Abstract
Background
While many clinical prediction models (CPMs) exist to guide valvular heart disease treatment decisions, the relative performance of these CPMs is largely unknown. We systematically describe the CPMs available for patients with valvular heart disease with specific attention to performance in external validations.
Methods and Results
A systematic review identified 49 CPMs for patients with valvular heart disease treated with surgery (n=34), percutaneous interventions (n=12), or no intervention (n=3). There were 204 external validations of these CPMs. Only 35 (71%) CPMs have been externally validated. Sixty‐five percent (n=133) of the external validations were performed on distantly related populations. There was substantial heterogeneity in model performance and a median percentage change in discrimination of −27.1% (interquartile range, −49.4%–−5.7%). Nearly two‐thirds of validations (n=129) demonstrate at least a 10% relative decline in discrimination. Discriminatory performance of EuroSCORE II and Society of Thoracic Surgeons (2009) models (accounting for 73% of external validations) varied widely: EuroSCORE II validation c‐statistic range 0.50 to 0.95; Society of Thoracic Surgeons (2009) Models validation c‐statistic range 0.50 to 0.86. These models performed well when tested on related populations (median related validation c‐statistics: EuroSCORE II, 0.82 [0.76, 0.85]; Society of Thoracic Surgeons [2009], 0.72 [0.67, 0.79]). There remain few (n=9) external validations of transcatheter aortic valve replacement CPMs.
Conclusions
Many CPMs for patients with valvular heart disease have never been externally validated and isolated external validations appear insufficient to assess the trustworthiness of predictions. For surgical valve interventions, there are existing predictive models that perform reasonably well on related populations. For transcatheter aortic valve replacement (CPMs additional external validations are needed to broadly understand the trustworthiness of predictions.
Keywords: clinical prediction models, risk, valvular heart disease
Subject Categories: Valvular Heart Disease
Clinical Perspective
What Is New?
Risk prediction is central to decision making for patients with advanced valvular heart disease; however, the performance of clinical predictive models in external validations is often substantially worse than expected based on derivation data set performance.
What Are the Clinical Implications?
Isolated external validations appear insufficient to broadly understand the performance of valvular heart disease clinical predictive models.
There are clinical predictive models for surgical valvular heart disease interventions that perform well across multiple external validations.
The trustworthiness of transcatheter aortic valve replacement predictions is largely unknown as these models have not been widely tested in external validations.
Introduction
Treatments for patients with advanced valvular heart disease (VHD) are increasingly offered to patients with advanced age and elevated pre‐procedural risk.1, 2, 3 Clinical predictive models (CPMs) have assumed a central role in clinical decision making and current guidelines link VHD treatment decisions to predicted risk.4, 5, 6 CPMs can potentially enhance shared decision making,7, 8 when they perform well and are appropriately matched to the correct decisional context, though there remain major questions about how well CPMs for patients with VHD perform in external validations.
It is well recognized that many of the best known (and most widely used) CPMs for VHD were derived on patients receiving surgical interventions9, 10 and do not accurately predict outcomes for patients treated with percutaneous interventions,11 though they continue to be used for this purpose. While there are newer efforts to create CPMs specific to percutaneous valve interventions, the relative performance of these models and their performances in external validations remains largely unknown. Generally, the performance of CPMs has often been underreported and incompletely assessed12 and since CPMs that perform poorly can yield misleading predictions that motivate harmful decision making,13 it is essential that clinicians understand CPM performance before leveraging outputs to inform decisions. This is especially important for VHD treatment decisions, given the importance of these tools.
Here, using the Tufts PACE (Predictive Analytics and Comparative Effectiveness) CPM Registry, we describe the available CPMs for patients with VHD treated with percutaneous or surgical interventions. This analysis focuses on comparative model performance during external validations.
Methods
General Approach
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study analyzed data from the Tufts PACE (Predictive Analytics and Comparative Effectiveness) CPM Registry, a database created to describe the CPM literature for patients at risk for and with known cardiovascular disease. The registry, which is free and available to the public at http://pace.tuftsmedicalcenter.org/cpm, encompasses a field synopsis of CPMs for patients with VHD. The methods have been previously reported.12 Briefly, we had previously searched PubMed for English‐language articles containing CPMs for cardiovascular disease published from January 1990 through 2015. We extended the search for VHD CPMs to January 2017 to include more recent CPM development (Table S1, Figure S1). Citations were reviewed to confirm completeness of our review. All citations and data fields were extracted in duplicate to ensure accuracy. Discrepancies were discussed until consensus was achieved.
For inclusion in the registry, articles had to meet the following criteria: (1) develop a CPM as a primary aim, (2) contain a model predicting the development of a specified clinical diagnosis (diagnostic models) or the probability of developing a clinical outcome (prognostic models), (3) contain at least 2 outcome predictors, and (4) present enough information to estimate the probability for an individual patient. Articles were excluded if they did not provide enough information to predict a patient's risk or if the described models predicted surrogate outcomes. We also excluded non‐English reports, pharmacology reports, cost‐effectiveness models, decision‐analysis models, systematic reviews, and editorials.
Model Selection
This report focuses on CPMs predicting outcomes for patients with VHD. CPMs predicting natural history outcomes and outcomes after surgical and percutaneous procedures were included. CPMs were grouped based on underlying valve pathology and procedure. CPMs were also included if they were derived on cardiac surgery cohorts where at least 50% of patients received treatment for VHD. CPMs derived exclusively on coronary artery bypass populations were excluded.
CPM Reporting
Information was extracted on CPM derivation and reporting. Collected fields included: index clinical condition, predicted outcome, timeframe of prediction, sample size, cohort size, and number of events. We calculated the events per variable (EPV) based on the number of variables included in the model. We also extracted information on modeling method and performance with specific attention to reporting of discrimination and calibration (Table S2).
Validation Search
Citations for each CPM article through September 2017 were identified using Scopus and reviewed for inclusion as external validations. An external validation was defined as any evaluation of CPM performance (assessment of either discrimination or calibration) on a data set distinct from the derivation data set.14 External validations included validations that were done on the same cohort but temporally or geographically distinct from the derivation cohort or on an entirely separate cohort. Each validation citation was reviewed by 2 investigators for inclusion and discrepancies were reviewed with an additional investigator to arrive at consensus.
Validation Reporting
Information on validation reporting was extracted, including sample size, continent of study, number of events, and reporting of measures of discrimination and calibration (Table S3). The validation performance analysis focused on whether CPM discrimination changed when compared with that seen in the derivation population. Because the c‐statistic ranges from 0.5 (no discriminatory ability) to 1.0 (perfect discrimination), it has been rescaled as Somer's D statistic15 (2×(c−0.5)) so that discrimination ranges from 0 (no discrimination) to 1.0 (perfect discrimination). We describe changes on this scale because it more intuitively reflects the true changes in discriminatory power. The percentage change in discrimination [(Validation AUC−0.5)−(Derivation AUC−0.5)/(Derivation AUC−0.5)×100] is presented. We also document whether validations include any assessment of CPM calibration. There is currently no literature standard for assessing calibration. Given this lack of consistency and interpretability, we have only reported on whether this dimension of performance was assessed. Calibration assessment included any comparison of observed versus expected outcomes. Examples include a Hosmer‐Lemeshow statistic or calibration plot. For this study we also included measures of global fit, where overall observed event rates are compared with predicted rates (ie, calibration‐in‐the‐large).
Relatedness
To assess the similarity between the derivation population and the validation population for each validation, we created a relatedness rubric to divide validations into 2 categories—”related” and “distantly related.” The rubric contained 3 domains: (1) type of intervention (ie, percutaneous or surgical), (2) percentage of the population undergoing isolated valve procedures (as opposed to valve procedures in combination with revascularization), and (3) calendar years of enrollment. We considered a validation population to be “related” if all of the following criteria were met: (1) same type of intervention (eg, both surgical populations), (2) ± 10% absolute difference in the proportion of isolated valve procedure (eg, derivation population was 100% isolated valve and validation population was 95% isolated valve), and (3) overlapping years of enrollment. Matches that did not meet all 3 criteria were deemed “distantly related.”
Results
VHD CPMs
We identified 49 CPMs predicting clinical outcomes for patients with VHD, which were cited a total of 1296 times (Table 1, Table S2).7, 9, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Thirty‐four (69%) predict outcomes following surgical interventions, 12 (24%) predict outcomes following percutaneous interventions, and 3 (6%) predict outcomes in the absence of intervention (Table 2). Overall, the most commonly predicted outcomes were 30‐day mortality (n=14, 29%) and in‐hospital mortality (n=14, 29%). Twenty‐four models (46%) were derived from patients in North America, followed by 12 (23%) from Europe and 8 (15%) from Asia (Figure 1). The median derivation sample size was 4510 (interquartile range [IQR], 1087–18 686), median event rate was 8.3% (IQR, 4.5%–14.8%), and median EPV was 40 (IQR, 20–92) (Table 2). The median number of covariates was 10 (IQR, 7–19).
Table 1.
De Novo VHD CPMs Overview
| Author, Model Name | Publication, y | Valve | Standardized Type of Intervention | Outcome | Model Method | C‐Statistic | Calibration Measure | Externally Validated? |
|---|---|---|---|---|---|---|---|---|
| Isolated valve | ||||||||
| Edwards,16 STS (original) Isolated Valve | 2001 | Aortic/Mitral | Surgery | 30 d operative mortality | Logistic regression | 0.766 | HL statistic, Calibration plot | Yes |
| Nowicki,17 NNE Aortic and Mitral Models | 2004 | Aortic | Surgery | In‐hospital mortality | Logistic regression, score | 0.75 | HL statistic | Yes |
| Mitral | Surgery | In‐hospital mortality | Logistic regression, score | 0.79 | HL statistic | Yes | ||
| Kuduvalli,18 NWQIP | 2007 | Aortic | Surgery | In‐hospital mortality | Logistic regression, score | 0.78 | HL statistic | Yes |
| Cruz‐Gonzalez,19 PMV Score | 2009 | Mitral | Percutaneous | Procedural success | Logistic regression, score | NR | HL statistic | Yes |
| Monin20 | 2009 | Aortic stenosis | Natural History | Composite (Non‐MACE) | Logistic regression, score | 0.90 | HL statistic | Yes |
| O'Brien,9 STS (2009)—Composite AEs | 2009 | Aortic/Mitral | Surgery | Composite (Non‐MACE) | Logistic regression | 0.721 | None | Yes |
| O'Brien,9 STS (2009)—DSWI | 2009 | Aortic/Mitral | Surgery | DSWI | Logistic regression | 0.704 | None | Yes |
| O'Brien,9 STS (2009)—Mortality | 2009 | Aortic/Mitral | Surgery | 30 d mortality | Logistic regression | 0.805 | None | Yes |
| O'Brien,9 STS (2009)—Prolonged LOS | 2009 | Aortic/Mitral | Surgery | Prolonged LOS | Logistic regression | 0.77 | None | Yes |
| O'Brien,9 STS (2009)—Prolonged Ventilation | 2009 | Aortic/Mitral | Surgery | Prolonged ventilation | Logistic regression | 0.77 | None | Yes |
| O'Brien,9 STS (2009)—Renal Failure | 2009 | Aortic/Mitral | Surgery | Renal failure | Logistic regression | 0.782 | None | Yes |
| O'Brien,9 STS (2009)—Reoperation | 2009 | Aortic/Mitral | Surgery | Reoperation | Logistic regression | 0.643 | None | Yes |
| O'Brien,9 STS (2009)—Short LOS | 2009 | Aortic/Mitral | Surgery | Prolonged LOS | Logistic regression | 0.738 | None | No |
| O'Brien,9 STS (2009)—Stroke | 2009 | Aortic/Mitral | Surgery | Stroke | Logistic regression | 0.694 | None | Yes |
| Guaragna,21 GuaragnaSCORE | 2010 | Aortic/Mitral | Surgery | In‐hospital mortality | Logistic regression, score | 0.82 | HL statistic, Calibration plot | Yes |
| Guo22 | 2010 | Aortic | Surgery | In‐hospital mortality | Logistic regression | NR | HL statistic | No |
| Mitral | Surgery | In‐hospital mortality | Logistic regression | NR | HL statistic | No | ||
| Elmariah,23 CRRAC the AV Score | 2011 | Aortic | Percutaneous | 30 d mortality | Cox regression, score | 0.754 | HL statistic | No |
| Bouleti24 | 2012 | Mitral | Percutaneous | Composite (MACE) | Cox regression, score | 0.74 | Calibration plot | No |
| Cioffi25 | 2012 | Aortic stenosis | Natural History | Composite (MACE) | Cox regression, score | NR | None | No |
| Holme,26 SEAS Score | 2012 | Aortic stenosis | Natural History | 5 y mortality | Cox regression | 0.722 | HL statistic, Calibration plot, Brier score | No |
| Kötting,27 German Aortic Valve Score | 2013 | Aortic | Percutaneous | In‐Hospital Mortality | Logistic regression, score | 0.808 | HL statistic | Yes |
| Arnold,28 6 mo and 1 y Models | 2014 | Aortic stenosis | Percutaneous | Composite (Non‐MACE) | Logistic regression | 0.66 | HL statistics, Calibration plot | Yes |
| Aortic stenosis | Percutaneous | Composite (Non‐MACE) | Logistic regression | 0.66 | HL statistics, Calibration plot | Yes | ||
| Capodanno,29 OBSERVANT Score | 2014 | Aortic stenosis | Percutaneous | 30 d mortality | Logistic regression, score | 0.73 | HL statistic, Calibration plot, Brier score | Yes |
| D'Ascenzo,30 Survival Post‐TAVI (STT)—30 d and 1 y Models | 2014 | Aortic | Percutaneous | 30 d mortality | Logistic regression, score | 0.66 | HL statistic | Yes |
| Aortic | Percutaneous | 1 y mortality | Logistic regression, score | 0.68 | HL statistic | Yes | ||
| Iung31 | 2014 | Aortic | Percutaneous | 30 d mortality | Logistic regression, score | 0.67 | HL statistic, Calibration in the large, Calibration plot | No |
| Debonnaire,32 TAVI2‐SCORe | 2015 | Aortic | Percutaneous | 1 y mortality | Cox regression, score | 0.715 | HL statistic, Calibration in the large | Yes |
| Edwards7 | 2016 | Aortic | Percutaneous | In‐hospital mortality | Logistic regression | 0.67 | HL statistics, Calibration in the large, Calibration plot | Yes |
| Isolated or multiple valve | ||||||||
| Koplan33 | 2003 | All | Surgery | Pacemaker placement | Logistic regression, score | NR | None | No |
| Ambler34 | 2005 | Aortic, mitral | Surgery | In‐hospital mortality | Logistic regression, Score | 0.77 | HL statistic, Calibration plot | Yes |
| Xu35 | 2006 | All | Surgery | Prolonged LOS | Logistic regression | 0.81 | Calibration in table form | No |
| Hannan36 | 2007 | Aortic, mitral | Surgery | In‐hospital mortality | Logistic regression, Score | 0.794 | HL statistic, Calibration plot | Yes |
| Xu,37 Fuwai Score | 2007 | All | Surgery | Prolonged LOS | Logistic regression, Score | 0.76 | HL statistic, Calibration plot | Yes |
| Shi38 | 2010 | Aortic, mitral | Surgery | In‐hospital mortality | Logistic regression | 0.7358 | None | No |
| Ariyaratne,39 Aus‐AVR Score | 2011 | Aortic, mitral | Surgery | 30 d mortality | Logistic regression, Score | 0.78 | HL statistic, Calibration in the large | Yes |
| Nashef,10 EuroSCORE II | 2012 | All | Surgery | In‐hospital mortality | Logistic regression | 0.8095 | None | Yes |
| Hannan,40 NY Operative Mortality Risk Score | 2013 | Aortic, mitral | Surgery | 30 d mortality | Logistic regression, Score | 0.781 | HL statistic | Yes |
| Wang41 | 2013 | All | Surgery | Prolonged ventilation | Logistic regression | 0.789 | HL statistic | No |
| Zheng42 | 2013 | Aortic, mitral | Surgery | In‐hospital mortality | Logistic regression, Score | 0.76 | HL statistic, Chi‐square statistic, Calibration plot | No |
| Multiple valve | ||||||||
| Guo22 | 2010 | Aortic, mitral | Surgery | In‐hospital mortality | Logistic regression | NR | HL statistic | No |
| Rankin,43 AM Preop | 2013 | Aortic, mitral | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
| Rankin,43 MT Preop | 2013 | mitral, tricuspid | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
| Rankin,43 AMT Preop | 2013 | Aortic, mitral, tricuspid | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
| Rankin,43 AM Preop + Intraop | 2013 | Aortic, mitral | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
| Rankin,43 MT Preop + Intraop | 2013 | Mitral, tricuspid | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
| Rankin,43 AMT Preop + Intraop | 2013 | Aortic, mitral, tricuspid | Surgery | 30 d mortality | Logistic regression | NR | Calibration plot | Yes |
AEs indicates adverse events; AM, aortic, mitral ; AMT, aortic, mitral, tricuspid; AV, aortic valvuloplasty; Aus‐AVR, Australian aortic valve replacement; CRRAC, critical status, renal dysfunction, right atrial pressure, and cardiac output; DSWI, deep sternal wound infections; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HL, Hosmer‐Lemeshow; LOS, length of stay; MACE, major adverse cardiovascular events; MT, mitral, tricuspid; NNE, Northern New England; NR, not reported; NWQIP, North West Quality Improvement Programme in Cardiac Interventions; NY, New York; OBSERVANT, Observational Study of Appropriateness, Efficacy and Effectiveness of AVR‐TAVR Procedures for the Treatment of Severe Symptomatic Aortic Stenosis; PMV, percutaneous mitral valvuloplasty; SEAS, Simvastatin and Ezetimibe in Aortic Stenosis; STS, Society of Thoracic Surgeons; STT, Survival posT‐TAVI; TAVI, transcatheter aortic valve implantation; TAVR, Transcatheter Aortic Valve Replacement.
Table 2.
Reported Characteristics of De Novo Valvular Heart Disease CPMs
| Characteristica | Overall (n=49) | Surgical (n=34) | Percutaneous (n=12) | Natural History (n=3) |
|---|---|---|---|---|
| Publication rangeb | 2001 to 2016 | 2001 to 2013 | 2009 to 2016 | 2009 to 2012 |
| Age, y | 69 (61–79) | 65 (58–70) | 82 (82–83) | 68 (67–70) |
| Sample size | 4510 (1087–18 686) | 12 079 (3125–92 563) | 1160 (752–2241) | 772 (440–1169) |
| Event rate | 0.08 (0.05–0.15) | 0.07 (0.04–0.11) | 0.14 (0.06–0.37) | 0.35 (0.23–0.47) |
| Events per variable | 40 (20–92) | 46 (25–110) | 42 (18–81) | 11 (10–45) |
| C‐statistic | 0.76 (0.72–0.78) | 0.77 (0.75–0.79) | 0.68 (0.67–0.74) | 0.81 (0.77–0.86) |
| % Externally validated | 71.4 | 73.5 | 83.3 | 33.3 |
CPM indicates clinical predictive models.
Values are reported as median (interquartile range), unless otherwise specified.
De novo CPM search spans January 1, 1990 to January 1, 2017.
Figure 1.
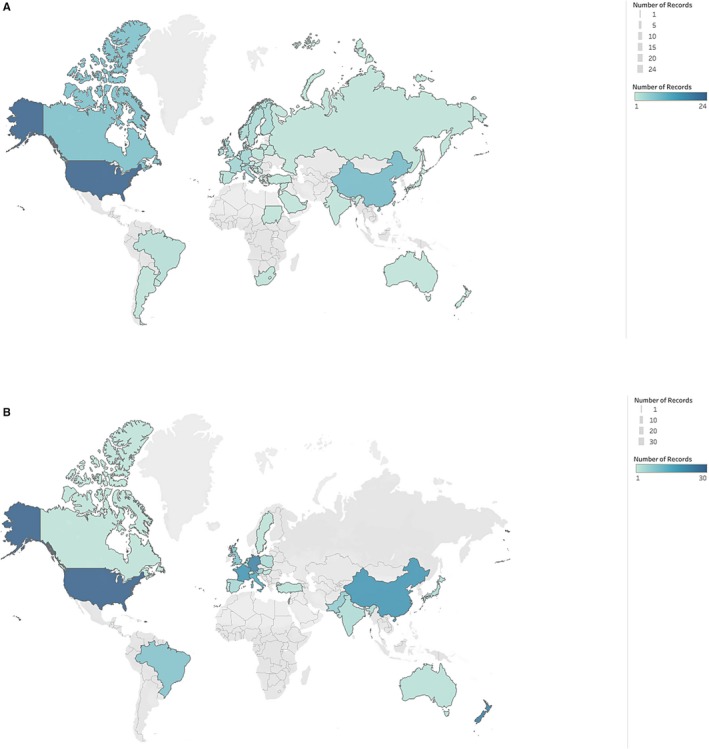
Geography of derivation and validation cohorts. Country of origin for derivation (A) and validation (B) populations. Maps created in Tableau Public.
Among models that reported a c‐statistic (n=37, 76%), the overall median ROC was 0.76 (IQR, 0.72–0.78) (Table 2). When stratified by intervention type, the median c‐statistic was 0.77 (IQR, 0.75–0.79) for CPMs predicting outcomes following surgical interventions, 0.68 (IQR, 0.67–0.74) for CPMs for percutaneous interventions, and 0.81 (IQR, 0.77–0.86) for CPMs predicting outcomes in the absence of intervention (Table 2).
CPMs for Isolated Valve Disease
There are 31 CPMs for isolated valve disease. Sixteen (52%) predict outcomes following surgical intervention and 12 (39%) predict outcomes following percutaneous interventions (transcatheter aortic valve replacement [TAVR], balloon aortic valvuloplasty, and percutaneous mitral balloon valvuloplasty). Three CPMs (10%) predict outcomes for patients with aortic stenosis in the absence of intervention. The median derivation sample size was 2552 (IQR, 1064–108 410) and the median age was 70 (IQR, 64–82). The median number of events was 360 (IQR, 104–2021) and the median EPV was 55 (IQR, 18–112). The median event rate was 10% (IQR, 4.6%–18.3%). For the 27 (87%) models reporting discrimination, the median c‐statistic was 0.74 (IQR, 0.69–0.78).
CPMs for Isolated or Multiple Valve Disease
There are 11 CPMs that predict outcomes for patients undergoing either single or multiple valve surgical procedures. The median derivation sample size was 3544 (IQR, 2297–12 079) and the median age was 60 (IQR, 54–65). The median number of events was 303 (IQR, 139–507) and the median EPV was 26 (IQR, 20–40). The median event rate was 5.1% (IQR, 4.1%–9.5%). For the 10 (91%) models reporting discrimination, the median c‐statistic was 0.78 (IQR, 0.76–0.79).
CPMs for Multiple Valve Disease
There are 7 CPMs that predict outcomes specifically for multiple valve surgical interventions. These CPMs include the Society of Thoracic Surgeons (STS) Multi‐Valve Risk Models43 and the median derivation sample size was 18 686 (IQR, 4510–22 861). The median number of events was 1420 (IQR, 591–1981) and the median EPV was 71 (IQR, 48–92). Median age was 70 (IQR, 70–71). The median event rate was 9.4% (IQR, 7.6%–11.3%). Median number of covariates was 20 (IQR, 14–23).
CPMs for Percutaneous VHD Interventions
Since 2009, there have been 12 CPMs presented that predict outcomes following percutaneous VHD interventions. Two models predict outcomes following mitral percutaneous mitral balloon valvuloplasty.24, 44 There were 9 CPMs that predict outcomes following TAVR with a median derivation sample size of 2130 (IQR, 10 642 552). The median age of patients in the TAVR CPMs was 82 (IQR, 82–83). TAVR CPMs had a median number of events of 253 (IQR, 80–704) and the median EPV was 28 (IQR, 20–70). The median event rate was 9.9% (IQR, 5.6%–15.7%). All of the CPMs predicting outcomes following TAVR reported discrimination with a median c‐statistic of 0.67 (IQR, 0.66–0.72).
External Validations
Two hundred and four external validations of these CPMs were identified, of which 190 (93%) report a c‐statistic. Overall, 35 (71%) of the VHD CPMs have been externally validated and 20 (37%) have been externally validated more than once. External validations were most commonly done in cohorts of patients from Europe (n=93, 46%), Asia (n=38, 19%), and North America (n=37, 18%) (Figure 1). Fifty‐three (26%) validations were performed on populations from the same continent as the derivation population, with a median c‐statistic of 0.71 (IQR, 0.66–0.77). Seventy‐one (35%) were done on populations from a different continent, with a median c‐statistic of 0.68 (IQR, 0.64–0.73). External validations overall had a median c‐statistic of 0.71 (IQR, 0.65– 0.77) (Table 3). For the models that were externally validated, we noted an overall median percentage change in discrimination of −27.1% (IQR, −49.4–−5.7). Just under two‐thirds of validations (n=129) demonstrate at least a 10% relative decline in discriminatory power, and 18 (9%) showed a decline of >80%. Thirty‐three (16%) validations showed CPM discrimination at or above that seen in the derivation cohort.
Table 3.
Reported Characteristics of Valvular Heart Disease External Validationsc
| Characteristica | Overall (n=204)b | Surgical (n=131) | Percutaneous (n=70) |
|---|---|---|---|
| Sample size | 450 (249–1495) | 809 (407–3306) | 304 (180–453) |
| Number of events | 38 (15–95) | 48 (14–119) | 38 (15–56) |
| Event rate | 0.06 (0.03–0.12) | 0.04 (0.03–0.07) | 0.11 (0.08–0.17) |
| % Men | 53 (47–62) | 57 (53–66) | 47 (43–52) |
| C‐statistic | 0.71 (0.65–0.77) | 0.74 (0.70–0.79) | 0.63 (0.57–0.68) |
Values are reported as median (interquartile range).
Validations done on populations treated with surgical and percutaneous interventions that did not disaggregate results (n=2) are only included in the overall count.
Validation search includes citations through September 8, 2017.
The distribution of number of validations was skewed towards a small number of CPMs. Two CPMs (EuroSCORE II and STS [2009] Models9) accounted for 73% of the external validations. EuroSCORE II has been validated 78 times across 5 continents (Table 4). Validation c‐statistics ranged from 0.50 to 0.95 with a median percentage change of −23.4% (range −100%–+46.7%). For the STS (2009) Models, validation c‐statistics ranged from 0.50 to 0.86 (Table 5). The median percentage change was −31.8% and ranged from −100% to +18%. The STS (2009) Models have been validated 70 times across 5 continents.
Table 4.
EuroSCORE II Population Compared With External Validation Populations, Stratified by Relatedness
| Statistica | EuroSCORE II | Validation Populationsb, c | |
|---|---|---|---|
| Related | Distantly Related | ||
| Total patients (n) | 16 828 | 14 382 | 98 744 |
| Total validations (n) | NA | 5 | 73 |
| Age, y | Mean (SD): 64.6 (12.5) | 63.4 (62.7–67.0) | 67.1 (61.1–80.5) |
| Number of events (n) | 656 | 123 (53–215) | 27 (12–57) |
| Event rate, % | 3.9 | 5.7 (5.7–6.1) | 6.3 (3.0–10.5) |
| Sex reported, n (%) | NA | 5 (100%) | 50 (68%) |
| Men, % | 69.1 | 65.2 (62.5–66.5) | 52.5 (46.8–64.1) |
| Type of intervention, n (%) | |||
| Surgery | 1 (100%) | 5 (100%) | 52 (71.2%) |
| Percutaneous | 0 (0%) | 0 (0%) | 20 (27.4%) |
| Both | 0 (0%) | 0 (0%) | 1 (1.4%) |
| Valve‐related, % | 53.3 | 56 (54.6–56.1) | 100 (100–100) |
| Enrollment, y (range) | 2010 | 2005 to 2013 | 1999 to 2015 |
| C‐statistic | 0.8095 | 0.82 (0.76–0.85) | 0.72 (0.67–0.78) |
| C‐statistic (range) | NA | 0.737 to 0.861 | 0.50 to 0.95 |
| Any calibration reported, n (%) | 0 (0%) | 4 (80%) | 65 (89%) |
| Change in discrimination,d % | NA | 2.6 (−16.0–13.1) | −28.9 (−45.3–−9.5) |
EuroSCORE indicates European System for Cardiac Operative Risk Evaluation.
All values are reported as median (interquartile range) unless otherwise specified.
Validation data is reported at the population level only; patient‐level data was not available.
Validation population are “related” if it meets all of the following criteria: (1) same type of intervention (eg, both surgical populations), (2) ±10% absolute difference in the proportion of isolated valve procedure (eg, derivation population was 100% isolated valve and validation population was 95% isolated valve), and (3) overlapping years of enrollment. A validation population that does not meet all 3 criteria is “distantly related.”
Change in discrimination is calculated as [(Validation AUC−0.5)−(Derivation AUC−0.5)]/(Derivation AUC−0.5)×100.
Table 5.
STS (2009) Population Compared With External Validation Populations, Stratified by Relatedness
| Statistica | STS Models (n=9) | Validation Populationsb,c | |
|---|---|---|---|
| Related | Distantly Related | ||
| Total patients, n | 109 759 | 37 395 | 49 530 |
| Total validations, n | NA | 33 | 37 |
| Age, y | Not Reported | 64.7 (56.6–73) | 81.6 (74.5–83) |
| Number of events, n | 9164 (3706–12 892) | 29 (12–82) | 38 (18–57) |
| Event rate, % | 8.3 (3.4–11.7) | 4.9 (2.7–12.6) | 9.1 (3.7–11.7) |
| Men, % | 55.4 | 56 (56.0–74.9) | 47.8 (43.6–55.3) |
| Type of intervention, n (%) | |||
| Surgery | 9 (100%) | 33 (100%) | 8 (21.6%) |
| Percutaneous | 0 (0%) | 0 (0%) | 28 (75.7%) |
| Both | 0 (0%) | 0 (0%) | 1 (2.7%) |
| Valve‐related, % | 100 | 100 (100–100) | 100 (100–100) |
| Enrollment, y (range) | 2002–2006 | 1997–2014 | 1999–2015 |
| C‐statistic, median, IQR | 0.74 (0.70–0.77) | 0.72 (0.67–0.79) | 0.65 (0.6–0.71) |
| C‐statistic (range) | 0.643 to 0.805 | 0.612 to 0.86 | 0.5 to 0.81 |
| Calibration reported, n (%) | 9 (100%) | 18 (54.5%) | 28 (75.7%) |
| Change in discrimination,d % | NA | −21.3 (−34.4–2.3) | −50.8 (−67.2–−25.1) |
IQR indicates interquartile range; STS, Society of Thoracic Surgeons.
All values are reported as median (interquartile range) unless otherwise specified.
Validation data is reported at the population level only; patient‐level data was not available.
Validation population is “related” if it meets all of the following criteria: (1) same type of intervention (eg, both surgical populations), (2) ±10% absolute difference in the proportion of isolated valve procedure (eg, derivation population was 100% isolated valve and validation population was 95% isolated valve), and (3) overlapping years of enrollment. A validation population that does not meet all 3 criteria is “distantly related.”
Change in discrimination is calculated as [(Validation AUC−0.5)−(Derivation AUC−0.5)]/(Derivation AUC−0.5)×100.
Of CPMs that have been validated at least 2 times (n=17) in related populations, the highest median validation c‐statistic was seen for EuroSCORE II (0.82 [IQR, 0.76–0.85]), followed by the North West Quality Improvement Programme in Cardiac Interventions model (0.78 [IQR, 0.77–0.78]), and the Northern New England Aortic model (0.76 [IQR, 0.75–0.77]) (Table 6).
Table 6.
CPMs that Have Been Validated ≥2 Times in Related Populations
| De Novo CPM | External Validations in Related Populations (n) | Validation C‐statistic, median (IQR) | % Change in Discrimination,a Median (IQR) | Any Calibration Reported (%) | |
|---|---|---|---|---|---|
| Pub., Y | Model Name | ||||
| 2001 | STS (original): Isolated Valve | 2 | 0.77 (0.77, 0.77) | 2.6 (2.6–2.6) | 100 |
| 2004 | NNE Aortic | 2 | 0.76 (0.76, 0.77) | 4.0 (2.0–6.0) | 100 |
| 2007 | NWQIP | 2 | 0.78 (0.77, 0.78) | −1.8 (−2.7–−0.9) | 100 |
| 2005 | Ambler | 4 | 0.73 (0.72, 0.76) | −15.2 (−18.9–−2.2) | 100 |
| 2009 | STS: Mortality | 19 | 0.74 (0.71, 0.79) | −21.3 (−31.5–−4.8) | 95 |
| 2009 | STS: Stroke | 2 | 0.65 (0.65, 0.66) | −20.9 (−23.8–−17.9) | 0 |
| 2009 | STS: Prolonged Ventilation | 2 | 0.72 (0.68, 0.75) | −20.2 (−33.8–−6.6) | 0 |
| 2009 | STS: Prolonged LOS | 2 | 0.67 (0.65, 0.68) | −38.1 (−43.5–−32.8) | 0 |
| 2009 | STS: Renal Failure | 2 | 0.76 (0.72, 0.79) | −9.6 (−22.5–3.4) | 0 |
| 2009 | STS: DSWI | 2 | 0.68 (0.65, 0.70) | −13.7 (−24.8–−2.7) | 0 |
| 2009 | STS: Composite AEs | 2 | 0.68 (0.65, 0.71) | −18.8 (−30.7–−6.9) | 0 |
| 2009 | STS: Reoperation | 2 | 0.64 (0.63, 0.65) | −2.1 (−11.9–7.7) | 0 |
| 2011 | Aus‐AVR Score | 3 | 0.72 (0.67, 0.72) | −22.9 (−40.4–−20.4) | 100 |
| 2012 | EuroSCORE II | 5 | 0.82 (0.76, 0.85) | 2.6 (−16.0–13.1) | 80 |
| 2013 | NY Operative Mortality Risk Score | 3 | 0.73 (0.71, 0.75) | −18.1 (−26.5–−9.8) | 66.7 |
| 2014 | OBSERVANT Score | 4 | 0.60 (0.58, 0.61) | −57.8 (−63.7–50.7) | 50 |
| 2014 | STT: 30 d | 2 | 0.66 | 0 | 50 |
AEs indicates adverse events; Aus‐AVR, Australian aortic valve replacement; CPM indicates clinical predictive models; DSWI, deep sternal wound infections; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IQR, interquartile range; LOS, length of stay; NNE, Northern New England; NWQIP, North West Quality Improvement Programme in Cardiac Interventions; NY, New York; OBSERVANT, Observational Study of Appropriateness, Efficacy and Effectiveness of AVR‐TAVR Procedures for the Treatment of Severe Symptomatic Aortic Stenosis; STS, Society of Thoracic Surgeons; STT, Survival posT‐TAVI; TAVI, transcatheter aortic valve implantation.
Change in discrimination is calculated as [(Validation AUC−0.5)−(Derivation AUC−0.5)]/(Derivation AUC−0.5)×100.
Forty‐five (22%) external validations did not report any measure of calibration. Of the 159 validations that did report calibration, 103 (65%) reported the Hosmer‐Lemeshow statistic, 87 (55%) reported calibration‐in‐the‐large, and 46 (29%) included a calibration plot. Median c‐statistic was 0.71 (IQR, 0.65–0.77) for validations that reported some measure of calibration and 0.68 (IQR, 0.63–0.74) for validations that did not report any calibration.
Clinical relatedness between the development and validation populations was assessed using a novel rubric. Seventy‐one validations (35%) were performed on related populations, while the remaining 133 (65%) were performed on distantly‐related populations. The median validation c‐statistic was 0.73 (IQR, 0.67–0.79) for related validations and 0.70 (IQR, 0.62–0.76) for distantly‐related validations (P=0.009). There was a significant difference in percentage change in discrimination: the median change in c‐statistic was −12.2% (IQR, −28.3%–+2.5%) for related validations and −32.1% (IQR, −54.9%–−12.8%) for distantly‐related validations (Figure 2, P<0.0001).
Figure 2.
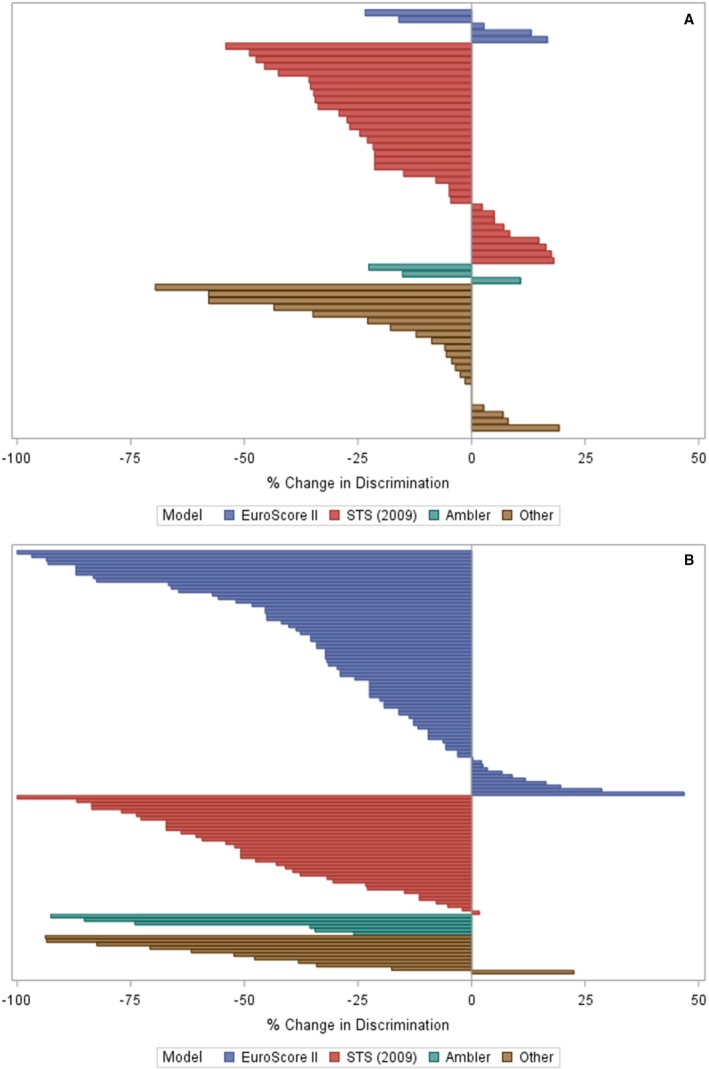
Percentage change in discrimination in external validations of valvular heart disease clinical prediction models, stratified by relatedness. Each bar represents a unique external validation that reports a c‐statistic (n=205). Society of Thoracic Surgeons (2009) Models. Percentage change in discrimination is calculated as ([validation c‐statistic–0.5]–[derivation c‐statistic–0.5])/[derivation c‐statistic–0.5]×100. STS indicates Society of Thoracic Surgeons
CPMs that were derived on percutaneously‐treated VHD populations and externally validated (n=9) underwent a total of 19 validations, almost all of which were on percutaneous populations. CPMs that were derived on surgical VHD populations and externally validated (n=25) underwent a total of 184 validations, of which 130 (71%) were on surgical populations, 52 (28%) were on percutaneous populations, and 2 (1%) were on populations including both surgical and percutaneous interventions. For validations of surgical VHD models discrimination was better when CPMs were tested on cohorts treated with surgical versus percutaneous interventions (median c‐statistic 0.74 versus 0.63, P<0.001).
Of the surgical VHD CPMs validated on percutaneous populations (n=52 validations), the CPM most often validated was the STS (2009) model predicting mortality (n=27, median c‐statistic 0.64 [IQR, 0.58–0.67]). EuroSCORE II (n=20) had the highest discrimination in this setting, with a median c‐statistic of 0.67 (IQR, 0.55–0.71).
Discussion
Here we show that there are many CPMs available for patients with VHD and that many of these CPMs have not been externally validated. For the CPMs that have been externally validated, models often perform substantially worse than expected based on performance in derivation data sets. Notably, isolated external validations of VHD CPMs appear insufficient for broadly understanding CPM performance in the context of specific clinical decisions as predictive models may have highly variable performance across various databases. For patients under consideration for surgical VHD interventions, there are CPMs that have been extensively validated. The fidelity of TAVR CPM predictions is largely unknown, as these models have not been widely tested in external validations.
Predicted risk is central to procedural decision making for patients with VHD, however. individual risk estimates using published CPMs for VHD appear more uncertain than originally thought, especially when prediction models are derived on patients who are not closely related to the patients being treated. CPM performance (specifically discrimination) substantially degrades from the derivation population to the validation population, particularly when populations are “distantly related” with respect to procedure type (percutaneous versus open surgical), therapeutic era, and the need for concurrent revascularization. Without attention to these patient‐level specifics, it is likely that there is widespread inappropriate use of CPMs that are informing treatment decisions for patients with VHD. While it is encouraging that newer models have been developed for TAVR patients, these CPMs have not been widely validated or integrated into contemporary guidelines, and have risk estimates that may become inaccurate as devices continue to improve and procedural techniques mature. The attenuated performance of these TAVR CPMs may also be related to the magnitude and significance of comorbid illnesses that are common for older treated adults and are rarely included as part of parsimonious modeling efforts.45 More work is needed to understand these risk factors.
The decrease in discrimination that is observed in this study may be attributable to model overfitting, differences in case mix (ie, narrower populations in the validation data set), and phenotypic heterogeneity. Ultimately, the relevant performance metrics for clinicians relate to the patients they are treating (with a specific intervention), not to performance measured at the time of CPM development. Rarely, discrimination appears to improve during validations. This is likely the result of differences between the derivation population and the validation population where some models are developed on more highly selected (narrow case‐mix) cohorts than they are testing on. The data presented here demonstrates that CPMs externally validated multiple times show substantial variation in performance. This strongly suggests that adequate performance demonstrated in a single external validation may be insufficient to assess the quality (and utility) of VHD CPMs and that a more tailored approach is needed to understand the trustworthiness of CPM predictions in specific settings.
There is increasing recognition of the central importance of CPM calibration. Surprisingly, calibration was reported in only 78% of the external validations of VHD CPMs. There is no agreed‐upon standard for reporting model calibration and no consensus on interpreting this metric. Moreover, there are well‐recognized limitations to the most commonly reported measure, the Hosmer‐Lemeshow statistic (eg, sample‐size dependence).46 Reporting of model calibration represents a major shortcoming in the assessment and overall trustworthiness of VHD CPMs, as adequate calibration can protect against harm.13 Ongoing efforts, including the Transparent Reporting of a Multivariable Model for Individual Prognosis or Diagnosis statement47 and the Prognosis Research Strategy,48 should lead to more consistent evaluation and better reporting of this important performance metric. For currently available CPMs, it is important for clinicians to know whether predictions over‐ or underestimate risk when compared with observed event rates in similar patients, especially when the decision for or against a particular intervention is informed by this output (as is the case for percutaneous VHD treatments). Poor calibration can often be overcome with a variety of updating techniques that can substantially improve accuracy of predictions.49
There is much that remains unknown about these predictive models. CPM performance has not been studied for patients treated in many areas of Eastern Europe, Asia, Central America, South America, and Africa. This is especially important considering the many regional differences in VHD etiology, access to technologies, care systems, and guidelines.50, 51, 52 The optimal number of validations required to adequately assess CPM performance remains unknown. Ideally, CPMs are serially validated and recalibrated (if necessary) to optimize performance for specific, local clinical decision making. Without addressing these limitations, clinical decisions that leverage CPM outputs may be inaccurate and lead to harmful decisions.
This analysis offers a structure to consider which CPMs are most accurate (discrimination and calibration) and trustworthy (consistent performance in multiple external validations). For patients being considered for surgical valve interventions, EuroSCORE II (median validation c‐statistic 0.82 [0.76, 0.85]), North West Quality Improvement Programme in Cardiac Interventions Model (median validation c‐statistic 0.78 [0.77, 0.78]), Northern New England Aortic Model (median validation c‐statistic 0.76 [0.76, 0.77]), Ambler (median validation c‐statistic 0.73 [0.72, 0.76]), and STS (2009) Mortality (median validation c‐statistic 0.74 [0.71, 0.79]) have reasonable discrimination and multiple assessments of discrimination and calibration in external data sets. There are no CPMs for patients treated with TAVR that demonstrate good performance across multiple related validation databases. The trustworthiness of these newer risk estimates for TAVR remains under‐studied.
There are several limitations to this work. Our review was limited to CPMs that provide enough information in the published report to calculate a risk prediction for a patient. Logistic regression models that did not report a full equation or intercept were not included. Cox regression models that did not report a point score or baseline hazard were excluded. The search for de novo VHD CPMs was last run in January 2017. While newer CPMs have been developed, there is often substantial delay before the publication of subsequent external validations. Notably, we present relative changes in discrimination to more accurately document changes on a clinically relevant scale, where small decreases in the C‐statistic can result in large changes in clinically relevant performance. Lastly, this study was limited in its examination of CPM calibration, which is an important measure of model performance, but often poorly reported and without a widely‐accepted summary measure.
While there are numerous available CPMs for patients with VHD, many have never been externally validated, and for those that have, discriminatory performance is often much worse than originally reported. We note that CPM performance is highly dependent on the cohort selected for study, suggesting that one‐off external validations may inadequately assess performance. Instead of new CPM development, robust external validations of established TAVR CPMs and an understanding of the updating techniques that best optimize model performance are needed.
Sources of Funding
This work was partially supported through a Patient‐Centered Outcomes Research Institute Methods Award (ME‐1606‐35555), as well as by the National Institutes of Health (R03 AG056447 GEMSSTAR Grant and K23 AG055667) and Bellows Foundation Grant from the American College of Cardiology.
Disclosures
None.
Supporting information
Table S1. Search strategy used to identify all CVD/Cerebrovascular and VHD‐specific CPMs
Table S2. De Novo Model Unabridged Overview
Table S3. External Validations Overview
Figure S1. Literature Search Overview.
Acknowledgments
The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute, its Board of Governors, or Methodology Committee.
(J Am Heart Assoc. 2019;8:e011972 DOI: 10.1161/JAHA.119.011972.)
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2. Muller DWM, Farivar RS, Jansz P, Bae R, Walters D, Clarke A, Grayburn PA, Stoler RC, Dahle G, Rein KA, Shaw M, Scalia GM, Guerrero M, Pearson P, Kapadia S, Gillinov M, Pichard A, Corso P, Popma J, Chuang M, Blanke P, Leipsic J, Sorajja P; Tendyne Global Feasibility Trial Investigators . Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol. 2017;69:381–391. [DOI] [PubMed] [Google Scholar]
- 3. Aboulhosn J, Cabalka AK, Levi DS, Himbert D, Testa L, Latib A, Makkar RR, Boudjemline Y, Kim DW, Kefer J, Bleiziffer S, Kerst G, Dvir D, McElhinney DB. Transcatheter valve‐in‐ring implantation for the treatment of residual or recurrent tricuspid valve dysfunction after prior surgical repair. JACC Cardiovasc Interv. 2017;10:53–63. [DOI] [PubMed] [Google Scholar]
- 4. Holmes DR Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 6. Nishimura RA, Otto CM, Bonow RO, Carabello BA, III Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, III Sundt TM, Thomas JD; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College Of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 7. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy R . Development and validation of a risk prediction model for in‐hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 8. Duke University . Optimizing health outcomes in patients with symptomatic aortic valve disease (pcori‐avr). National Institutes of Health. NCT number NCT02266251; 2014.
- 9. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task F . The society of thoracic surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 10. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. Euroscore ii. Eur J Cardiothorac Surg. 2012;41:734–744; discussion 744–735. [DOI] [PubMed] [Google Scholar]
- 11. Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high‐risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. [DOI] [PubMed] [Google Scholar]
- 12. Wessler BS, Lai YhL, Kramer W, Cangelosi M, Raman G, Lutz JS, Kent DM. Clinical prediction models for cardiovascular disease: tufts predictive analytics and comparative effectiveness clinical prediction model database. Circ Cardiovasc Qual Outcomes. 2015;8:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Calster B, Vickers AJ. Calibration of risk prediction models: impact on decision‐analytic performance. Med Decis Making. 2015;35:162–169. [DOI] [PubMed] [Google Scholar]
- 14. Siontis GC, Tzoulaki I, Castaldi PJ, Ioannidis JP. External validation of new risk prediction models is infrequent and reveals worse prognostic discrimination. J Clin Epidemiol. 2015;68:25–34. [DOI] [PubMed] [Google Scholar]
- 15. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer‐Verlag; 2001. [Google Scholar]
- 16. Edwards FH, Peterson ED, Coombs LP, DeLong ER, Jamieson WR, Shroyer ALW, Grover FL. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885–892. [DOI] [PubMed] [Google Scholar]
- 17. Nowicki ER, Birkmeyer NJ, Weintraub RW, Leavitt BJ, Sanders JH, Dacey LJ, Clough RA, Quinn RD, Charlesworth DC, Sisto DA, Uhlig PN, Olmstead EM, O'Connor GT; Northern New England Cardiovascular Disease Study Group and the Center for Evaluative Clinical Sciences, Dartmouth Medical School . Multivariable prediction of in‐hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg. 2004;77:1966–1977. [DOI] [PubMed] [Google Scholar]
- 18. Kuduvalli M, Grayson AD, Au J, Grotte G, Bridgewater B, Fabri BM, Fabri BM; North West Quality Improvement Programme in Cardiac Interventions . A multi‐centre additive and logistic risk model for in‐hospital mortality following aortic valve replacement. Eur J Cardiothorac Surg. 2007;31:607–613. [DOI] [PubMed] [Google Scholar]
- 19. Cruz‐Gonzalez I, Sanchez‐Ledesma M, Sanchez PL, Martin‐Moreiras J, Jneid H, Rengifo‐Moreno P, Inglessis‐Azuaje I, Maree AO, Palacios IF. Predicting success and long‐term outcomes of percutaneous mitral valvuloplasty: a multifactorial score. Am J Med. 2009;122:581.e511–581.e519. [DOI] [PubMed] [Google Scholar]
- 20. Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Pierard L, Gueret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 21. Guaragna JC, Bodanese LC, Bueno FL, Goldani MA. Proposed preoperative risk score for patients candidate to cardiac valve surgery. Arq Bras Cardiol. 2010;94:541–548. [DOI] [PubMed] [Google Scholar]
- 22. Guo LX, Meng X, Zhang ZG, Bai T. Analysis of risk factors for valve replacements in 5,128 cases from a single heart center in China. Chin Med J (Engl). 2010;123:3509–3514. [PubMed] [Google Scholar]
- 23. Elmariah S, Lubitz SA, Shah AM, Miller MA, Kaplish D, Kothari S, Moreno PR, Kini AS, Sharma SK. A novel clinical prediction rule for 30‐day mortality following balloon aortic valuloplasty: the CRRAC the AV score. Catheter Cardiovasc Interv. 2011;78:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bouleti C, Iung B, Laouenan C, Himbert D, Brochet E, Messika‐Zeitoun D, Detaint D, Garbarz E, Cormier B, Michel PL, Mentre F, Vahanian A. Late results of percutaneous mitral commissurotomy up to 20 years: development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125:2119–2127. [DOI] [PubMed] [Google Scholar]
- 25. Cioffi G, Cramariuc D, Dalsgaard M, Davidsen ES, Egstrup K, Rossebo AB, de Simone G, Gerdts E. Left atrial systolic force and outcome in asymptomatic mild to moderate aortic stenosis. Echocardiography. 2012;29:1038–1044. [DOI] [PubMed] [Google Scholar]
- 26. Holme I, Pedersen TR, Boman K, Egstrup K, Gerdts E, Kesaniemi YA, Malbecq W, Ray S, Rossebo AB, Wachtell K, Willenheimer R, Gohlke‐Barwolf C. A risk score for predicting mortality in patients with asymptomatic mild to moderate aortic stenosis. Heart. 2012;98:377–383. [DOI] [PubMed] [Google Scholar]
- 27. Kotting J, Schiller W, Beckmann A, Schafer E, Dobler K, Hamm C, Veit C, Welz A. German aortic valve score: a new scoring system for prediction of mortality related to aortic valve procedures in adults. Eur J Cardiothorac Surg. 2013;43:971–977. [DOI] [PubMed] [Google Scholar]
- 28. Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes‐Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ; PARTNER Investigators . Predictors of poor outcomes after transcatheter aortic valve replacement: results from the partner (placement of aortic transcatheter valve) trial. Circulation. 2014;129:2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capodanno D, Barbanti M, Tamburino C, D'Errigo P, Ranucci M, Santoro G, Santini F, Onorati F, Grossi C, Covello RD, Capranzano P, Rosato S, Seccareccia F; OBSERVANT Research Group . A simple risk tool (the observant score) for prediction of 30‐day mortality after transcatheter aortic valve replacement. Am J Cardiol. 2014;113:1851–1858. [DOI] [PubMed] [Google Scholar]
- 30. D'Ascenzo F, Capodanno D, Tarantini G, Nijhoff F, Ciuca C, Rossi ML, Brambilla N, Barbanti M, Napodano M, Stella P, Saia F, Ferrante G, Tamburino C, Gasparetto V, Agostoni P, Marzocchi A, Presbitero P, Bedogni F, Cerrato E, Omede P, Conrotto F, Salizzoni S, Biondi Zoccai G, Marra S, Rinaldi M, Gaita F, D'Amico M, Moretti C. Usefulness and validation of the survival post TAVI score for survival after transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol. 2014;114:1867–1874. [DOI] [PubMed] [Google Scholar]
- 31. Iung B, Laouenan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau‐Gouge P, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M; FRANCE 2 Investigators . Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–1023. [DOI] [PubMed] [Google Scholar]
- 32. Debonnaire P, Fusini L, Wolterbeek R, Kamperidis V, van Rosendael P, van der Kley F , Katsanos S, Joyce E, Tamborini G, Muratori M, Gripari P, Bax JJ, Marsan NA, Pepi M, Delgado V. Value of the “TAVI2‐SCORe” versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2015;115:234–242. [DOI] [PubMed] [Google Scholar]
- 33. Koplan BA, Stevenson WG, Epstein LM, Aranki SF, Maisel WH. Development and validation of a simple risk score to predict the need for permanent pacing after cardiac valve surgery. J Am Coll Cardiol. 2003;41:795–801. [DOI] [PubMed] [Google Scholar]
- 34. Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112:224–231. [DOI] [PubMed] [Google Scholar]
- 35. Xu J, Ge Y, Pan S, Liu F, Shi Y. A preoperative and intraoperative predictive model of prolonged intensive care unit stay for valvular surgery. J Heart Valve Dis. 2006;15:219–224. [PubMed] [Google Scholar]
- 36. Hannan EL, Wu C, Bennett EV, Carlson RE, Culliford AT, Gold JP, Higgins RS, Smith CR, Jones RH. Risk index for predicting in‐hospital mortality for cardiac valve surgery. Ann Thorac Surg. 2007;83:921–929. [DOI] [PubMed] [Google Scholar]
- 37. Xu J, Ge Y, Hu S, Song Y, Sun H, Liu P. A simple predictive model of prolonged intensive care unit stay after surgery for acquired heart valve disease. J Heart Valve Dis. 2007;16:109–115. [PubMed] [Google Scholar]
- 38. Shi JH, Meng X, Han J, Li Y, Wang JG, Zhang HB, Jia YX, Gurbanov E, Zhuang XJ. A mortality risk assessment model for cardiac valve replacement surgery and its application in the use of prophylactic extracorporeal membrane oxygenation. Int Surg. 2010;95:227–231. [PubMed] [Google Scholar]
- 39. Ariyaratne TV, Billah B, Yap CH, Dinh D, Smith JA, Shardey GC, Reid CM. An Australian risk prediction model for determining early mortality following aortic valve replacement. Eur J Cardiothorac Surg. 2011;39:815–821. [DOI] [PubMed] [Google Scholar]
- 40. Hannan EL, Racz M, Culliford AT, Lahey SJ, Wechsler A, Jordan D, Gold JP, Higgins RS, Smith CR. Risk score for predicting in‐hospital/30‐day mortality for patients undergoing valve and valve/coronary artery bypass graft surgery. Ann Thorac Surg. 2013;95:1282–1290. [DOI] [PubMed] [Google Scholar]
- 41. Wang C, Zhang GX, Lu FL, Li BL, Zou LJ, Han L, Xu ZY. A local risk prediction model for prolonged ventilation after adult heart valve surgery in a Chinese single center. Heart Lung. 2013;42:13–18. [DOI] [PubMed] [Google Scholar]
- 42. Zheng Z, Fan H, Gao H, Li X, Yuan X, Meng J, Xu J, Song Y, Sun H, Hu S. Mortality risk model for heart valve surgery in China. J Heart Valve Dis. 2013;22:93–101. [PubMed] [Google Scholar]
- 43. Rankin JS, He X, O'Brien SM, Jacobs JP, Welke KF, Filardo G, Shahian DM. The society of thoracic surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg. 2013;95:1484–1490. [DOI] [PubMed] [Google Scholar]
- 44. Cruz‐Gonzalez I, Sanchez‐Ledesma M, Sanchez PL, Martin‐Moreiras J, Jneid H, Rengifo‐Moreno P, Inglessis‐Azuaje I, Maree AO, Palacios IF. Predicting success and long‐term outcomes of percutaneous mitral valvuloplasty: a multifactorial score. Am J Med. 2009;122:581.e511–581.e519. [DOI] [PubMed] [Google Scholar]
- 45. Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, Kappetein AP, Stepinska J, Kaden JJ, Naber CK, Acarturk E, Gohlke‐Barwolf C. ESC working group on valvular heart disease position paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2012;33: 822–828,;828a, 828b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 48. Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, Riley RD, Hemingway H, Altman DG; Group P . Prognosis research strategy (progress) 3: prognostic model research. PLoS Med. 2013;10:e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009. [Google Scholar]
- 50. Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–1216. [DOI] [PubMed] [Google Scholar]
- 51. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Sjogren J, Mas PT, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Rev Esp Cardiol (Engl Ed). 2018;71:110. [DOI] [PubMed] [Google Scholar]
- 52. Agarwal SK, Kasula S, Edupuganti MM, Raina S, Shailesh F, Almomani A, Payne JJ, Pothineni NV, Uretsky BF, Hakeem A. Clinical decision making for the hemodynamic “Gray Zone” (FFR 0.75‐0.80) and long‐term outcomes. J Invasive Cardiol. 2017;29:371–376. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy used to identify all CVD/Cerebrovascular and VHD‐specific CPMs
Table S2. De Novo Model Unabridged Overview
Table S3. External Validations Overview
Figure S1. Literature Search Overview.


