Abstract
Background
Cardiovascular and cerebrovascular diseases (CBVDs) and cancer are leading causes of death. Short sleep is a potential contributor to health; however, its role in predicting mortality associated with cardiometabolic risk factors (CMRs) and CBVD remains poorly understood. We tested whether objective short sleep duration increases the risk of mortality associated with CMRs and CBVD.
Methods and Results
A total of 1654 adults (aged 20–74 years) from the Penn State Adult Cohort (47.5 years, 52.5% women, and 89.8% white) whose cause of death was determined after 19.2 years (5.2 years). CMR was defined as stage 2 hypertension and/or type 2 diabetes mellitus on the basis of blood pressure and glucose levels or a report of diagnosis or treatment for these conditions. CBVD was defined as a report of diagnosis or treatment for heart disease and/or stroke. Objective short sleep duration was defined as polysomnographic total sleep time <6 hours. Cox proportional hazard models estimated multivariable‐adjusted hazard ratios (HRs) and 95% CIs. Risk of all‐cause mortality associated with CMR or CBVD was significantly modified by objective sleep duration (P<0.05), and it was significantly higher in subjects who slept <6 hours (HR, 2.14 [95% CI, 1.52–3.02] and HR, 3.17 [95% CI=2.16–4.65], respectively). In subjects who slept <6 hours, CMR was associated with a 1.83 higher (95% CI, 1.07–3.13) risk of CBVD mortality and CBVD with a 2.92 higher (95% CI, 1.28–6.65) risk of cancer mortality. In subjects who slept ≥6 hours, CMR was not significantly associated with CBVD mortality (HR, 1.35; 95% CI, 0.70–2.63) nor was CBVD significantly associated with cancer mortality (HR, 0.55; 95% CI, 0.18–1.64).
Conclusions
Objective short sleep duration predicts the all‐cause mortality prognosis of middle‐aged adults with CMR and the cancer‐specific mortality prognosis of those with CBVD.
Keywords: cancer, cardiovascular disease, diabetes mellitus, hypertension, mortality, sleep, survival analysis
Subject Categories: Mortality/Survival, Epidemiology, Lifestyle, Risk Factors, Cardiovascular Disease
Clinical Perspective
What Is New?
Identification of short sleep duration as an effect modifier of mortality and cause of death in people with hypertension, diabetes mellitus, heart disease, or stroke.
Ascertainment of sleep duration via objective in‐laboratory study (ie, polysomnography) in a longitudinal, population‐based cohort.
What Are the Clinical Implications?
Cardiovascular risk factors and diseases as well as sleep disturbances are highly prevalent in the outpatient practice of general practitioners, such as family physicians, internists, and cardiologists, who often refer for an objective in‐laboratory study to rule out sleep apnea.
Clinicians should become aware that the risk of all‐cause and cancer mortality associated with hypertension, diabetes mellitus, heart disease, or stroke is greater in patients with objective short sleep duration, a potentially modifiable risk factor.
Patients with hypertension, diabetes mellitus, heart disease, or stroke, who sleep objectively short, may benefit from targeted treatments to lengthen sleep and improve their long‐term prognosis.
Introduction
Despite increased preventive efforts and campaigns, cardiovascular and cerebrovascular diseases (CBVDs) remain the primary cause of death in the United States and worldwide.1 Similarly, hypertension and type 2 diabetes mellitus remain as the most prevalent cardiometabolic risk factors (CMRs) for the development of CBVD. It is estimated that ≈45% of the US population has CMRs, whereas another 14% have already developed CBVD, making them healthcare problems of epidemic proportions.1
Recent studies increasingly recognize sleep as an additional important contributor to health. Although there is a clear association between obstructive sleep apnea (OSA) with CMRs, CBVD, and mortality,2, 3, 4 we still lack a comprehensive understanding of the association of short sleep, independent of OSA, with adverse health outcomes. This is despite 35% of the population reporting short sleep duration5 and ≈50% of the population sleeping objectively <6 hours, as assessed with in‐laboratory or at‐home polysomnography.6 In 2016, short sleep was identified as a novel contributor to CMRs and CBVD,7 whereas its association with mortality has been modest and inconsistent across studies.8, 9, 10, 11, 12 A limitation of previous epidemiologic studies is the reliance on self‐reported data, which do not allow controlling for the presence of OSA and testing the role of objectively measured sleep duration. Also, the scientific paradigm used in previous studies has been the sole, independent association of sleep duration with mortality.12 These limitations have led to the understanding that measures of short sleep duration may not be useful yet in predicting risk of mortality.
We have shifted this paradigm and conceptualize objective sleep duration as an effect modifier of the association between CMRs and CBVD with mortality. Our scientific premise holds that the main risk factors and causes of mortality are well known; however, studies of potential effect modifiers that are clinically meaningful predictors of the prognosis of individuals with a given risk factor (ie, CMR) or who have already developed diseases that lead to death (ie, CBVD) have not been comprehensive as they pertain to sleep. Support for this effect modification paradigm comes from previous studies in which objectively measured short sleep duration was associated with cardiac, stress, and immune system biomarkers as well as CMRs and mortality in individuals with chronic insomnia.13, 14, 15, 16, 17, 18, 19, 20, 21 Specifically, our theoretical framework posits that objective short sleep duration in individuals with traditional CMRs may worsen cardiovascular, metabolic, and inflammatory functions and increase the risk of mortality, given previous evidence that impaired cardiac autonomic modulation, glucose metabolism, increased inflammation, and progressive changes in endothelial function at subclinical levels have all been associated with short sleep duration under experimental conditions. On the basis of this framework, the previously demonstrated link between inadequate sleep, CBVD, and mortality may be amplified in those with objective short sleep duration and, thus, objective measures of sleep are likely to identify populations or patients who are more vulnerable to the long‐term adverse effect of CMRs and CBVD known to lead to early death.
Thus, in the present study, we hypothesized that objective short sleep duration increases the risk of mortality associated with CMRs and CBVD in middle‐aged adults. To test this novel hypothesis, we examined the effect modification by objective sleep duration on the increased risk of all‐cause and cause‐specific mortality.
Methods
Because of the sensitive nature of the mortality data collected for this study, the data that support the findings of this study are available from the corresponding author on reasonable request by qualified researchers trained in human subject confidentiality protocols.
Participants
Detailed descriptions of the Penn State Adult Cohort and sampling procedures are described elsewhere.19, 22, 23, 24 Briefly, telephone interviews were conducted between January 1990 and August 1991 with 16 583 age‐eligible men and women with response rates of 73.5% and 74.1%, respectively, in the first phase. In the second phase, 741 men and 1000 women were randomly selected from the first phase and studied in the sleep laboratory between January 1990 and March 1999, with response rates of 67.8% and 65.8%, respectively. From 2014 to 2017, vital status and cause of death were extracted yearly from the National Death Index of the US Centers for Disease Control and Prevention. Written informed consent was obtained at baseline, and all study protocols were approved by the Institutional Review Board at Penn State College of Medicine.
Mortality
Death certificates for deceased individuals as of December 31, 2016, were retrieved from the US Centers for Disease Control and Prevention. Participants were linked by the US Centers for Disease Control and Prevention to death records from the National Death Index for the years 1992 through 2016, and vital status was determined through a rigorous process of probabilistic matching and death certificate review on the basis of participants’ social security number, full name, date of birth, and sex.25, 26 The primary cause of death was abstracted from raw files for case definition, which was classified using International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10), for deaths occurring before 1998 and 1999 and beyond, respectively.27, 28, 29 CBVD mortality was defined as ICD‐9 codes 390 to 459 and ICD‐10 codes I00 to I99, whereas mortality from other non‐CBVD causes was defined according to their respective ICD‐9 and ICD‐10 codes, including cancer (ICD‐9 codes 140‐239 and ICD‐10 codes C00‐D49), the second leading cause of death.27, 28, 29 Other non‐CBVD causes of death with low rates (ie, <70 deceased cases) included respiratory, neurological, endocrine, genitourinary, digestive, or any other causes (eg, suicide or accidents), all based on their respective ICD‐9 or ICD‐10 codes.27, 28, 29 Standard guidelines and algorithms were followed to avoid data misclassification.30, 31 Of the 1741 participants, a total of 1145 subjects were alive and 596 were deceased as of December 31, 2016. Survival time was calculated from the time of the baseline in‐laboratory evaluation to the date of death for those deceased or to December 31, 2016, for those alive. The average survival time in the entire cohort was 18.77±5.56 years, with a median of 20.05 (range, 0.09–30.20) years. Participants who were aged ≥75 years at baseline (n=87) were excluded from the analyses, given their potential for excess mortality as a consequence of the natural process of aging with such a long follow‐up; in fact, the median survival time for individuals who were aged ≥75 years at baseline was 9.1 years and only 3 were still alive after 20 years. Thus, of the 1654 participants aged <75 years, 1142 were alive and 512 were deceased at follow‐up, of whom 209 died of CBVD and 131 died of cancer.
Cardiometabolic Conditions
During the clinical history and physical examination at baseline, blood pressure was measured in the evening ≈2 hours before the start of the polysomnography following standard procedures (ie, average of 3 consecutive readings during a 5‐minute period after 10 minutes of rest in the supine position).24 Fasting glucose was assayed from blood drawn in the morning after the polysomnography.24
We established the presence of cardiometabolic conditions based on 3 mutually exclusive clinically meaningful groups. First, the presence of CBVD at baseline was defined by a report of a physician diagnosis or treatment for heart disease and/or stroke during the clinical history and physical examination. Second, the presence of CMRs was defined by stage 2 hypertension (ie, a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or use of antihypertensive medication) and/or type 2 diabetes mellitus (ie, fasting glucose levels ≥126 mg/dL or receiving treatment for diabetes mellitus), and none in the CMR group had a history of CBVD. Finally, the reference group was defined as the absence of either of these 2 categories (ie, none in the reference group had either CBVD or CMR). Our primary independent variable was the presence of CMR or CBVD, whereas our secondary independent variable was the presence of CMR and CBVD as separate categories.
Effect Modifier
All subjects were evaluated for one night in the sleep laboratory in sound‐attenuated, light‐ and temperature‐controlled rooms. Each subject was continuously monitored for 8 hours using 16‐channel polysomnography, including electroencephalography, electrooculography, and electromyography. Sleep was recorded between 10 pm to 11 pm and 6 am to 7 am to conform to subjects’ usual sleep pattern. The sleep recordings were subsequently scored independently, according to standardized criteria.32 On the basis of the distribution of polysomnography‐measured total sleep time, we categorized the entire study sample into 2 groups: ≥50th percentile (ie, ≥6 hours) and <50th percentile (ie, <6 hours). This cutoff of 6 hours of sleep was previously shown to be associated with significant morbidity and mortality.5, 7, 19, 33, 34
Covariables
As part of the physical examination at baseline, height (cm) and weight (kg) were measured and body mass index was calculated. During the polysomnography, respiration was monitored throughout the night using thermocouples at the nose and mouth and thoracic strain gauges. All‐night recordings of hemoglobin oxygen saturation were obtained with an oximeter attached to the finger. The presence of OSA was defined as an apnea‐hypopnea index ≥5 events per hour of sleep.22, 23 A standardized questionnaire, administered during the clinical history and physical examination, assessed for the presence of physical and mental health conditions.24 The presence of physical health conditions was defined as a binary variable, including any positive response to a past or current history of a physician diagnosis or treatment for allergies/asthma, anemia, birth defects, cancer/tumor, colitis, encephalitis, epilepsy, kidney/bladder disorders, migraine, Parkinson disease, rheumatism, thyroid, or ulcer. The presence of mental health conditions was also defined by a past or current history of a physician diagnosis or treatment for depression, suicidal ideation or attempts, loneliness, marital problems, alcohol abuse, or drug abuse.24 Participants’ sex, age, and race as well as daily consumption of caffeine (number of cups/day), tobacco (number of cigarettes/day), and alcohol (number of drinks/day) were also obtained via the standardized questionnaire during the clinical history and physical examination.24
Statistical Analyses
The primary independent variable was the presence of CMR or CBVD at baseline, and the primary outcome was all‐cause mortality with objective short sleep duration (ie, <6 hours) as the effect modifier. The secondary independent variable was the presence of CMR and CBVD at baseline as separate categories, whereas the secondary outcomes were CBVD and non‐CBVD mortality, including cancer mortality. To quantify the excess risk of all‐cause and cause‐specific mortality associated with CMRs or CBVD compared to the otherwise healthy reference group, multivariable‐adjusted Cox proportional hazards regression models were used. We evaluated the significance of the interaction term between the primary independent variable (CMR/CBVD) and log‐transformed survival time to test whether the proportional hazards assumption was violated. The P value for this interaction term was 0.495, which indicates that the proportional hazards assumption was not violated. All results are presented as hazard ratios (HRs) with their 95% CIs, adjusted for age, race, sex, education, body mass index, smoking, alcohol use, apnea‐hypopnea index, mental health conditions, other physical health conditions, and objective sleep duration as covariables. We tested the interaction between CMR or CBVD and objective sleep duration to investigate the hypothesized effect modification and presented the associations on the basis of 2 strata: those who slept ≥6 hours and those who slept <6 hours at baseline. We projected the survival functions using Kaplan‐Meier curves across all objective sleep duration subgroups. These survival curves were based on the aforementioned multivariable‐adjusted Cox models, with the average sample characteristics presented in Table 1. Also, projected multivariable‐adjusted all‐cause mortality rates at 15 and 20 years after baseline are presented in Table S1 to enhance the clinical interpretation of the results. Finally, sensitivity analyses tested whether subjective sleep duration, measured with the question “How many hours of sleep do usually get at night?,” acted as an effect modifier of the relationship between CMR or CBVD with mortality in 1467 participants with available data. P≤0.05 was used to determine the significance for all analyses. All analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC).
Table 1.
Demographic and Clinical Characteristics of the Overall Sample and Stratified by the Presence of CMRs and CBVD
| Characteristics | Overall (N=1654) | Reference (n=594) | CMR or CBVD (n=1060) | P Valuea | CMR (n=828) | CBVD (n=232) | P Valueb |
|---|---|---|---|---|---|---|---|
| Age, y | 47.5 (12.3) | 43.4 (14.0) | 53.1 (9.5) | <0.01 | 52.7 (9.3) | 54.2 (10.4) | <0.01 |
| Men, % | 47.5 | 42.5 | 54.4 | <0.01 | 52.0 | 61.3 | <0.01 |
| White, % | 89.8 | 91.2 | 88.0 | 0.03 | 86.9 | 91.5 | 0.02 |
| Education, y | 13.6 (2.8) | 14.0 (3.4) | 13.2 (2.4) | <0.01 | 13.2 (2.4) | 13.2 (2.2) | <0.01 |
| BMI, kg/m2 | 27.6 (5.8) | 26.1 (6.1) | 29.8 (5.1) | <0.01 | 29.9 (5.1) | 29.5 (4.9) | <0.01 |
| CMR or CBVD, % | |||||||
| Hypertension | 32.9 | 0.0 | 78.4 | 85.7 | 56.4 | ||
| Diabetes mellitus | 13.9 | 0.0 | 33.0 | 34.0 | 29.8 | ||
| Heart disease | 9.2 | 0.0 | 21.9 | 0.0 | 87.9 | ||
| Stroke | 1.6 | 0.0 | 3.7 | 0.0 | 15.1 | ||
| Smoker, % | 23.7 | 23.5 | 24.1 | 0.78 | 24.0 | 24.3 | 0.96 |
| Alcohol use, drinks/d | 1.1 (5.5) | 1.3 (8.7) | 0.9 (2.0) | 0.15 | 0.9 (2.0) | 0.8 (1.9) | 0.30 |
| Physical health conditions, % | 54.3 | 52.2 | 57.3 | 0.03 | 55.6 | 62.6 | 0.03 |
| Mental health conditions, % | 22.5 | 20.5 | 25.4 | 0.02 | 23.8 | 30.3 | 0.01 |
| AHI, events/h | 2.3 (7.5) | 1.2 (6.4) | 3.8 (7.9) | <0.01 | 3.6 (7.6) | 4.5 (8.8) | <0.01 |
| OSA, % | 10.6 | 6.2 | 16.6 | <0.01 | 15.9 | 18.7 | <0.01 |
| Objective sleep duration, h | 5.9 (1.2) | 6.2 (1.4) | 5.6 (1.0) | <0.01 | 5.6 (0.9) | 5.7 (1.1) | <0.01 |
| <6 h, % | 44.1 | 33.6 | 54.9 | <0.01 | 57.0 | 48.8 | <0.01 |
| Total deaths, % | 30.9 | 16.2 | 39.2 | <0.01 | 35.1 | 53.9 | <0.01 |
| CBVD cause, % | 12.6 | 5.7 | 16.5 | <0.01 | 12.8 | 29.7 | <0.01 |
| Non‐CBVD cause, % | 18.3 | 10.4 | 22.7 | <0.01 | 22.3 | 24.1 | <0.01 |
| Cancer cause, % | 7.9 | 5.7 | 9.2 | 0.01 | 9.2 | 9.1 | 0.03 |
| Other causes, % | 10.4 | 4.7 | 13.6 | <0.01 | 13.2 | 15.1 | <0.01 |
| Survival time, y | 19.2 (5.2) | 20.5 (3.9) | 18.5 (5.7) | <0.01 | 19.1 (5.4) | 16.7 (6.4) | <0.01 |
| Alive, y | 21.6 (2.3) | 21.5 (2.3) | 21.6 (2.3) | 0.55 | 21.7 (2.3) | 21.4 (2.1) | 0.51 |
| Deceased, y | 14.0 (6.0) | 14.9 (5.6) | 13.8 (6.0) | 0.09 | 14.2 (6.0) | 12.4 (6.0) | 0.01 |
Data are mean (SD) for continuous variables and percentage for binary variables. The reference group was absent of CMR and CBVD. AHI indicates apnea‐hypopnea index; BMI, body mass index; CBVD, cardiovascular and cerebrovascular disease (ie, heart disease and/or stroke); CMR, cardiometabolic risk factor (ie, hypertension and/or diabetes mellitus); OSA, obstructive sleep apnea (ie, AHI ≥5 events per hour of sleep).
P value comparing reference vs CMR or CBVD (t‐test and χ2 test were used to compare continuous and binary variables, respectively)
P value comparing reference vs CMR vs CBVD (ANOVA or Cochran‐Mantel‐Haenszel test was used to compare continuous and binary variables among the 3 groups, respectively).
Results
The baseline demographic and clinical characteristics of the cohort are summarized in Table 1. Among the 1654 participants, 594 (35.9%) were free of any CMRs or CBVD, whereas 1060 (64.1%) had either CMR (n=828) or CBVD (n=232). By December 31, 2016, ≈31% (n=512) of the cohort were deceased, of which 209 (40.8%) died of CBVD causes and 303 (59.2%) died of other non‐CBVD causes, including 131 (25.6%) who died of cancer. As expected, the crude mortality rates were positively related to the presence of CMRs or CBVD; subjects with CBVD had the highest mortality rate (53.9%; n=125), followed by those with CMRs (35.1%; n=291), whereas the reference group showed the lowest mortality rate (16.2%; n=92). Moreover, subjects who slept objectively <6 hours had a higher crude mortality rate (40.2%; n=336) compared with those who slept objectively ≥6 hours (21.5%; n=176).
The multivariable‐adjusted HRs and their 95% CIs for the association between CMRs or CBVD with all‐cause mortality are shown in Table 2. The association between CMRs or CBVD with all‐cause mortality was significantly modified by objective short sleep duration (P‐interaction=0.05). The risk of all‐cause mortality associated with CMRs or CBVD was 2.23 (95% CI, 1.60–3.12) times higher among those who slept <6 hours, whereas this risk was 1.39‐fold (95% CI, 0.97–1.99) among those who slept ≥6 hours. The survival curves presented in Figure 1 illustrate the significant effect modification by objective short sleep duration on all‐cause mortality. When examined separately, the risks of all‐cause mortality associated with CMRs and CBVD were 2.14 and 3.17 times higher, respectively, among those who slept <6 hours, whereas the risks were 1.38 and 2.34 times higher, respectively, among those who slept ≥6 hours (Table 2).
Table 2.
HRs and 95% CIs for All‐Cause and Cause‐Specific Mortality Associated With CMRs and CBVD: Role of Objective Sleep Duration
| Mortality | No. of Deaths | Overall | ≥6 h | <6 h |
|---|---|---|---|---|
| CMR or CBVD | ||||
| All cause | 416 | 1.82 (1.42–2.33)a | 1.39 (0.97–1.99) | 2.23 (1.60–3.12)a |
| CBVD cause | 175 | 2.06 (1.38–3.08)a | 1.77 (0.95–3.30) | 2.27 (1.35–3.81)a |
| Non‐CBVD cause | 241 | 1.68 (1.23–2.29)a | 1.21 (0.78–1.89) | 2.20 (1.42–3.40)a |
| Cancer | 97 | 1.49 (0.96–2.32) | 0.89 (0.49–1.61) | 2.62 (1.29–5.33)a |
| Other | 144 | 2.08 (1.34–3.23)a | 2.03 (1.01–4.10)a | 2.11 (1.21–3.68)a |
| CMR | ||||
| All cause | 291 | 1.77 (1.38–2.28)a | 1.38 (0.95–2.01) | 2.14 (1.52–3.02)a |
| CBVD cause | 106 | 1.64 (1.08–2.49)a | 1.35 (0.70–2.63) | 1.83 (1.07–3.13)a |
| Non‐CBVD cause | 185 | 1.64 (1.19–2.26)a | 1.22 (0.77–1.92) | 2.12 (1.36–3.32)a |
| Cancer | 76 | 1.50 (0.95–2.36) | 0.98 (0.54–1.79) | 2.53 (1.23–5.22)a |
| Other | 109 | 1.97 (1.25–3.09)a | 1.83 (0.90–3.84) | 2.02 (1.14–3.58)a |
| CBVD | ||||
| All cause | 125 | 2.75 (2.06–3.69)a | 2.34 (1.48–3.69)a | 3.17 (2.16–4.65)a |
| CBVD cause | 69 | 3.69 (2.34–5.80)a | 3.61 (1.77–7.38)a | 3.81 (2.14–6.76)a |
| Non‐CBVD cause | 56 | 1.83 (1.24–2.72)a | 1.22 (0.65–2.31) | 2.45 (1.46–4.12)a |
| Cancer | 21 | 1.45 (0.80–2.62) | 0.55 (0.18–1.64) | 2.92 (1.28–6.65)a |
| Other | 35 | 2.51 (1.47–4.28)a | 2.80 (1.17–6.69)a | 2.40 (1.23–4.65)a |
HRs adjusted for age, race, sex, education, body mass index, smoking, alcohol use, apnea‐hypopnea index, other physical health conditions, and mental health conditions. CBVD indicates cardiovascular and cerebrovascular disease; CMR, cardiometabolic risk factor; HR, hazard ratio.
P<0.05.
Figure 1.
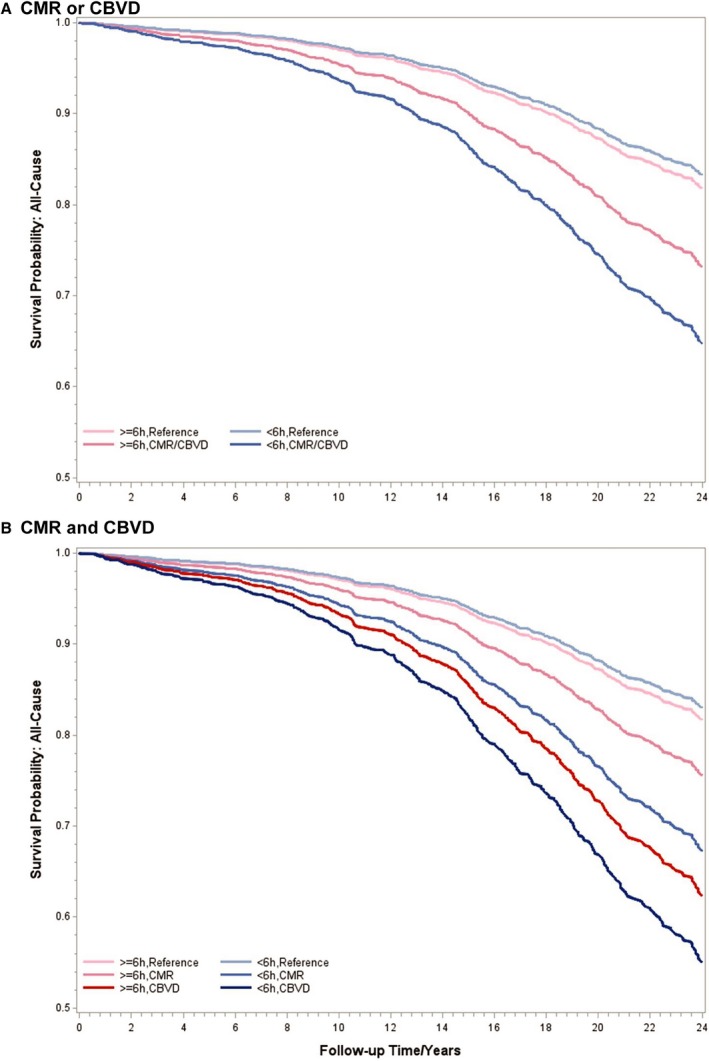
Multivariable‐adjusted survival curves for all‐cause mortality associated with cardiometabolic risk factors (CMRs) and cardiovascular and cerebrovascular disease (CBVD) at baseline. A, Survival curves for all‐cause mortality associated with CMRs or CBVD. B, Survival curves for all‐cause mortality associated with CMRs and CBVD. All data adjusted for age, race, sex, education, body mass index, smoking, alcohol use, apnea‐hypopnea index, other physical health conditions, and mental health conditions.
In terms of cause‐specific mortality, we found differential effect modifications by objective short sleep duration for CBVD mortality (P‐interaction=0.54) versus non‐CBVD mortality (P‐interaction=0.06), specifically, cancer mortality (P‐interaction=0.02). Table 2 presents the HRs of CBVD and cancer mortality stratified by objective sleep duration. CMR was significantly associated with CBVD mortality among those who slept <6 hours (HR, 1.83; 95% CI, 1.07–3.13), whereas this risk was not significant (HR, 1.35; 95% CI, 0.70–2.63) among those who slept ≥6 hours (Table 2). CBVD was significantly associated with CBVD mortality regardless of the objective sleep duration at baseline (HR, 3.61 [95% CI, 1.77–7.38] among those who slept ≥6 hours and HR, 3.81 [95% CI, 2.14–6.76] among those who slept <6 hours). In contrast, the excess risk of cancer mortality associated with CBVD was significantly higher among those who slept <6 hours (HR, 2.92; 95% CI, 1.28–6.65) but not among those who slept ≥6 hours (HR, 0.55; 95% CI, 0.18–1.64). This significant association of CBVD with cancer mortality among those who slept <6 hours remained strong and in the same direction, even after excluding the 147 subjects who already had cancer at baseline (Table S2). The survival curves in Figure 2 illustrate the effect modification by objective short sleep duration on mortality from CBVD and non‐CBVD causes.
Figure 2.
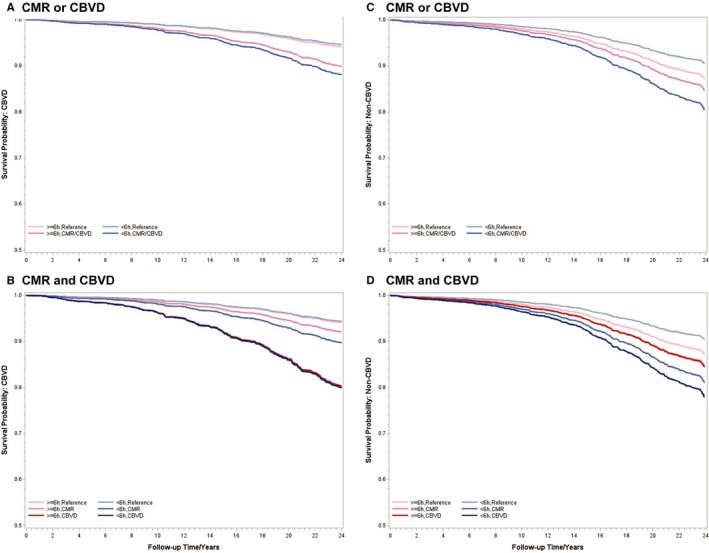
Multivariable‐adjusted survival curves for cause‐specific mortality associated with cardiometabolic risk factors (CMRs) and cardiovascular and cerebrovascular disease (CBVD) at baseline. A and B, Survival curves for CBVD mortality. C and D, Survival curves for mortality from other non‐CBVD causes. All data adjusted for age, race, sex, education, body mass index, smoking, alcohol use, apnea‐hypopnea index, other physical health conditions, and mental health conditions.
Finally, sensitivity analyses showed that subjective sleep duration did not act as an effect modifier (P‐interaction=0.840), and the risk of mortality associated with CMRs or CBVD was similar in those who reported sleeping ≥7 hours (HR, 1.92; 95% CI, 1.31–2.79) and those who reported sleeping <7 hours (HR, 1.82; 95% CI, 1.26–2.62) or <6 hours (HR, 1.78; 95% CI, 1.06–2.99).
Discussion
Our novel findings show that objective short sleep duration increases the mortality risk of middle‐aged adults with CMRs and those who have already developed CBVD. Middle‐aged adults with CMR who slept <6 hours were at a high risk of dying from CBVD, whereas middle‐aged adults with CBVD who slept <6 hours were at a high risk of dying from cancer. These associations were independent of sex, age, race, smoking, obesity, OSA, or other physical or mental health conditions. In middle‐aged adults without CMR or CBVD, sleeping <6 hours was not associated with an increased risk of dying. If these findings are replicated in other large cohorts with objective sleep measures, short sleep duration should be included in the prediction of the mortality prognosis of middle‐aged adults with CMR or CBVD.
The primary finding of the current study indicated that there was an ≈2‐fold risk for all‐cause, CBVD, and non‐CBVD mortality in participants who had CMRs at baseline and demonstrated short sleep duration in the sleep laboratory. Individuals who had CMRs and normal sleep duration at baseline, on the other hand, did not show a significantly increased risk on any of the mortality outcomes. This finding suggests that obtaining an adequate amount of sleep may minimize the adverse effect of CMRs on multiple mortality outcomes. For instance, participants with both CMRs and short sleep at baseline showed an 83% higher risk of dying from CBVD, whereas their CMR counterparts with normal sleep duration had a modest 35% nonsignificant higher risk of CBVD mortality (see Table S1 for mortality rates up to 20 years). Unlike individuals with CMRs, those with baseline CBVD and normal sleep duration did not show better mortality prognosis, as the presence of CBVD yielded similar HRs (Table 2) and mortality rates (Table S1) among those who slept <6 or ≥6 hours for dying from CBVD. This progression from the risk factor level (CMR) to developing the actual disease and dying from it (CBVD) deserves discussion in terms of its potential underlying mechanisms. Previous studies have shown that short sleep duration is associated with impaired cardiac autonomic modulation, sympathetic activation, endothelial dysfunction, subclinical atherosclerosis, and chronic low‐grade inflammation.13, 14, 15, 16, 17, 18, 19, 20, 21, 33, 34 It is likely that objective short sleep duration helps identify who among those with CMRs are at greater risk of developing and dying from CBVD because short sleep duration may be accelerating the process of cardiovascular and metabolic dysfunction. However, this process does not appear to apply to those individuals who had already developed CBVD at baseline; it is most likely that, if an adult has already developed CBVD, the end‐organ damage has already occurred through the vascular mechanisms mentioned above and that normal sleep duration does not preclude the adverse mortality outcome expected for CBVD. Together, these data highlight the need of identifying short sleep and improving sleep duration as early as the person develops CMR as part of our preventative public health strategies.
Moreover, the excess risk of cancer mortality was different between individuals with CBVD who slept ≥6 and <6 hours. Recent studies have indicated a strong association between CBVD with the development of cancer morbidity and mortality.35 In our study, the risk of cancer mortality was not significantly increased in individuals with CMR or CBVD who had normal sleep duration, whereas this risk was ≈3‐fold higher in individuals with CMR or CBVD who slept <6 hours. The discrepancy between the role of objective sleep duration in predicting CBVD mortality versus cancer mortality in those who already had CBVD at baseline may be explained by differences in disease causes. Although cardiovascular dysfunction (eg, myocardial infarction, congestive heart failure, and pathological arrhythmias) may be the main mechanism of CBVD mortality, in which short sleep duration has limited impact once CBVD has been developed, short sleep duration may significantly contribute to an altered immune response in individuals with CBVD. In‐laboratory and population‐based studies have shown that the sleep and circadian systems exert a strong regulatory influence on immune functions, including immune suppression and low‐grade inflammation in response to short sleep.36, 37, 38, 39 Our data suggest that preservation of normal sleep duration in individuals with CBVD may result in a lower cancer mortality rate (Table S1) and that short sleep duration enhances this risk even when associated with new‐onset cancer (Table S2). The potential role of short sleep duration in increasing the risk of cancer mortality in individuals with CBVD in our study was further supported by the data showing that the risk of mortality attributable to other causes (neither CBVD nor cancer) in individuals with CBVD at baseline was similar regardless of their objective sleep duration (Table 2). These novel findings highlight the role of short sleep in individuals who have already developed CBVD as it pertains to their progression toward cancer and associated mortality.
An important issue to be addressed in future longitudinal studies is whether objective short sleep duration may be associated with specific disturbances in sleep architecture or regulation. For example, an underlying deficit in slow‐wave sleep40 or circadian misalignment41 may be in the causal pathways examined herein.
This study has some limitations that should be taken into account when interpreting our results. First, the objective sleep duration in this study was based on one night of polysomnography, which may be affected by the first night effect, a phenomenon by which individuals sleep significantly worse on the first night in the laboratory compared with other consecutive nights after adaptation to the laboratory conditions and environment has occurred.42 Also, the study lacked an in‐laboratory follow‐up visit before death to examine the trajectory of sleep duration over time. Nevertheless, our study reinforces the need of objective sleep measures when predicting all‐cause and cause‐specific mortality given that subjective sleep duration did not act as an effect modifier in sensitivity analyses. Future studies should make use of multiple night recordings as well as examine the longitudinal trajectory of objective sleep duration. Second, we did not have the ability to confirm the type of diagnoses for either CBVD or other physical or mental health conditions, as reported by the subjects during the clinical history and physical examination using medical record data. Third, although we examined all‐cause mortality as well as the 2 leading causes of death, CBVD and cancer, we did not have the ability to examine other non‐CBVD causes of death beyond cancer given the small number of deceased individuals. Nevertheless, the associations found for those other clustered non‐CBVD causes of death had the expected HRs and provided confidence about the reliability and validity of our findings.
In conclusion, objective short sleep duration is an effect modifier of the mortality risk associated with CMR or CBVD. More important, our data suggest that short sleep may operate through different mechanisms on CBVD versus cancer mortality. The findings of this study set the foundation for future studies that can tease out the underlying mechanisms of the effect modification of short sleep on CBVD and cancer mortality. Such studies should incorporate multiple night recordings and circadian assessments and test our hypotheses in more ethnically diverse samples, thereby extending the generalizability of our findings. Clinically, these data further support the inclusion of short sleep duration as a novel, modifiable factor in assessing the prognosis of individuals with CMR and CBVD. Our findings suggest that individuals with CMR and CBVD with sleep complaints should undergo a sleep study, not only to rule out sleep apnea, but also to quantify their objective sleep duration, which can help ascertain the biological severity of their sleep impairment. Individuals with CMR or CBVD who demonstrate objective short sleep duration may experience greater impairment in sympathetic and immune regulation. Randomized clinical trials are warranted to examine whether lengthening sleep via pharmacological or behavioral therapies improves cardiovascular, metabolic, and immune outcomes in individuals with CMR or CBVD. Given the high prevalence of CMR and CBVD and the need to better predict their prognosis, including their evolution into cancer morbidity and mortality, the introduction of novel, modifiable factors should be a primary public health target.
Source of Funding
This work was supported by the American Heart Association (14SDG19830018 [principal investigator: Dr Fernandez‐Mendoza]) and the National Institutes of Health (R01HL51931 and R01HL40916 [principal investigator: Dr Bixler]).
Disclosures
None.
Supporting information
Table S1. Projected Multivariable‐Adjusted Mortality Rates Associated With Cardiometabolic Risk Factors (CMR) and Cardio‐Cerebrovascular Disease (CBVD): Role of Objective Sleep Duration
Table S2. Hazard Ratios and 95% Confidence Intervals for Cancer Mortality Associated With Cardiometabolic Risk Factors (CMR) and Cardio‐Cerebrovascular Disease (CBVD) Among Subjects Free of Cancer at Baseline: Role of Objective Sleep Duration
Acknowledgments
The work was performed at the Sleep Research and Treatment Center at the Penn State University Milton S. Hershey Medical Center, and the staff are especially commended for their efforts.
(J Am Heart Assoc. 2019;8:e013043 DOI: 10.1161/JAHA.119.013043.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–224. [DOI] [PubMed] [Google Scholar]
- 3. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing: in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all‐cause mortality: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;169:207–214. [DOI] [PubMed] [Google Scholar]
- 5. Grandner MA, Alfonso‐Miller P, Fernandez‐Mendoza J, Shetty S, Shenoy S, Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016;31:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, Quan SF. Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS). J Clin Sleep Med. 2007;3:622–630. [PMC free article] [PubMed] [Google Scholar]
- 7. St‐Onge MP, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all‐cause mortality: a systematic review and meta‐analysis of prospective studies. Sleep. 2010;33:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta‐analysis of prospective studies. Diabetes Care. 2015;38:529–537. [DOI] [PubMed] [Google Scholar]
- 10. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 11. Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta‐analysis. J Sleep Res. 2009;18:148–158. [DOI] [PubMed] [Google Scholar]
- 12. Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all‐cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013;23:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall MH, Fernandez‐Mendoza J, Kline CH, Vgontzas AN. Insomnia and health In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. New York, NY: Elsevier Academic Press; 2016:794–803. [Google Scholar]
- 14. Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self‐reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, Li SX, Yu MW, Ho CS, Chan MH, Zhang B, Wing YK. Relationship of sleep quantity and quality with 24‐hour urinary catecholamines and salivary awakening cortisol in healthy middle‐aged adults. Sleep. 2011;34:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mullington JM, Simpson NS, Meier‐Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castro‐Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, Shea S. Sleep duration and quality in relation to autonomic nervous system measures: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39:1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela‐Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez‐Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela‐Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Crosssectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vasisht KP, Kessler LE, Booth JN III, Imperial JG, Penev PD. Differences in insulin secretion and sensitivity in short‐sleep insomnia. Sleep. 2013;36:955–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men, I: prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. [DOI] [PubMed] [Google Scholar]
- 23. Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela‐Bueno A, Kales A. Prevalence of sleep‐disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. [DOI] [PubMed] [Google Scholar]
- 24. Bixler EO, Vgontzas AN, Lin HM, Vela‐Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53:589–592. [DOI] [PubMed] [Google Scholar]
- 25. National Center for Health Statistics . 2003 Revision of the U.S. Standard Certificate of Death. 2003. Available at: http://www.cdc.gov/nchs/data/dvs/DEATH11-03final-acc.pdf. Accessed December 13, 2018.
- 26. National Center for Health Statistics . Report of the panel to evaluate the U.S. standard certificates. 2000. Available at: http://www.cdc.gov/nchs/data/dvs/panelreport_acc.pdf. Accessed December 13, 2018.
- 27. World Health Organization . Manual of the International Classification of Diseases, Injuries, and Causes of Death, Ninth Revision. Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 28. World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 29. World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. 2008th ed Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 30. Vital Statistics, Instructions for Classifying the Underlying Cause of Death: NCHS Instruction Manual, Part 2a. Hyattsville, MD: Public Health Service. Published annually.
- 31. National Center for Health Statistics . ICD–10 cause‐of‐death lists for tabulating mortality statistics, updated March 2011: NCHS instruction manual, part 9. Hyattsville, MD: Public Health Service. 2011. Available at: http://www.cdc.gov/nchs/nvss/instruction_manuals.htm. Accessed December 13, ?2018.
- 32. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: National Institutes of Health; 1968. [Google Scholar]
- 33. Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vgontzas AN, Fernandez‐Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. [DOI] [PubMed] [Google Scholar]
- 37. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watson NF, Buchwald D, Delrow JJ, Altemeier WA, Vitiello MV, Pack AI, Bamshad M, Noonan C, Gharib SA. Transcriptional signatures of sleep duration discordance in monozygotic twins. Sleep. 2017;40:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta‐analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brindle RC, Duggan KA, Cribbet MR, Kline CE, Krafty RT, Thayer JF, Mulukutla SR, Hall MH. Cardiovascular stress reactivity and carotid intima‐media thickness: the buffering role of slow‐wave sleep. Psychosom Med. 2018;80:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agnew HW Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Projected Multivariable‐Adjusted Mortality Rates Associated With Cardiometabolic Risk Factors (CMR) and Cardio‐Cerebrovascular Disease (CBVD): Role of Objective Sleep Duration
Table S2. Hazard Ratios and 95% Confidence Intervals for Cancer Mortality Associated With Cardiometabolic Risk Factors (CMR) and Cardio‐Cerebrovascular Disease (CBVD) Among Subjects Free of Cancer at Baseline: Role of Objective Sleep Duration


