Abstract
Background
Some, but not all, studies report associations between shift work and hypertension, suggesting that particular subgroups may be at risk. We examined moderating effects of sleep duration and circadian preference on the relationship between shift work and new blood pressure (BP) medicine use at follow‐up.
Methods and Results
Baseline and 5‐year follow‐up data from the UK Biobank cohort (N=9200) were used to generate logistic regression models for shift workers and nonshift workers. The moderating effects of sleep duration (short ≤6 hours; adequate 7–8 hours; long ≥9 hours) and circadian preference (morning “larks;” intermediate; evening “owls”) at baseline were examined with new BP medicine use at follow‐up, adjusting for age, sex, race, education, employment, urban/rural, cardiovascular disease family history, depression, alcohol intake, physical activity, diet, smoking, and body mass index. The sample was predominately middle aged (55.3±7.4), female (57.3%), and white (97.9%). Most reported adequate sleep duration (7–8 hours, 73.7%) and were intermediate type (65.3%); 8.0% were shift workers at baseline. Only 6.5% reported new BP medicine use at follow‐up. Short sleep duration was a significant moderator of new BP medicine use in shift workers. Among short sleepers, shift workers had a 2.1‐fold increased odds of new BP medicine use compared with nonshift workers (odds ratio=2.08, 95% CI=1.21–3.58, P=0.008). In those reporting adequate (odds ratio=0.82, 95% CI=0.54–1.25, P=0.35) and long sleep (odds ratio=0.64, 95% CI=0.11–3.54, P=0.60), this relationship was protective but nonsignificant. Interaction between circadian preference and shift work on BP medicine use was nonsignificant.
Conclusions
Shift workers with short sleep duration may be at risk for hypertension.
Keywords: circadian rhythm, hypertension, risk factors, shift work schedule, sleep
Subject Categories: Lifestyle, Primary Prevention, Risk Factors
Clinical Perspective
What Is New?
Particular subgroups of shift workers appear to be at risk for the development of hypertension.
Shift workers with short sleep duration may be at risk for hypertension
The interaction between circadian preference and shift work on blood pressure medicine use was not significant.
What Are the Clinical Implications?
Monitor blood pressure routinely in shift workers.
Vary blood pressure monitoring to capture work days and nonwork days.
Ask patients about shift work and their tolerance to shift work schedules.
Introduction
Shift work professions are a cornerstone of a functioning society and rates of shift work are rising in our 24/7 society.1 But shift workers bear a disproportionate burden of chronic diseases.2 Some,3, 4, 5, 6 but not all,7, 8, 9 studies have reported associations between shift work and hypertension, suggesting that particular subgroups of shift workers may be more at risk than others. Specifically, a systematic review and meta‐analysis of studies involving 394 793 individuals found that shift work appeared to play an important role in hypertension, but there was no significant association between working permanent night shift and the risk of hypertension.10 The dimensions of sleep identified as risk factors for hypertension in shift workers include short sleep duration,11 poor sleep quality,4, 12 and circadian disruption.13, 14 Identifying a sleep phenotype of risk for hypertension among shift workers could inform hypertension prevention strategies.
Background
Shift work is defined as work hours that rotate to different times of the day (eg, morning, afternoon, and night shift) or work at constant but unusual hours of the day (eg, permanent night shift). In most cases, shift work involves “irregular, odd, flexible, variable, unusual, non‐standard working hours” (p.563).15 In the United Kingdom (UK) where this cohort was enrolled, 17% of the populace are shift workers.16 In the United States (US), 15% of citizens are shift workers.17 The UK Office of National Statistics reports that shift workers are predominantly young males (ages 16–24 years) who work two 8‐hour shifts in a day, which are alternated weekly or over other intervals.18
Shift work is associated with physiological, psychosocial, and behavioral consequences that may cause cardiovascular disease (CVD) such as hypertension.10 Sleep is a pathway with both physiological and behavioral elements linking shift work to hypertension. Poor sleep quality and short sleep duration (≤6 hours) may stem from circadian disruption or sleeping outside of the times normally dictated by one's circadian preference as required by shift work.19 Circadian disruption has been shown to increase blood pressure (BP) in shift workers.13 Circadian disruption and short sleep have also been associated with hypertension risk factors including weight gain, low fruit and vegetable consumption, sedentary behavior, and smoking.20, 21, 22, 23, 24, 25, 26, 27, 28 Adequate sleep duration lowers risk beyond what is achieved by meeting recommendations for physical activity, healthy diet, alcohol consumption, and tobacco use alone.29 Short sleep duration is associated with hypertension,30 with prolonged intervals of short sleep particularly detrimental.30 However, in a sample of adult Chinese males, short sleep duration was not associated with hypertension except when sleep quality was poor.31
In summary, the research conducted to date demonstrates that shift work may be a risk factor for hypertension. However, the inconsistent evidence suggests the need to identify subgroups of shift workers who are at risk. In this study we sought to determine whether sleep duration and circadian preference moderated the relationship between shift work and hypertension. We hypothesized that shift workers who were short sleepers and preferred mornings (“larks”) would have an increase in BP medicine use at follow‐up.
Methods
We examined the moderating effects of sleep duration and circadian preference on the relationship between shift work and BP medicine use at follow‐up 5 years later. People with known CVD at baseline were excluded. We adjusted the analyses for age, sex, race, education, employment, urban/rural, family history of CVD, depression status, alcohol intake, physical activity, diet, smoking, and body mass index. The data that support the findings of this study are available from UK Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The UK Biobank study was approved by the North West Multi‐centre Research Ethics Committee (reference 16/NW/0274), the England and Wales Patient Information Advisory Group, and the Scottish Community Health Index Advisory Group. All participants provided written informed consent before data collection.
Sample
Population data for these secondary analyses were collected by the UK Biobank (project # 16 153),32 a 20‐year, prospective cohort study that began in 2005. Eligible participants were (1) registered users of the National Health Service, (2) aged 40 to 69 years, and (3) living within a 25‐mile radius of one of the UK Biobank assessment centers.33 Between 2006 and 2010, 503 325 eligible and consenting adults were enrolled (5.47% response rate).34, 35 Volunteers provided informed consent and completed touchscreen questionnaires on lifestyle, environment, and medical history. From this cohort, 5‐year follow‐up data were collected on ≈20 000 participants.
For the current analysis, we started with a sample of 20 002 adults with data at the baseline and 5‐year follow‐up. Participants who had a history of CVD (ie, angina, stroke, or myocardial infarction; N=922) and those who used medication to treat CVD (N=4104) were excluded because of our interest in predicting the development of new hypertension. We also excluded anyone pregnant at the time of enrollment because of potential effects on sleep (N=3).36 Participants with responses of “do not know” or “prefer not to answer” (N=3714) on any of the core questions (eg, shift work, sleep duration, circadian preference, and BP medicine use) were excluded. Furthermore, participants with missing data for any of the core questions or adjusting variables (age, sex, race, education, employment, urban/rural, family history of CVD, depression status, alcohol intake, physical activity, diet, smoking, and body mass index) were excluded (N=2059), leaving 9200 participants in the final analytic sample. Although statistically significant differences between participants with complete and incomplete data were observed for some of the study variables (Table 1), this is likely because of our large sample size and high power to detect even the slightest difference. As a result, Cohen's d and Cramer's V effect sizes, as appropriate, were calculated. Cohen's d were interpreted as small (0.20), medium (0.50), and large (0.80).37 According to Rea and Parker,38 Cramer's V was interpreted as negligible to weak (0–0.20), moderate (0.20–0.39), and strong (0.40–1). As such, effect sizes were deemed small or weak for all study variables, indicating negligible differences between participants with complete and incomplete data. Thus, all analyses in this study reflect data from 9200 participants with complete information for all core questions and covariates.
Table 1.
UK Biobank Sample Characteristics
| Measure | Complete Data (N=9200) | Incomplete Data (N=2059) | P Valuea | Effect Sizeb |
|---|---|---|---|---|
| Sociodemographic characteristics at baseline | ||||
| Age (y), mean±SD | 55.3±7.4 | 54.8±7.2 | 0.006c | 0.07 |
| Sex, n (%) | ||||
| Female | 5272 (57.3%) | 1213 (58.9%) | 0.18 | 0.01 |
| Male | 3928 (42.7%) | 846 (41.1%) | ||
| Race/ethnicity, n (%) | ||||
| Mixed/other | 70 (0.8%) | 24 (1.2%) | 0.17 | 0.02 |
| Asian | 88 (1.0%) | 26 (1.3%) | ||
| Black | 35 (0.4%) | 7 (0.3%) | ||
| White | 9007 (97.9%) | 1999 (97.1%) | ||
| Missing | 0 | 3 (0.2%) | ||
| Education, n (%) | ||||
| Attended college | 4699 (51.1%) | 787 (38.2%) | <0.001c | 0.03 |
| No college | 4501 (48.9%) | 899 (43.7%) | ||
| Missing | 0 | 373 (18.1%) | ||
| Employment status, n (%) | ||||
| Employed | 6062 (65.9%) | 1428 (69.4%) | <0.001c | 0.17 |
| Retired | 2681 (29.1%) | 460 (22.3%) | ||
| Not employed/not retired | 457 (5.0%) | 106 (5.2%) | ||
| None of the above/prefer not to answer | 0 | 62 (3.0%) | ||
| Missing | 0 | 3 (0.2%) | ||
| Shift work, n (%) | ||||
| Yes | 734 (8.0%) | 189 (9.2%) | 0.03a | 0.02 |
| No | 8466 (92.0%) | 1805 (87.7%) | ||
| Missing | 0 | 65 (3.2%) | ||
| Residence, n (%) | ||||
| Urban | 7792 (84.7%) | 1775 (86.2%) | <0.001c | 0.06 |
| Rural | 1408 (15.3%) | 201 (9.8%) | ||
| Missing | 0 | 83 (4.0%) | ||
| Alcohol frequency intake, n (%) | ||||
| Daily or almost daily | 2013 (21.9%) | 437 (21.2%) | <0.001c | 0.05 |
| Three or 4 times a wk | 2636 (28.7%) | 528 (25.6%) | ||
| Once or twice a wk | 2361 (25.7%) | 509 (24.7%) | ||
| One to 3 times a mo | 992 (10.8%) | 248 (12.0%) | ||
| Special occasions only/never | 1198 (13.0%) | 333 (16.2%) | ||
| Prefer not to answer | 0 | 1 (0.1%) | ||
| Missing | 0 | 3 (0.2%) | ||
| Depression status, n (%) | ||||
| Yes | 458 (5.0%) | 72 (3.5%) | <0.001c | 0.07 |
| No | 1293 (14.1%) | 186 (9.0%) | ||
| Unknown | 7449 (81.0%) | 1801 (87.5%) | ||
| Family history of CVD, n (%) | ||||
| Yes | 5089 (55.3%) | 1030 (50.0%) | 0.24 | 0.02 |
| No | 4083 (44.4%) | 880 (42.7%) | ||
| Do not know/prefer not to answer | 28 (0.3%) | 9 (0.4%) | ||
| Missing | 0 | 140 (6.8%) | ||
| Cardiovascular outcomes at 5‐y follow‐up | ||||
| Myocardial infarction, n (%) | ||||
| Yes | 43 (0.5%) | 14 (0.7%) | 0.29 | 0.01 |
| No | 9153 (99.5%) | 2039 (99.0%) | ||
| Prefer not to answer | 4 (0.04%) | 2 (0.1%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Angina, n (%) | ||||
| Yes | 55 (0.6%) | 19 (0.9%) | 0.16 | 0.02 |
| No | 9141 (99.4%) | 2034 (98.8%) | ||
| Prefer not to answer | 4 (0.04%) | 2 (0.1%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Stroke, n (%) | ||||
| Yes | 46 (0.5%) | 15 (0.7%) | 0.28 | 0.02 |
| No | 9150 (99.5%) | 2038 (99.0%) | ||
| Prefer not to answer | 4 (0.04%) | 2 (0.1%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Any CVD event, n (%) | ||||
| Yes | 135 (1.5%) | 46 (2.2%) | 0.03a | 0.03 |
| No | 9061 (98.5%) | 2007 (97.5%) | ||
| Prefer not to answer | 4 (0.04%) | 2 (0.1%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Cardiovascular medication use at 5‐y follow‐up | ||||
| Cholesterol‐lowering medication use, n (%) | ||||
| Yes | 700 (7.6%) | 244 (11.9%) | <0.001c | 0.06 |
| No | 8500 (92.4%) | 1811 (88.0%) | ||
| Missing | 0 | 4 (0.2%) | ||
| BP medicine use, n (%) | ||||
| Yes | 602 (6.5%) | 223 (10.8%) | <0.001c | 0.06 |
| No | 8598 (93.5%) | 1832 (89.0%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Insulin use, n (%) | ||||
| Yes | 4 (0.04%) | 7 (0.3%) | <0.001c | 0.04 |
| No | 9196 (99.9%) | 2048 (99.5%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Any CVD medication use, n (%) | ||||
| Yes | 1090 (11.9%) | 369 (17.9%) | <0.001c | 0.07 |
| No | 8110 (88.2%) | 1686 (81.9%) | ||
| Missing | 0 | 4 (0.2%) | ||
| Self‐reported sleep measures at baseline | ||||
| Sleep duration, n (%) | ||||
| Short | 1899 (20.6%) | 505 (24.5%) | <0.001c | 0.04 |
| Adequate | 6777 (73.7%) | 1417 (68.8%) | ||
| Long | 524 (5.7%) | 134 (6.5%) | ||
| Missing | 0 | 3 (0.2%) | ||
| Circadian preference, n (%) | ||||
| Morning | 2359 (25.6%) | 407 (19.8%) | <0.001c | 0.05 |
| Intermediate | 6004 (65.3%) | 1053 (51.1%) | ||
| Evening | 837 (9.1%) | 226 (11.0%) | ||
| Missing | 0 | 373 (18.1%) | ||
| AHA simple‐7 measures at baseline | ||||
| Physical activity, n (%) | ||||
| Ideal | 5636 (61.3%) | 163 (7.9%) | 0.27 | 0.02 |
| Intermediate | 3346 (36.4%) | 87 (4.2%) | ||
| Poor | 10 (0.1%) | 1 (0.1%) | ||
| Do not know/prefer not to answer | 208 (2.3%) | 9 (0.4%) | ||
| Missing | 0 | 1799 (87.4%) | ||
| Diet, n (%) | ||||
| Ideal | 10 (0.1%) | 0 | 0.05 | 0.02 |
| Intermediate | 4889 (53.1%) | 1046 (50.8%) | ||
| Poor | 4301 (46.8%) | 1010 (49.1%) | ||
| Missing | 0 | 3 (0.2%) | ||
| Tobacco use at baseline, n (%) | ||||
| Ideal | 5906 (64.2%) | 1295 (62.9%) | 0.005c | 0.03 |
| Intermediate | 2766 (30.1%) | 600 (29.1%) | ||
| Poor | 517 (5.6%) | 158 (7.7%) | ||
| Prefer not to answer | 11 (0.1%) | 3 (0.2%) | ||
| Missing | 0 | 3 (0.2%) | ||
| BMI, n (%) | ||||
| Ideal | 2026 (22.0%) | 403 (19.6%) | <0.001c | 0.05 |
| Intermediate | 3956 (43.0%) | 792 (38.5%) | ||
| Poor | 3218 (35.0%) | 845 (41.0%) | ||
| Missing | 0 | 19 (0.9%) | ||
| BP, n (%) | ||||
| Ideal | 1669 (18.1%) | 347 (16.9%) | 0.51 | 0.01 |
| Intermediate | 3719 (40.4%) | 727 (35.3%) | ||
| Poor | 3543 (38.5%) | 736 (35.8%) | ||
| Missing | 269 (2.9%) | 249 (12.1%) | ||
AHA indicates American Heart Association; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease.
P<0.05.
Effect sizes based on Cohen's d (small: 0.20, medium 0.50, large: 0.80) for continuous variables and Cramer's V (negligible to weak: 0–0.20, moderate: 0.20–0.39, strong: 0.40–1) for categorical variables.
P<0.01.
Measurement
Shift workers were identified based on the question “Does your work involve shift work?”. Responses were coded as yes (always, usually, sometimes) or no (never/rarely). The outcome of interest was new‐onset hypertension, measured as self‐reported BP medicine newly prescribed during the course of follow‐up. Responses were coded as yes or no.32 Sleep duration was estimated by participant responses at baseline to the query “About how many hours sleep do you get in every 24 hours? (Please include naps).” Sleep duration was coded as a 3‐level variable (short ≤6 hours; adequate 7–8 hours; or long ≥9 hours).39 Circadian preference was estimated from participant responses to the query “Do you consider yourself to be… definitely a morning person, more a morning than evening person, more an evening than a morning person, definitely an evening person.” Responses were coded into a 3‐level variable: morning (definitely morning), intermediate (more a morning than evening person, more an evening than morning person), or evening (definitely evening).40
Covariates (age, sex, race, education, employment, urban/rural, family history of CVD, depression status, alcohol intake, physical activity, diet, smoking, and body mass index) were self‐reported. Race was adjusted as a covariate because short‐sleeping black shift workers, but not short‐sleeping white shift workers, have been found to have an increased odds of hypertension.11 Family history of CVD was coded as yes if at least 1 biological parent reported heart disease, stroke, or high BP and was otherwise coded as no. Depression was coded as a dichotomous variable. Participants reporting a “probable recurrent major depression” either severe or moderate or a “single probable major depression episode” were coded as yes for depression.
Analysis
Data were analyzed using descriptive statistics such as means and standard deviations for continuous variables and percentages and frequencies for categorical variables. Moderation analysis with sleep duration and circadian preference as the moderators was used to evaluate the relationship between shift workers and new BP medicine at 5‐year follow‐up. The primary effect of interest was the interaction between the moderators (sleep duration, circadian preference) and the predictor (shift worker) on newly prescribed BP medicine at 5‐year follow‐up. If the interaction was statistically significant, separate stratified logistic regression models according to shift work status were completed to aid with interpretation of the results. Of note, the Firth bias correction was applied to all stratified models because of small cells for adjusting variables. All analyses were conducted with SAS Version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was prespecified at a P<0.05.
Results
The final sample of 9200 adults with complete data was mostly white, middle‐aged, employed females and more than half had attended college (Table 1). Shift work was reported by 8.0% of this sample. About one fourth reported inadequate (ie, short or long) sleep duration and 9.1% identified as evening‐type (“owls”). Fewer than 2% of the sample reported any CVD event (eg, angina, stroke, and myocardial infarction) at follow‐up but 6.5% reported the new use of a BP medicine at the 5‐year follow‐up.
We observed statistically significant moderating effects of the 3‐level sleep duration variable on the relationship between shift work and new BP medicine use at 5‐year follow‐up (Table 2). In the stratified models, among short sleepers, those reporting shift work had a 2.1‐fold increased odds of using a new BP medicine as compared with those without shift work (Table 3: odds ratio=2.08, 95% CI=1.21–3.58, P=0.008). This relationship was protective among adequate (odds ratio=0.82, 95% CI=0.54–1.25, P=0.35) and long sleepers (odds ratio=0.64, 95% CI=0.11–3.54, P=0.60), but was not statistically significant (Tables 4 and 5, respectively).
Table 2.
Model Results for BP Medication Use at 5YFU Regressed on the Interaction of 3‐Level Sleep Duration and Shift Work and Their Main Effects (N=9200)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 0.78 | 0.51–1.18 | 0.24 |
| No shift work | REF | REF | REF |
| Sleep duration | |||
| Short | 0.98 | 0.79–1.22 | 0.88 |
| Adequate | REF | REF | REF |
| Long | 0.87 | 0.58–1.28 | 0.47 |
| Sleep duration × shift work | |||
| Short sleep—shift work | 2.49 | 1.27–4.86 | 0.008b |
| Adequate sleep—no shift work | REF | REF | REF |
| Long sleep—shift work | 0.74 | 0.09–6.03 | 0.78 |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
P<0.01.
Table 3.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Short Sleepers (N=1899)
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
P<0.01.
Table 4.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Adequate Sleepers (N=6777)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 0.82 | 0.54–1.25 | 0.35 |
| No shift work | REF | REF | REF |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
Table 5.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Long Sleepers (N=524)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 0.64 | 0.11–3.54 | 0.60 |
| No shift work | REF | REF | REF |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
While the interaction between circadian preference and shift work was statistically significant for morning‐type (Table 6, P=0.04), shift work was not a significant predictor of new BP medicine use in models stratified by circadian preference (Tables 7, 8 through 9). However, the odds ratios are in different directions for each group (ie, increased odds in larks and owls, and protective in intermediate circadian preference types) (Figures 1 and 2). Of note, model results for all adjusting variables have been provided in Tables S1 through S8.
Table 6.
Model Results for BP Medication Use at 5YFU Regressed on the Interaction of Circadian Preference and Shift Work and Their Main Effects (N=9200)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 0.80 | 0.51–1.25 | 0.32 |
| No shift work | REF | REF | REF |
| Circadian preference | |||
| Morning | 0.97 | 0.79–1.19 | 0.74 |
| Intermediate | REF | REF | REF |
| Evening | 1.05 | 0.77–1.44 | 0.75 |
| Circadian preference×shift work | |||
| Morning—shift work | 2.05 | 1.02–4.13 | 0.04b |
| Intermediate—no shift work | REF | REF | REF |
| Evening—shift work | 1.26 | 0.47–3.40 | 0.65 |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
P<0.01.
Table 7.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Morning Types (N=2359)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 1.53 | 0.88–2.67 | 0.14 |
| No shift work | REF | REF | REF |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, and urban/rural,
Family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
Table 8.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Intermediate Types (N=6004)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 0.83 | 0.53–1.29 | 0.40 |
| No shift work | REF | REF | REF |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
Table 9.
Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Evening Types (N=837)
| Variable | ORa | 95% CI | P Value |
|---|---|---|---|
| Shift work | |||
| Shift work | 1.21 | 0.49–2.96 | 0.68 |
| No shift work | REF | REF | REF |
BP indicates blood pressure; OR, odds ratio; REF, reference category; 5YFU, 5‐year follow‐up.
Model was adjusted for age, sex, race, education, employment status, urban/rural, family history of cardiovascular disease, depression status, alcohol intake, physical activity, diet, smoking, and body mass index.
Figure 1.
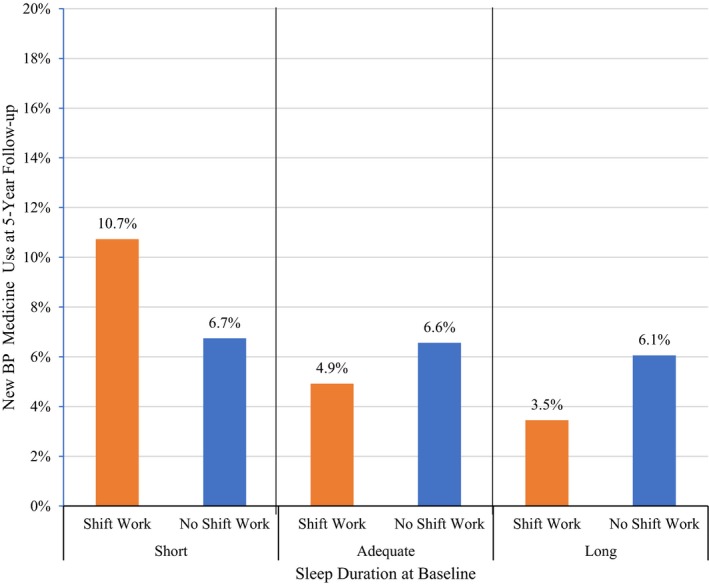
Percent of UK Biobank participants with new blood pressure (BP) medicine use at 5‐year follow‐up according to shift work status stratified by sleep duration (N=9200).
Figure 2.
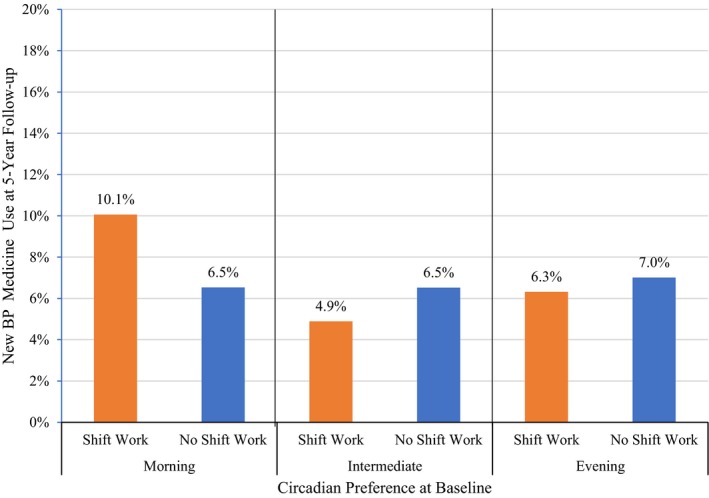
Percent of UK Biobank participants with new blood pressure (BP) medicine use at 5‐year follow‐up according to shift work status stratified by circadian preference (N=9200).
Discussion
The purpose of this study was to examine the moderating effects of sleep duration and circadian preference on the relationship between shift work and new BP medicine use at 5‐year follow‐up. In this large population sample, 6.5% of the sample reported new BP medicine use 5 years later. This percentage of new BP medicine use over a relatively short period of time underscores the growing burden of hypertension, particularly among older adults.41, 42 Reducing hypertension rates hinges on identifying at‐risk subgroups and on developing effective nonpharmacologic interventions for these at‐risk groups. Our results suggest that shift workers who are short sleepers might be an at‐risk group worth targeting for intervention.
Prospective studies have reported that shift work is associated with incident hypertension in some, but not all, studies43 (for a review). Disparate findings stem from different definitions of shift work, different durations of participants’ exposure to shift work, and the inclusion of different covariates in the data analyses in these prospective studies. Additionally, the “healthy worker effect” may explain the lack of significant associations between shift work and hypertension in some studies.44 The “healthy worker effect” explains insignificant associations between shift work and hypertension because shift workers diagnosed with chronic conditions, such as hypertension, retire or transition to nonshift work, and the remaining shift workers are healthier than their same‐aged nonshift worker counterparts.44 Short‐sleeping shift workers were found to have a greater odds of new‐onset BP medicine use at follow‐up compared with short‐sleeping nonshift workers in this UK population sample. Others have reported that short sleep was a significant predictor of incident hypertension in black, but not white, female shift workers.7 Our finding extends the link between short sleep and shift work with new BP medication use to middle‐aged white males and females in the United Kingdom. Our finding suggests that a subgroup of shift workers (eg, short sleepers) are at greater risk for new BP medicine use than other shift workers (eg, adequate sleepers) and that intervening to promote adequate sleep duration among shift workers may be an important prevention strategy.
The increased odds for new BP medicine use in short‐sleeping shift workers compared with short‐sleeping nonshift workers at follow‐up suggests that circadian disruption contributes to new BP medicine use above and beyond short sleep duration. Well‐controlled laboratory studies have provided consistent evidence for a biological pathway linking circadian disruption to hypertension. These studies have shown that circadian disruption, induced by simulating shift work, increases nocturnal sympathetic nervous system activation independently from sleep restriction alone in healthy young adults.45, 46 This laboratory evidence has been corroborated in shift workers by evidence of an elevated BP and heart rate along with reduced heart rate variability and incomplete BP recovery following a 12‐hour shift of night work.47 Although some well‐controlled laboratory studies have demonstrated an increase in BP during simulated shift work conditions,45 others have not.46 Yet, well‐controlled laboratory studies are devoid of environmental and behavioral factors that may also contribute to sympathetic nervous stimulation in shift workers, such as exposure to light, noise, caffeine consumption, and occupational stress during the night.48 Collectively, these biological, environmental, and behavioral factors are plausible pathways that support an increased odds of new BP medicine use in short‐sleeping shift workers compared with short‐sleeping nonshift workers.
Circadian preference moderated the relationship between shift work and new BP medicine use; however, the odds of new BP medicine use in shift workers with morning preferences were not significantly different from shift workers with evening preferences. Although not significantly different, shift workers with an evening preference were less likely to report new BP medicine use at follow‐up and shift workers with a morning preference were more likely to report new BP medicine use at follow‐up. The opposing relationship between morning and evening shift workers with new BP medicine use in our sample may stem from the effect of circadian preference on sleep duration, sleep quality, and sleep regularity in shift workers. Morning‐preference shift workers have reported shorter sleep duration, poorer sleep quality, and greater sleep irregularity when working night shifts compared with evening‐preference shift workers.49 Sleep duration, sleep quality, and sleep regularity have been reported to improve by eliminating night work for shift workers with morning preferences and by eliminating early morning work for shift workers with evening preferences.50 Thus, adjusting shift work according to circadian preference can yield sleep health benefits. Whether or not similar benefits for reducing hypertension may be garnered for shift workers is uncertain. Alves et al51 found that shift workers with less irregular sleep patterns report more physical activity and less sedentary behavior compared with shift workers with more irregular sleep patterns. More work is needed to determine whether aligning shift work with circadian preferences reduces new BP medicine use and hypertension‐related risk behaviors.
Limitations
These analyses were limited by several factors. All variables were measured using self‐reported data, which is only moderately correlated with objectively measured sleep duration.52, 53 The duration, frequency, and specific types of shifts worked were not included in these data. Our analyses were restricted to a predominately white population, so results need to be replicated in other racial and ethnic groups. Although we restricted analyses to participants reporting no BP medicine use at baseline, it is possible that some participants had untreated hypertension at enrollment. Future analyses should include objective measures of sleep and healthcare provider confirmation of new‐onset hypertension. Other limitations include a possible underestimation of hypertension risk because not every increase in BP needs to be treated pharmacologically. Also, we were not able to control for external factors that may influence BP medicine use, such as the regularity of healthcare appointments, provider prescribing practices, and/or the overall healthcare delivery system. Those who visit the doctor more often are more likely to receive treatment for their hypertension. Furthermore, it was not possible to break down the medications to individual medication classes, so we cannot confirm that a specific compound was initiated for the treatment of hypertension, but this is what the participants were told. Another limitation is that the data set did not allow us to distinguish between behavioral short sleep duration and short sleep duration because of other reasons. We also did not control for sleep‐disordered breathing, a common cause of poor sleep quality and a known cause of hypertension in middle‐ and older‐aged adults.54 Finally, a limitation of the UK Biobank data is that only 5.47% of eligible individuals participated in the study. Future research is needed to explore the match between circadian preference and the shift worked, as we did not have data available to answer this specific question.
Perspective
These results underscore the increasing prevalence and health burden of hypertension, and suggest that short sleep duration in shift workers may contribute to this burden. Our finding that short‐sleeping shift workers had an increased odds of a new need to take a BP medicine on follow‐up has several clinical implications. First, clinicians should consider questioning patients about their exposure to shift work. Second, ask shift‐working patients about their tolerance to shift work schedules in terms of sleep duration, sleep quality, and sleep regularity. Third, BP monitoring for shift workers should include checking BP on work days and after nonwork days.43 Fourth, interventions to promote sleep health for shift workers are needed.
Current demands for a 24/7 society and growth in the percentage of people reporting shift work suggest that the success of policies limiting the number of night shift personnel to safely perform essential tasks will be challenging to promote. Nonetheless, eliminating economic policies that incentivize night shift work may ensure that the burden of chronic diseases linked to shift work is not disproportionately shouldered by the socioeconomically disadvantaged. Future research that advances our understanding of the relationships between circadian disruption and CVD risk in shift workers may guide equitable interventions by aligning work schedules according to preferences.
Sources of Funding
Research reported in this publication was supported by the National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Number R01MD012734 (Patterson), the National Institute of Nursing Research (T32NR007104‐20) (Daus), the National Institute of Nursing Research (K99NR017416) (Malone) and the National Heart, Lung, and Blood Institute (T32HL7953) (Malone). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Table S1. Model Results for BP Medication Use at 5YFU Regressed on the Interaction of 3‐Level Sleep Duration and Shift Work and Their Main Effects (N=9200)
Table S2. Model Results for BP Medication Use at 5YFU Regressed on the Interaction of Circadian Preference and Shift Work and Their Main Effects (N=9200)
Table S3. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Short Sleepers (N=1899)
Table S4. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Adequate Sleepers (N=6777)
Table S5. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Long Sleepers (N=524)
Table S6. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Morning Types (N=2359)
Table S7. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Intermediate Types (N=6004)
Table S8. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Evening Types (N=837)
Acknowledgments
The authors acknowledge the UK Biobank resource.
(J Am Heart Assoc. 2019;8:e013269 DOI: 10.1161/JAHA.119.013269.)
References
- 1. Pickering TG. Could hypertension be a consequence of the 24/7 society? The effects of sleep deprivation and shift work. J Clin Hypertens (Greenwich). 2006;8:819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pryce C. Impact of shift work on critical care nurses. Can J Crit Care Nurs. 2016;27:17–21. [PubMed] [Google Scholar]
- 3. Attarchi M, Dehghan F, Safakhah F, Nojomi M, Mohammadi S. Effect of exposure to occupational noise and shift working on blood pressure in rubber manufacturing company workers. Ind Health. 2012;50:205–213. [DOI] [PubMed] [Google Scholar]
- 4. Guo Y, Liu Y, Huang X, Rong Y, He M, Wang Y, Yuan J, Wu T, Chen W. The effects of shift work on sleeping quality, hypertension and diabetes in retired workers. PLoS One. 2013;8:e71107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kubo T, Fujino Y, Nakamura T, Kunimoto M, Tabata H, Tsuchiya T, Kadowaki K, Odoi H, Oyama I, Matsuda S. An industry‐based cohort study of the association between weight gain and hypertension risk among rotating shift workers. J Occup Environ Med. 2013;55:1041–1045. [DOI] [PubMed] [Google Scholar]
- 6. Nazri SM, Tengku MA, Winn T. The association of shift work and hypertension among male factory workers in Kota Bharu, Kelantan, Malaysia. Southeast Asian J Trop Med Public Health. 2008;39:176–183. [PubMed] [Google Scholar]
- 7. Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African‐Americans. J Hypertens. 2012;30:61–66. [DOI] [PubMed] [Google Scholar]
- 8. Murata K, Yano E, Hashimoto H, Karita K, Dakeishi M. Effects of shift work on qtc interval and blood pressure in relation to heart rate variability. Int Arch Occup Environ Health. 2005;78:287–292. [DOI] [PubMed] [Google Scholar]
- 9. Ohlander J, Keskin MC, Stork J, Radon K. Shift work and hypertension: prevalence and analysis of disease pathways in a german car manufacturing company. Am J Ind Med. 2015;58:549–560. [DOI] [PubMed] [Google Scholar]
- 10. Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: a systematic review and meta‐analysis. J Hypertens. 2017;35:1929–1937. [DOI] [PubMed] [Google Scholar]
- 11. Ceide ME, Pandey A, Ravenell J, Donat M, Ogedegbe G, Jean‐Louis G. Associations of short sleep and shift work status with hypertension among Black and White Americans. Int J Hypertens. 2015;2015:697275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, Peat GM, Cleator J, Chew‐Graham C, Loke YK, Mamas MA. Self‐reported sleep duration and quality and cardiovascular disease and mortality: a dose‐response meta‐analysis. J Am Heart Assoc. 2018;7:e008552 Doi: 10.1161/JAHA.118.008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. Circadian misalignment increases c‐reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smolensky MH, Hermida RC, Reinberg A, Sackett‐Lundeen L, Portaluppi F. Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int. 2016;33:1101–1119. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization .Painting, firefighting, and shiftwork. IARC Monogr Eval Carcinog Risks Hum. 2010;98:9–764. [PMC free article] [PubMed] [Google Scholar]
- 16. Statistics OfN . People aged over 16 in employment who do shift work, by region of the UK.2014.
- 17. Labor USDo . Workers on flexible and shift schedules in May 2004. 1‐14;2004.
- 18. Office for National Statistics . Changes in shift work patterns over the last ten years (1999 to 2009).2011.
- 19. Stone JE, Sletten TL, Magee M, Ganesan S, Mulhall MD, Collins A, Howard M, Lockley SW, Rajaratnam SMW. Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: role of light‐dark exposure. J Physiol. 2018;596:2381–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puttonen S, Harma M, Hublin C. Shift work and cardiovascular disease—pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36:96–108. [DOI] [PubMed] [Google Scholar]
- 21. Merikanto I, Lahti T, Seitsalo S, Kronholm E, Laatikainen T, Peltonen M, Vartiainen E, Partonen T. Behavioral trait of morningness‐eveningness in association with articular and spinal diseases in a population. PLoS One. 2014;9:e114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schubert E, Randler C. Association between chronotype and the constructs of the three‐factor‐eating‐questionnaire. Appetite. 2008;51:501–505. [DOI] [PubMed] [Google Scholar]
- 23. Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, Csako G, Cizza G;Sleep Extension Study G .Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8:e56519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and bmi. Obesity. 2011;19:1374–1381. [DOI] [PubMed] [Google Scholar]
- 25. Krueger PM, Friedman EM. Sleep duration in the United States: a cross‐sectional population‐based study. Am J Epidemiol. 2009;169:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehari A, Weir NA, Gillum RF. Gender and the association of smoking with sleep quantity and quality in American adults. Women Health. 2014;54:1–14. [DOI] [PubMed] [Google Scholar]
- 27. Léger D, Beck F, Richard J‐B, Sauvet F, Faraut B. The risks of sleeping “too much”. Survey of a national representative sample of 24671 adults (inpes health barometer). PLoS ONE. 2014;9:e106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krueger PM, Reither EN, Peppard PE, Burger AE, Hale L. Cumulative exposure to short sleep and body mass outcomes: a prospective study. J Sleep Res. 2015;24:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoevenaar‐Blom MP, Spijkerman AM, Kromhout D, Verschuren WM. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: the morgen study. Eur J Prev Cardiol. 2014;21:1367–1375. [DOI] [PubMed] [Google Scholar]
- 30. Cappuccio FP, Miller MA. Sleep and cardio‐metabolic disease. Curr Cardiol Rep. 2017;19:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu K, Chen J, Wang L, Wang C, Ding R, Wu S, Hu D. Association of sleep duration, sleep quality and shift‐work schedule in relation to hypertension prevalence in chinese adult males: a cross‐sectional survey. Int J Environ Res Public Health. 2017;14 pii: E210. DOI: 10.3390/ijerph14020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biobank U . Uk biobank: Protocol for a large‐scale prospective epidemiological resource.2007.
- 33. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. Uk biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, Gallacher J, Green J, Matthews P, Pell J, Sprosen T, Collins R. Uk biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1:123–126. [Google Scholar]
- 35. UK Biobank . Available at: https://www.ukbiobank.ac.uk/. Accessed August 15, 2015.
- 36. Sedov ID, Cameron EE, Madigan S, Tomfohr‐Madsen LM. Sleep quality during pregnancy: a meta‐analysis. Sleep Med Rev. 2018;38:168–176. [DOI] [PubMed] [Google Scholar]
- 37. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, MI:Lawrence Erlbaum Associates;1988. [Google Scholar]
- 38. Rea LM, Parker RA. Designing and conducting survey research.San Francisco, CA:Jossey‐Bass;1992. [Google Scholar]
- 39. Hoevenaar‐Blom MP, Spijkerman AMW, Kromhout D, van den Berg JF, Verschuren WMM. Sleep duration and sleep quality in relation to 12‐year cardiovascular disease incidence: the morgen study. Sleep. 2011;34:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torsvall L, Åkerstedt T. A diurnal type scale. Construction, consistency and validation in shift work. Scand J Work Environ Health. 1980;6:283–290. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization .A global brief on hypertension: Silent killer, global public health crisis.2013.
- 42. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon‐Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017. Oct;289:1‐8. [PubMed] [Google Scholar]
- 43. Esquirol Y, Perret B, Ruidavets JB, Marquie JC, Dienne E, Niezborala M, Ferrieres J. Shift work and cardiovascular risk factors: new knowledge from the past decade. Arch Cardiovasc Dis. 2011;104:636–668. [DOI] [PubMed] [Google Scholar]
- 44. Arrighi HM, Hertz‐Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–196. [DOI] [PubMed] [Google Scholar]
- 45. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grimaldi D, Carter J, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su TC, Lin L, Baker D, Schnall P, Chen MF, Hwang WC, Chen CF, Hwang JD. Elevated blood pressure, decreased heart rate variability, and incomplete blood pressure recovery after a 12‐hour night shift work. J Occup Health. 2008;50:380–386. [DOI] [PubMed] [Google Scholar]
- 48. Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24‐hour blood pressure regulation and patterning. Sleep Med Rev. 2017;33:4–16. [DOI] [PubMed] [Google Scholar]
- 49. Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift‐workers. J Biol Rhythms. 2013;28:141–151. [DOI] [PubMed] [Google Scholar]
- 50. Vetter C, Fischer D, Matera JL, Roenneberg T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. 2015;25:907–911. [DOI] [PubMed] [Google Scholar]
- 51. Alves MS, Andrade RZ, Silva GC, Mota MC, Resende SG, Teixeira KR, Goncalves BF, Crispim CA. Social jetlag among night workers is negatively associated with the frequency of moderate or vigorous physical activity and with energy expenditure related to physical activity. J Biol Rhythms. 2017;32:83–93. [DOI] [PubMed] [Google Scholar]
- 52. Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, Hall MH, Loredo JS, Mossavar‐Rahmani Y, Ramos AR, Reid KJ, Shah NA, Sotres‐Alvarez D, Zee PC, Wang R, Patel SR. Comparison of self‐reported sleep duration with actigraphy: results from the hispanic community health study/study of latinos sueno ancillary study. Am J Epidemiol. 2016;183:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self‐reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caples SM, Garcia‐Touchard A, Somers VK. Sleep‐disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Model Results for BP Medication Use at 5YFU Regressed on the Interaction of 3‐Level Sleep Duration and Shift Work and Their Main Effects (N=9200)
Table S2. Model Results for BP Medication Use at 5YFU Regressed on the Interaction of Circadian Preference and Shift Work and Their Main Effects (N=9200)
Table S3. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Short Sleepers (N=1899)
Table S4. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Adequate Sleepers (N=6777)
Table S5. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Long Sleepers (N=524)
Table S6. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Morning Types (N=2359)
Table S7. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Intermediate Types (N=6004)
Table S8. Relationship Between Shift Work and the Odds of New BP Medication Use at 5YFU Among Evening Types (N=837)


