Abstract
Parathyromatosis is a rare entity and usually appears as a consequence of the seeding on previous parathyroid surgery which was applied for the secondary hyperparathyroidism. A 63-year-old woman presented with a history of subtotal thyroidectomy 20 years ago and parathyroidectomy due to primary hyperparathyroidism (PHPT) four years ago. Imaging methods revealed multiple parathyromatosis foci on subcutaneous tissue of the neck. En-bloc resection was performed and pathological examination confirmed the diagnosis of parathyromatosis. After an uneventful 10 months, biochemical and radiological tests revealed recurrence on bilateral thyroid lodges. En-bloc resection was performed. The patient has remained well for 24 months after the second operation and has been followed-up with normal parathormone and serum calcium values. To the best of our knowledge, this report describes the twenty-first case of parathyromatosis in PHPT setting in the literature. It should be kept in mind that parathyromatosis may recur at different sites in the neck even in patients with PHPT.
Keywords: Parathyroidectomy, Parathyromatosis, Primary hyperparathyroidism, Recurrent parathyromatosis
Background
Parathyromatosis is a rare cause of recurrent hyperparathyroidism (HPT), which is characterised by hyperfunctional parathyroid foci in the neck and mediastinum.1 Although there are various theories for the development of parathyromatosis, it usually is a consequences of spillage of parathyroid tissue during the surgery for the secondary HPT.2 Continuing stimulus for the residual parathyroid cells leads to many hyperfunctioning small foci, which results in parathyromatosis. Parathyromatosis is extremely rare in the setting of primary HPT cases and the mechanism of development is still obscure. The treatment of the disease is also difficult and challenging. We present a successfully treated patient with parathyromatosis and review the literature of parathyromatosis in the setting of primary hyperparathyroidism.
Case history
A 63-year-old woman was admitted to the emergency department with complaints of nausea and vomiting. She had no history of chronic disease excluding type 2 diabetes mellitus. The patient had a history of subtotal thyroidectomy 20 years ago and a pathology report revealed nodular goitre and left completion lobectomy and upper-left parathyroidectomy due to primary HPT three years ago in different hospitals. The pathology revealed parathyroid adenoma and nodular thyroid tissue. On physical examination, there was a Kocher incision scar in the neck and subcutaneous centimetric nodules near the right side of the incision (Fig 1). Laboratory results revealed that her serum calcium level was 16.4 mg/dl (normal range 8.5–10.5 mg/dl) and parathormone (PTH) was 1776 pg/ml (normal range 17–79 pg/ml). Neck ultrasonography showed six nodule structures suspicious for parathyromatosis, ranging in diameter from 5 mm to 16 mm were found in subcutaneous localisation on the anterior of the right sternocleidomastoid muscle (Figure 2a). Tc99m sestamibi scintigraphy showed no focus consistent with hyperfunctional parathyroid tissue (Figure 2b). Computed tomography (CT) of the neck and upper mediastinum provided similar results to those shown on ultrasound (Figure 2c). The PTH value in the nodule aspiration fluid was 352,590 pg/ml.
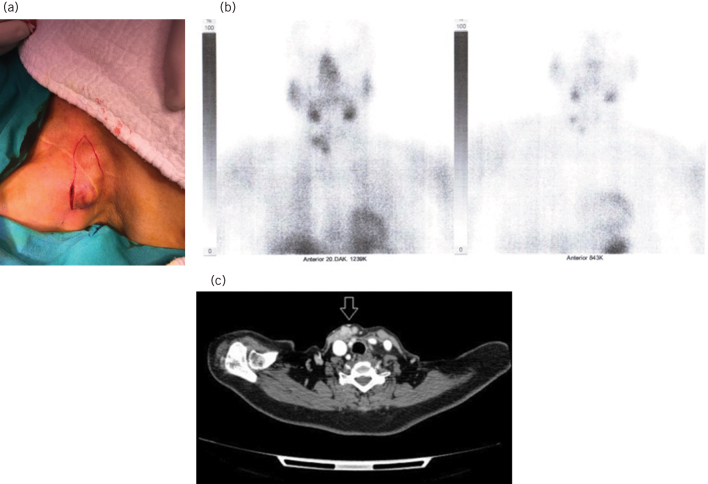
Figure 1.
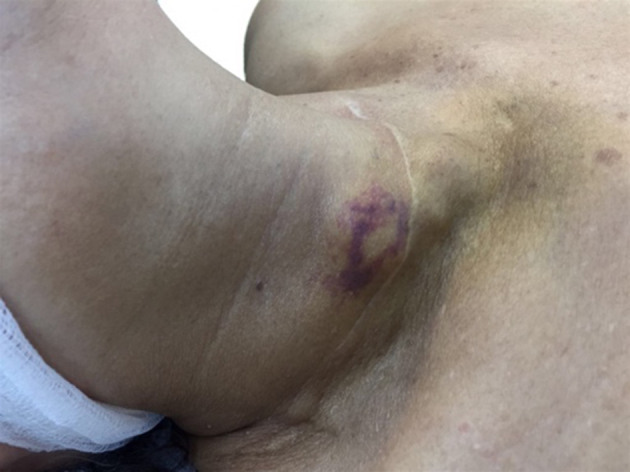
The parathyromatosis foci with ecchymotic appearance due to preoperative nodule aspiration, located at the edge of the old Kocher incision scar.
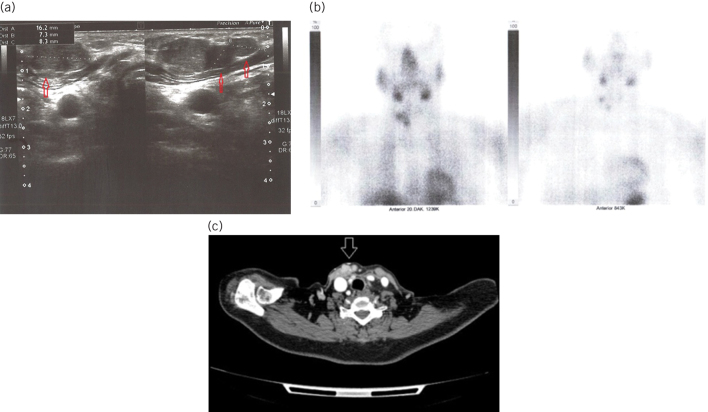
Figure 2.
a) Subcutaneous localised parathyromatosis foci (arrows) with dimensions of 16 mm, 7 mm and 8 mm, respectively, on ultrasound. b) Images of Tc99m sestamibi scintigraphy at 0th and 20th minutes. At the 20th-minute, the initial physiological involvement is seen to be disappeared. c) Contrast-enhanced cervical tomography revealed contrast enhanced multiple parathyromatosis foci (arrows) on the anterior and medial sides of the right sternocleidomastoid muscle.
The patient was first treated with intravenous hydration and forced diuresis with furosemide and then underwent en-bloc resection including all nodules and surrounding tissue without entering the thyroid lodge (Fig 3) and intraoperative PTH decreased adequately (from 1326 pg/ml to 245 pg/ml). The patient was discharged with normal serum calcium and PTH values. The histopathological examination revealed a final diagnosis of parathyromatosis (Fig 4).
Figure 3.
a) Incision parallel to the sternocleidomastoid muscle. b, c) En-bloc excision with adjacent skin and subcutaneous tissue of the parathyromatosis foci over the right sternocleidomastoid muscle.
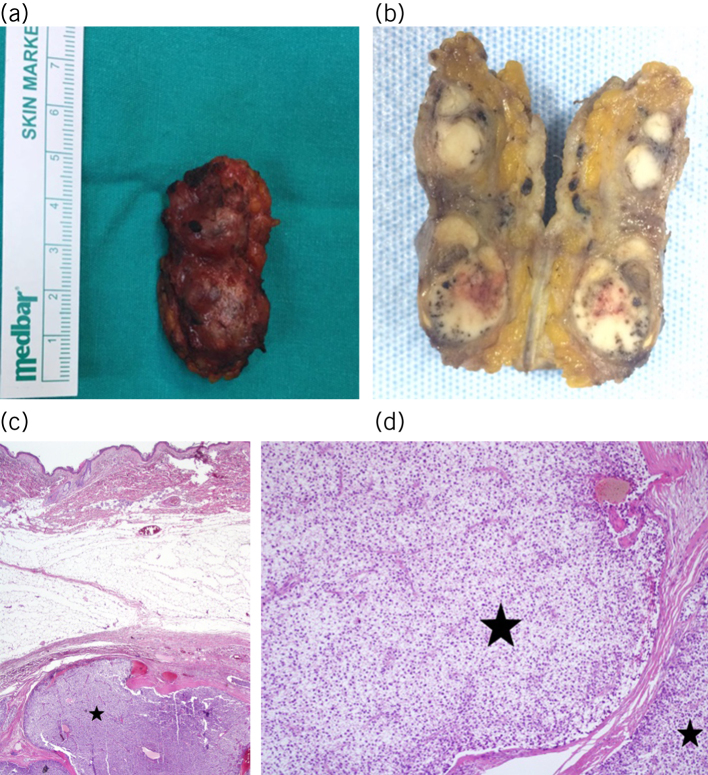
Figure 4.
a) Excised tissue, dimensions 5 × 2 cm. b) Multiple parathyromatosis foci in a dirty white colour on the macroscopic section of the specimen. c) Parathyroid tissue (star) in subcutaneous adipose tissue (haematoxylin and eosin × 4). d) Parathyromatosis foci (stars) separated with trabeculae (haematoxylin and eosin × 40).
The patient was followed-up for 10 months and was normocalcaemic and asymptomatic. However, 10 months after surgery, she was admitted to the outpatient clinic complaining of increased bone pain. Physical examination was normal but her PTH was 376 pg/ml and serum calcium value was 12.6 mg/dl. Ultrasound of the neck revealed multiple parathyroid tissue foci, including a single focus of 9 mm in the right thyroid lodge and four foci with a maximum diameter of 11 mm in the left thyroid gland. Tc99m sestamibi scintigraphy showed no focus consistent with hyperfunctional parathyroid tissue. Suspicious foci revealed on neck CT were described as residual thyroid tissue in the right thyroid lodge and parathyroid tissue in the left (Fig 5).
Figure 5.
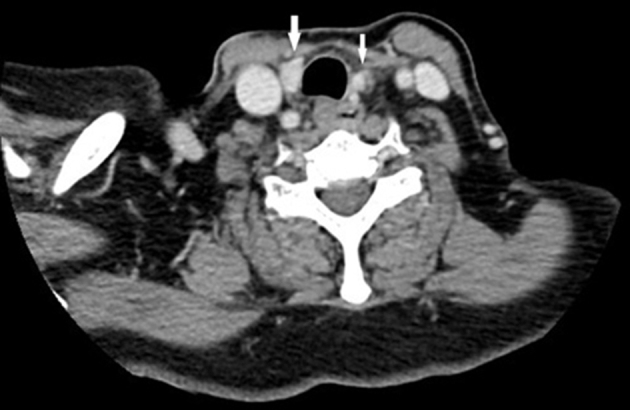
Computed tomography of the neck revealed contrast-enhanced lesions (arrows) on both sides of the trachea. The radiologists described the lesions as the residual thyroid tissue on the right and as parathyroid tissue on the left.
Bilateral neck exploration was performed and all foci detected in the imaging methods were resected. Interestingly, on the left side, the pathological gland was the upper one and no lower gland was identified, which led us to think the gland removed previously had been the lower gland. On the right side, we resected the remnant nodular thyroid and identified the normal appearing upper and lower glands but found an extra enlarged parathyroid gland adherent to the thyroid capsule, which we resected en-bloc. During surgery, an adequate PTH drop was achieved (349–110 pg/ml).
The postoperative course was uneventful and the patient was discharged with normal calcium and PTH values on the second postoperative day. Histopathological examination revealed four hypercellular parathyroid foci with necrosis on the left site and one on the right site, all of which were consistent with parathyromatosis. The pathological criteria did not fulfil the diagnosis of parathyroid cancer (Fig 6). The patient has remained asymptomatic for 24 months after the second operation and has been followed-up with normal PTH and serum calcium values with no evidence of recurrence.
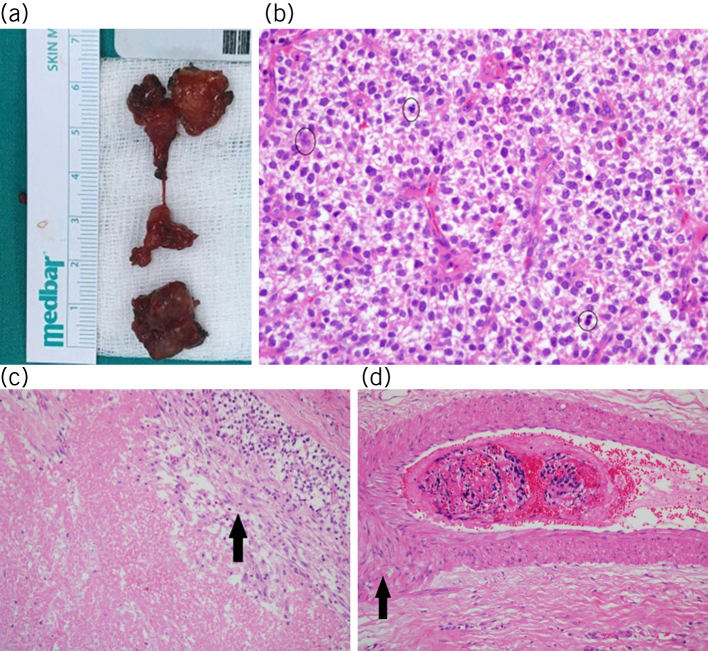
Figure 6.
a) Macroscopic appearance of the parathyromatosis foci excised from the right thyroid lodge (inferior) and left thyroid lodge (superior). b) Multiple cell mitoses (circles) were detected in parathyroid tissue (haematoxylin and eosin × 40). c) Presence of necrosis (arrow) was obtained on microscopic examination (haematoxylin and eosin × 40). d) A suspected vascular invasion image (arrow) was detected in one section (haematoxylin and eosin × 40).
Discussion
Parathyromatosis is extremely rare in the primary HPT setting and very few cases have been reported in the English literature. We searched PubMed with the keywords ‘primary’, ‘hyperparathyroidism’ and ‘parathyromatosis’, and found only 20 reported cases (Table 1).3–18
Table 1.
Reported parathyromatosis cases in patients with primary hyperparathyroidism from 1975 to September 2018 in English literature.
| Authors, Year (Reference number) | Cases (n) | Treatment | Outcome |
| Reddick et al, 19773 | 1 | 3 reoperations | Remission |
| Rattner et al, 19854 | 2 | 1 and 3 reoperations | Remission |
| Akerstrom et al, 19885 | 3 | 1/2/2 reoperations | Remission |
| Sokol et al, 19936 | 1 | 2 reoperations | Persistence |
| Evans et al, 20057 | 1 | 1 reoperation | Remission |
| Tublin et al, 20078 | 1 | 2 reoperations | Remission |
| Diaconescu et al, 20119 | 1 | 1 reoperation | Remission |
| Mohammadi et al, 201210 | 1 | 1 reoperation | Remission |
| Hage et al, 201211 | 1 | 4 reoperations + cinacalcet | Mild remission |
| Twigt et al, 201312 | 2 | 1 and 3 reoperations | Remission |
| Pinnamaneni et al, 201313 | 1 | 2 reoperations | Persistence |
| Scorza et al, 201414 | 1 | 3 reoperations + cinacalcet | Persistence |
| Edling et al, 201415 | 1 | 1 reoperation + cinacalcet | Remission |
| Sharma et al, 201616 | 1 | 1 reoperation | Persistence |
| Jain et al, 201717 | 1 | 2 reoperations | Remission |
| Aggarwal et al, 201718 | 1 | 1 reoperation | Remission |
Three theories have been proposed for the development of parathyromatosis: low-grade malignancy, seeding during parathyroidectomy, and activation of embryological parathyroid remnants.19 Parathyromatosis is more common in patients with chronic renal failure, as in the case of secondary HPT due to continuous stimulation.2 Renal function was normal in our patient, and it was not known whether seeding of parathyroid tissue had occurred in previous surgical procedures. Despite the fact that the thyroid lodge was not opened in the first parathyromatosis operation 10 months earlier, relapsed parathyromatosis in the bilateral thyroid lodge suggests surgery-related seeding during the earlier parathyroid operation in the external centre, but also we think that parathyromatosis recurrence located in a different foci of the neck within a short interval supported the low-grade malignancy theory. Moreover we consider that the hyperfunctioning large foci may supress the relatively small foci and the removal of these large tumours may facilitate the overgrowth of other foci.
The most common locations for parathyromatosis are the thyroid lodge and sites of parathyroid gland autotransplantation such as forearm or sternocleidomastoid muscle. It has also been described in subcutaneous adipose tissue, adjacent to recurrent laryngeal nerve, retrosternal area, carotid sheath, thymus, upper mediastinum and tracheoesophageal groove.1 In our patient, in the first operation all the foci were in the contralateral subcutaneous tissue of the parathyroid surgery, and in the bilateral thyroid lodge in the second operation.
Parathyromatosis foci can be seen on ultrasound, doppler ultrasound, Tc99m sestamibi scintigraphy and CT.1 Small-sized foci and fused foci with surrounding tissue may not be visible on ultrasound and Tc99m sestamibi scintigraphy. In ultrasound, foci may be seen to be hypoechoic or hypervascular, such as benign lymph nodes and normal parathyroid tissues. Tublin et al showed the diagnostic efficacy of Doppler ultrasound.8 In our patient, multiple hypoechoic foci were seen on ultrasound before both operations and contrast enhancement of foci were observed on contrast-enhanced CT. The Tc99m sestamibi scintigraphy was insignificant twice in the diagnosis. In addition, very high PTH values were obtained on ultrasound-guided aspiration before the first operation and diagnostic efficacy was seen.
Treatment is the surgical removal of all foci.19 However, complete excision is thought to be difficult, owing to the difficulty of intraoperatively detecting the foci not observed in the preoperative localisation.8 Moreover, to achieve a complete remission, multiple surgeries may be needed. The presented case was operated on with a diagnosis of parathyromatosis four years following parathyroidectomy due to primary HPT and about one year later, silent foci of parathyromatosis became active in bilateral thyroid lodge.
Conclusion
Parathyromatosis extremely rarely develops after parathyroidectomy in the setting of primary HPT. Only 20 cases have been reported in the English literature. After appropriate preoperative localisation studies, en-bloc resection in which all foci are removed is the only curative treatment. After surgery, patients should be closely followed for recurrence and laboratory and radiological screening should be performed. It should be kept in mind that in patients undergoing surgery for parathyromatosis, recurrence may develop at different foci during the follow-up period.
Acknowledgements
This manuscript was presented as a poster in eighth National Endocrine Surgery Congress, 27–30 April 2017, Antalya, Turkey. The authors thank all general surgery staff for their cooperation. Written informed consent was obtained from the patient who participated in this study.
References
- 1.Fernandez-Ranvier GG, Khanafshar E, Jensen K et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer 2007; : 255–264. [DOI] [PubMed] [Google Scholar]
- 2.Baloch ZW, Fraker D, LiVolsi VA. Parathyromatosis as cause of recurrent secondary hyperparathyroidism: a cytologic diagnosis. Diagn Cytopathol 2001; : 403–405. [DOI] [PubMed] [Google Scholar]
- 3.Reddick RL, Costa JC, Marx SJ. Parathyroid hyperplasia and parathyromatosis. Lancet 1977; (i): 549. [DOI] [PubMed] [Google Scholar]
- 4.Rattner DW, Marrone GC, Kasdon K et al. Recurrent hyperparathyroidism due to implantation of parathyroid tissue. Am J Surg 1985; : 745–748. [DOI] [PubMed] [Google Scholar]
- 5.Akerstrom G, Rudberg C, Grimelius K et al. Recurrent hyperparathyroidism due to peroperative seeding of neoplastic or hyperplastic parathyroid tissue: case report. Acta Chir Scand 1988; : 549–552. [PubMed] [Google Scholar]
- 6.Sokol MS, Kavolius J, Schaaf K et al. Recurrent hyperparathyroidism from benign neoplastic seeding: a review with recommendations for management. Surgery 1993; : 456–461. [PubMed] [Google Scholar]
- 7.Evans CF, Mansfield L, Sharma AK. Recurrent hyperparathyroidism caused by parathyromatosis. Hosp Med 2005; : 424–425. [DOI] [PubMed] [Google Scholar]
- 8.Tublin ME, Yim JH, Carty SE. Recurrent hyperparathyroidism secondary to parathyromatosis: clinical and imaging findings. J Ultrasound Med 2007; : 847–851. [DOI] [PubMed] [Google Scholar]
- 9.Diaconescu MR, Glod M, Grigorovici K et al. Parathyromatosis coexisting with papillary thyroid microcarcinoma. Chirurgia (Bucur) 2011; (5): 669–672. [PubMed] [Google Scholar]
- 10.Mohammadi A, Ghasemi-Rad M. Parathyromatosis or recurrent multiple parathyroid adenomas? A case report. Maedica (Buchar) 2012; (1): 66–69. [PMC free article] [PubMed] [Google Scholar]
- 11.Hage MP, Salti I, Fuleihan GEH. Parathyromatosis: a rare yet problematic etiology of recurrent and persistent hyperparathyroidism. Metabolism 2012; (6): 762–775. [DOI] [PubMed] [Google Scholar]
- 12.Twigt BA, van Dalen T, Vroonhoven K et al. Recurrent hyperparathyroidism caused by benign neoplastic seeding: two cases of parathyromatosis and a review of the literature. Acta Chir Belg 2013; (3): 228–232. [DOI] [PubMed] [Google Scholar]
- 13.Pinnamaneni N, Shankar PR, Muthukrishnan A. 99mTc MIBI SPECT findings in parathyromatosis: a rare entity causing recurrent hyperparathyroidism. Clin Nucl Med 2013; : e443–5. [DOI] [PubMed] [Google Scholar]
- 14.Scorza AB, Moore AG, Terry K et al. Secondary parathyromatosis in a patient with normal kidney function: review of diagnostic modalities and approaches to management. Endocr Pract 2014; (1 Suppl): e4–7. [DOI] [PubMed] [Google Scholar]
- 15.Edling KL, Korenman SG, Janzen K et al. A pregnant dilemma: primary hyperparathyroidism due to parathyromatosis in pregnancy. Endocr Pract 2014; : e14–17. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Dey P, Gude G, Saikia UN. Parathyromatosis: a rare occurrence along the endoscopic tract detected on fine needle aspiration cytology. Diagn Cytopathol 2016; : 1,125–1,127. [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Krasne DL, Singer K et al. Recurrent primary hyperparathyroidism due to type 1 parathyromatosis. Endocrine 2017; (2): 643–650. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Wadhwa R, Aggarwal V. Parathyromatosis following endoscopic parathyroid surgery: a Rare occurrence. Indian J Endocrinol Metab 2017; : 641–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksoy-Altinboga A, Akder Sari A, Rezanko K et al. Parathyromatosis: critical diagnosis regarding surgery and pathologic evaluation. Korean J. Pathol 2012; (2): 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]