Abstract
This randomized clinical trial (www.clinicaltrials.gov ID# ) examined the impact of a peer-mediated, theatre-based social skills intervention, SENSE Theatre®, on social cognition and behavior in 77 youth (ages 8-16) with high-functioning autism spectrum disorder. Analysis of Covariance models revealed that post-treatment, the experimental group (n= 44) performed significantly better than the controls (n= 33) on NEPSY theory of mind (verbal) subtest, demonstrated increased neural evidence of memory for faces, and engaged in more cooperative play and verbal interaction with novel peers. The study extends previous findings showing that SENSE Theatre® contributes to improvement in social cognition and behavior.
Keywords: autism, face, intervention, social, theory of mind
Introduction
Autism Spectrum Disorder (ASD) is an archetypal disorder of social cognition and social interaction. Children and adolescents with ASD exhibit core impairment in many areas of social competence (APA, 2013) and show limited improvement without intervention (Seltzer, Shattuck, Abbeduto, & Greenberg, 2004).
Social competence is dependent on the successful integration of cognition and behavior (Semrud-Clikeman, 2007; Kennedy & Adolphs, 2012). A recent meta-analysis (Gates, Kang, & Lerner, 2017) showed that social cognition and social behavior are distinct processes, and knowledge of how to act in a social situation does not always translate to effectively maneuvering in real-life complex social interactions. Due to this intersection, considerable research has been aimed at elucidating the social cognitive phenotype in individuals with ASD.
The ability to remember faces of conspecifics (Adolphs, 1999) is one of the early emerging social cognition skills necessary for age appropriate social competence (Gauthier & Nelson, 2001; Nelson, 2001). Face recognition, the extent to which one remembers or discriminates facial identity, has long been implicated in ASD (e.g., Boucher & Lewis, 1992; O’Hearn, Schroer, Minshew, & Luna, 2010). Behavioral and neuroimaging data indicate that many individuals with ASD exhibit difficulty in face recognition (e.g., Langdell, 1978; McPartland, Dawson, Webb, Panagiotides, & Carver, 2004) and face memory (Arkush, Smith-Collins, Fiorentini, & Skuse, 2013; Key & Corbett, 2014; Langdell, 1978). Methodical review indicates that impairment is especially notable on face recognition tasks which have a delay (Weigelt, Koldewyn, & Kanwisher, 2012). Moreover, face recognition deficits are domain-specific (manifest as primary deficit in face memory) and process-specific such that impairment is in face memory rather than face perception (Weigelt, Koldewyn, & Kanwisher, 2013). Such deficits in ASD are unlikely causal and possibly due to reduced social motivation (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012) or limited face-to-face interactions (Dawson, Webb, & McPartland, 2005). It is important to note that better face memory has been associated with fewer ASD symptoms (Arkush et al., 2013) and more reciprocal social play (Corbett, Newsom, Key, Qualls, & Edmiston, 2014).
Another social cognition skill necessary to successfully participate in social interaction is Theory of Mind (TOM) (Kita, 2014). TOM is the ability to understand another point of view and use this knowledge to predict how others may act (Baron-Cohen, 1995). In a recent longitudinal study, early differences in TOM predicted later peer relationships, with pro-social behavior as a mediator (Caputi, Lecce, Pagnin, & Banerjee, 2012). Treatments have been developed to directly train TOM skills (Fisher & Happe, 2005), such as explicitly teaching social cognitive processes (Begeer et al., 2015; de Veld et al., 2017; Hoddenbach et al., 2012). However, the results show that improvement in TOM may not coincide with demonstrable change in social communication skills (Hadwin, Baron-Cohen, Howlin, & Hill, 1997; Ozonoff & Miller, 1995). Conversely, training designed to specifically enhance social communication may not enhance TOM (Chin & Bernard-Opitz, 2000).
Since social cognition and social behavior abilities can be modified through treatment, then greater and more generalizable gains in social competence could be achieved through intervention approaches that engage both cognition and behavior. A relatively small, but growing, body of literature supports the promise of theatre approaches incorporating modeling, role-playing, and games in social skills programs for children and adolescents with ASD (e.g., Lerner, Mikami, & Levine, 2011; Webb, Miller, Pierce, Strawser, & Jones, 2004; Williams, 1989). Specifically, theatrical performance includes taking on the role of another (Hull, 1985), and understanding or predicting their cognitive processes/behavior (Noice, 1991). Practice of acting techniques has been linked to improvement in TOM skills in typical individuals (Goldstein, 2011; Goldstein & Winner, 2012) and in children with ASD (Corbett et al., 2011; Corbett, Swain, Coke, et al., 2014b; Guli, Semrud-Clikeman, Lerner, & Britton, 2013). Furthermore, acting exercises with typical peers create an opportunity for the child with ASD to observe competent models. Therefore, the training in and practice of acting techniques has the potential to target social cognition and behavior, which in turn can affect social competence in ASD (Corbett, Swain, Coke, et al., 2014b).
A recently developed intervention, called SENSE Theatre®, combines multiple theatre techniques with trained typical peers who serve as expert models and interventionists. Preliminary efficacy studies reported improvements in social cognition, behavior, and broader functioning (Corbett et al., 2011; Corbett, Qualls, Valencia, Fecteau, & Swain, 2014; Corbett, Swain, Coke, et al., 2014b). A small randomized clinical trial (RCT) of this peer-mediated, theatre-based intervention in 30 children and adolescents with ASD (Corbett et al., 2016) identified group differences between the treatment and control group on social behavior (e.g., group play with novel peers) and social cognition (e.g., immediate and delayed memory for faces, TOM). While promising, the sample size was relatively small and behavioral coding was not conducted by blind raters. In addition, that RCT did not explore potential moderators of treatment response to help identify “for whom” the treatment may be most effective.
The goal of the current study was to more rigorously examine the impact of SENSE Theatre® intervention on social cognition and behavior in a larger sample of youth with ASD. Specifically, we predicted that the experimental (EXP) group would demonstrate improvements in social cognition reflected in better TOM (Verbal and Contextual) and memory for faces following treatment. Differences between the EXP and waitlist control (WLC) Group were also predicted for social behavior: we hypothesized that the EXP group would show more cooperative play and verbal interaction during peer interaction with novel peers after treatment. No changes were expected for the WLC group. Exploratory analyses also examined potential predictors of treatment response.
Method
Participants
Initial enrollment included 102 youth (8-16 years old) with ASD in the Southeastern United States, recruited via local clinics and support organizations. Of the 87 children that met inclusion criteria, 10 were lost to follow-up prior to post-testing; however, there were no significant differences on any of the diagnostic and demographic variables (e.g., sex, p= 0.78; ADOS, p= 0.27, IQ, p= 0.92) or the pre-test variables (TOM Verbal, p= 0.57, TOM Contextual, p = 0.95), between those lost and participants who completed the study. Three cohorts (from three consecutive implementations of the study) of 29, 28, and 20 participants were allocated to groups based on simple randomization by a non-affiliated statistician. The final sample included 77 youth with high-functioning ASD, 44 in the EXP and 33 in the CON (see Figure 1 for CONSORT participant flow). Participants in the EXP group received the SENSE Theatre® Intervention during the study period; those in the WLC group were placed on a waitlist for a SENSE Theatre® Intervention (completed 6 months later). Both groups received the intervention at no cost. The study is registered with www.clinicaltrials.gov ID# .
Figure 1.
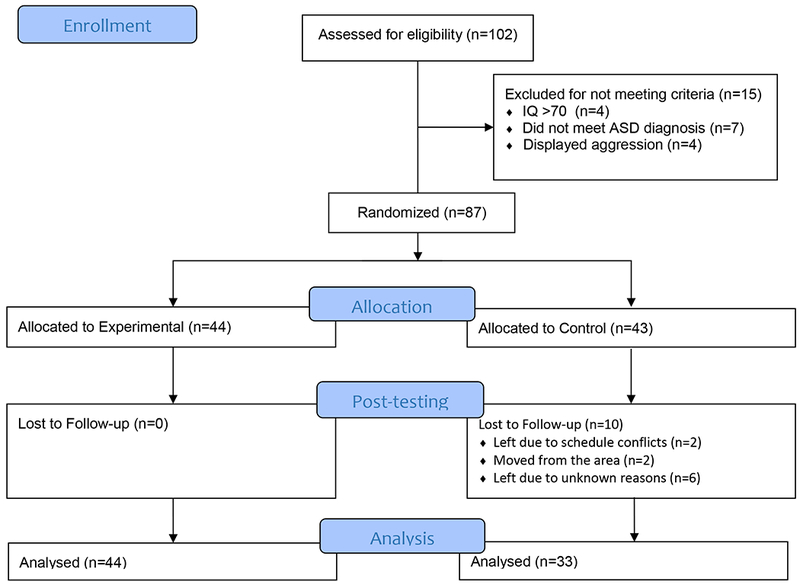
CONSORT Flow of participants.
The diagnosis of ASD was made in accordance with the Diagnostic and Statistical Manual-5 (APA, 2013) based on three criteria: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with autism expertise; (2) current clinical judgment (BAC); and (3) corroboration by the Autism Diagnostic Observation Schedule-2 (ADOS) (Lord et al., 2000), administered by research-reliable personnel. Selection criteria also required participants have an IQ ≥ 70 as measured by the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). To maintain the safety of all participants and staff, exclusionary criteria included children who displayed aggression defined as verbal or physical threats to harm other children or adults, which was based on parental report or clinical observation occurring over the last six months. Demographic information is presented in Table 1. The Vanderbilt Institutional Review Board approved the study. Informed written consent was obtained from parents or guardians and assent was obtained from child participants prior to inclusion in the study.
Table 1.
Demographic and Diagnostic Characteristics for Experimental (EXP) and Waitlist Control (WLC) Group
| Variable | EXP | WLC | df | χ2/t | p value |
|---|---|---|---|---|---|
| Demographics | |||||
| Race | |||||
| Caucasian | 36 | 29 | 1 | 0.53 | 0.47 |
| African American | 6 | 2 | 1 | 1.16 | 0.28 |
| Asian/Pacifc Islander | 2 | 2 | 1 | 0.09 | 0.77 |
| Ethnicity | |||||
| Hispanic/ Latino |
2 | 3 | 1 | 0.64 | 0.42 |
| Not Hispanic/ Latino |
42 | 30 | |||
| Medication: Psychotropic | 23 | 14 | 1 | 0.73 | 0.39 |
| Medications: Psychotropic > 1 | 11 | 6 | 1 | 0.51 | 0.48 |
| Gender F/M | 11/33 | 7/26 | 1 | 0.15 | 0.70 |
| Age | 11.12 (2.54) | 10.58 (2.32) | 1,75 | −0.96 | 0.34 |
| ADOS Algorithm | 10.57 (4.73) | 11.83 (5.43) | 1,75 | 1.00 | 0.32 |
| WASI | 104.18 (19.27) | 96.49 (17.50) | 1,75 | −1.80 | 0.07 |
| SCQ | 20.95 (6.70) | 20.69 (7.18) | 1,75 | −.162 | 0.87 |
Note: Means (Standard Deviations) by group. F/M= Female/Male, ADOS = Autism Diagnostic Observation Schedule, WASI= Wechsler Abbreviated Scale of Intelligence, SCQ= Social Communication Questionnaire.
Participants completed three research visits (see Table 2). Visit 1 included diagnostic, cognitive, and neuropsychological assessments. During visit 2, real-life social behavior was assessed using the Peer Interaction Paradigm (Corbett, Swain, Newsom, et al., 2014). The children also completed the ERP task evaluating memory for faces. The same neuropsychological, ERP, and social interaction measures were repeated post-treatment (visit 3). All assessments were conducted during the same period of time for both the EXP and the WLC groups; specifically, the fall for pre-test and the winter (after the EXP received the treatment) for post-test. Each child was tested individually.
Table 2.
Study events
| Pre-test | SENSE Theatre Intervention | Post-test | ||
|---|---|---|---|---|
| Visit 1 | Visit 2 | 10 weeks | Visit 3 | |
| Events | Diagnostic: ADOS, WASI | Role play, improvisation, play rehearsal, play performance | Neuropsychological: NEPSY, SCQ | |
| Neuropsychological: NEPSY, SCQ | ERP (face memory) | ERP (face memory) | ||
| Peer Interaction (playground) | Peer Interaction (playground) | |||
Note: ADOS = Autism Diagnostic Observation Schedule, WASI = Wechsler Abbreviated Scale of Intelligence, SCQ = Social Communication Questionnaire, ERP = Event-Related Potentials
Intervention
SENSE Theatre® incorporates theatre, established learning theory behavioral strategies, and peer-mediation. To explore and practice social interaction skills, the SENSE model uses theatre games, role-play exercises, improvisation, and character development, while putting on a play (Corbett et al., 2011; Corbett, Swain, Coke, et al., 2014b). A primary objective of social interventions is to help children learn to interact more competently with peers in natural settings (DiSalvo & Oswald, 2002); thus, the inclusion of trained peers in treatment is logical, beneficial, and economical (Lang et al., 2011; Odom & Strain, 1984).
The trained peer actors serve as expert models of reciprocal social communication, empathy, and flexible thinking and behavior. In the SENSE Theatre® program, peers are conceptualized as the primary agents of change thus serving as teachers and recipients of the reciprocal social exchange (Corbett, Qualls, et al., 2014). Selected peers demonstrated age-appropriate social communication skills and expressed an interest in working with participants with ASD. The majority of the peers came from the University School of Nashville (USN) Theatre Guild and had previous acting experience. All peers completed a training program that included a review of ASD, behavioral strategies (i.e., positive reinforcement) and introduction to the core principles of the intervention (e.g., provide social support, model warm, appropriate social interaction, advance learning (Corbett et al., 2016).
The treatment was implemented over 10, 4-hour group sessions, held at a school auditorium. A schedule of each day was provided to the participants and peers in advance and displayed on a white board in the rehearsal space. Initial sessions included theatre games, role-playing activities, and improvisation. Subsequent sessions included character development, role playing, learning songs and choreography, as well as blocking and rehearsing the play. The EXP group received the treatment during winter months on consecutive Saturdays, which culminated in two public performances of a 45-minute play with music.
Fidelity
Fidelity was measured using behaviorally-anchored rating scales (Corbett et al., 2016) by trained undergraduate and graduate students, supervised by a psychology intern or postdoctoral fellow. Delivery of trained behavioral techniques and core principles was monitored using a 5-point Likert Scale and reported as percentages. Booster sessions (involving review of behavioral and core principles) were provided if fidelity fell below 80%. Mean scores across all ratings for the beginning, middle, and end of the treatment for behavioral techniques were 91.29%, 78.75%, and 87.86% respectively. Mean scores across all ratings for the beginning, middle, and end of the treatment for application of core principles were 88.86%, 78.75%, and 89.1% respectively. Diminished values during the middle resulted from a low scoring peer who received booster training immediately after fidelity was calculated.
Dependent Measures
Social cognition.
Both neuropsychological and neurophysiological measures were used to assess social cognition. Measures of brain activity, such as event-related potentials (ERP), allowed us to examine social information processing without the requirement of overt behavioral responses, thus reducing participants’ cognitive load and motivation-related confounds (e.g., differences in the cooperative engagement with the task).
Theory of Mind (TOM).
The TOM subtests from NEPSY (Korkman, Kirk, & Kemp, 2007) were administered to assess changes in social perception. The TOM task presents a variety of perspective-taking tasks that result in a total score used for clinical purpose, consisting of separate verbal and contextual subtests. TOM internal consistency ranges from .84-.86 and test-retest reliability ranges from .76- 87. The verbal portion (TOM-V) requires the child to demonstrate the ability to understand that others have their own thoughts, ideas, and feelings. It includes 15 items that assess 1st and 2nd order false belief, recognizing mental states, imitation, and understanding figurative language. The contextual portion (TOM-C) assesses the ability to relate emotion to social context. It requires the child to identify a picture that most represents the feelings of a character depicted in 6 different scenarios. Previous research has shown that these portions measure separate constructs and therefore are evaluated separately (Corbett et al., 2016). The NEPSY-II has been evaluated for test-criterion validity with children with ASD; as expected, this group demonstrated deficits on the TOM subtest compared to the normative sample, indicating the TOM subtest’s discernment to clinical differences (Korkman, Kirk, & Kemp, 2007). Other work has confirmed these findings (e.g., Loukusa, Makinen, Kuusikko-Gauffin, Ebeling, & Moilanen, 2014; Miranda, Berenguer, Rosello, Baixauli, & Colomer, 2017). The NEPSY –II TOM subtest has also been utilized as an outcome measure for intervention research in youth with ASD (e.g. Corbett et al., 2016; Rice, Wall, Fogel, & Shic, 2015) and shows sensitivity to changes in TOM abilities concurrent with other social cognition skills.
Incidental face memory (Key & Corbett, 2014).
Persons with ASD may engage face-specific perceptual mechanisms, but are less successful than typical peers in face recognition (see Weigelt et al., 2012 for review). Repeated presentations of identical images in a stream of continuously varying stimuli result in increased familiarity (Jessen et al., 2002), which is a basic form of memory (Yonelinas, 2002) and can be reflected in neural responses. Recently, we developed the incidental face memory ERP paradigm and demonstrated that the ability to spontaneously detect repetition of a socially salient stimulus (face) among unfamiliar images during passive viewing is associated with more adaptive social functioning (Key & Corbett, 2014). Briefly, participants were presented with color photographs of 51 unfamiliar young adult faces (Radboud Faces Database; Langner et al., 2010) and 51 unfamiliar houses displayed on a computer monitor. One image in each category was randomly selected and repeated 50 times throughout the experiment, yielding a unique set of repeated stimuli for each participant. The remaining photographs were presented once. All stimuli were presented by E-prime (v.2.0, PST, Inc., Pittsburgh, PA) in random order for 1500 ms with a varied inter-stimulus interval of 1300-1600 ms. Participants were not instructed to memorize the images or to detect repetitions. To encourage looking at the stimuli, participants were asked to press a response button when they saw the yellow smiley face (10 attention probe trials presented randomly throughout the test session). The entire task included 210 trials and lasted approximately 12 minutes. From the viewing distance of 90 cm, the stimuli subtended visual angles of 19° (h) x 16°(w) (9.21° for the attention probe).
EEG was acquired using a 128-channel Geodesic Sensor Net (EGI, Inc., Eugene, OR) with a vertex reference. Data were sampled at 250Hz with the filters set to .1-100 Hz. Electrode impedances were kept at or below 40 kOhm. Data were re-referenced offline to an average reference (Picton et al., 2000). A researcher was present in the room to monitor participants’ behavior. If participants became restless, stimulus presentation was suspended until the participant was ready to continue with the task. For the test of treatment efficacy, the ERP data were quantified as the mean amplitude difference score at the parietal electrodes between 300-500 ms contrasting repeated and single stimulus conditions.
ERP variable derivation.
Collected EEGs were filtered using a 30Hz low-pass filter and segmented on stimulus onset to include a 100-ms prestimulus baseline and a 900 ms post-stimulus interval. All trials contaminated by ocular and movement artifacts were excluded from further analysis using an automated screening algorithm in NetStation followed by a manual review. Data for electrodes with poor signal quality within a trial were reconstructed using spherical spline interpolation procedures. If more than 20% of the electrodes within a trial were deemed bad, the entire trial was discarded. The mean retention rates per condition were comparable across groups and test sessions (EXP: T1= 20.22, SD= 6.20; T2= 21.26, SD= 7.21; WLC: T1= 20.71, SD= 7.19; T2=19.09, SD= 7.04; all p-values >.05), exceeded the minimum number of trials considered acceptable in prior studies of memory (e.g., Curran & Cleary, 2003), and were comparable to those reported using this measure (Key & Corbett, 2014; Key & Dykens, 2016, 2017).
Artifact-free individual ERPs were averaged, re-referenced to an average reference, and baseline-corrected. The analyses focused on the mean amplitude differences between repeated faces and faces seen once at the parietal electrode cluster within 300-500ms after stimulus onset. The specific scalp locations and time interval indexed incidental memory for faces and were selected a priori based on results in previous studies using this paradigm (Corbett et al., 2016; Key & Corbett, 2014; Key & Dykens, 2016).
Social behavior.
The Peer Interaction Paradigm (PIP) is an established paradigm consisting of a 20-minute semi-structured interaction, in which the participant with ASD engages in play with two trained sex-and age-matched confederates. The 20-minute interaction is divided into times of solicited and free play (Corbett, Swain, Newsom, et al., 2014). Confederates provide behavioral structure to the play by allowing key interactive sequences (i.e., cooperative play) to occur in an otherwise natural interaction and setting (Corbett, Swain, Newsom, et al., 2014). Confederates are typical children who demonstrate interest in working in research, have age-appropriate social communication skills, and do not know the participant (i.e., not involved in SENSE Theatre intervention). Interactions were video recorded using four professional 70 Sony PTZ remotely operated cameras housed in glass cases and affixed to the four corners of the external fence of the 130 ft. x 120 ft. playground. Audio communication was obtained by Sennheiser body pack and Audio-Technica transmitters and receivers, which functioned as battery-operated microphones. The Observer XT was used for the collection, analysis, and presentation of observational data (Noldus, 2008). Continuous timed-event coding of two primary duration behaviors (Cooperative Play and Verbal Interaction) was conducted from synced video recordings. Cooperative play was defined as the percentage of time the participant with ASD was engaged in a reciprocal activity for enjoyment that both involved and relied on the participation of two or more children (e.g., throwing a ball back-and-forth). Verbal interaction was defined as an engagement between two or more children, including the participant with ASD, that begins with a verbal overture and continues in a reciprocal sequence of to-and-fro communication. The PIP has been used in previous interventions demonstrating good reliability (e.g., k = .85) and robust treatment effects in social communication and play behaviors in children with ASD (Corbett et al., 2016).
Interrater reliability was examined for a random sample of all coded videos. The primary coders consisted of three research assistants and three students. Random participants from the EXP and WLC group were selected, comprised of an equal distribution of pre-test and post-test sessions. There were 154 coded sessions, 18 of which were randomly selected for reliability. No specific subject characteristics were identified prior to random selection of reliability. The Noldus program (Noldus, 2008) has a reliability statistical program embedded in the software that calculates Cohen’s kappa. Reliability was k= .82 and k= .88 for Cooperative Play and Verbal Interaction, respectively, which is comparable to previous studies using the PIP (Corbett, Swain, Newsom, et al., 2014).
Statistical Analysis
Independent sample t-tests were conducted to examine baseline group differences in the demographic and diagnostic variables (see Table 1) and in all pre-test dependent variables. To examine between-group treatment effects, a series of linear mixed Analysis of Covariance (ANCOVA) models were used to test the post-intervention between-group differences on each social cognition and behavior dependent variable (e.g., TOM, Cooperative/Uncooperative Play and Verbal Interaction). The post-test (after intervention) score for each dependent variable served as the outcome variable, group (EXP/WLC) as the main independent variable while controlling for baseline (pre-test) score and Cohort (i.e., 1, 2, 3), which served as covariates. To examine the extent to which diagnostic (e.g., symptom severity, IQ) and demographic (age,sex) variables predicted treatment response, a series of moderator analyses were performed. Statistical analyses were performed using SPSS 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Results
Preliminary
The two groups did not differ on age, sex, IQ, or symptom profile (p > 0.05; see Table 1). Participants also did not differ on the primary dependent variables at baseline (Pre-test, p> 0.05; see Table 3 first column).
Table 3.
Performance on measures of social cognition and behavior at pretest and posttest for the Experimental (EXP) and Waitlist Control (WLC) Groups
| Pre-test | Post-test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | EXP | WLC | df | F | p | d | EXP | WLC | df | F | p | d |
| Social Cognition | ||||||||||||
| TOM-Verbal | 15.64 (4.87) | 14.06 (5.54) | 1,75 | 1.75 | 0.19 | .31 | 17.65 (4.59) | 15.50 (4.95) | 1,72 | 4.06 | 0.04 | 0.45 |
| TOM-Contextual | 4.84 (1.13) | 4.5 (1.5) | 1,73 | 1.23 | 0.27 | .26 | 4.98 (0.89) | 4.59 (1.19) | 1,72 | 3.03 | 0.09 | 0.38 |
| Social Behavior | ||||||||||||
| Cooperative Play-Solicited | 40.30 (27.86) | 34.38 (34.11) | 1,74 | 0.69 | 0.41 | 0.19 | 52.81 (30.23) | 34.83 (32.14) | 1,73 | 5.48 | 0.02 | 0.58 |
| Cooperative Play-Unsolicited | 7.51 (21.62) | 0.95 (3.22) | 1,74 | 02.98 | 0.09 | 0.4 | 14.39 (25.01) | 4.62 (11.80) | 1,73 | 2.49 | 0.12 | 0.48 |
| Verbal Interaction | 58.19 (38.76) | 56.10 (40.65) | 1,74 | .05 | 0.82 | 0.05 | 65.40 (33.46) | 48.78 (37.00) | 1,73 | 4.51 | 0.04 | 0.47 |
Note: TOM – Theory of Mind.
Pre-test data were analyzed using independent sample t-tests. Post-test data were analyzed controlling for baseline (pre-test). Data were missing for TOM-V, Cooperative Play and Verbal Interaction resulting in approximately 4% loss. TOM-C had 5% missing data.
Primary
Social cognition.
In regards to social cognition, significant differences were observed between the EXP and the WLC group at post-treatment (Table 3 Post-test). Specifically, the EXP group showed better performance on TOM-V, F(1,72)= 4.30, p= 0.04, d= 0.62 (Table 3). TOM-C differences did not reach statistical significance, F(1,71)= 2.97, p= 0.09, d= 0.38.
Significant group differences were also present in the ERP markers of incidental face memory between the EXP and the WLC groups at post-treatment, F(1,76)= 6.321, p= 0.01. Follow-up analyses using paired-sample t-tests indicated that neither the EXP nor the WLC group differentiated between the repeated faces and those seen once at pre-test, t(43)=−.424, p= 0.67 and t(32)= 1.41, p= 0.17, respectively. However, following treatment, the EXP group demonstrated significantly increased ERP amplitudes for the repeated faces compared to faces seen once, t(42)= −2.82, p= 0.01, while such response was not detected in the WLC group, t(32)= 0.65, p= 0.52 (see Figure 2).
Figure 2.
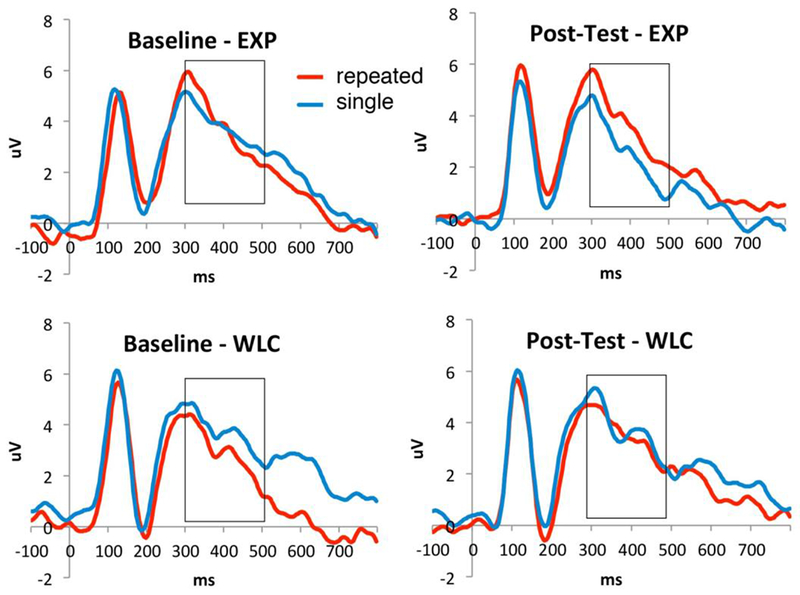
Incidental memory for single and repeated faces at baseline and post-test.
Social behavior.
In the domain of social behavior, at post-test while controlling for pre-test performance, the EXP group engaged in more cooperative play during solicited play, F(1,73)= 5.48, p= 0.02, d= 0.58 (Table 3). However, there was not sufficient evidence for an effect of the treatment on unsolicited play, F (1,73)= 2.49, p= 0.12 d= 0.48. Additionally, Verbal Interactions during solicited play increased by 12.39% in the EXP group, whereas the WLC showed a 13.05% decrease compared to baseline levels, F(1,73)= 4.51, p= 0.04, d= 0.47.
Exploratory Analysis to Identify Potential Predictors of Treatment Response
We examined age, autism severity, IQ, and pre-test dependent measures scores as potential predictors of response to treatment using a moderator analysis. None of the statistical interactions of the putative moderators with treatment group membership predicting the post-test dependent variables were significant.
Discussion
The primary aim of the current study was to examine the impact of SENSE Theatre® on social cognition and behavior in a relatively large sample of youth with ASD. We extended previous findings demonstrating that the theatre-based intervention contributes to clinically-meaningful improvement in both cognitive and behavioral aspects of social competence in youth with ASD (Corbett et al., 2011; Corbett et al., 2016; Corbett, Swain, Coke, et al., 2014a; Schaller & Rauh, 2017).
Regarding social cognition, there were strong treatment effects such that the EXP group showed better performance on verbal TOM skills. Youth that participated in the treatment were better able to verbalize and attribute mental states and behaviors to others. It is plausible that engaging in theatre promotes the ability to understand what others are thinking and to use this knowledge to predict how others may act (Noice, 1991). These results are consistent with previous research examining the effect of acting and theatre technique on TOM skills in typically developing children (Goldstein, 2011; Goldstein & Winner, 2012) as well as in children and adolescents with ASD (Corbett et al., 2011; Corbett, Swain, Coke, et al., 2014b; Guli et al., 2013). Moreover, some TOM treatments that incorporate various aspects of theatre, such as role-playing, (e.g., Begeer et al., 2015; Hoddenbach et al., 2012) report significant gains in TOM skills, providing additional support for the inclusion of theatre-based activities in interventions for children with ASD.
It was somewhat surprising that the TOM contextual subtest did not reach statistical significance as this task addresses more implicit and affective aspects of perspective taking often shown to be impaired in ASD (Schaller & Rauh, 2017; Schuwerk, Vuori, & Sodian, 2015). The limited number of only 6 items may have resulted in relatively lower reliability, leading to lower statistical power to carefully sample the construct. Future studies that include additional items, as well as measure other important aspects of TOM (such as false beliefs or imaginative play) are needed to uncover the TOM skills that theatre intervention may enhance.
Another fundamental aspect of social cognition, face memory, is often impaired in children with ASD based on behavioral and neuroimaging studies. Children with ASD also exhibit difficulty in face recognition especially when involving a delay (Arkush et al., 2013; Key & Corbett, 2014; Langdell, 1978). Neural measures of incidental face memory in the current study demonstrated treatment-related improvements as shown in previous studies (Corbett et al., 2016). Although there were no between-group differences at baseline, at post-test ERPs of children who participated in SENSE Theatre® demonstrated the expected increase in amplitudes for the repeated faces compared to the faces that were seen only once. The comparable findings at baseline across the groups support the notion that children with ASD have quanititative deficits in face recognition, especially on tasks that include even a brief delay (Weigelt et al., 2012). The results also emphasize that they are domain-specific, evidenced by the observed differences between faces vs. houses (Weigelt et al., 2013). Despite the often observed deficit in memory for faces, the results highlight that it is amenable to treatment and lend support for the idea that theatre engagement with peers has the potential to increase salience of social information, such that changes are observable on objective neuropsychological and neurophysiological measures (Corbett et al., 2016).
In addition to changes in social cognition, significant treatment effects were present for social behavior. Changes were apparent in the participants’ motivation to engage with other peers during a proxy of an everyday social encounter (Corbett, Swain, Newsom, et al., 2014). Specifically, youth with ASD in the EXP group showed more cooperative play during solicited but not unsolicited play. This finding highlights the importance of peer solicitation to facilitate social engagement, which has been shown in previous research (Corbett, Swain, Newsom, et al., 2014). Thus, while there is more reciprocal social interaction with novel peers following treatment, the peer continues to play a pivotal role in that process. Similarly, it was shown that participants in the EXP group engaged in more verbal interactions when solicited by peers following treatment. The data from the WLC group demonstrated that the mere re-exposure to the playground setting did not result in a maintenance or increase in verbal communication with peers. Based on the moderate effect sizes, these behavioral changes are likely to be clinically meaningful even though a standard is not yet established in the field. Of note, the effect sizes for all behavioral measures reported in this paper meet or exceed effect sizes reported in similar intervention studies, such as Dolan et al. (2016).
The use of theatre is only recently emerging as a promising form of intervention for individuals with ASD (Lerner et al., 2011; Webb et al., 2004; Williams, 1989) which include studies showing observable changes on objective neuropsychological and neurophysiological measures (Corbett et al., 2016). The inclusion of acting techniques and reciprocal role play provides a natural foundation in which to build social skills in a less structured therapeutic setting. Among the unique features of the SENSE Theatre® program compared to other theatre approaches (Lerner et al., 2011; Webb et al., 2004; Williams, 1989) is peer-mediation - the inclusion of expert social models in the form of trained peer actors who are able to provide clear exemplars of social engagement, communication and emotional expression. Another unique aspect of the intervention is that it culminates in a theatrical performance in which the participants are able to demonstrate their acquired skills in a public forum. The inclusion of this vital component seems to serve as a powerful reinforcing agent by rewarding not only the completion of the goal (play) but the generalized reinforcement from the audience increases the likelihood that the individual will engage in such social interaction with other peers in the future.
Strengths and Limitations
Strengths of the RCT include a relatively large cohort of carefully characterized children and adolescents with ASD and the use of randomization and a multi-method approach demonstrating changes in social information processing at the behavioral and neural levels. Nevertheless, limitations exist, such as the inclusion of only high-functioning children with ASD with variability in cognitive functioning. Additionally, the sample was 84% Caucasian (see Table 1); future research with more diverse and heterogenous samples will increase applicability. Another limitation is the lack of follow-up data to examine long-term maintenance of reported social cognitive and behavioral effects. For the current study, participants did not complete follow-up testing on the neuropsychological or physiolological data. Subsequent RCTs are underway which include this important outcome data to determine the maintenance or loss of treatment effects. Additionally, the second aim of the study was to identify potential demographic/diagnostic variables predicting treatment response; however, none of the selected variables moderated response. Thus, the question “for whom” SENSE Theatre® is most beneficial remains an open one, warranting future exploration. Finally, the statistical approach was used to be consistent with previous studies (Corbett et al., 2016) ; however, future studies comparing across different treatments and more robust statistical designs may be needed.
Future Directions
The structure of social cognition may be distinguished between the ability to use a skill and the propensity to use the skill in everyday life (Happe, Cook, & Bird, 2017). While the current study measured the extent to which participants socially interacted with peers during play, an interesting next step might be to evaluate the content of the interaction and whether youth with ASD show measurable changes in their ability to take on the perspective of others in more natural contexts. Moreover, the use of a comprehensive battery of TOM tasks and more ecologically valid measures that consider the complexity of social cognition (Schaller & Rauh, 2017), are warranted.
The intervention closely resembles the experience of acting in theatre, which includes elements of role play, observing behavior in others, and social interaction. As such, it remains unclear what specific aspects of theatre contribute to the observed changes in social cognition and behavior. Future studies aimed at disentangling these core components may be meaningful in identifying the active and necessary ingredients for improvement in social competence in youth with ASD.
In summary, the results demonstrate that theatre-based treatments are emerging as a clinically meaningful intervention for high-functioning children and youth with ASD. While speculative, the findings suggest at least two aspects inherent in the SENSE Theatre® intervention may be driving the findings; namely, increase in social salience due to positive, reciprocal engagement with peers, and improved TOM as a result of enhanced perspective taking using theatre techniques.
Acknowledgements
We are grateful to the University School of Nashville for their support and community partnership. We are indebted to the dedicated, supportive and creative peers that are vital to the SENSE program. Finally, we appreciate the commitment of the children and families that participate in our intervention research, some of whom travel great distances to participate.
Funding: This work was supported, in part, by NIMH R34 MH097793 (Corbett), NICHD Grant U54HD083211 to Vanderbilt Kennedy Center, a VKC Hobbs Discovery Award (Corbett & Key), and donations to SENSE Theatre® to assist some families with travel costs.
Footnotes
Conflict of Interest: Blythe Corbett is the founder of SENSE Theatre® but derives no financial compensation from the nonprofit 501(c)(3) entity.
Dedication: This paper is dedicated to our long-standing theatre director and friend, Catherine Coke. Her dedication to theatre as a vital means of communication, social engagement, and support has been a cornerstone of SENSE Theatre®
References
- Adolphs R (1999). Social cognition and the human brain. Trends in Cognitive Neuroscience, 3, 469–479. [DOI] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-5). Washinton, D.C: American Psychiatric Association. [Google Scholar]
- Arkush L, Smith-Collins AP, Fiorentini C, & Skuse DH (2013). Recognition of Face and Non-Face Stimuli in Autistic Spectrum Disorder. Autism Res. 10.1002/aur.1318 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S (1995). Minblindness: an essay on autism and theory of mind. Cambridge, MA: MIT Press. [Google Scholar]
- Begeer S, Howlin P, Hoddenbach E, Clauser C, Lindauer R, Clifford P, … Koot HM (2015). Effects and Moderators of a Short Theory of Mind Intervention for Children with Autism Spectrum Disorder: A Randomized Controlled Trial. Autism Res, 8(6), 738–748. 10.1002/aur.1489 [DOI] [PubMed] [Google Scholar]
- Boucher J, & Lewis V (1992). Unfamiliar face recognition in relatively able autistic children. J Child Psychol Psychiatry, 33(5), 843–859. [DOI] [PubMed] [Google Scholar]
- Caputi M, Lecce S, Pagnin A, & Banerjee R (2012). Longitudinal effects of theory of mind on later peer relations: the role of prosocial behavior. Dev Psychol, 48(1), 257–270. 10.1037/a0025402 [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, & Schultz RT (2012). The social motivation theory of autism. Trends Cogn Sci, 16(4), 231–239 10.1016/j.tics.2012.02.007S1364-6613(12)00052-6[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin HY, & Bernard-Opitz V (2000). Teaching conversational skills to children with autism: effect on the development of a theory of mind. J Autism Dev Disord, 30(6), 569–583. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Gunther JR, Comins D, Price J, Ryan N, Simon D, … Rios T (2011). Brief report: theatre as therapy for children with autism spectrum disorder. J Autism Dev Disord, 41(4), 505–511. 10.1007/s10803-010-1064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Key AP, Qualls L, Fecteau S, Newsom C, Coke C, & Yoder P (2016). Improvement in Social Competence Using a Randomized Trial of a Theatre Intervention for Children with Autism Spectrum Disorder. J Autism Dev Disord, 46(2), 658–672. 10.1007/s10803-015-2600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Newsom C, Key AP, Qualls LR, & Edmiston EK (2014). Examining the relationship between face processing and social interaction behavior in children with and without autism spectrum disorder. Journal of Neurodevelopmental Disorders, 6 doi:Artn 35 Doi 10.1186/1866-1955-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Qualls LR, Valencia B, Fecteau SM, & Swain DM (2014). Peer-mediated theatrical engagement for improving reciprocal social interaction in autism spectrum disorder. Front Pediatr, 2, 110 10.3389/fped.2014.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Coke C, Simon D, Newsom C, Houchins-Juarez N, … Song Y. (2014a). Improvement in social deficits in autism spectrum disorders using a theatre-based, peer-mediated intervention. Autism Res, 7(1), 4–16. 10.1002/aur.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Coke C, Simon D, Newsom C, Houchins-Juarez N, … Song Y. (2014b). Improvement in Social Deficits in Autism Spectrum Disorders Using a Theatre-Based, Peer-Mediated Intervention. Autism Res, 7, 4–16. 10.1002/aur.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Newsom C, Wang L, Song Y, & Edgerton D (2014). Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. J Child Psychol Psychiatry, 55(8), 924–934. 10.1111/jcpp.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, & Cleary AM (2003). Using ERPs to dissociate recollection from familiarity in picture recognition. Brain Research. Cognitive brain research, 15(2), 191–205. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol, 27(3), 403–424. 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- de Veld DMJ, Howlin P, Hoddenbach E, Mulder F, Wolf I, Koot HM, … Begeer S (2017). Moderating Effects of Parental Characteristics on the Effectiveness of a Theory of Mind Training for Children with Autism: A Randomized Controlled Trial. J Autism Dev Disord, 47(7), 1987–1997. 10.1007/s10803-017-3117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSalvo C, & Oswald D (2002). Peer-mediated interventions to increase the social interaction of children with autism: Consideration of peer expectancies. Focus on Autism & Other Developmental Disabilities, 17(4), 198–207. [Google Scholar]
- Fisher N, & Happe F (2005). A training study of theory of mind and executive function in children with autistic spectrum disorders. J Autism Dev Disord, 35(6), 757–771. 10.1007/s10803-005-0022-9 [DOI] [PubMed] [Google Scholar]
- Gates JA, Kang E, & Lerner MD (2017). Efficacy of group social skills interventions for youth with autism spectrum disorder: A systematic review and meta-analysis. Clin Psychol Rev, 52, 164–181. 10.1016/j.cpr.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, & Nelson CA (2001). The development of face expertise. Curr Opin Neurobiol, 11(2), 219–224. [DOI] [PubMed] [Google Scholar]
- Goldstein TR (2011). Correlations among social-cognitive skills in adolescents involved in acting or arts classes. Mind, Brain & Education, 5, 97–103. [Google Scholar]
- Goldstein TR, & Winner E (2012). Enhancing Empathy and Theory of Mind. Journal of Cognition and Development, 13(1), 19–37. doi:Doi 10.1080/15248372.2011.573514 [DOI] [Google Scholar]
- Guli LA, Semrud-Clikeman M, Lerner MD, & Britton N (2013). Social Competence Intervention Program (SCIP): A pilot study of a creative drama program for youth with social difficulties. The Arts in Psychotherapy, 40(1), 47–44. [Google Scholar]
- Hadwin J, Baron-Cohen S, Howlin P, & Hill K (1997). Does teaching theory of mind have an effect on the ability to develop conversation in children with autism? J Autism Dev Disord, 27(5), 519–537. [DOI] [PubMed] [Google Scholar]
- Happe F, Cook JL, & Bird G (2017). The Structure of Social Cognition: In(ter)dependence of Sociocognitive Processes. Annu Rev Psychol, 68, 243–267. 10.1146/annurev-psych-010416-044046 [DOI] [PubMed] [Google Scholar]
- Hoddenbach E, Koot HM, Clifford P, Gevers C, Clauser C, Boer F, & Begeer S (2012). Individual differences in the efficacy of a short theory of mind intervention for children with autism spectrum disorder: a randomized controlled trial. Trials, 13. doi:Artn 20610.1186/1745-6215-13-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LS (1985). Strasberg’s method: As taught by Lorrie Hull. Woodbridge, CT: Ox Bow Publishing, Inc. [Google Scholar]
- Jessen F, Manka C, Scheef L, Granath DO, Schild HH, & Heun R (2002). Novelty detection and repetition suppression in a passive picture viewing task: a possible approach for the evaluation of neuropsychiatric disorders. Hum Brain Mapp, 17(4), 230–236. 10.1002/hbm.10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, & Adolphs R (2012). The social brain in psychiatric and neurological disorders. Trends Cogn Sci, 16(11), 559–572. 10.1016/j.tics.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Corbett BA (2014). ERP responses to face repetition during passive viewing: a nonverbal measure of social motivation in children with autism and typical development. Dev Neuropsychol, 39(6), 474–495. 10.1080/87565641.2014.940620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Dykens EM (2016). Face repetition detection and social interest: An ERP study in adults with and without Williams syndrome. Soc Neurosci, 11(6), 652–664. 10.1080/17470919.2015.1130743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Dykens EM (2017). Incidental memory for faces in children with different genetic subtypes of Prader-Willi syndrome. Soc Cogn Affect Neurosci. 10.1093/scan/nsx013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Y (2014). Face recognition in children with autism spectrum disorders. International Journal of Psychophysiology, 94(2), 141–142. 10.1016/j.ijpsycho.2014.08.649 [DOI] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (2007). NEPSY 2nd Edition. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Lang R, Kuriakose S, Lyons G, Mulloy A, Boutot A, Britt C, … Lancioni G (2011). Use of school recess time in the education and treatment of children with autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders, 5(4), 1296–1305. doi:DOI 10.1016/j.rasd.2011.02.012 [DOI] [Google Scholar]
- Langdell T (1978). Recognition of faces: an approach to the study of autism. J Child Psychol Psychiatry, 19(3), 255–268. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus D, Hawk S, & van Knippenberg A (2010). Presentation and validation of the Radboud Faces Database. Cognition and Emotion, 24(8), 1377–1388. [Google Scholar]
- Lerner MD, Mikami AY, & Levine K (2011). Socio-dramatic affective-relational intervention for adolescents with asperger syndrome & high functioning autism: pilot study. Autism, 15(1), 21–42. doi:1362361309353613 [pii] 10.1177/1362361309353613 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, … Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord, 30(3), 205–223. [PubMed] [Google Scholar]
- Loukusa S, Makinen L, Kuusikko-Gauffin S, Ebeling H, & Moilanen I (2014). Theory of mind and emotion recognition skills in children with specific language impairment, autism spectrum disorder and typical development: group differences and connection to knowledge of grammatical morphology, word-finding abilities and verbal working memory. Int J Lang Commun Disord, 49(4), 498–507. 10.1111/1460-6984.12091 [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, & Carver LJ (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry, 45(7), 1235–1245. 10.1111/j.1469-7610.2004.00318.xJCPP318[pii] [DOI] [PubMed] [Google Scholar]
- Miranda A, Berenguer C, Rosello B, Baixauli I, & Colomer C (2017). Social Cognition in Children with High-Functioning Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. Associations with Executive Functions. Front Psychol, 8, 1035 10.3389/fpsyg.2017.01035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA (2001). The development and neural bases of face recognition. Infant Child Development, 10(1–2), 3–18. [Google Scholar]
- Noice H (1991). The Role of Explanations and Plan Recognition in the Learning of Theatrical Scripts. Cognitive Science, 15(3), 425–460. doi:DOI 10.1207/s15516709cog1503_4 [DOI] [Google Scholar]
- Noldus. (2008). The Observer XT (Vol. 10.5). Wageningen, The Netherlands: Noldus Information Technology. [Google Scholar]
- O’Hearn K, Schroer E, Minshew N, & Luna B (2010). Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia, 48(13), 3955–3960. 10.1016/j.neuropsychologia.2010.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom SL, & Strain PS (1984). Peer-mediated approaches to promoting children’s social interaction: a review. Am J Orthopsychiatry, 54(4), 544–557. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, & Miller JN (1995). Teaching theory of mind: a new approach to social skills training for individuals with autism. J Autism Dev Disord, 25(4), 415–433. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, … Taylor MJ (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(2), 127–152. [PubMed] [Google Scholar]
- Rice LM, Wall CA, Fogel A, & Shic F (2015). Computer-Assisted Face Processing Instruction Improves Emotion Recognition, Mentalizing, and Social Skills in Students with ASD. J Autism Dev Disord, 45(7), 2176–2186. 10.1007/s10803-015-2380-2 [DOI] [PubMed] [Google Scholar]
- Schaller UM, & Rauh R (2017). What Difference Does It Make? Implicit, Explicit and Complex Social Cognition in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 47(4), 961–979. 10.1007/s10803-016-3008-x [DOI] [PubMed] [Google Scholar]
- Schuwerk T, Vuori M, & Sodian B (2015). Implicit and explicit Theory of Mind reasoning in autism spectrum disorders: The impact of experience. Autism, 19(4), 459–468. 10.1177/1362361314526004 [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, & Greenberg JS (2004). Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev, 10(4), 234–247. 10.1002/mrdd.20038 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M (2007). Social competence in children. New York, NY: Springer. [Google Scholar]
- Webb BJ, Miller SP, Pierce TB, Strawser S, & Jones PS (2004). Effects of social skill instruction for high-functioning adolescents iwth Autism Spectrum Disorders. Focus on Autism and Other Developmental Disabilties, 19(1), 53–62. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weigelt S, Koldewyn K, & Kanwisher N (2012). Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev, 36(3), 1060–1084. 10.1016/j.neubiorev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, & Kanwisher N (2013). Face recognition deficits in autism spectrum disorders are both domain specific and process specific. PLoS One, 8(9), e74541 10.1371/journal.pone.0074541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TI (1989). A social skills group for autistic children. J Autism Dev Disord, 19(1), 143–155. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517. doi:DOI 10.1006/jmla.2002.2864 [DOI] [Google Scholar]


