Abstract
Background
Real‐time video communication software such as Skype and FaceTime transmits live video and audio over the Internet, allowing counsellors to provide support to help people quit smoking. There are more than four billion Internet users worldwide, and Internet users can download free video communication software, rendering a video counselling approach both feasible and scalable for helping people to quit smoking.
Objectives
To assess the effectiveness of real‐time video counselling delivered individually or to a group in increasing smoking cessation, quit attempts, intervention adherence, satisfaction and therapeutic alliance, and to provide an economic evaluation regarding real‐time video counselling.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register, CENTRAL, MEDLINE, PubMed, PsycINFO and Embase to identify eligible studies on 13 August 2019. We searched the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov to identify ongoing trials registered by 13 August 2019. We checked the reference lists of included articles and contacted smoking cessation researchers for any additional studies.
Selection criteria
We included randomised controlled trials (RCTs), randomised trials, cluster RCTs or cluster randomised trials of real‐time video counselling for current tobacco smokers from any setting that measured smoking cessation at least six months following baseline. The real‐time video counselling intervention could be compared with a no intervention control group or another smoking cessation intervention, or both.
Data collection and analysis
Two authors independently extracted data from included trials, assessed the risk of bias and rated the certainty of the evidence using the GRADE approach. We performed a random‐effects meta‐analysis for the primary outcome of smoking cessation, using the most stringent measure of smoking cessation measured at the longest follow‐up. Analysis was based on the intention‐to‐treat principle. We considered participants with missing data at follow‐up for the primary outcome of smoking cessation to be smokers.
Main results
We included two randomised trials with 615 participants. Both studies delivered real‐time video counselling for smoking cessation individually, compared with telephone counselling. We judged one study at unclear risk of bias and one study at high risk of bias. There was no statistically significant treatment effect for smoking cessation (using the strictest definition and longest follow‐up) across the two included studies when real‐time video counselling was compared to telephone counselling (risk ratio (RR) 2.15, 95% confidence interval (CI) 0.38 to 12.04; 2 studies, 608 participants; I2 = 66%). We judged the overall certainty of the evidence for smoking cessation as very low due to methodological limitations, imprecision in the effect estimate reflected by the wide 95% CIs and inconsistency of cessation rates. There were no significant differences between real‐time video counselling and telephone counselling reported for number of quit attempts among people who continued to smoke (mean difference (MD) 0.50, 95% CI –0.60 to 1.60; 1 study, 499 participants), mean number of counselling sessions completed (MD –0.20, 95% CI –0.45 to 0.05; 1 study, 566 participants), completion of all sessions (RR 1.13, 95% CI 0.71 to 1.79; 1 study, 43 participants) or therapeutic alliance (MD 1.13, 95% CI –0.24 to 2.50; 1 study, 398 participants). Participants in the video counselling arm were more likely than their telephone counselling counterparts to recommend the programme to a friend or family member (RR 1.06, 95% CI 1.01 to 1.11; 1 study, 398 participants); however, there were no between‐group differences on satisfaction score (MD 0.70, 95% CI –1.16 to 2.56; 1 study, 29 participants).
Authors' conclusions
There is very little evidence about the effectiveness of real‐time video counselling for smoking cessation. The existing research does not suggest a difference between video counselling and telephone counselling for assisting people to quit smoking. However, given the very low GRADE rating due to methodological limitations in the design, imprecision of the effect estimate and inconsistency of cessation rates, the smoking cessation results should be interpreted cautiously. High‐quality randomised trials comparing real‐time video counselling to telephone counselling are needed to increase the confidence of the effect estimate. Furthermore, there is currently no evidence comparing real‐time video counselling to a control group. Such research is needed to determine whether video counselling increases smoking cessation.
Keywords: Humans, Communications Media, Behavior Therapy, Counseling, Counseling/methods, Randomized Controlled Trials as Topic, Smoking, Smoking/therapy, Smoking Cessation, Smoking Cessation/methods
Plain language summary
How does real‐time video counselling compare to telephone counselling for helping people quit smoking?
Background
Video communication software like Skype and FaceTime allows counsellors to see and hear people over the Internet to help them quit smoking. Video counselling could help large numbers of people to quit smoking because more than four billion people use the Internet, and video communication software is free.
Study characteristics
We searched for studies on 13 August 2019, and found two that met our inclusion criteria. Our main focus was to learn if video counselling delivered individually or to a group could help people quit smoking and to learn how it compared with other types of support to help people quit. We also studied the effect of real‐time video counselling on the number of times people tried to quit, the number of sessions they completed, their satisfaction with the counselling, their relationship or bond with the counsellor and the costs of using video communication to help people quit smoking. Both studies took place in the USA, and included people from rural areas or women with HIV. Both studies gave one‐to‐one video sessions to individuals. There were eight video sessions in one study and four video sessions in the other study. Both studies compared video counselling to telephone counselling and looked at whether people quit smoking, the number of sessions they completed and their satisfaction with the programme. One study examined the number of times people tried to quit and one study looked at the relationship or bond with the counsellor.
Key findings
It is unclear how video counselling compares with telephone counselling in terms of helping people to quit smoking. People who used video counselling were more likely than those who used telephone counselling to recommend the programme to a friend or someone in their family, but we found no differences in how satisfied they were, the number of video or telephone sessions completed, whether all sessions were completed and in the relationship or bond with the counsellor.
Quality of evidence
We rated the quality of the evidence for smoking cessation to be very low. There were only two studies, and the limitations in these studies made it difficult to draw reliable conclusions about whether video counselling can help people to quit smoking. This should be taken into account when looking at these findings.
Summary of findings
Summary of findings for the main comparison. Real‐time video counselling compared with telephone counselling for smoking cessation.
| Real‐time video counselling compared with telephone counselling for smoking cessation | ||||||
|
Population: smokers Settings: community and healthcare Intervention: real‐time video counselling Comparison: telephone counselling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Telephone counselling | Real‐time video counselling | |||||
|
Smoking cessation (strictest definition and longest follow‐up) Follow‐up: 6–12 months |
71 per 1000 | 153 per 1000 (27 to 855) | RR 2.15 (0.38 to 12.04) | 608 (2 randomised trials) | ⊕⊝⊝⊝a,b,c Very low | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level because of risk of bias: high risk of attrition bias for one of two studies and unclear risk of selection bias for both studies. bDowngraded one level because of imprecision: imprecision in the effect estimate, illustrated by the wide 95% confidence intervals. cDowngraded one level because of inconsistency of results: inconsistency in cessation rates.
Background
Description of the condition
Tobacco use is a leading preventable cause of premature death and disease worldwide (WHO 2019). Globally, there are an estimated 1.1 billion current tobacco smokers and tobacco use is responsible for the death of approximately eight million people each year (WHO 2019). Subgroups that are at high risk of tobacco use include: lower socioeconomic groups (Jamal 2018; Santero 2019); adults with mental illness (Jamal 2018; Lê Cook 2014); people who reside in remote or very remote areas (AIHW 2018; Doogan 2017); indigenous populations (AIHW 2018; Jetty 2017); lesbian, gay, bisexual, transgender and queer populations (Jamal 2018; Wheldon 2018); and ethnic minority groups (Jamal 2018; Odani 2018). Current tobacco smokers are estimated to die a mean of 10 years earlier than non‐smokers (Banks 2015; Jha 2013). Tobacco use harms nearly every organ of the body, causes numerous diseases including coronary heart disease, chronic obstructive pulmonary disease, stroke, cancers and respiratory diseases, and causes adverse reproductive outcomes (HSS 2014). Quitting smoking reduces the risk of tobacco‐related mortality and morbidity even among long‐term smokers (Jha 2013). Smoking cessation before 40 years of age reduces the risk of death associated with continued smoking by approximately 90% (Jha 2013). Effective behavioural interventions and pharmacotherapies for smoking cessation include print‐based self‐help materials (Livingstone‐Banks 2019), Internet‐based programmes (Taylor 2017), proactive telephone counselling (Matkin 2019), healthcare professional advice (Stead 2013), individual counselling (Lancaster 2017), group therapy (Stead 2017), nicotine replacement therapy (Hartmann‐Boyce 2018), bupropion (Hughes 2014), and varenicline (Cahill 2016).
The global economic cost of smoking in 2012 was estimated to be USD 1.4 trillion, comprised of USD 422 billion in direct healthcare costs, USD 357 billion for morbidity and USD 657 billion for mortality (NCI and WHO 2016). In the US, the cost of tobacco use between 2001 and 2004 totalled USD 193 billion per year, consisting of USD 96 billion in direct healthcare costs and USD 97 billion for productivity losses (CDC 2008). There are limited data about the economic costs of tobacco use in low‐ and middle‐income countries, but available evidence suggests that as a percentage of the total healthcare costs, tobacco‐related healthcare costs for low‐ and middle‐income countries are comparable to those of high‐income countries (NCI and WHO 2016).
Description of the intervention
Real‐time video counselling uses video conferencing technology (also referred to as 'telemedicine' or 'telehealth'), consisting of a video camera connected to a computer or mobile device, to securely transmit live video and audio of the counsellor and client to one another over the Internet. There are more than four billion Internet users worldwide (Internet Society 2019), suggesting that real‐time video counselling has the potential for widespread implementation. Freely available software such as Skype, FaceTime, Facebook Messenger or Google+Hangouts allows real‐time, interactive video communication between users via personal computers or mobile devices, or both. Real‐time video counselling differs from telephone counselling because telephone services including quitlines transmit only the sound of the counsellor's and client's voices to each other (Matkin 2019). The Cochrane systematic review of telephone counselling for smoking cessation included interventions that were delivered via audio only over the telephone (Matkin 2019). In contrast, real‐time video counselling includes a visual mode of communication because it transmits both live video and audio of the interaction between the counsellor and client over the Internet.
How the intervention might work
Healthcare providers have used video conferencing technology to deliver smoking cessation care (Richter 2015), as well as consultations for medical conditions including preoperative anaesthesia (Roberts 2015), ophthalmology (Johnson 2015), mental health (Saurman 2014), and remote supervision of chemotherapy administration to people with cancer (Sabesan 2012). Real‐time video consultations are potentially valuable because despite the success of behavioural interventions, such as individual counselling (Lancaster 2017), group therapy (Stead 2017), and proactive telephone counselling (Matkin 2019), in increasing smokers' chances of quitting successfully, the use of in‐person cessation services (Matcham 2014) and quitlines (Wilson 2010) is low. Real‐time video interventions allow health care to be delivered to people who may otherwise have limited access to health care and specialist services, and thus may increase the uptake of behavioural support. For example, people who live in rural areas may not receive treatment or may delay seeking treatment because of fewer healthcare services near their home, and people with mobility problems may find it difficult to attend in‐person consultations, resulting in poorer health outcomes. The evaluation of a telehealth‐delivered smoking cessation support group found that 86% of rural participants were only able to take part in the programme because it was offered via video‐conferencing. The participants reported that travel time and associated costs to attend a face‐to‐face group would have been prohibitive (Carlson 2012). The limited access to effective smoking cessation support and healthcare services in rural areas may have an associated negative impact on health.
In addition, low‐ and middle‐income countries have experienced an increase in the use of Internet technologies, with technology‐enabled health programmes emerging in lower‐income countries (Lewis 2012). The reasons for using technology to deliver health care in low‐ and middle‐income countries include improving access to, and the quality of, care, in diverse geographical locations where there may be a shortage of healthcare professionals, facilitating communications outside regular health visits, and improving diagnosis and treatment (Lewis 2012). These could all lead to better health outcomes for people.
As well as being used as an additional treatment option, real‐time video counselling could also be implemented in existing community and healthcare settings, to expand their reach or reduce the burden on overstretched services. For instance, real‐time video counselling could be delivered through existing quitline services or other smoking cessation services in settings such as general practice, hospitals or other treatment centres, to clients in their own homes. Smoking cessation counsellors or healthcare professionals (or both) could provide behavioural support via video sessions to assist smokers to quit as either a primary intervention or as an adjunct to other smoking cessation treatments.
Similar to face‐to‐face smoking cessation interventions, the live video images transmitted during real‐time video counselling sessions allow counsellors to deliver behavioural support via a visual mode and be responsive to the smoker's verbal and non‐verbal cues. During real‐time video counselling sessions, counsellors can use evidence‐based techniques, such as cognitive behavioural therapy (CBT) (Clark 1997), and motivational interviewing (Miller 1991) to support smokers to quit. As there is evidence that behavioural interventions are generally effective across a range of media (Lancaster 2017; Stead 2017; Matkin 2019), it is reasonable to assume that this may translate to the medium of real‐time video counselling.
However, the limitations of real‐time video counselling also need to be considered. Potential disadvantages of this approach include that the smoker or healthcare professional (or both) or counsellor may not feel adequately skilled to operate the video‐conferencing equipment (O'Connell 2015), there may be insufficient bandwidth (Winters 2007), and low quality audio and video transmission may impede clear communication (Winters 2007). Despite the virtual face‐to‐face capabilities of real‐time video counselling, either in‐person communication or the greater anonymity of non‐visual contact may be preferred by the provider and client.
Why it is important to do this review
To our knowledge, there are no systematic reviews that have examined the effectiveness of real‐time video counselling for smoking cessation in any smoking population or setting. Real‐time video counselling is a scalable intervention that may increase access to a wider variety of smoking cessation services particularly for people living in rural and remote areas and people with mobility issues. If found to be effective, real‐time video counselling for smoking cessation could be included in the suite of smoking cessation services offered by any smoking cessation service provider worldwide.
Objectives
To assess the effectiveness of real‐time video counselling delivered individually or to a group in increasing smoking cessation, quit attempts, intervention adherence, satisfaction and therapeutic alliance, and to provide an economic evaluation regarding real‐time video counselling.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), randomised trials (i.e. comparing multiple treatment groups), cluster RCTs or cluster randomised trials that measure smoking cessation at least six months from baseline.
Types of participants
Current tobacco smokers (daily or occasional) recruited from a community, healthcare or any other setting. There were no restrictions based on age, gender, level of nicotine dependence or comorbidities.
Types of interventions
We included interventions where real‐time video counselling was delivered by smoking cessation advisors or healthcare professionals as either the primary intervention or an adjunct to other smoking cessation treatments. Studies were eligible where administration of the intervention occurred via telemedicine video conferencing technology or other platforms such as Skype, FaceTime, Google+Hangouts, Talky Core, Facebook Messenger, Viber, Tango (or both) or alternative forms of video communication.
The real‐time video counselling intervention could have been compared with either a control intervention or another smoking cessation intervention, or both. Therefore, eligible comparison arms could have included: no intervention control; health information or brief advice; written self‐help materials; proactive telephone counselling; individual face‐to‐face support; group face‐to‐face support; web‐based interventions or any other smoking cessation intervention.
The meta‐analyses included only studies where the isolation of the video component could be achieved (e.g. video counselling plus telephone counselling versus telephone counselling alone).
Types of outcome measures
Primary outcomes
Smoking cessation (e.g. point prevalence, continuous or prolonged abstinence) measured at least six months from baseline. Where a study measured cessation in several ways, we used the most stringent measure for meta‐analyses. The most stringent measure was the one that required smoking cessation to have been achieved for the longest duration (i.e. prolonged abstinence was judged to be more stringent than point prevalence abstinence). Where biochemically validated cessation rates were not available, we included self‐reported measures of cessation in the analysis.
Secondary outcomes
Self‐reported number of quit attempts (i.e. quitting smoking intentionally for one day or longer).
Intervention adherence (e.g. number of completed sessions) and duration of consultations.
Satisfaction, including ease of use (e.g. satisfaction with counselling, connectivity and quality of audio and video, satisfaction with usability of video conferencing equipment).
Therapeutic alliance (e.g. affective bond, client‐therapist collaboration, mutual goals).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: the Cochrane Tobacco Addiction Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), PubMed (from 1966), PsycINFO (from 1806) and Embase (from 1947) to identify eligible studies published to 13 August 2019. The search strategy, including MeSH terms and keywords, for MEDLINE is presented in Appendix 1. We also searched the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) and ClinicalTrials.gov (clinicaltrials.gov/) to identify ongoing trials registered by 13 August 2019.
Searching other resources
We checked the reference lists of included articles and relevant systematic reviews to identify any additional eligible publications. We contacted 10 researchers who had published studies of behavioural smoking cessation interventions, including the first authors of the included studies, and asked if they were aware of any other randomised trials of real‐time video counselling for smoking cessation.
Data collection and analysis
Selection of studies
One author (FT) implemented the search strategy and imported all identified references from each electronic database into EndNote reference management software and removed duplicates. Two authors (FT and CP or TR or JD or RH or EB or JB) independently screened the titles and abstracts in duplicate to determine if they met the inclusion criteria. For articles that appeared relevant or where we could not determine eligibility from the title or abstract, we obtained the full‐text article. The same two authors independently reviewed full‐text articles in duplicate for possible inclusion. The two authors discussed any inconsistencies until they reached consensus.
Data extraction and management
Two authors (FT and CG) independently extracted data in duplicate from all eligible and ongoing trials. We used a standardised data collection form, adapted from the Cochrane Effective Practice and Organisation of Care (EPOC) Group's template (EPOC 2015), which was tailored to this review's objectives. We pilot‐tested the data collection form and incorporated the feedback. We extracted the following information from the eligible studies: authors and year of publication; setting and location/country; population; recruitment method and consent rate; sample size and sociodemographic characteristics (e.g. age, gender, ethnicity, level of education, socioeconomic status); smoking status and history (e.g. current or occasional smoker, level of nicotine dependence, interest in quitting), inclusion criteria and exclusion criteria; study design; video counselling intervention (e.g. number of contacts, duration, frequency, type of provider); comparison arm (e.g. control or other smoking cessation treatment, number of contacts/doses, duration, frequency, type of provider); biochemically validated smoking cessation outcomes (where available) and self‐reported smoking cessation outcomes; self‐reported number of quit attempts; satisfaction and therapeutic alliance measures for video intervention and comparison arms; and costs.
The two authors discussed discrepancies in data extraction until they reached consensus.
Assessment of risk of bias in included studies
Two authors (FT and CW) independently assessed the risk of bias for included trials using the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed the following six study characteristics: random sequence generation (selection bias); allocation concealment (selection bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias) and other biases (e.g. contamination, baseline imbalances, inappropriate administration of the intervention) (Higgins 2011). In addition, for cluster RCTs we planned to assess the risk of: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis and comparability with individually randomised trials (Higgins 2011). We rated each of these features as 'low', 'high' or 'unclear' risk using the criteria for judging risk of bias described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The two authors discussed the inconsistencies in ratings to reach consensus.
Measures of treatment effect
For the dichotomous primary outcome, we calculated a risk ratio (RR) and 95% confidence interval (CI) for each study. The RR for each study was calculated as (number of participants who reported smoking abstinence in the intervention group/number of participants randomised to the intervention group)/(number of participants who reported smoking abstinence in the control (comparison) group/number of participants randomised to the control (comparison) group). For dichotomous secondary outcomes, we calculated the RR with 95% CI for each study. We analysed continuous secondary outcomes by calculating mean differences (MD) if the trials used the same method of measurement or standardised mean difference (SMD) if each study used different measures, with 95% CI.
Unit of analysis issues
For cluster RCTs, we planned to extract individual level data that adjusted for clusters using an intracluster correlation coefficient (ICC). If clusters were not controlled for in the analyses, we planned to contact the trial's authors and request the ICC information. If this information was unavailable, we planned to obtain an estimate of the ICC from similar studies where appropriate, and perform an approximate analysis.
Dealing with missing data
We reported the number and percentage of participants lost to follow‐up in each relevant arm, and considered this in the 'Risk of bias' assessment. Where primary outcome data were missing at follow‐up, we used a conservative approach commonly used in the tobacco control field that assumes that people with missing data continue to smoke tobacco (Hedeker 2007). We recorded if the trial's authors conducted sensitivity analyses using different assumptions to deal with missing data. Analysis was based on the intention‐to‐treat principle and participants remained in the group they were randomly allocated to, irrespective of the extent to which they received the intervention/comparison.
We contacted the first authors of included studies to ask for further data when required in relation to the primary and secondary outcomes.
Assessment of heterogeneity
We inspected the characteristics of included studies and considered whether there was clinical or methodological (or both) heterogeneity across included trials. We also used forest plots to visually inspect statistical heterogeneity among studies' effect estimates. When there were sufficient homogenous studies, we pooled the data and quantified statistical heterogeneity using the I2 statistic. The I2 statistic is a measure of inconsistency that describes the percentage of variation between studies that is due to heterogeneity rather than sampling error (chance) (Higgins 2011). We considered I2 statistic of 50% or greater as representative of substantial heterogeneity (Higgins 2011). Due to the limited studies included, we were unable to explore reasons for such variability by conducting subgroup and sensitivity analyses.
Assessment of reporting biases
If there were at least 10 studies, we planned to use funnel plots to assess publication bias. Asymmetrical funnel plots may be indicative of publication bias, although other potential explanations for asymmetry in funnel plots include methodological flaws or true heterogeneity (Egger 1997). However, given that there were fewer than 10 included studies, we did not test for funnel plot asymmetry, as the power would have been too low to distinguish chance from real asymmetry (Higgins 2011).
Data synthesis
Where meta‐analysis of outcomes was deemed appropriate, following assessment of heterogeneity, we pooled studies by calculating RR, MD and SMD for each outcome using a random‐effects model. We analysed continuous secondary outcomes by calculating MD if the same method of measurement was used across trials for an outcome, or SMD if different measures were employed.
For the primary outcome, a pooled RR greater than 1 indicated that more participants in the real‐time video counselling arm achieved tobacco abstinence than participants in the control/comparison arm.
Where RCTs or cluster RCTs included multiple arms, we included only the arms that met the inclusion criteria. If multiple intervention or control arms were eligible, we combined all relevant and comparable intervention arms into a single intervention group and all relevant and comparable control arms into a single control group to create a single pair‐wise comparison.
In trials with multiple follow‐up points of six months or greater, we analysed the most stringent cessation outcome measured at the longest follow‐up.
We planned to report any studies not suitable for meta‐analysis using a narrative synthesis, categorising studies based on the intervention versus control/comparison group and the population type, and summarising the primary outcome (smoking cessation) followed by each of the secondary outcomes.
Subgroup analysis and investigation of heterogeneity
We planned to investigate any potential heterogeneity between studies by conducting subgroup analyses, categorising studies by population (e.g. general population, people with cancer or people with mental health conditions), intensity of support (e.g. number of sessions), type of provider (e.g. healthcare provider, smoking cessation counsellor) and type of control/comparison group (e.g. no intervention control, proactive telephone counselling). We planned to compare pooled summary statistics across groups and run statistical tests for subgroup differences.
Sensitivity analysis
If there were sufficient studies, we planned to perform sensitivity analyses to examine the impact of removing trials from the meta‐analyses that were judged at high risk of bias (i.e. rated as high risk of bias on one or more domains). Abstinence misreporting rates have been found not to differ significantly between intervention and control conditions (Lantini 2015), and there is often substantial non‐response to biochemical validation in studies of remote interventions, therefore we planned to perform a sensitivity analysis to examine the impact of using self‐reported cessation rates only.
'Summary of findings' table
The 'Summary of findings' table describes the certainty of evidence for the primary outcome using information and approaches recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two authors (FT and TM) assessed the certainty of the body of evidence for the primary outcome (i.e. smoking cessation) using the GRADE approach (Guyatt 2011). The same was planned for any studies reporting our secondary outcome: 'therapeutic alliance'. This involved consideration of: risk of bias (methodological quality); directness of evidence; heterogeneity; precision of effect estimates and risk of publication bias. We assigned each outcome a GRADE rating of 'very low', 'low', 'moderate' or 'high'. The two authors discussed any disagreements until they reached consensus.
Incorporating economic evidence
We developed a brief economic evaluation based on current methods guidelines (Shemilt 2011), to summarise the availability and principal findings of trial‐based economic evaluations (cost analyses, cost‐effectiveness analyses, cost‐utility analyses and cost–benefit analyses) that compare real‐time video counselling to no intervention control or other smoking cessation treatments, among current tobacco smokers. This economic evaluation focused on the extent to which principal findings of eligible economic evaluations indicate that an intervention might be judged favourably (or unfavourably) from an economic perspective, when implemented in different settings. The eligibility criteria for the studies that were included in the economic evaluation (with respect to the population, intervention, comparator(s) and primary health outcome) were the same as those for the main systematic review of treatment effects.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
We identified 5764 records from the electronic database searches and 317 records from trial registries. After removing duplicates, we screened 2974 records and retrieved 38 full‐text articles for further review. We excluded 23 of these full‐text articles (reasons listed in Excluded studies). Two completed trials (Kim 2018; Richter 2015), and one ongoing trial (Tzelepis 2018) met this review's inclusion criteria. We linked secondary reports related to these completed and ongoing trials to each study's primary reference in the references. The PRISMA flow diagram illustrating the process for study selection is presented in Figure 1 (Moher 2009).
1.
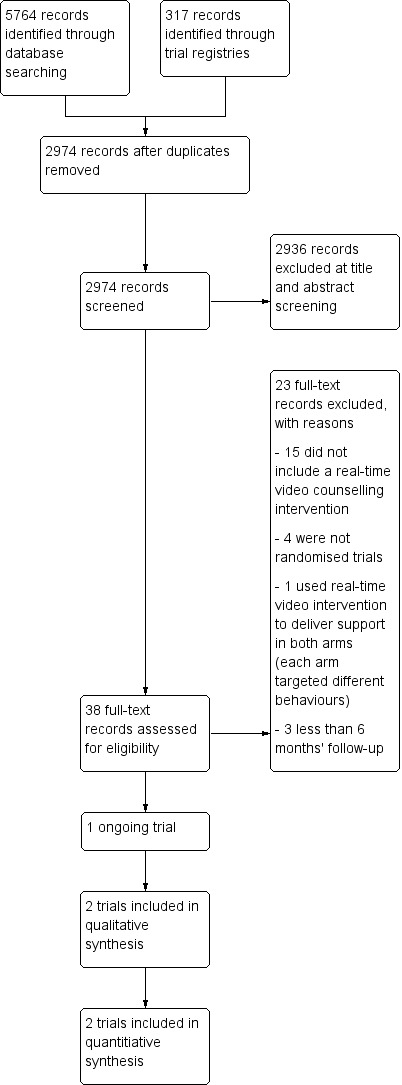
Study flow diagram.
Included studies
Types of studies
This review includes two randomised trials that compared smoking cessation treatments and randomised 615 participants (Kim 2018; Richter 2015). Both studies were conducted in the USA (Kim 2018; Richter 2015). The trials were supported by government (Richter 2015) or university (Kim 2018) funding.
Participants
Richter 2015 recruited participants from 20 primary care clinics located in rural counties and via community‐based activities, and Kim 2018 recruited people living with HIV from communities across the USA using professional networks of healthcare providers and study adverts placed on a free website. Eligible smokers were defined as those who smoked five or more cigarettes per day for at least one year and had smoked 25 out of the past 30 days in Richter 2015, and as those who smoked at least five cigarettes a day for the past six months in Kim 2018. Kim 2018 required participants to be willing to set a quit date within four weeks from the first counselling session, and Richter 2015 included all smokers regardless of their motivation to quit. The number of participants randomised in the two trials was 49 (Kim 2018) and 566 (Richter 2015), which represented 88% (Kim 2018) and 37% (Richter 2015) of participants who met eligibility criteria. Kim 2018 included women only, while Richter 2015 had no restrictions on gender. In Kim 2018, most participants were black and there was a significant proportion of Hispanic (28.6%) participants. In Richter 2015, more than 60% of participants had an income less than 200% of the Federal Poverty Level which means that their annual income was less than twice the income of people on the poverty line (DeNavas‐Walt 2010). The mean number of cigarettes smoked per day in each trial was 14.2 (Kim 2018) and 19.7 (Richter 2015), and mean scores on the Fagerström Test for Nicotine Dependence were 4.9 (Richter 2015) and 5.6 (Kim 2018).
Interventions
Both studies delivered real‐time video counselling for smoking cessation individually and not in a group format. In Kim 2018, the video counselling intervention consisted of eight sessions, while in Richter 2015 the intervention comprised four sessions. The length of the video treatment was eight weeks in both studies. Kim 2018 used 10‐ to 30‐minute video sessions, while Richter 2015 did not report video session duration. Richter 2015 delivered the real‐time video counselling intervention via videoconferencing equipment located at primary care clinics, so participants were required to travel to receive video counselling, while Kim 2018 delivered the video counselling intervention via software installed on the participants' own smartphones. Richter 2015 delivered the video counselling intervention between 2009 and 2011 and Kim 2018 between 2016 and 2017.
Both studies compared the real‐time video counselling intervention with a telephone counselling intervention for smoking cessation, and delivered the telephone counselling interventions directly to participants via their own telephones. The intended number and timing of sessions, length of treatment and duration of sessions in the telephone counselling interventions were identical to those of the video counselling interventions delivered in each study.
Kim 2018 based the counselling content for both the video counselling and telephone counselling interventions on a behavioural therapy foundation, guided by Bandura's Social Cognitive Theory and, Richter 2015 on a combination of motivational interviewing and CBT. A tobacco treatment specialist and a trained graduate student delivered the counselling in Kim 2018, and trained counsellors delivered the counselling in Richter 2015.
Kim 2018 provided all participants with nicotine patches. Richter 2015 provided information about the cessation medications covered by participants' insurance plan or public assistance programme, and study staff assisted income‐eligible participants with no insurance coverage to apply for cessation medications from the pharmacy assistance programmes of pharmaceutical drug companies.
Outcomes
Both studies reported cessation outcomes at short‐term (less than six months) and long‐term (six months or greater) follow‐up assessments. The long‐term follow‐up assessments ranged from six months to 12 months. Both studies reported prolonged abstinence and point prevalence abstinence outcomes. Given that we judged prolonged abstinence to be a more stringent measure of cessation than point prevalence abstinence, we included the prolonged abstinence outcome data in the meta‐analysis for both studies. Both studies described self‐reported and biochemically validated cessation outcomes. Both studies biochemically validated smoking cessation outcomes using a salivary cotinine test.
Richter 2015 assessed number of quit attempts and did so among participants who continued to smoke at 12 months. Both studies examined intervention adherence, which Kim 2018 defined as the completion of all eight counselling sessions and Richter 2015 as the mean number of counselling sessions. Both studies measured satisfaction using self‐reported measures, and Kim 2018 reported acceptable internal consistency. Richter 2015 reported on therapeutic alliance in a secondary data analysis, and Kim 2018 did not assess therapeutic alliance.
Excluded studies
We excluded 23 full‐text records as they did not meet the review's inclusion criteria. Reasons for exclusion included: did not assess a real‐time video counselling intervention (15 studies); were not randomised trials (four studies); used real‐time video intervention to deliver support in both arms (each arm targeted different behaviours) (one study); and follow‐up was less than six months postbaseline (three studies). See Characteristics of excluded studies table.
Risk of bias in included studies
For each included study, we described the authors' judgements and support for judgements in the 'Risk of bias' section of the Characteristics of included studies table. Figure 2 summarises the results for each risk of bias item across all included studies. We judged both studies at low risk of detection bias and reporting bias and at unclear risk of selection bias (Kim 2018; Richter 2015). In relation to attrition bias, we rated Kim 2018 at high risk and Richter 2015 at low risk. Figure 3 presents the risk of bias assessments for each individual study.
2.
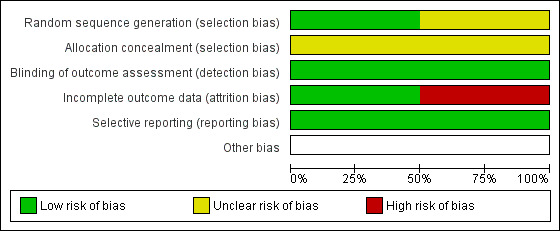
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
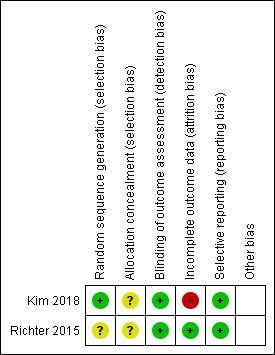
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In relation to random sequence generation, Kim 2018 used a computer‐generated number and was at low risk of bias, while Richter 2015 did not describe the method used to generate the random sequence and was at unclear risk of bias. Both studies were at unclear risk of bias for allocation concealment because there was no description about whether the sealed envelopes were opaque.
Outcome assessment (detection bias)
The risk of detection bias for the smoking cessation outcome was low in both studies as smoking cessation was biochemically verified.
Incomplete outcome data
Richter 2015 reported 12% attrition at 12‐month follow‐up with similar losses across arms and was at low risk of attrition bias. In Kim 2018, the risk of attrition bias was high because attrition at the six‐month postquit follow‐up was significantly higher in the telephone counselling arm (52.4%) than in the video counselling arm (19%).
Selective reporting
Both studies were at low risk of reporting bias as the primary and secondary outcomes reported aligned to those prespecified in the published study protocols.
Other potential sources of bias
There was no evidence of other potential sources of bias.
Effects of interventions
See: Table 1
See Table 1.
Primary outcome
Smoking cessation
There was no evidence of a difference for smoking cessation (using the strictest definition and longest follow‐up) across the two included studies when real‐time video counselling was compared to telephone counselling (RR 2.15, 95% CI 0.38 to 12.04; 2 studies, 608 participants; I2 = 66%; Analysis 1.1). Although Kim 2018 randomised 49 participants, seven were excluded from the meta‐analysis because six did not meet inclusion criteria (no access to video software or ex‐smokers) and one died. We rated the certainty of the evidence for smoking cessation as very low due to methodological limitations in the design, imprecision of the effect estimate and inconsistency of cessation rates. In Richter 2015, the prolonged smoking cessation rates were 8.2% for video counselling and 7.3% for telephone counselling (RR 1.12, 95% CI 0.63 to 1.97; 566 participants) at 12 months follow‐up, while for Kim 2018 the prolonged smoking cessation rates were 33.3% for video counselling and 4.8% for telephone counselling (RR 7.0, 95% CI 0.94 to 52.04; 42 participants) at six‐month postquit. Given both studies reported biochemically verified cessation rates, we did not perform sensitivity analysis to examine the impact of using self‐reported cessation rates only.
1.1. Analysis.
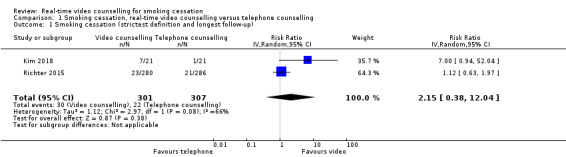
Comparison 1 Smoking cessation, real‐time video counselling versus telephone counselling, Outcome 1 Smoking cessation (strictest definition and longest follow‐up).
Secondary outcomes
Number of quit attempts
Richter 2015 examined quit attempts among participants who continued to smoke at 12 months and reported no significant difference in mean number of quit attempts between the video counselling group (mean 4.8, standard deviation (SD) 6.8) and the telephone counselling group (mean 4.3, SD 5.7) (MD 0.50, 95% CI –0.60 to 1.60; 499 participants; Analysis 2.1). Only participants who continued to smoke at the 12‐month assessment were included in this analysis.
2.1. Analysis.
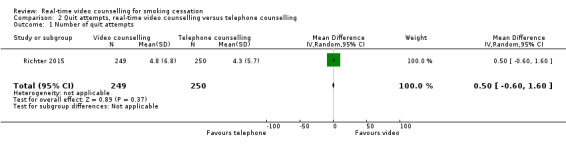
Comparison 2 Quit attempts, real‐time video counselling versus telephone counselling, Outcome 1 Number of quit attempts.
Intervention adherence
Both studies assessed intervention adherence using either a continuous measure (number of counselling sessions, Richter 2015) or a dichotomous measure (per cent completing all counselling sessions, Kim 2018). Given the differences in the measures, we did not pool data from these two studies. Richter 2015 found no evidence of a difference in mean number of counselling sessions between participants randomised to the video counselling arm (mean 2.4, SD 1.5) and participants randomised to the telephone counselling arm (mean 2.6, SD 1.5) (MD –0.20, 95% CI –0.45 to 0.05; 566 participants; Analysis 3.1). In Kim 2018, there was no evidence of a difference between participants who completed all sessions in the video counselling arm (66.7%) or the telephone counselling arm (59.1%) (RR 1.13, 95% CI 0.71 to 1.79; 43 participants; Analysis 3.2). While Kim 2018 excluded the woman who died from the smoking cessation outcome data, she was included in the intervention adherence data which was part of the process evaluation and so there were 43 rather than 42 participants included in this analysis.
3.1. Analysis.
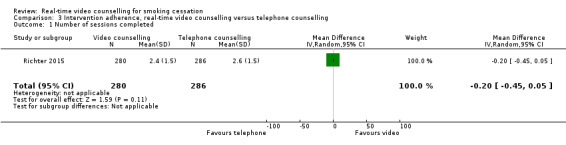
Comparison 3 Intervention adherence, real‐time video counselling versus telephone counselling, Outcome 1 Number of sessions completed.
3.2. Analysis.
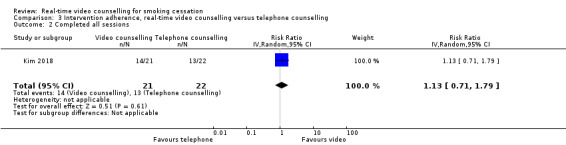
Comparison 3 Intervention adherence, real‐time video counselling versus telephone counselling, Outcome 2 Completed all sessions.
Satisfaction
Both studies assessed satisfaction; however, due to differences in the measures used, we were unable to pool the data. Both studies included only participants who completed the satisfaction measures in the follow‐up surveys in the analysis. In Richter 2015, those in the video counselling arm (97%) were significantly more likely than their telephone counselling counterparts (91.9%) to recommend the programme to a friend or family member (RR 1.06, 95% CI 1.01 to 1.11; 398 participants; Analysis 4.1). There were no other between‐group differences on other satisfaction items reported by Richter 2015. Kim 2018 reported no significant differences in the mean scores of the Client Satisfaction Questionnaire between the video counselling arm (mean 29.6, SD 2.5) and telephone counselling arm (mean 28.9, SD 2.6) (MD 0.70, 95% CI –1.16 to 2.56; 29 participants; Analysis 4.2).
4.1. Analysis.
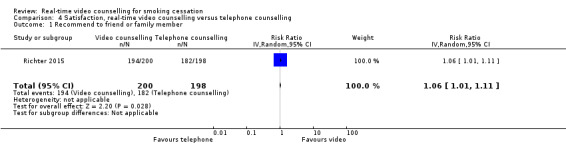
Comparison 4 Satisfaction, real‐time video counselling versus telephone counselling, Outcome 1 Recommend to friend or family member.
4.2. Analysis.
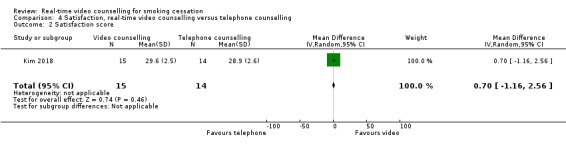
Comparison 4 Satisfaction, real‐time video counselling versus telephone counselling, Outcome 2 Satisfaction score.
Therapeutic alliance
Only Richter 2015 examined therapeutic alliance, and reported no significant difference in mean scores of therapeutic alliance between the video counselling group (mean 70.38, SD 5.8) and the telephone counselling group (mean 69.25, SD 8) (MD 1.13, 95% CI –0.24 to 2.50; 398 participants; Analysis 5.1). The analysis included only participants who completed the follow‐up assessment.
5.1. Analysis.
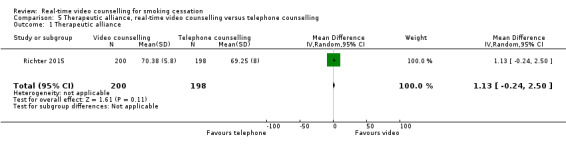
Comparison 5 Therapeutic alliance, real‐time video counselling versus telephone counselling, Outcome 1 Therapeutic alliance.
Discussion
Summary of main results
This review included two randomised trials that compared the effectiveness of real‐time video counselling to telephone counselling for smoking cessation. A meta‐analysis that pooled the findings from the two randomised trials found no evidence of a difference for smoking cessation between real‐time video counselling and telephone counselling. Using the GRADE approach, we rated the certainty of the evidence comparing real‐time video counselling to telephone counselling for smoking cessation as very low due to methodological limitations in design, imprecision of results and inconsistency in cessation rates. There was very low confidence in the meta‐analysis result for smoking cessation due to such limitations and further research is needed to produce greater certainty in this effect estimate. With regards to the secondary outcomes, there were no differences between the real‐time video counselling and telephone counselling groups for mean number of quit attempts and therapeutic alliance in Richter 2015 and intervention adherence for each of the two studies. In terms of satisfaction, video counselling participants were significantly more likely than their telephone counselling counterparts to recommend the programme to a friend or family member in Richter 2015. However, there was no evidence of between‐group differences for other satisfaction measures in either of the included studies.
Economic evaluation
To supplement the main systematic review of efficacy of real‐time video counselling for smoking cessation, we sought to identify economic evaluations of the approach; however economic evidence regarding real‐time video counselling for smoking cessation is currently lacking. The following information was available from the two included studies relating to the costs of the intervention. Richter 2015 reported the costs of providing the video and telephone counselling interventions from the perspective of the provider, participant and society, as well as the cost per quit. To receive the intervention, participants in the video counselling group had to travel to a clinic where the videoconferencing equipment was located, whereas the telephone counselling intervention was delivered to participants directly in their homes. When calculating costs for the video counselling intervention, the cost of clinic space was calculated using: rental rate and physician office visit rates. The mean cost of the video counselling intervention from a provider perspective was USD 47.04 (SD 32.59) when clinic space was valued at rental rate and USD 272.65 (SD 178.29) when valued at physician office visit rates compared to USD 53.25 (SD 35.82) for the telephone counselling intervention. For the video counselling intervention, the total participant variable costs without pharmacotherapy was USD 119.44 (SD 341) and with pharmacotherapy was USD 124.55 (SD 259.10) compared to USD 28.36 (SD 27.80) without pharmacotherapy and USD 75.29 (SD 169.10) with pharmacotherapy in the telephone counselling intervention. The total participant variable costs for the video counselling arm included travel time costs and mileage costs and there was larger variation in participant costs than in the telephone counselling group. From a societal perspective, the cost of the video counselling intervention was USD 166.04 (SD 347.90) when clinic space was valued at rental rate and USD 390.20 (SD 415.40) when valued at physician office visit rates compared to USD 81.61 (SD 58.70) for the telephone counselling intervention. The cost per quit from the provider perspective was USD 480/quit for the video counselling intervention compared to USD 444/quit for the telephone counselling intervention. The cost per quit increased to USD 1694/quit for the video counselling intervention and USD 680/quit for the telephone counselling intervention when participant costs were added (Richter 2015). Kim 2018 reported no economic data. Future studies should undertake economic evaluations of the real‐time video counselling model for smoking cessation.
Overall completeness and applicability of evidence
There is very limited evidence about the effectiveness of real‐time video counselling for smoking cessation. Only two randomised trials were included in this review and these studies examined the comparative effectiveness of real‐time video counselling to telephone counselling. None of the studies examined real‐time video counselling for smoking cessation compared to a no intervention control or a behavioural smoking cessation intervention other than telephone counselling (e.g. face‐to‐face counselling). The findings of the smoking cessation meta‐analysis should be interpreted with caution due to evidence of substantial heterogeneity, the limited studies pooled and the small sample size in Kim 2018. There was also inadequate information of the effect of real‐time video counselling on the secondary outcomes. Two trials assessed intervention adherence and satisfaction; however, these results could not be pooled because of differences in measures (e.g. continuous or dichotomous measures), only one trial examined number of quit attempts (Richter 2015), and one explored therapeutic alliance (Richter 2015). Richter 2015 reported cost findings; however, participants had to travel to the clinic to use the video‐conferencing equipment, and so costs would be different if real‐time video counselling was delivered to smokers at home. Furthermore, the requirement to travel to the clinic meant that video counselling lacked the convenience of telephone counselling, despite both these interventions being able to be delivered directly to participants at home. This may have had an impact on intervention adherence and smoking cessation, which may have differed if participants received video sessions at home. Due to insufficient studies, we were unable to conduct planned subgroup and sensitivity analyses to address the heterogeneity between studies, or the impact of risk of bias and using self‐reported cessation rates only. Furthermore, given that only one study measured therapeutic alliance, this secondary outcome was not included in the Table 1 (as per the protocol; Tzelepis 2017), because it could not be assessed in terms of features that contribute to the GRADE rating such as heterogeneity. The two included studies were conducted in the USA (Kim 2018, Richter 2015), so the findings may have limited generalisability to low‐ and middle‐income countries and other high‐income countries with different ethnic groups.
Certainty of the evidence
Based on the GRADE approach (Guyatt 2011), we judged the certainty of the evidence for smoking cessation to be very low. The GRADE rating was downgraded by one level for each of the following factors: methodological limitations in the design; imprecision of results and inconsistency in cessation rates. The wide 95% CIs around the effect estimate and substantial heterogeneity across the studies illustrate the imprecision of this result. Potential sources of heterogeneity may include the differences in type of participants (i.e. rural residents versus people living with HIV), intensity of the video counselling intervention (four sessions versus eight sessions), the manner in which pharmacotherapies were provided (i.e. directly versus indirectly), the way in which the video counselling was delivered (i.e. videoconferencing equipment at the clinic versus via participants' smartphones) and the longest follow‐up (six months postquit versus 12 months). The methodological limitations included that both studies were assessed at unclear risk of bias for allocation concealment. Kim 2018 was at high risk of attrition bias because the attrition rate at the six‐month postquit follow‐up was significantly higher in the telephone counselling arm (52.4%) than in the video counselling arm (19%). The methodological strengths included that both studies biochemically validated smoking cessation and were assessed at low risk of detection bias. In relation to the inconsistency in cessation rates, in Richter 2015, the prolonged smoking cessation rates were 8.2% for video counselling and 7.3% for telephone counselling at 12 months' follow‐up, while for Kim 2018, the prolonged smoking cessation rates were 33.3% for video counselling and 4.8% for telephone counselling at 6 months postquit.
Potential biases in the review process
To reduce the risk of potential biases, we performed comprehensive searches of electronic databases and trial registries, checked reference lists of included studies, and contacted smoking cessation researchers. As per Cochrane recommendations (Higgins 2011), two authors independently screened titles, abstracts and full‐text articles, and completed data extraction and risk of bias assessments. Despite this rigorous approach, it is possible that relevant literature, particularly unpublished/grey literature, may have been missed. It is also possible that non‐reporting of information in the published articles may have influenced the risk of bias assessments. Because of the lack of studies, we were unable to check for reporting bias as planned in our protocol (Tzelepis 2017).
Agreements and disagreements with other studies or reviews
To our knowledge this is the first systematic review to investigate the effectiveness of real‐time video counselling for smoking cessation in any smoking population or setting. As part of a systematic review that examined the effectiveness of various technology‐based smoking cessation interventions, the effectiveness of real‐time video counselling was investigated in low socioeconomic status or disadvantaged populations (Boland 2018). The Boland 2018 review included only one study of video counselling (Richter 2015), and, consistent with the current review, there was no significant treatment effect reported between video counselling and telephone counselling. Further randomised trials of real‐time video counselling for smoking cessation are needed to inform future systematic reviews and advance the limited literature currently available.
Authors' conclusions
Implications for practice.
There is very little evidence on the effectiveness of real‐time video counselling for smoking cessation to guide the policies and practices of service providers such as quitlines. The meta‐analysis of the effect of video counselling compared to telephone counselling on smoking cessation included data from only two studies and there was evidence of methodological limitations in the design, imprecision of the effect estimate and inconsistency in the cessation rates, so there is very low certainty about the effect. Importantly, there is no evidence that compares real‐time video counselling to a control group to determine whether video counselling increases smoking cessation. There is insufficient evidence from which to draw reliable conclusions regarding the effectiveness of integrating real‐time video counselling into the routine practices of quitlines and other smoking cessation services.
Implications for research.
Further randomised trials of real‐time video counselling for smoking cessation are necessary to inform the evidence base. This review included only two studies from the US that compared video counselling to telephone counselling and focused on particular subgroups of smokers such as smokers in rural locations (Richter 2015), and women with HIV (Kim 2018). There is no evidence about the effectiveness of real‐time video counselling for smoking cessation compared to a no intervention control or any behavioural smoking cessation intervention other than telephone (e.g. face‐to‐face), suggesting that randomised trials that address these gaps in the literature are needed. To increase the generalisability of the evidence, future studies could be conducted outside of the US in other high‐income countries as well as in low‐ and middle‐income countries and could target smokers from the broader general population or other subgroups at high risk of tobacco use such as lower socioeconomic groups (Jamal 2018; Santero 2019); adults with mental illness (Jamal 2018; Lê Cook 2014); indigenous populations (AIHW 2018; Jetty 2017); lesbian, gay, bisexual, transgender and queer populations (Jamal 2018; Wheldon 2018); and ethnic minority groups (Jamal 2018; Odani 2018). There was limited evidence of the effect of video counselling on quit attempts, intervention adherence, satisfaction and therapeutic alliance. Future randomised trials could be designed to address these gaps in the literature. Furthermore, only one study reported the cost of delivering real‐time video counselling for smoking cessation and because the videoconferencing equipment was located at clinics included the cost of clinic space (Richter 2015). Further studies should examine the cost‐effectiveness of real‐time video counselling for smoking cessation delivered directly to smokers at home, as such information will be important for informing the policies and practices of smoking cessation services. Since Richter 2015, which commenced in 2009, there has been increased availability of video communication software that can be downloaded for personal use onto people's personal computers or mobile devices (Oduor 2013). As a result of increased access to video communication software, future research will no longer require participants to access a video communication intervention via equipment located at clinics, as was done in Richter 2015. Instead, real‐time video counselling for smoking cessation can be delivered directly to participants at home or a location of their choice via their personal computers or mobile devices. This may increase use of the video counselling intervention and reduce barriers to accessing treatment.
None of the existing studies delivered the real‐time video counselling via a real‐world quitline service or face‐to‐face smoking cessation service. Future research should test real‐time video counselling within such services to strengthen the likelihood of developing a sustainable model of service delivery and to facilitate rapid and direct translation of the research into the practices of real‐world smoking cessation service providers.
Acknowledgements
We thank Dr Jonathan Livingstone‐Banks for conducting the searches in the Cochrane Tobacco Addiction Group Specialised Register and the trial registries and for editorial support. We would also like to thank Professor Kimber Richter, Dr Edward Liebmann and Associate Professor Kim for providing further information about the included studies to the review team. We also wish to thank Felix Naughton, University of East Anglia, Dr Abi Eccles, Academic Primary Care, University of Warwick, and Lee Bromhead for peer‐review of this manuscript and for their helpful comments.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. MEDLINE search strategy
| 1 | exp Smoking/ |
| 2 | exp Smoking Cessation/ |
| 3 | exp Tobacco/ |
| 4 | exp Tobacco Products/ |
| 5 | exp “Tobacco Use”/ |
| 6 | exp “Tobacco Use Cessation”/ |
| 7 | exp “Tobacco Use Cessation Products”/ |
| 8 | exp Nicotine/ |
| 9 | smok*.mp |
| 10 | tobacco.mp |
| 11 | cigar*.mp |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
| 13 | exp Videoconferencing/ |
| 14 | exp Remote consultation/ |
| 15 | exp Telemedicine/ |
| 16 | tele?health.mp |
| 17 | tele?medicine.mp |
| 18 | video*.mp |
| 19 | Skype.mp |
| 20 | Facetime.mp |
| 21 | Google+Hangouts.mp |
| 22 | Talky Core.mp |
| 23 | Messages.mp |
| 24 | Viber.mp |
| 25 | Tango.mp |
| 26 | 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 |
| 27 | exp Randomized Controlled Trial/ |
| 28 | exp Randomized Controlled Trials as Topic/ |
| 29 | exp Clinical Trial |
| 30 | exp Clinical Trials as Topic/ |
| 31 | exp Pragmatic Clinical Trial/ |
| 32 | exp Pragmatic Clinical Trials as Topic/ |
| 33 | exp Random Allocation/ |
| 34 | random*.mp |
| 35 | RCT*.mp |
| 36 | trial*.mp |
| 37 | 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 |
| 38 | 12 and 26 and 37 |
Data and analyses
Comparison 1. Smoking cessation, real‐time video counselling versus telephone counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (strictest definition and longest follow‐up) | 2 | 608 | Risk Ratio (IV, Random, 95% CI) | 2.15 [0.38, 12.04] |
Comparison 2. Quit attempts, real‐time video counselling versus telephone counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of quit attempts | 1 | 499 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.60, 1.60] |
Comparison 3. Intervention adherence, real‐time video counselling versus telephone counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of sessions completed | 1 | 566 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.45, 0.05] |
| 2 Completed all sessions | 1 | 43 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.71, 1.79] |
Comparison 4. Satisfaction, real‐time video counselling versus telephone counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recommend to friend or family member | 1 | 398 | Risk Ratio (IV, Random, 95% CI) | 1.06 [1.01, 1.11] |
| 2 Satisfaction score | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.16, 2.56] |
Comparison 5. Therapeutic alliance, real‐time video counselling versus telephone counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Therapeutic alliance | 1 | 398 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐0.24, 2.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kim 2018.
| Methods |
Design: 2‐arm, parallel group, randomised pilot trial Setting: US communities – most participants recruited from Massachusetts and New York. Recruitment: professional networks of healthcare providers who were working with people living with HIV and study adverts placed on the free website, Craigslist. Callers were screened via a brief telephone interview and those who were eligible were informed of the study purpose and procedures. Of the 94 callers screened for eligibility, 56 were eligible and 49 were randomised (88% of eligible). Inclusion criteria: women; English‐speaking ability; HIV‐positive serostatus and CD4 cell count and viral load; 18–75 years old; smokers reporting ≥ 5 cigarettes a day for the past 6 months; access to video calling, via smartphone; willingness to set a quit date within 4 weeks from the first counselling session and usage of an approved form of contraception during the study period. Exclusion criteria: unable to speak English; pregnant or lactating; self‐reported and active skin disease; serious alcohol use (≥ 26 on the Alcohol Use Disorders Identification Test); history of serious mental illness (psychotic and bipolar disorders) or illegal substance use, excluding marijuana. |
|
| Participants |
Total number randomised: 49 randomised; 25 to video counselling arm, 24 to telephone counselling arm Withdrawals and exclusions: in video counselling arm, 4 excluded (3 could not install a video call app, 1 had already quit). In telephone counselling arm, 3 excluded (2 had already quit, 1 died). 42 included in analysis; 21 in video counselling arm, 21 in telephone counselling arm Sociodemographic characteristics: 100% women, mean age 51.12 years (SD 7.65), 30.95% married or living with partner, 28.57% Hispanic, 73.81% black, 57.14% had 12 years of education, 80.5% employed Smoking status and history: mean age at smoking onset 18.08 years (SD 6.82), mean years of smoking 33.14 years (SD 10.00), mean number of cigarettes per day 14.23 (SD 6.73), mean nicotine dependence score 5.57 (SD 1.84), mean self‐efficacy in quitting smoking score 23.90 (SD 8.29) |
|
| Interventions |
Video counselling: 8 weekly individualised counselling sessions with duration of 10–30 minutes each delivered via IMO or other video communication software. Quit date set during the first counselling session (participant encouraged to choose a quit date between the third and fifth sessions). Counselling content based on a cognitive behavioural therapy foundation, guided by Bandura's Social Cognitive Theory. Telephone counselling: 8 weekly individualised counselling sessions with duration of 10–30 minutes delivered via telephone. Quit date set during the first counselling session (participant encouraged to choose a quit date between the third and fifth sessions). Counselling content based on a cognitive behavioural therapy foundation, guided by Bandura's Social Cognitive Theory. All participants: 8‐week supply of nicotine patches Providers: tobacco treatment specialist and a trained graduate student |
|
| Outcomes |
Cessation: prolonged abstinence at 6 months postquit (self‐report at each follow‐up and biochemical validation at 3 and 6 months postquit), point prevalence abstinence at end of intervention (self‐report), 3 months (biochemically validated) and 6 months postquit (biochemically validated) Intervention adherence: 8 counselling sessions completed Satisfaction: Client Satisfaction Questionnaire |
|
| Notes |
Funding: partially supported by a Joseph P. Healey Research Grant awarded by the University of Massachusetts (UMass) Boston and the UMass Boston – Dana Farber Harvard Cancer Center U54 Partnership grant. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated using a computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Random number along with the corresponding group was enclosed in a sealed envelope; however, it was unclear if the envelope was opaque. |
| Blinding of outcome assessment (detection bias) Smoking cessation | Low risk | Smoking cessation was biochemically verified and so low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The attrition rate at the 6‐month postquit follow‐up was significantly higher in the telephone counselling arm (52.4%) than in the video counselling arm (19%). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the paper aligned to those prespecified in the protocol paper. |
Richter 2015.
| Methods |
Design: 2‐arm randomised trial Setting: 20 primary care clinics in Kansas, USA. Half the clinics were in cities with a population < 1800, and 3 were federally qualified health clinics for the medically underserved. Recruitment: people recruited at clinics by clinic staff and via mailings from clinic directors. Study staff conducted community‐based recruitment activities that targeted Latino people through radio interviews, health fairs, community newsletters and staff recruitment tables at Latino worksites, religious organisations and businesses. Of 1544 eligible smokers, 566 (37%) provided consent and were randomised. Inclusion criteria: had a primary care physician who was participating in the study; ≥ 18 years of age; smoke ≥ 5 cigarettes per day for at least 1 year; smoke 25 out of the past 30 days; spoke English or Spanish and had a telephone. All smokers regardless of level of motivation to quit were eligible. Exclusion criteria: used other tobacco products; currently taking smoking cessation medications or participating in another quit smoking programme; breastfeeding, pregnant or planning to become pregnant; planning to relocate in the next year or lived with a smoker already enrolled in the study. |
|
| Participants |
Total number randomised: 566 randomised; 280 to integrated telemedicine (video counselling arm), 286 to telephone counselling arm Withdrawals and exclusions: no withdrawals reported, 874 not eligible, 828 declined to participate, 155 excluded for other reasons Sociodemographic characteristics: 64.8% women, mean age 47.4 years (SD 12.9), 42.6% married, 82.9% Caucasian, 9% Hispanic/Latino, 56.8% high school education or less, 64.5% had income < 200% Federal Poverty Level, 41.7% employed full‐time Smoking status and history: mean age started smoking regularly 17.1 years (SD 5.0), mean number of cigarettes per day 19.7 (SD 10.3), mean nicotine dependence score 4.9 (SD 2.3), mean number of quit attempts in past 12 months 2.0 (SD 3.1), mean longest period of past abstinence 382.9 days (SD 902.9), 73.4% prior use of cessation pharmacotherapy, 58.5% preparation stage to stop smoking, 39.0% contemplation, 2.5% precontemplation, mean perceived competence for cessation score 5.0 (SD 1.5) |
|
| Interventions |
Video counselling: 4 video telemedicine counselling sessions over 8 weeks (weeks 0, 1, 4, 8) delivered via Polycom PVX software, computer and webcam located at the clinic. The counselling approach was adapted to smokers' level of motivation and based on Combined Behavioural Intervention, a combination of motivational interviewing and cognitive behavioural therapy. If the participant created a quit plan or expressed interest in pharmacotherapy (or both), the quit plan and a medication prescription request form were faxed to the receptionist for placement in the participants' medical record and for review/prescription approval by the participants' primary care provider. Telephone counselling: 4 telephone counselling sessions over 8 weeks (weeks 0, 1, 4, 8) delivered via their home or mobile phones. The counselling approach was adapted to smokers' level of motivation and based on Combined Behavioural Intervention, a combination of motivational interviewing and cognitive behavioural therapy. If the participant created a quit plan or expressed interest in pharmacotherapy (or both), the quit plan and a medication prescription request form were mailed to the participant, with instructions to take the forms to their healthcare provider for placement in their medical records and review/prescription approval by their primary care providers. All participants: mailed study materials that included educational materials on smoking cessation and a pharmacotherapy guidance form, which provided individually tailored information on what medications were covered by the participants' insurance plan or public assistance programme. Study staff assisted income‐eligible participants with no insurance coverage to apply for cessation medication from the pharmacy assistance programmes of pharmaceutical drug companies. Providers: 3 full‐time equivalent trained counsellors |
|
| Outcomes |
Cessation: biochemically verified 7‐day point prevalence abstinence at 12 months; self‐reported point prevalence abstinence at 3‐, 6‐ and 12‐months follow‐ups; prolonged abstinence Quit attempts: number of quit attempts at 12 months (among participants who continued to smoke) Intervention adherence: mean number of counselling sessions Satisfaction: satisfaction with the counselling and overall intervention at 3‐month follow‐up Therapeutic alliance: Working Alliance Inventory, short‐form at 3‐month follow‐up Costs: cost from provider perspective, participant perspective, societal perspective, cost per quit |
|
| Notes |
Funding: National Heart, Lung, and Blood Institute Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generated by the study statistician and database manager. The process used to generate the random sequence, however, was not specified and, therefore, the risk of bias was unclear. |
| Allocation concealment (selection bias) | Unclear risk | The project director allocated enrolled participants to study arm by opening sealed envelopes that contained randomly generated group assignments created in advance by the study statistician and database manager. It was not specified if the envelopes were opaque and, therefore, the risk of bias was unclear. |
| Blinding of outcome assessment (detection bias) Smoking cessation | Low risk | Smoking cessation was biochemically verified and so low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% attrition rate at 12 months' follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the paper align to those prespecified in the protocol paper. |
SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2013 | No real‐time video counselling intervention |
| Battaglia 2016 | No real‐time video counselling intervention |
| Calhoun 2016 | No real‐time video counselling intervention |
| Carlson 2012 | Not a randomised trial |
| Garrison 2015 | No real‐time video counselling intervention |
| Gerbert 2003 | No real‐time video counselling intervention with counsellor (computer‐generated interaction using algorithms and pretaped videos) |
| Graham 2013 | No real‐time video counselling intervention |
| Graham 2016 | No real‐time video counselling intervention |
| Gritz 2013 | No real‐time video counselling intervention |
| Houston 2010 | No real‐time video counselling intervention |
| Japuntich 2012 | No real‐time video counselling intervention |
| Kim 2016 | Follow‐up < 6 months |
| Kong 2017 | Not a randomised trial |
| Marhefka 2018 | Not a randomised trial |
| NCT03290430 | Not a randomised trial |
| Nomura 2019 | Follow‐up < 6 months |
| Peterson 2015 | No real‐time video counselling intervention |
| Price 1991 | No real‐time video counselling intervention |
| Prochaska 2018 | Used real‐time video intervention to deliver support in both arms (each arm targeted different behaviours) |
| Stanczyk 2016 | No real‐time video counselling intervention |
| Tanigawa 2019 | Follow‐up < 6 months |
| Toll 2007 | No real‐time video counselling intervention |
| Tsoh 2010 | No real‐time video counselling intervention with counsellor (computer‐generated interaction using algorithms and pretaped videos) |
Characteristics of ongoing studies [ordered by study ID]
Tzelepis 2018.
| Trial name or title | Real‐time video counselling for smoking cessation in regional and remote areas |
| Methods |
Design: 3‐arm, parallel group, randomised trial Setting: inner regional, outer regional, remote and very remote areas of New South Wales, Australia based on the Accessibility and Remoteness Index of Australia (ARIA+) Recruitment: online (e.g. Facebook, Twitter, e‐mail) and traditional recruitment strategies (e.g. newspaper, radio, posters). Inclusion criteria: daily tobacco use; aged ≥ 18 years; access to video‐communication (e.g. Skype, FaceTime); Internet access; telephone access; e‐mail address; and residing in inner/outer regional or remote/very remote areas of New South Wales |
| Participants | Target number: 1842; approximately 614 in video counselling arm, 614 in telephone counselling arm, 614 in written materials (control) arm |
| Interventions |
Video counselling: up to 6 video sessions, with mean duration 15–20 minutes delivered via participant's preferred form of video communication (e.g. Skype, FaceTime). The first video session scheduled within 7 days of participant enrolment. For those who nominate a quit date within 1 month during the initial session, subsequent video sessions offered on the quit date and 3, 7, 14 and 30 days after the quit date. Those who are not ready to quit within 1 month during the initial session offered video sessions at 2, 4 and 6 weeks. Counselling content based on quitline protocols and cognitive behavioural therapy and motivational interviewing used to support smokers to quit. Telephone counselling: up to 6 telephone sessions, with mean duration 15–20 minutes delivered via telephone. The first telephone session scheduled within 7 days of participant enrolment. For those who nominate a quit date within 1 month during the initial session, subsequent telephone sessions offered on the quit date and 3, 7, 14 and 30 days after the quit date. Those who are not ready to quit within 1 month during the initial session offered telephone sessions at 2, 4 and 6 weeks. Counselling content based on quitline protocols and cognitive behavioural therapy and motivational interviewing used to support smokers to quit. Written materials (control): mailed one‐off written materials (i.e. a quit kit) Providers: advisors with university qualifications |
| Outcomes |
Cessation: self‐reported 7‐day point prevalence abstinence at 4, 7 and 13 months postbaseline, prolonged abstinence at 4, 7 and 13 months postbaseline Quit attempts: quit attempt at 4, 7 and 13 months postbaseline Intervention adherence: number and duration of counselling sessions Therapeutic alliance: 5‐item Agnew Relationship measure (ARM‐5) at 4 months postbaseline Costs: cost‐effectiveness analyses at 13 months postbaseline |
| Starting date | 25 May 2017 |
| Contact information | Flora Tzelepis, School of Medicine and Public Health & Hunter New England Population Health, Locked bag 10, Wallsend, NSW 2287, Australia e‐mail address: Flora.Tzelepis@newcastle.edu.au |
| Notes |
Funding: Cancer Institute New South Wales Conflicts of interest: none declared |
Differences between protocol and review
The meta‐analyses were conducted using random‐effects models rather than fixed‐effect models as specified in the protocol (Tzelepis 2017), because random‐effects models allow for variability between studies.
We did not assess blinding of participants and personnel (performance bias) as part of the 'Risk of bias' assessment as specified in the protocol (Tzelepis 2017), because blinding of this behavioural intervention is not possible.
Due to the limited number of eligible studies, we did not conduct subgroup analyses or sensitivity analyses to examine potential heterogeneity between studies, the impact of risk of bias and using self‐reported cessation rates only.
Given there was only one study that measured the effect of real‐time video counselling on therapeutic alliance, we did not include this secondary outcome in the 'Summary of findings' table as specified in the protocol because it could not be assessed in terms of features that contribute to the GRADE rating such as heterogeneity.
Contributions of authors
FT, CP, TR, JD, RH, EB and JB screened the titles, abstracts and full‐texts of potentially eligible articles.
FT and CG completed data extraction from included and ongoing trials.
FT and CW assessed the risk of bias.
FT and TM rated the certainty of evidence using the GRADE approach.
FT drafted the manuscript.
All authors provided feedback and approved the final version of the manuscript.
Sources of support
Internal sources
-
University of Newcastle, Australia.
Salary for review authors
-
Hunter New England Population Health, Australia.
Salary for review authors
External sources
-
Cancer Institute New South Wales, Australia.
Flora Tzelepis was supported by a Cancer Institute New South Wales Early Career Fellowship (15/ECF/1‐44) in 2016–2018.
-
National Health and Medical Research Council (NHMRC), Australia.
Flora Tzelepis is supported by an NHMRC Career Development Fellowship (APP1143269), Christine L Paul was supported by an NHMRC Career Development Fellowship (2014–2018) and Christopher M Williams by an NHMRC Early Career Clinical Research Fellowship.
Declarations of interest
FT: none.
CLP: none.
CMW: none.
CG: none.
TR: none.
JD: none.
RKH: none.
EB: none.
JB: none.
TM: none.
JW: none.
New
References
References to studies included in this review
Kim 2018 {published data only}
- Kim SS, Darwish S, Lee SA, DeMarco RF. A pilot study of a smoking cessation intervention for women living with HIV: study protocol. Open Access Journal of Clinical Trials 2017;9:11‐20. [Google Scholar]
- Kim SS, Darwish S, Lee SA, Sprague C, Demarco RF. A randomized controlled pilot trial of a smoking cessation intervention for US women living with HIV: telephone‐based video call vs voice call. International Journal of Women's Health 2018;10:545‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02898597. Smoking cessation intervention for women living with HIV (SoCIWHIV). clinicaltrials.gov/ct2/show/nct02898597 (first received 25 September 2017).
Richter 2015 {published data only}
- Hunt J, Leon Salas A, Nazir N, Ellerbeck E, Lambart L, Richter K. Process and outcomes of a novel pharmacotherapy guidance procedure to increase medication use for smoking cessation. Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 Mar 13‐16, 2012; Houston (TX).
- Lambart L, Casey G, Nazir N, Richter K. Satisfaction with video versus phone counseling in a rural smoking cessation trial. Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 Mar 13‐16; Houston (TX).
- Leon‐Salas A, Hunt JJ, Richter KP, Nazir N, Ellerbeck EF, Shireman TI. Pharmaceutical assistance programs to support smoking cessation medication access. Journal of the American Pharmacists Association : JAPhA 2017;57:67‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann EP, Preacher KJ, Richter KP, Cupertino AP, Catley D. Identifying pathways to quitting smoking via telemedicine‐delivered care. Health Psychology 2019;38:638‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussulman L, Ellerbeck EF, Cupertino AP, Preacher KJ, Spaulding R, Catley D, et al. Design and participant characteristics of a randomised‐controlled trial of telemedicine for smoking cessation among rural smokers. Contemporary Clinical Trials 2014;38:173‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00843505. Evaluating a telemedicine smoking cessation program in rural primary care practices. clinicaltrials.gov/show/nct00843505 (first received 5 October 2012).
- Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Catley D, Cox LS, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. Journal of Medical Internet Research 2015;17:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
An 2013 {published data only}
- An LC, Demers MR, Kirch MA, Considine‐Dunn S, Nair V, Dasgupta K, et al. A randomized trial of an avatar‐hosted multiple behavior change intervention for young adult smokers. Journal of the National Cancer Institute. Monographs 2013;47:209‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Battaglia 2016 {published data only}
- Battaglia C, Peterson J, Whitfield E, Min SJ, Benson SL, Maddox TM, et al. Integrating motivational interviewing into a home telehealth program for veterans with posttraumatic stress disorder who smoke: a randomized controlled trial. Journal of Clinical Psychology 2016;72:194‐206. [DOI] [PubMed] [Google Scholar]
Calhoun 2016 {published data only}
- Calhoun PS, Datta S, Olsen M, Smith VA, Moore SD, Hair LP, et al. Comparative effectiveness of an Internet‐based smoking cessation intervention versus clinic‐based specialty care for veterans. Journal of Substance Abuse Treatment 2016;69:19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 2012 {published data only}
- Carlson LE, Lounsberry JJ, Maciejewski O, Wright K, Collacutt V, Taenzer P. Telehealth‐delivered group smoking cessation for rural and urban participants: feasibility and cessation rates. Addictive Behaviors 2012;37:108‐14. [DOI] [PubMed] [Google Scholar]
Garrison 2015 {published data only}
- Garrison KA, Pal P, Rojiani R, Dallery J, O'Malley SS, Brewer JA. A randomized controlled trial of smartphone‐based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerbert 2003 {published data only}
- Gerbert B, Berg‐Smith S, Mancuso M, Caspers N, McPhee S, Null D, et al. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promotion Practice 2003;4:249‐61. [DOI] [PubMed] [Google Scholar]
Graham 2013 {published data only}
- Graham AL, Chang Y, Fang Y, Cobb NK, Tinkelman DS, Niaura RS, et al. Cost‐effectiveness of internet and telephone treatment for smoking cessation: an economic evaluation of The iQUITT Study. Tobacco Control 2013;22:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2016 {published data only}
- Graham AL, Jacobs MA, Cohn AM, Cha S, Abroms LC, Papandonatos GD, et al. Optimising text messaging to improve adherence to web‐based smoking cessation treatment: a randomised control trial protocol. BMJ Open 2016;6:e010687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gritz 2013 {published data only}
- Gritz ER, Danysh HE, Fletcher FE, Tami‐Maury I, Fingeret MC, King RM, et al. Long‐term outcomes of a cell phone‐delivered intervention for smokers living with HIV/AIDS. Clinical Infectious Diseases 2013;57:608‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2010 {published data only}
- Houston TK, Sadasivam RS, Ford DE, Richman J, Ray MN, Allison JJ. The QUIT‐PRIMO provider‐patient Internet‐delivered smoking cessation referral intervention: a cluster‐randomized comparative effectiveness trial: study protocol. Implementation Science 2010;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Japuntich 2012 {published data only}
- Japuntich SJ, Regan S, Viana J, Tymoszczuk J, Reyen M, Levy DE, et al. Comparative effectiveness of post‐discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial. Trials 2012;13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim SS, Sitthisongkram S, Bernstein K, Fang H, Choi WS, Ziedonis D. A randomized controlled trial of a videoconferencing smoking cessation intervention for Korean American women: preliminary findings. International Journal of Women's Health 2016;8:453‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2017 {published data only}
- Kong G, Goldberg AL, Dallery J, Krishnan‐Sarin S. An open‐label pilot study of an intervention using mobile phones to deliver contingency management of tobacco abstinence to high school students. Experimental and Clinical Psychopharmacology 2017;25:333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marhefka 2018 {published data only}
- Marhefka SL, Turner, Lockhart E, Rivara A, Wang W, Shuter J. Meeting our patients "where they are": video‐group smoking cessation for people living with HIV. Journal of the Association of Nurses in AIDS Care 2018;29:338‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03290430 {published data only}
- NCT03290430. End nicotine dependence clinic. clinicaltrials.gov/show/nct03290430 (first received 21 September 2017).
Nomura 2019 {published data only}
- Nomura A, Tanigawa T, Muto T, Oga T, Fukushima Y, Kiyosue A, et al. Clinical efficacy of telemedicine compared to face‐to‐face clinic visits for smoking cessation: multicenter open‐label randomized controlled noninferiority trial. Journal of Medical Internet Research 2019;21:e13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 2015 {published data only}
- Peterson J, Prochazka AV, Battaglia C. Smoking cessation and care management for veterans with posttraumatic stress disorder: a study protocol for a randomized controlled trial. BMC Health Services Research 2015;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 1991 {published data only}
- Price JH, Krol RA, Desmond SM, Losh DP, Roberts SM, Snyder FF. Comparison of three antismoking interventions among pregnant women in an urban setting: a randomized trial. Psychological Reports 1991;68:595‐604. [DOI] [PubMed] [Google Scholar]
Prochaska 2018 {published data only}
- NCT02137902. Healing and Empowering Alaskan Lives Towards Healthy‐Hearts Study (HEALTHH). clinicaltrials.gov/show/nct02137902 (first received 14 May 2014).
- Prochaska JJ, Epperson A, Skan J, Oppezzo M, Barnett P, Delucchi K, et al. The Healing and Empowering Alaskan Lives Toward Healthy‐Hearts (HEALTHH) Project: study protocol for a randomized controlled trial of an intervention for tobacco use and other cardiovascular risk behaviors for Alaska Native People. Contemporary Clinical Trials 2018;71:40‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanczyk 2016 {published data only}
- Stanczyk N, Bolman C, Adrichem M, Candel M, Muris J, Vries H. Comparison of text and video computer‐tailored interventions for smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2014;16:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk N, Vries H, Candel M, Muris J, Bolman C. Effectiveness of video‐ versus text‐based computer‐tailored smoking cessation interventions among smokers after one year. Preventive Medicine 2016;82:42‐50. [DOI] [PubMed] [Google Scholar]
- Stanczyk NE, Bolman C, Muris JW, Vries H. Study protocol of a Dutch smoking cessation e‐health program. BMC Public Health 2011;11:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Influence of delivery strategy on message‐processing mechanisms and future adherence to a Dutch computer‐tailored smoking cessation intervention. Journal of Medical Internet Research 2013;15:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk NE, Smit ES, Schulz DN, Vries H, Bolman C, Muris JW, et al. An economic evaluation of a video‐ and text‐based computer‐tailored intervention for smoking cessation: a cost‐effectiveness and cost‐utility analysis of a randomized controlled trial. PloS One 2014;9:e110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanigawa 2019 {published data only}
- Tanigawa T, Nomura A, Kuroda M, Muto T, Hida E, Satake K. Comparing telemedicine and face‐to‐face consultation based on the standard smoking cessation program for nicotine dependence: protocol for a randomized controlled trial. JMIR Research Protocols 2019;8:e12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toll 2007 {published data only}
- Toll BA, O'Malley SS, Katulak NA, Wu R, Dubin JA, Latimer A, et al. Comparing gain‐ and loss‐framed messages for smoking cessation with sustained‐release bupropion: a randomized controlled trial. Psychology of Addictive Behaviors 2007;21:534‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoh 2010 {published data only}
- Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trial. Acta Obstetricia et Gynecologica Scandinavica 2010;89:515‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Tzelepis 2018 {published data only}
- ACTRN12617000514303. Real‐time video counselling for smoking cessation in regional and remote areas. www.anzctr.org.au/ACTRN12617000514303.aspx (first received 30 March 2017).
- Byaruhanga J, Tzelepis F, Paul C, Wiggers J, Byrnes E. Connectivity of real‐time video counseling versus telephone counseling for smoking cessation. Asia‐Pacific Journal of Clinical Oncology 2018;14(Suppl 6):28. [Google Scholar]
- Byaruhanga J, Tzelepis F, Paul C, Wiggers J, Byrnes E, Bowman J, et al. The short‐term effectiveness of real‐time video counseling on smoking cessation among smokers residing in regional and remote areas. Asia‐Pacific Journal of Clinical Oncology 2018;14(Suppl 6):16. [Google Scholar]
- Tzelepis F, Paul C, Wiggers J, Byrnes E, Byaruhanga J, Mitchell A, et al. The long‐term effectiveness of real‐time video counseling for smoking cessation among regional and remote residents. Asia‐Pacific Journal of Clinical Oncology 2018;14(Suppl 6):10. [Google Scholar]
- Tzelepis F, Wiggers J, Paul CL, Byaruhanga J, Byrnes E, Bowman J, et al. A randomised trial of real‐time video counselling for smoking cessation in regional and remote locations: study protocol. Contemporary Clinical Trials 2018;74:70‐5. [DOI] [PubMed] [Google Scholar]
Additional references
AIHW 2018
- Australian Institute of Health and Welfare. Australia's Health 2018. In Brief. Canberra (Australia): Australian Institute of Health and Welfare, 2018. [Google Scholar]
Banks 2015
- Banks E, Joshy G, Weber MF, Liu B, Grenfell R, Egger S, et al. Tobacco smoking and all‐cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Medicine 2015;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boland 2018
- Boland VC, Stockings EA, Mattick RP, McRobbie H, Brown J, Courtney RJ. The methodological quality and effectiveness of technology‐based smoking cessation interventions for disadvantaged groups: a systematic review and meta‐analysis. Nicotine & Tobacco Research 2018;20(3):276‐85. [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2008
- Centers for Disease Control and Prevention. Smoking‐attributable mortality, years of potential life lost, and productivity losses – United States, 2000–2004. MMWR. Morbidity and Mortality Weekly Report 2008;57:1226‐8. [PubMed] [Google Scholar]
Clark 1997
- Clark D, Fairburn CG, Gelder MG. Science and Practice of Cognitive Behaviour Therapy. Oxford (UK): Oxford University Press, 1997. [Google Scholar]
DeNavas‐Walt 2010
- DeNavas‐Walt C, Proctor BD, Smith JC, US Census Bureau. Income, Poverty and Health Insurance Coverage in the United States: 2009. Washington (DC): US Government Printing Office, 2010. [Google Scholar]
Doogan 2017
- Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, et al. A growing geographic disparity: rural and urban cigarette smoking trends in the United States. Preventive Medicine 2017;104:79‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Cochrane Effective Practice and Organisation of Care Group (EPOC). EPOC Resources for Review Authors. Oslo (Norway): Norwegian Knowledge Centre for the Health Services; Available at: epoc.cochrane.org/epoc‐specificresources‐review‐authors, 2015. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedeker 2007
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction 2007;102(10):1564‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
HSS 2014
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Internet Society 2019
- Internet Society. 2019 Internet Society Global Internet Report: Consolidation in the Internet Economy. Reston (VA): Internet Society, 2019. [Google Scholar]
Jamal 2018
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current cigarette smoking among adults – United States, 2016. MMWR. Morbidity and Mortality Weekly Report 2018;67:53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jetty 2017
- Jetty R. Tobacco use and misuse among Indigenous children and youth in Canada. Paediatrics and Child Health (Canada) 2017;22:395‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jha 2013
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st‐century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine 2013;368(4):341‐50. [DOI] [PubMed] [Google Scholar]
Johnson 2015
- Johnson KA, Meyer J, Yazar S, Turner AW. Real‐time teleophthalmology in rural Western Australia. Australian Journal of Rural Health 2015;23(3):142‐9. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Lantini 2015
- Lantini R, McGrath AC, Stein LA, Barnett NP, Monti PM, Colby SM. Misreporting in a randomized clinical trial for smoking cessation in adolescents. Addictive Behaviors 2015;45:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012
- Lewis T, Synowiec C, Lagomarsino G, Schweitzer J. E‐health in low‐ and middle‐income countries: findings from the center for health market innovations. Bulletin of the World Health Organization 2012;90:332‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingstone‐Banks 2019
- Livingstone‐Banks J, Ordóñez‐Mena JM, Hartmann‐Boyce J. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD001118.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lê Cook 2014
- Lê Cook B, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. Journal of the American Medical Association 2014;311:172‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matcham 2014
- Matcham F, McNally L, Vogt F. A pilot randomized controlled trial to increase smoking cessation by maintaining National Health Service Stop Smoking Service attendance. British Journal of Health Psychology 2014;19(4):795‐809. [DOI] [PubMed] [Google Scholar]
Matkin 2019
- Matkin W, Ordóñez‐Mena JM, Hartmann‐Boyce J. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 5. [DOI: 10.1002/14651858.CD002850.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1991
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York (NY): Guilford Press, 1991. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCI and WHO 2016
- U.S. National Cancer Institute and World Health Organization. The Economics of Tobacco and Tobacco Control. National Cancer Institute Tobacco Control Monograph 21. NIH Publication No. 16‐CA‐8029A. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; and Geneva (CH): World Health Organization, 2016. [Google Scholar]
O'Connell 2015
- O'Connell P. Advantages and challenges to using telehealth medicine. Global Journal of Medical Research 2015;15(4):19‐22. [Google Scholar]
Odani 2018
- Odani S, Armour BS, Agaku IT. Racial/ethnic disparities in tobacco product use among middle and high school students – United States, 2014–2017. MMWR. Morbidity and Mortality Weekly Report 2018;67:952‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oduor 2013
- Oduor E, Neustaedter C, Venolia G, Judge TK. The future of personal video communication: moving beyond talking heads to shared experiences. Conference on Human Factors in Computing Systems – Proceedings 2013;April:3247‐50. [Google Scholar]
Roberts 2015
- Roberts S, Spain B, Hicks C, London J, Tay S. Telemedicine in the Northern Territory: an assessment of patient perceptions in the preoperative anaesthetic clinic. Australian Journal of Rural Health 2015;23(3):136‐41. [DOI] [PubMed] [Google Scholar]
Sabesan 2012
- Sabesan S, Larkins S, Evans R, Varma S, Andrews A, Beuttner P, et al. Telemedicine for rural cancer care in North Queensland: bringing cancer care home. Australian Journal of Rural Health 2012;20(5):259‐64. [DOI] [PubMed] [Google Scholar]
Santero 2019
- Santero M, Melendi S, Hernandez‐Vasquez A, Irazola V. Socio‐economic inequalities in smoking prevalence and involuntary exposure to tobacco smoke in Argentina: analysis of three cross‐sectional nationally representative surveys in 2005, 2009 and 2013. PloS One 2019;14(6):e0217845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saurman 2014
- Saurman E, Lyle D, Perkins D, Roberts R. Successful provision of emergency mental health care to rural and remote New South Wales: an evaluation of the Mental Health Emergency Care‐Rural Access Program. Australian Health Review 2014;38(1):58‐64. [DOI] [PubMed] [Google Scholar]
Shemilt 2011
- Shemilt I, Mugford M, Byford S, Drummond M, Eisenstein E, Knapp M, et al. Chapter 15: Incorporating economics evidence. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2017
- Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2017
- Taylor GMJ, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD007078.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wheldon 2018
- Wheldon CW, Kaufman AR, Kasza KA, Moser RP. Tobacco use among adults by sexual orientation: findings from the Population Assessment of Tobacco and Health Study. LGBT Health 2018;5:33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2019: Offer Help to Quit Tobacco Use. Geneva: World Health Organization, 2019. [Google Scholar]
Wilson 2010
- Wilson N, Weerasekera D, Borland R, Edwards R, Bullen C, Li J. Use of a national quitline and variation in use by smoker characteristics: ITC project New Zealand. Nicotine & Tobacco Research 2010;12:S78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winters 2007
- Winters JM, Winters JM. Videoconferencing and telehealth technologies can provide a reliable approach to remote assessment and teaching without compromising quality. Journal of Cardiovascular Nursing 2007;22:51‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tzelepis 2017
- Tzelepis F, Paul CL, Williams CM, Gilligan C, Regan T, Daly J, et al. Real‐time video counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012659] [DOI] [PMC free article] [PubMed] [Google Scholar]


