Abstract
The sigma-2 receptor is a steroid-binding membrane-associated receptor which has been implicated in cell survival. Sigma-2 has recently been shown to bind amyloid-β (Aβ) oligomers in Alzheimer's disease (AD) brain. Furthermore, blocking this interaction was shown to prevent or reverse the effects of Aβ to cause cognitive impairment in mouse models and synaptic loss in neuronal cultures. In the present work, the density of sigma-2 receptors was measured in a double transgenic mouse model of amyloid-β deposition (APP/PS1). Comparisons were made between males and females and between transgenic and wt animals.
Sigma-2 receptor density was assessed by quantitative autoradiography performed on coronal brain slices using [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5-methyl-benzamide ([3H]RHM-1), which has a 300-fold selectivity for the sigma-2 receptor over the sigma-1 receptor. The translocator protein of 18 kDa (TSPO) is expressed on activated microglia and is a marker for neuroinflammation. TSPO has been found to be upregulated in neurodegenerative disorders, including AD. Therefore, in parallel with the sigma-2 autoradiography experiments, we measured TSPO expression using the selective radioligand, [3H]PBR28. We also quantified Aβ plaque burden in the same animals using a monoclonal antibody raised against aggregated Aβ.
Sigma-2 receptor density was significantly decreased in piriform and motor cortices as well as striata of 16-month old female, but not male, APP/PS1 mice as compared to their wt counterparts. [3H]PBR28 binding and immunostaining for Aβ plaques were significantly increased in piriform and motor cortices of both male and female transgenic mice. In striatum however, significant increases were observed only in females.
Keywords: Sigma-2 receptor, PGRMC1, Alzheimer's disease, Transgenic mice, Peripheral benzodiazepine receptor
1. Introduction
Alzheimer's disease (AD) is the most common cause of dementia and presents, due to the aging global population, a growing challenge to the world's health systems. The pathological hallmarks of this neurodegenerative disease include extracellular amyloid plaques, intracellular neurofibrillary tangles of hyperphosphorylated tau and neuroinflammatory activation of microglia [1] and [2]. The amyloid plaques are aggregates of amyloid-β (Aβ) peptides formed from the amyloid precursor protein (APP) by proteolytic cleavage. Loss of dendritic spines, a sign of synaptotoxicity, is seen in early-stage AD. Synapse loss is believed to underlie cognitive impairment in early stages of the disease, but in contrast to the neuronal death which becomes apparent in late-stage AD, it is thought to be reversible [2] and might thus represent a window of therapeutic opportunity. Moreover, these early synaptotoxic effects are likely caused by water-soluble, oligomeric forms of Aβ, rather than the insoluble plaques [3].
The risk of contracting AD is higher in women than in men [4]. It has been suggested that sex steroids play a protective role against AD and that the low levels of progesterone following menopause may contribute to the increased risk of AD in females [5] and [6]. Progesterone is known to possess neuroprotective properties and has been shown to reduce Aβ levels in transgenic (TG) AD mouse models [7], to improve cognitive performance in aged wt and TG AD mice [8] and is being considered as preventative treatment in postmenopausal women at risk of developing AD [6].
The sigma receptors, sigma-1 and sigma-2, were originally identified as membrane-associated binding sites for opiate-like ligands. While they do not bind endogenous opioids, the sigma receptors have been demonstrated to possess physiologically relevant affinities for steroid hormones including progesterone [9]. Whereas the sigma-1 receptor was cloned in 1996, the molecular identity of the sigma-2 receptor has been unknown until recently [10]. However, using photoaffinity labeling, the sigma-2 receptor was identified as a previously known cytochrome-like single-transmembrane protein; progesterone receptor membrane component-1 (PGRMC1, also known as 25-Dx, VEMA, Hrp6 and IZAg) [10]. Interestingly, progesterone has been demonstrated to exert neurogenic and neuroprotective effects via sigma-2/PGRMC1 [11]. It has further been shown that sigma-2/PGRMC1 binds Aβ oligomers in AD brain tissue and that this interaction mediates synaptotoxic effects of Aβ in vitro [12] and [13]. Moreover, it was demonstrated that sigma-2 ligands which compete with Aβ oligomers for binding to the receptor can block both spine loss in vitro and cognitive impairment in a TG mouse model of AD [12] and [13]. Additionally, sigma-2/PGRMC1 ligands have been shown to reduce neurotoxic microglia activation and apoptosis induced by Aβ fragments [14].
Given the links between AD, sigma-2/PGRMC1, neuroprotection and synaptogenesis outlined above, we wanted to investigate whether sigma-2 receptor expression might be altered in a commonly used mouse model of Aβ deposition. Thus, in the present work, brain sigma-2 receptor densities were measured in double TG APP/PS1 mice (see Savonenko et al., [15]) which express a humanized form of APP containing the Swedish mutation (APPswe) together with mutant human presenilin-1 (PS1dE9; which forms part the enzyme which cleaves APP into Aβ peptides). Both mutations have been associated with familial AD and the transgenes promote the prominent formation of amyloid plaques in these animals [15]. However, whereas loss of dendritic spines is evident, neuronal death is not observed in this and similar APP TG mouse models [2]. Thus, APP TG mice have been proposed to model early-rather than late-stage AD [2].
The translocator protein of 18 kDa (TSPO; also known as PBR, peripheral benzodiazepine receptor) is expressed on activated microglia and is a marker for neuroinflammation. TSPO has been found to be upregulated in neurodegenerative disorders, including AD [16] [17], and [18], and neuroinflammation is widely regarded as a process which significantly contributes to synaptotoxicity [19]. In order to compare the extent of Aβ pathology and neuro-inflammation with a putative change in sigma-2 receptor binding, Aβ plaque burden and TSPO binding was examined in parallel with the sigma-2 autoradiography experiments in tissue from the same animals.
2. Methods
2.1. Compounds and radiotracers
[3H]RHM-1 and [3H]PBR28 were custom synthesized by American Radiolabeled Chemicals, Inc. (St. Louis, MO) via O-alkylation of the corresponding phenol precursors [20]. The specific activity of the radioligands was 80 Ci mmol−1. PK11195 was purchased from Sigma–Aldrich (St. Louis, MO). Siramesine was synthesized by our group using published methods [21].
2.2. Animals
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University in St. Louis. Male and female APPswe/PS1dE9 mice (APP/PS1; The Jackson Laboratory, Bar Harbor, ME) and wt C57BL/6J counterparts were sacrificed at 16 months of age and their brains were immediately removed and fresh-frozen on powdered dry ice. Brains were sectioned at 20 μm on a cryostat and thaw-mounted onto Fisher Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA), with 12 sets of 12 sections per slide taken from the rostral through caudal striatum, corresponding approximately to Bregma +2.0 to −1.0 mm. Slides were stored at −80 °C until processing for receptor autoradiography or immunohistochemistry.
2.3. Autoradiography and quantification of radioligand binding
Quantitative autoradiography was performed according to previously described procedures [22]. Slides containing brain sections were incubated for 30 min in an open staining jar with autoradiography buffer (50 mM TrisHCl pH 7.8, 50 mM NaCl, 33 mM EDTA) and the respective radiotracers (4 nM [3H]RHM-1 for sigma-2 receptor binding and 2 nM [3H]PBR28 for TSPO binding). Nonspecific binding was determined in the presence of 1 μM Siramesine for sigma-2 receptor and 1 μM PK11195 for TSPO autoradiography, respectively. The slides were subsequently rinsed five times at 1 min intervals with ice-cold buffer (10 mM Tris HCl pH 7.4,150 mM NaCl), air dried and made conductive by coating the free side with a copper foil tape. The slides were then placed into a gas chamber containing a mixture of argon and triethylamine (Sigma–Aldrich, St. Louis, MO) as part of a detector apparatus, the Beta Imager 2000Z Digital Beta Imaging System (Biospace, Nesles la Vallée, France). After the gas was well mixed and a homogenous state was reached, further exposure for 24 h yielded high-quality images. A [3H]Microscale (American Radiolabeled Chemicals, St. Louis, MO) was counted simultaneously as a reference for total radioactivity quantitative analysis. Quantitative analysis was performed with the program Beta-Vision Plus (BioSpace, Nesles la Vallée, France) for the anatomical regions of interest (ROIs). A total of 4—8 brain sections were chosen for each animal. Using known neuroanatomical landmarks, bilateral ROIs were drawn freehand on serial sections from each individual mouse brain to define the representative binding densities. Data were linearly fitted to a standard slope which was used for calibration, thereby converting counts per minute per mm2 into nCi per mg tissue. Finally, the radioligand binding densities were calculated using the specific activity of each radioligand as previously described [22].
2.4. Immunohistochemical quantification of Aβ plaque deposition
Sections were permeabilized with 0.3% Tween-20 in Trisbuffered saline (TBS-T20) for 10 min, and endogenous peroxidase activity was quenched by a 10-min treatment with 0.3% H2O2 solution in TBS. Tissue was washed with TBS, blocked with 3% dry milk in TBS-T20 for 1 h, and incubated with biotinylated mouse anti-Aβ antibody (HJ3.4B, 1:1000; [23]) overnight. A fresh solution of streptavidin and horseradish peroxidase-conjugated biotin (1:400, Vector Laboratories, Burlingame, CA) was applied to tissue for 90 min, followed by 0.025% 3-3′-diaminobenzadine tetrachloride in 0.25% NiCl and 0.05% H2O2 for 15 min. The slices were placed on glass slides, dried overnight, dehydrated, and mounted. Stained brain sections were scanned with a NanoZoomer slide scanner (Hamamatsu Photonics, Hamamatsu City, Japan). For quantitative analyses of the staining, scanned images were exported with NDP viewer software (Hamamatsu Photonics, Hamamatsu City, Japan) and converted to 8 bit grayscale. Converted images were thresholded to highlight plaques. ROIs were drawn freehand in ImageJ (National Institutes of Health, Bethesda, MD) to select the relevant areas and plaque density was analyzed using the “Measure” function. Two brain sections per mouse were used for quantification. These sections correspond approximately to Bregma −0.8 to −1.0 mm. The average of the two sections was used to represent plaque load for each mouse.
2.5. Statistical analysis
Analyses of variance (ANOVA) and linear regression were performed using GraphPad Prism (Graphpad software, San Diego, CA). ANOVAs were corrected for multiple comparisons using Bonferroni's post-hoc test.
3. Results
3.1. Sigma-2 receptor binding density
Sigma-2 receptor density was assessed by quantitative autoradiography on coronal brain sections using [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5-methyl-benzamide ([3H]RHM-1), which has a 300-fold selectivity for the sigma-2 receptor over sigma-1 [24]. The relative binding density of [3H]RHM-1 was highest in cerebral cortical tissue (in motor cortex; 44 ± 6 and 22 ± 1 fmol/mg tissue in wt females and wt males, respectively), and somewhat lower in striatal tissue (35 ± 4 and 18 ± 1 fmol/mg tissue in wt females and wt males, respectively; see Fig. 1), which is comparable to the results reported by Søby et al. [25] for rat brain binding of the sigma-2 receptor ligand [3H]Siramesine. The distribution of [3H]RHM-1 binding is also in good agreement with recent autoradiography results using a novel sigma-2 receptor-selective dihydroisoquinolinone derivative [26] and with the brain expression pattern of PGRMC1 mRNA in rat, as determined by in situ hybridization [27]. We found sigma-2 receptor density to be significantly decreased in the motor- and piriform cortices, as well as the striata, of female, but not male, TG mice as compared to their wt counterparts. Furthermore, female wt mice exhibited significantly higher binding than male wt animals (Fig. 1).
Fig. 1.
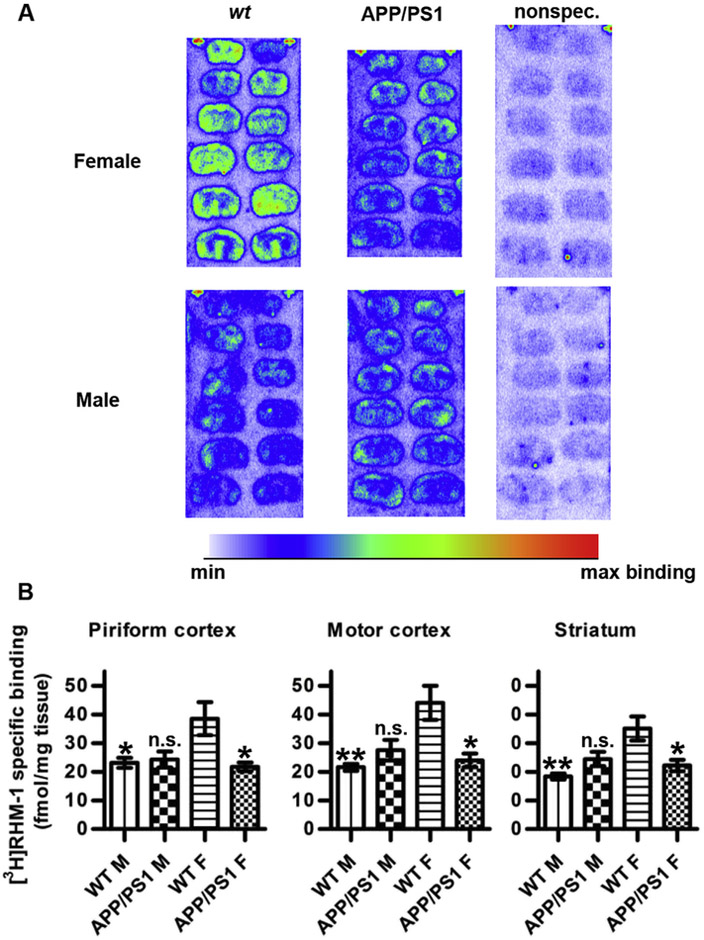
Quantitative autoradiographic analysis of the binding of [3H]RHM-1 in APP/PS1 and wt mouse brain. (A) Representative autoradiograms showing [3H]RHM-1 binding in wt (left column) and APP/PS1 (middle column) brain sections from females (top row) and males (bottom row). [3H]RHM-1 was used at 4 nM. Nonspecific binding was determined by co-incubation with 1 μM Siramesine (right column). (B) Specific RHM-1 binding in the different experimental groups (n = 4 in each group). Asterisks indicate statistical significances in [3H]RHM-1 binding compared to that observed in wt female brain; **; p < 0.01, *; p < 0.05, n.s.; not significant (1-way ANOVA with Bonferroni's post-hoc correction for multiple comparisons between all experimental groups).
3.2. Aβ plaque accumulation
Aβ plaque load was assessed by immunohistochemistry on coronal brain sections using a monoclonal antibody raised against aggregated human Aβ [23]. Immunostaining for Aβ plaques was significantly increased in motor- and piriform cortices in both male and female 16-month old TG mice compared to wt (Fig. 2). In the striatum, Aβ immunostaining was much weaker and significantly increased only in female, and not in male mice (Fig. 2).
Fig. 2.
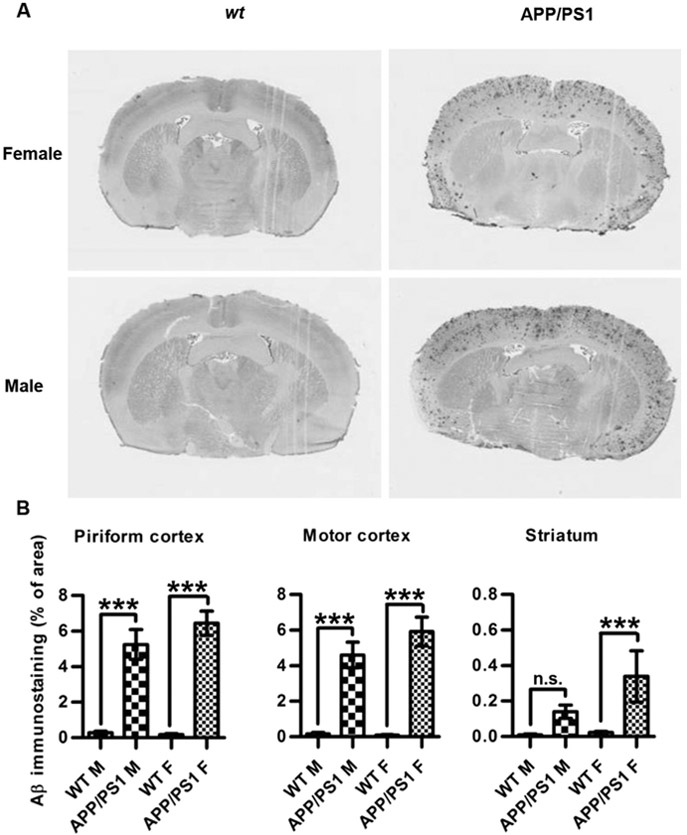
Aβ immunohistochemical staining in APP/PS1 and wt mouse brain sections. (A) Representative slide micrographs showing Aβ staining in wt (left column) and APP/PS1 (right column) brain sections from females (top row) and males (bottom row). (B) Aβ staining in the different experimental groups (n = 4 in each group). Asterisks indicate statistical significances in Aβ staining densities between wt and TG male and female brains, respectively; ***; p < 0.001, *; p < 0.05, n.s.; not significant (1-way ANOVA with Bonferroni's post-hoc correction for multiple comparisons between wt and TG within male and female groups, respectively).
3.3. TSPO binding density
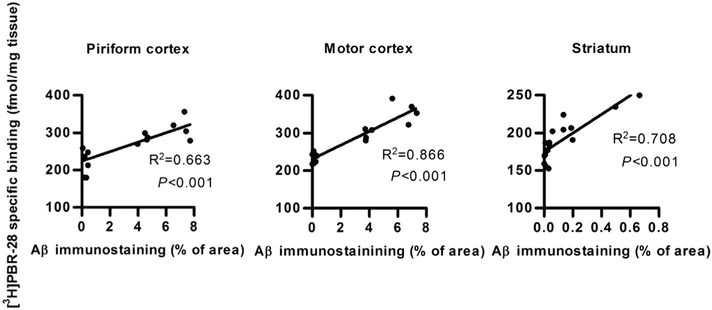
TSPO expression was evaluated by quantitative autoradiography on brain sections from the same animals incubated with the selective radioligand, [3H]N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5-pyridinamine ([3H]PBR28) [28]. [3H]PBR28 binding in the motor- and piriform cortices was significantly increased in both male and female TG mice, whereas in the striatum, [3H]PBR28 binding was significantly higher only in female TG animals (Fig. 3). A significant positive correlation was observed between Aβ plaque load and [3H]PBR28 binding in motor- and piriform cortices and striatum (Fig. 4).
Fig. 3.
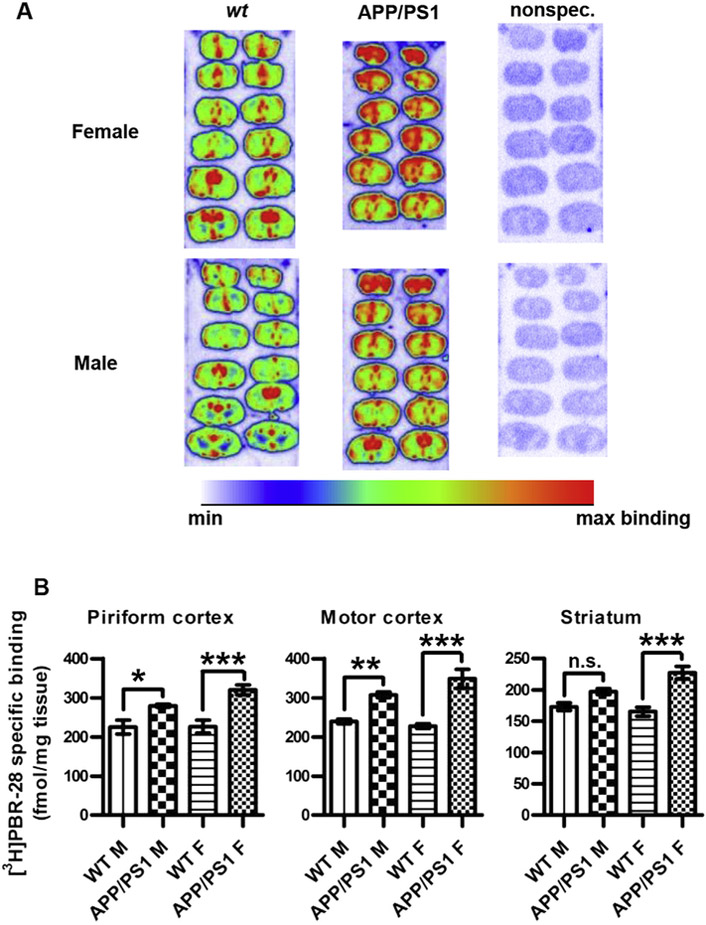
Quantitative autoradiographic analysis of the binding of [3H]PBR28 in APP/PS1 and wt mouse brain. (A) Representative autoradiograms showing [3H]PBR28 binding in wt (left column) and APP/PS1 (middle column) brain sections from females (top row) and males (bottom row). [3H]PBR28 was used at 2 nM. Nonspecific binding was determined by co-incubation with 1 μM cold PK11195 (right column). (B) Specific PBR28 binding in the different experimental groups (n = 4 in each group). Asterisks indicate statistical significances in [3H]PBR28 binding between wt and TG male and female brains, respectively; ***; p < 0.001, **; p < 0.01, *; p < 0.05, n.s.; not significant (1-way ANOVA with Bonferroni's post-hoc correction for multiple comparisons between wt and TG within male and female groups, respectively).
Fig. 4.
Correlation of [3H]PBR28 binding with Aβ immunohistochemical staining. Data from piriform cortex (left column), motor cortex (middle column) and striatum (right column) from APP/PS1 and wt mice. The correlation lines were fitted by linear regression; R2 ~ 0.66,0.87,0.71, from left to right. The slopes of the fitted lines are significantly non-zero; p < 0.001, for all three fits.
4. Discussion
The present study found [3H]RHM-1 binding to be decreased in female, but not in male TG mice, pointing to a sex-specific effect of the transgenes on the availability of sigma-2 receptor sites. Moreover, [3H]RHM-1 binding was higher in wt females as compared to wt and TG males. Thus, the present results may indicate that sigma-2 receptor expression in higher in wt females than wt males and that Aβ pathology is accompanied by a reduction of sigma-2 receptor availability in female, but not in male APP/PS1 mice. Sex differences in PGRMC1 expression has been reported for the choroid plexus and subcomissural organ in rats, where immunoreactivity for this protein is higher in pseudopregnant females than in males [29], whereas Krebs et al. [30] and Sakamoto et al. [31] reported similar PGRMC1 expression in both sexes in the hypothalamus and cerebellum, respectively.
Izzo et al. [13] found no difference in sigma-2 binding in postmortem brain tissue from late-stage AD patients compared to non-AD controls; however, the effect of gender was not specifically examined in that study. The same study reported that treatment with Aβ oligomers upregulated sigma-2/PGRMC1 in cultured neurons. The picture is further complicated by the fact that sigma-2/PGRMC1 is expressed not only on neurons, but is also induced in activated microglia [32], the latter of which increase in number in AD and in relevant mouse models as discussed further below. Since neuronal loss is evident in late-stage AD, the maintenance of sigma-2 binding in AD patients might thus reflect upregulation of sigma-2/PGRMC1 on remaining neurons, an increase of activated microglia, or both. While neuronal death is not observed in APP/PS1 mice [2], further experiments will be required to distinguish the relative contributions of neurons and microglia to [3H]RHM-1 binding in brain.
It has been demonstrated that regulation of PGRMC1 expression by progesterone, acting via the nuclear progesterone receptor, occurs only in females [30], pointing to the presence of sex-specific regulatory mechanisms which may explain the sex differences observed in the present study. Although the brain sections were washed before binding experiments were performed, it remains a possibility that the reduced [3H]RHM-1 binding observed in APP/PS1 females is due to increased levels of an endogenous sigma-2 receptor ligand, such as neurosteroids or Aβ oligomers, rather than to decreased receptor expression. As discussed below, Aβ plaque deposition was found to be higher in females than in males in the present study. However, assuming that plaque deposition is proportional to the presence of soluble oligomers, the relative Aβ sex difference does not seem large enough to account for the sex difference in the effect of genotype on [3H]RHM-1 binding.
Several studies have reported an increase in TSPO binding in AD patients compared to age-matched controls in vivo [16] and [17] and in post mortem brain tissue [18]. Co-localization of TSPO with Aβ plaques has been shown in AD mouse models [33]. The present study examined this relation in more quantitative terms and demonstrates a positive correlation between Aβ plaque load and TSPO expression in cortical and striatal brain regions. It is noteworthy that across brain regions, both Aβ plaque load and TSPO binding was higher in TG females than in males in the present study, suggesting that TG females have a more severe phenotype. Consistent with these findings, recent studies have reported that both plaque load and accumulation of soluble Aβ is higher in female than in male APP/PS1 mice [23] and [34].
In conclusion, the sex differences in sigma-2 receptor binding densities in wt and APP/PS1 animals demonstrated in the present study could be relevant to understanding the differences in susceptibility to AD between men and women and warrant further study of the role of sigma-2/PGRMC1 in neurodegenerative disease. It will be important to determine whether the reduction in sigma-2 receptor binding sites represents a consequence of the greater increase in Aβ deposition and TSPO expression seen in female TG animals as compared to males, and how this reduction might contribute to synaptotoxicity in these animals.
Acknowledgments
The authors would like to thank Dr. Marcus Raichle for help helpful suggestions and comments. KS is a recipient of postdoctoral grants from the Swedish Brain Foundation, the Swedish Society of Medicine and the Swedish Society for Medical Research. This study was also funded in part by the Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Alzheimer's Disease Research Center (RHM). The funding sources had no role in study design, in the collection, analysis, and interpretation of data, in manuscript writing, or in the decision to submit the manuscript.
Abbreviations:
- AD
Alzheimer's disease
- Aβ
amyloid-β
- ANOVA
analysis of variance
- APP
amyloid precursor protein
- PBR
peripheral benzodiazepine receptor
- PGRMC1
progesterone receptor membrane component-1
- PS1
presenilin-1
- ROI
region of interest
- TBS
Tween-20 in Tris-buffered saline
- TG
transgenic
- TSPO
translocator protein of 18 kDa
Footnotes
Conflict of interest
None declared.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2015.03.052.
References
- [1].Petrella JR, Neuroimaging and the search for a cure for Alzheimer disease, Radiology 269 (2013) 671–691. [DOI] [PubMed] [Google Scholar]
- [2].Mucke L, Selkoe DJ, Neurotoxicity of amyloid β-protein: synaptic and network dysfunction, Cold Spring Harb. Perspect. Med 2 (2012) a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L, High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation, J. Neurosci 20 (2000) 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A, Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group, Neurology 53 (1999) 1992–1997. [DOI] [PubMed] [Google Scholar]
- [5].Barron AM, Pike CJ, Sex hormones, aging, and Alzheimer's disease, Front. Biosci (Elite Ed.) 4 (2012) 976–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh M, Su C, Progesterone and neuroprotection, Horm. Behav 63 (2013) 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carroll JC, Rosario ER, Villamagna A, Pike CJ, Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer's disease mice, Endocrinology 151 (2010) 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frye CA, Walf AA, Effects of progesterone administration and APPswe+-PSEN1Deltae9 mutation for cognitive performance of mid-aged mice, Neurobiol. Learn. Mem 89 (2008) 17–26. [DOI] [PubMed] [Google Scholar]
- [9].Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB, Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels, Am. J. Physiol. Cell. Physiol 300 (2011) C328–C337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH, Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site, Nat. Commun 2 (2011) 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD, Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2, Endocrinology 150 (2009) 3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Izzo NJ, Staniszewski A, To L, Fa M, Teich AF, Saeed F, Wostein H, Walko T 3rd, Vaswani A, Wardius M, Syed Z, Ravenscroft J, Mozzoni K, Silky C, Rehak C, Yurko R, Finn P, Look G, Rishton G, Safferstein H, Miller M, Johanson C, Stopa E Windisch M, Hutter-Paier B Shamloo M Arancio O LeVine H 3rd, Catalano SM, Alzheimer's therapeutics targeting amyloid Beta 1-42 oligomers I: abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits, PLoS One 9 (2014)e111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R, Look G, Rishton G, Safferstein H, Cruchaga C, Goate A, Cahill MA, Arancio O, Mach RH, Craven R, Head E, LeVine H 3rd, Spires-Jones TL, Catalano SM, Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers II: sigma-2/PGRMC1 receptors mediate abeta 42 oligomer binding and synaptotoxicity, PLoS One 9 (2014) e111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J, Stimulation of sigma receptors with Afobazole blocks activation of microglia and reduces Toxicity caused by amyloid-β25-35, J. Pharmacol. Exp. Ther 347 (2013) 458–467. [DOI] [PubMed] [Google Scholar]
- [15].Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price L, Tang F, Markowska AL, Borchelt DR, Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to β-amyloid deposition and neurotransmitter abnormalities, Neurobiol. Dis 18 (2005) 602–617. [DOI] [PubMed] [Google Scholar]
- [16].Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB, In-vivo measurement of activated microglia in dementia, Lancet 358 (2001) 461–467. [DOI] [PubMed] [Google Scholar]
- [17].Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T, Increased binding of peripheral benzodiazepine receptor in Alzheimer's disease measured by positron emission tomography with [11C]DAA1106, Biol. Psychiatry 64 (2008) 835–841. [DOI] [PubMed] [Google Scholar]
- [18].Gulyás B, Makkai B, Kása P, Gulya K, Bakota L, Várszegi S, Beliczai Z, Andersson J, Csiba L, Thiele A, Dyrks T, Suhara T, Suzuki K, Higuchi M, Halldin C, A comparative autoradiography study in post mortem whole hemisphere human brain slices taken from Alzheimer patients and age-matched controls using two radiolabelled DAA1106 analogues with high affinity to the peripheral benzodiazepine receptor (PBR) system, Neurochem. Int 54 (2009) 28–36. [DOI] [PubMed] [Google Scholar]
- [19].Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA, Neuroinflammation and synaptic loss, Neurochem. Res 37 (2012) 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tu Z, Dence CS, Ponde DE, Jones L, Wheeler KT, Welch MJ, Mach RH, Carbon-11 labeled sigma2 receptor ligands for imaging breast cancer, Nucl. Med. Biol 32 (2005) 423–430. [DOI] [PubMed] [Google Scholar]
- [21].Perregaard J, Moltzen EK, Meier E, Sanchez C, Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omegaaminoalkyl)-1H-indoles, J. Med. Chem 38 (1995) 1998–2008. [DOI] [PubMed] [Google Scholar]
- [22].Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH, [3H]4-(Dimethylamino)-N-(4-(4-(2-methoxyphenyl) piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D3 receptors. II. Quantitative analysis of dopamine D3 and D2 receptor density ratio in the caudate-putamen, Synapse 64 (2010) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM, Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance, Neuron 64 (2009) 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RM, [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5-methylbenzamide: a novel sigma-2 receptor probe, Eur. J. Pharmacol 525 (2005) 8–17. [DOI] [PubMed] [Google Scholar]
- [25].Søby KK, Mikkelsen JD, Meier E, Thomsen C, Lu 28-179 labels a sigma(2)-site in rat and human brain, Neuropharmacology 43 (2002) 95–100. [DOI] [PubMed] [Google Scholar]
- [26].Abate C, Selivanova SV, Müller A, Krämer SD, Schibli R, Marottoli R, Perrone R, Berardi F, Niso M, Ametamey SM, Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the Positron Emission Tomography (PET) imaging of σ2 receptors, Eur. J. Med. Chem 69 (2013) 920–930. [DOI] [PubMed] [Google Scholar]
- [27].Intlekofer KA, Petersen SL, Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain, Neuroscience 172 (2011) 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Briard E, Hong J, Musachio JL, Zoghbi SS, Fujita M, Imaizumi M, Cropley V, Innis RB, Pike VW, Synthesis and evaluation of two candidate 11C-labeled radioligands for brain peripheral benzodiazepine receptors, J. Label. Compd. Radiopharm 48 (2005) S71. [Google Scholar]
- [29].Meffre D, Delespierre B, Gouezou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R, The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury, J. Neurochem 93 (2005) 1314–1326. [DOI] [PubMed] [Google Scholar]
- [30].Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW, A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors, Proc. Natl. Acad. Sci. U S A 97 (2000) 12816–12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K, Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell, Neuroscience 126 (2004) 325–334. [DOI] [PubMed] [Google Scholar]
- [32].Bali N, Morgan TE, Finch CE, Pgrmc1: new roles in the microglial mediation of progesterone-antagonism of estradiol-dependent neurite sprouting and in microglial activation, Front. Neurosci 7 (2013) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ji B, Maeda J, Sawada M, Ono M, Okauchi T, Inaji M, Zhang MR, Suzuki K, Ando K, Staufenbiel M, Trojanowski JQ, Lee VM, Higuchi M, Suhara T, Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer's and other CNS pathologies, J. Neurosci 28 (2008) 12255–12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bengtsson SK, Johansson M, Bäckströam T, Wang MJ, Chronic allopreg-nanolone treatment accelerates Alzheimer's disease development in AβPP(Swe)PSEN1(ΔE9) mice, Alzheimers Dis. 31 (2012) 71–84. [DOI] [PubMed] [Google Scholar]