Arterioscler Thromb Vasc Biol. 2006;26:398–405. [DOI] [PubMed] [Google Scholar]
- 13. Meigs JB, O'Donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D'Agostino RB, Wilson PW. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530–537. [DOI] [PubMed] [Google Scholar]
- 14. Odegaard AO, Jacobs DR Jr, Sanchez OA, Goff DC Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song Y, Manson JE, Tinker L, Rifai N, Cook NR, Hu FB, Hotamisligil GS, Ridker PM, Rodriguez BL, Margolis KL, Oberman A, Liu S. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56:1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wild PS, Zeller T, Beutel M, Blettner M, Dugi KA, Lackner KJ, Pfeiffer N, Munzel T, Blankenberg S. The Gutenberg Health Study [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:824–829. [DOI] [PubMed] [Google Scholar]
- 17. Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross‐sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. [DOI] [PubMed] [Google Scholar]
- 18. Schnabel RB, Wild PS, Schulz A, Zeller T, Sinning CR, Wilde S, Kunde J, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T; Gutenberg Health Study Investigators . Multiple endothelial biomarkers and noninvasive vascular function in the general population: the Gutenberg Health Study. Hypertension. 2012;60:288–295. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Collaborating centre for drug statistics methodology. 2018. Available at: https://www.whocc.no/atc/structure_and_principles/. Accessed September 20, 2019.
- 20. Campbell N, Gaston A, Gray C, Rush E, Maddison R, Prapavessis H. The short questionnaire to assess health‐enhancing (SQUASH) physical activity in adolescents: a validation using doubly labeled water. J Phys Act Health. 2016;13:154–158. [DOI] [PubMed] [Google Scholar]
- 21. Lampert T, Kroll LE, Muters S, Stolzenberg H. Measurement of the socioeconomic status within the German health update 2009 (GEDA) [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:131–143. [DOI] [PubMed] [Google Scholar]
- 22. Grossmann V, Schmitt VH, Zeller T, Panova‐Noeva M, Schulz A, Laubert‐Reh D, Juenger C, Schnabel RB, Abt TG, Laskowski R, Wiltink J, Schulz E, Blankenberg S, Lackner KJ, Munzel T, Wild PS. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38:1356–1364. [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl):S8–S16. [DOI] [PubMed] [Google Scholar]
- 24. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. [DOI] [PubMed] [Google Scholar]
- 25. Tousoulis D, Kampoli AM, Stefanadis C. Diabetes mellitus and vascular endothelial dysfunction: current perspectives. Curr Vasc Pharmacol. 2012;10:19–32. [DOI] [PubMed] [Google Scholar]
- 26. Nohria A, Gerhard‐Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006;101:545–548. [DOI] [PubMed] [Google Scholar]
- 27. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 28. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 29. Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta‐analysis. Arterioscler Thromb Vasc Biol. 2012;32:3082–3094. [DOI] [PubMed] [Google Scholar]
- 30. Eringa EC, Serne EH, Meijer RI, Schalkwijk CG, Houben AJ, Stehouwer CD, Smulders YM, van Hinsbergh VW. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord. 2013;14:39–48. [DOI] [PubMed] [Google Scholar]
- 31. Bonadonna RC, Saccomani MP, Del Prato S, Bonora E, DeFronzo RA, Cobelli C. Role of tissue‐specific blood flow and tissue recruitment in insulin‐mediated glucose uptake of human skeletal muscle. Circulation. 1998;98:234–241. [DOI] [PubMed] [Google Scholar]
- 32. Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–E258. [DOI] [PubMed] [Google Scholar]
- 33. Tal MG. Type 2 diabetes: microvascular ischemia of pancreatic islets? Med Hypotheses. 2009;73:357–358. [DOI] [PubMed] [Google Scholar]
- 34. Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, Scotney P, Nyqvist D, Samen E, Lu L, Stone‐Elander S, Proietto J, Andrikopoulos S, Sjoholm A, Nash A, Eriksson U. Targeting vegf‐b as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. [DOI] [PubMed] [Google Scholar]
- 35. Giroix MH, Irminger JC, Lacraz G, Noll C, Calderari S, Ehses JA, Coulaud J, Cornut M, Kassis N, Schmidlin F, Paul JL, Kergoat M, Janel N, Halban PA, Homo‐Delarche F. Hypercholesterolaemia, signs of islet microangiopathy and altered angiogenesis precede onset of type 2 diabetes in the goto‐kakizaki (gk) rat. Diabetologia. 2011;54:2451–2462. [DOI] [PubMed] [Google Scholar]
- 36. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes. 1997;46(suppl 2):S9–S13. [DOI] [PubMed] [Google Scholar]
- 38. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. [DOI] [PubMed] [Google Scholar]
- 39. Jax TW. Metabolic memory: a vascular perspective. Cardiovasc Diabetol. 2010;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, Dejong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Meta‐analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, McNamara M, Thom SA, Klein R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981. [DOI] [PubMed] [Google Scholar]
- 42. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women: the atherosclerosis risk in communities study. JAMA. 2002;287:1153–1159. [DOI] [PubMed] [Google Scholar]
- 43. McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual‐participant meta‐analysis. Am J Epidemiol. 2009;170:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina‐Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome‐wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dubowitz N, Xue W, Long Q, Ownby JG, Olson DE, Barb D, Rhee MK, Mohan AV, Watson‐Williams PI, Jackson SL, Tomolo AM, Johnson TM II, Phillips LS. Aging is associated with increased hba1c levels, independently of glucose levels and insulin resistance, and also with decreased hba1c diagnostic specificity. Diabet Med. 2014;31:927–935. [DOI] [PubMed] [Google Scholar]
- 46. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow‐mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond). 2001;101:629–635. [PubMed] [Google Scholar]


