Abstract
Purpose:
Despite tumor resection being the frontline clinical care for glioblastoma (GBM) patients, nearly all preclinical immune therapy models intends to treat established GBM tumor. Characterizing cytoreductive surgery-induced immune-response combined with the administration of immune cytokines has the potential of offering a new treatment paradigm of immune therapy for GBMs.
Experimental design:
We developed syngeneic orthotopic mouse GBM models of tumor-resection and characterized the immune response of intact and resected tumors. We also created a highly secretable variant of immune cytokine IFNβ to enhance the its release from engineered mouse mesenchymal stem cells (MSC-IFNβ) and assessed whether surgical resection of intracranial GBM tumor significantly enhance the anti-tumor efficacy of targeted on-site delivery of encapsulated-MSC-IFNβ.
Results:
We show that tumor debulking results in substantial reduction of myeloid-derived suppressor cells (MDSCs) and simultaneous recruitment of CD4/CD8 T cells. This immune response significantly enhanced the anti-tumor efficacy of locally delivered encapsulated-MSC-IFNβ. IFNβ enhanced selective post-surgical infiltration of CD8 T cells and directly induced cell-cycle arrest in tumor cells resulting in increased survival of mice. Utilizing encapsulated-MSC-IFNβ in resected orthotopic tumor xenografts of patient-derived GBM, we further show that IFNβ induces cell-cycle arrest followed by apoptosis resulting in increased survival in immune-compromised mice despite their absence of an intact immune system.
Conclusions:
This study demonstrates the importance of syngeneic tumor resection models in developing cancer immunotherapies and emphasizes the translational potential of local delivery of immune-therapeutic agents in treating cancer.
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults and is associated with a very poor prognosis (1). Current treatment for GBM consists of maximal surgical tumor resection followed by radiation and chemotherapy (2). Despite the current advances in therapeutic interventions, GBM almost always recurs and the associated patient mortality is nearly 100%. Given the overall survival benefit observed with immunotherapies in melanoma and prostate cancer patients (3–5), there is an earnest need for evaluating immunotherapies for GBM.
Recent advances in cancer immunology has led to an increased understanding of the concept of cancer immune surveillance and immunoediting: newly emerging tumor cells can potentially be recognized and eliminated by the immune system, but tumors escape eradication via a process of immunoediting, thereby tilting the immune balance towards the development of an immunosuppressive tumor microenvironment (6). Immune checkpoint blockade with monoclonal antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) or PD-1 ligand (PD-L1) is a promising strategy to overcome immunosuppressive tumor microenvironments and has recently shown favorable results in the clinical therapy of multiple cancer types (7). However despite the importance of tumor resection as a frontline treatment for the most of solid tumors, surprisingly, the specific influence of tumor resection in tumor microenvironment and over immunomodulatory therapy has been poorly explored. Immune response to the damage of healthy, non-tumorous tissue was studied and is known to initiate activation of the innate immune response followed by vigorous infiltration of T cells into the lesion resulting in anti-inflammatory response (8). We hypothesized that a front line treatment of maximal surgical tumor resection would invoke an acute immune reaction, possibly enough to break the immune tolerance within the tumor microenvironment and that administration of immunomodulatory agents post-tumor resection would have superior therapeutic efficacy in treating GBM. In this study, we therefore developed syngeneic mouse tumor models of GBM resection and characterized the immune response of intact and resected tumors. Our results indicate that the resection-induced immune reaction can be further modulated towards a tumor-specific immune response via local delivery of IFNβ, leading to significantly increased survival of mice. Modulating the non-specific immune reaction post-tumor debulking towards a tumor-specific immune response might be an ideal immunotherapy strategy in GBM treatment.
IFNβ belongs to type I interferons that bind to the interferon-α/β cell surface receptor complex (IFNAR) (9) and induces the classical JAK-STAT pathway as well as the phosphatidylinositol 3-kinase (PI3K) and p38 MAPK pathways (10). A number of pre-clinical studies have shown that IFNβ has direct anti-tumor activity on many tumor cell types (11–13). Additionally, IFNβ acts as an immunostimulatory molecule, which is known to indirectly provoke an antitumor response via modulation of the immune system (14–16). However, despite these bi-functional modes of action, the clinical translation of IFNβ treatments for cancer so far has been restricted by its short half-life and systemic toxicity (17–19).
In our previous studies, we have shown that stem cells have the inherent ability to migrate towards malignant lesions and can be engineered to continuously express therapeutic agents making them ideal pathotropic delivery agents (20–24). We therefore thought that the stem cell based on-site delivery of therapeutic molecules can address the issues related to the short half-life of IFNβ, while achieving therapeutic concentrations locally without causing systemic toxicity (25). In this study, we created a highly secretable variant of IFNβ by replacing its endogenous signal sequence with that of Flt3 ligand to enhance the secretion from engineered stem cells and tested our hypothesis whether targeted delivery of IFNβ can simultaneously inhibit tumor cell proliferation and boost immune response in tumor microenvironment when combined with surgical resection of intracranial GBM tumor in syngeneic mouse models. We show that mouse mesenchymal stem cells (MSC) secreting IFNβ, encapsulated in synthetic extracellular matrix (sECM) and placed in the tumor resection cavity increase the survival of mice by direct induction of cell cycle arrest in tumor cells and significantly enhance CD8+ T cell infiltration into the resected peritumoral microenvironment. Moreover, IFNβ shows greater direct effect on human than mouse GBM cells by driving cells from cell cycle arrest towards apoptosis, which has great potential in future clinical translation.
Materials and Methods
Viral Vector Generation:
The following lentiviral (LV) and retroviral (RV) vectors were used in this study: LV-pico2-Fluc-mCherry expressing Firefly luciferase and mCherry (FmC), a kind gift from Dr. Andrew Kung (Dana Farber Cancer Institute; Boston, MA); RV-MGRi-GFP-Fluc, RV-MGRi-HSV-TK, and MFGi-GFP as previously described (26) To construct viral IFNβ vectors, the human and mouse IFNβ genes were amplified from human and mouse cDNA of IFNβ (Sino Biological Inc., Beijing, China) by PCR using the forward primer with XhoI site (5’cggcctcgagatgacagtgctggcgccagcctggagcccaacaacctatctcctcctgctgctgct gctgagcatgagctacaacttgcttgga3’) for a secretable variant of human IFNβ, in which endogenous signal sequence was replaced with that of Flt3L, (5’cggcctcgagatgaccaacaagtgtctcctc3’) for human IFN with its own signal sequence and the reverse primer with BamHI site (5’ ggcggatcctcagtttgcgaggtaacctct 3’). Secretable mouse IFNβ was amplified the same way with the forward primer with XhoI site (5’ cggcctcgaggctagcatgacagtgctggcgccagcctggagcccaacaacctatctcctcctgctgctgctgctgagcgaattcatcaactataagcagctccagc 3’), (5’cggcctcgagatgaacaacaggtggatcctc3’) and the reverse primer with BamHI site (5’ ggcggatcctcagttttggaagtttctggtaag 3’). The amplified cDNA of human and mouse IFNβ were cloned into retroviral MFGi-GFP vector with XhoI/BamHI digestion respectively. Lentiviral vector cloning were performed similarly using primers with NheI and XhoI sites and ligating the PCR products into the pLV-CSC-IG bearing IRES-GFP (27).
Establishing syngeneic mouse models of mouse GBM surgical resection:
Female C56/B6 or female SCID (both 8–9 weeks of age; Charles River Laboratories) 25–30g in weight were immobilized on a stereotactic frame to create a cranial window 7 to 10 days prior to tumor cell implantation. Using a SZX stereo-microscope system (Olympus), a small circular portion of the skull covering the right cerebral hemisphere (~7 mm in diameter) was removed. In 7 to 10 days, the mice were again immobilized on a stereotactic frame, the, the previously established cranial window was exposed and CT2A-FmC or Gl261-FmC cells (1×105 cells/mouse) were superficially implanted into the right cerebral cortex (2.5mm lateral from bregma, 0.5–0.7 mm deep) of C57/B6 mice. GBM4-FmC cells were implanted in SCID mice same way. In the following weeks, tumor growth was non-invasively monitored via bioluminescence imaging (BLI) of the tumor cells Fluc signal activity. For fluorescence-guided tumor resection, mice were immobilized on a stereotactic frame, the skin was opened and the superficial tumor was exposed. A dissecting Leica surgical microscope with ×20 magnification was used for mechanical resection to reduce the tumor volume up to the tumor-tissue interface, leaving margins of the dura intact. Finally, the wound was copiously irrigated and the skin closed with 4–0 Vicryl suture. GBM tumor burden was followed by Fluc imaging over time and mice were sacrificed when neurological symptoms became apparent. All in vivo procedures were approved by the subcommittee on Research Animal Care at Massachusetts General Hospital.
Immune cell isolation and flow cytometry analysis:
Mononuclear cells were isolated from mouse brains using a discontinuous percoll gradient as previously described (28). The following primary antibodies directly conjugated to fluorophores were used: Gr-1 (Ly6C and Ly6G)-BV421, CD11b-PECY7, CD11c-PE, CD45.2-APCCY7, CD4-BV711, CD8-PERCPCY5.5, IFN-γ-BV421, (BD Biosciences, San Jose, CA), Granzyme B-eFlour660, CD38-FITC, and Egr2-APC, (Thermo Fisher, Waltham, MA). Single cell suspensions were stained with antibodies against surface molecules and counter-stained post fixation and permeablisation with intracellular markers. For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 50ng/ml), ionomycin (1μg/ml) and Brefeldin A (5μg/ml) (Sigma Aldrich, St. Louis, MO) for four hours prior to staining with antibodies against surface proteins followed by fixation and permeablisation and staining with antibodies against IFN-γ and Granzyme B. Stained mononuclear cell preparations were analyzed by flow cytometry (BD Biosciences LSRFORTESSA, San Jose, CA).
Statistical analysis:
Data were analyzed by Student t-test when comparing two groups and expressed as mean ± s.d for in vitro analysis and mean ± s.e.m for BLI imaging analysis of tumor volumes. Differences were considered significant at P<0.05 (*); P<0.01 (**). Survival times of mouse groups were analyzed using Prism 5 software (GraphPad Software) and compared using a Mantel Cox log-rank test.
Results
Surgical tumor resection reduces MDSCs and enhances infiltration of DC and T cells into the tumor resected area
To assess the immunological impact of surgical resection in a clinically relevant context, syngeneic GBM resection models were established in immunocompetent C57BL/6 mice. Both CT2A and GL261 syngeneic mouse GBM cells were engineered to express fluorescent (mCherry) and bioluminescent (firefly luciferase (Fluc)) proteins, herein referred to CT2A-FmC and GL261-FmC, and characterized with respect to their growth in vitro and in vivo (Figs. 1A–B, S1 and S2A–B). Surgical resection of established intracranial tumors derived from engineered GBM lines resulted in a small but significant survival benefit compared to mice with unresected tumors (Figs. 1C–D, S2C; P = 0.039 in CT2A- and GL261-FmC tumors).
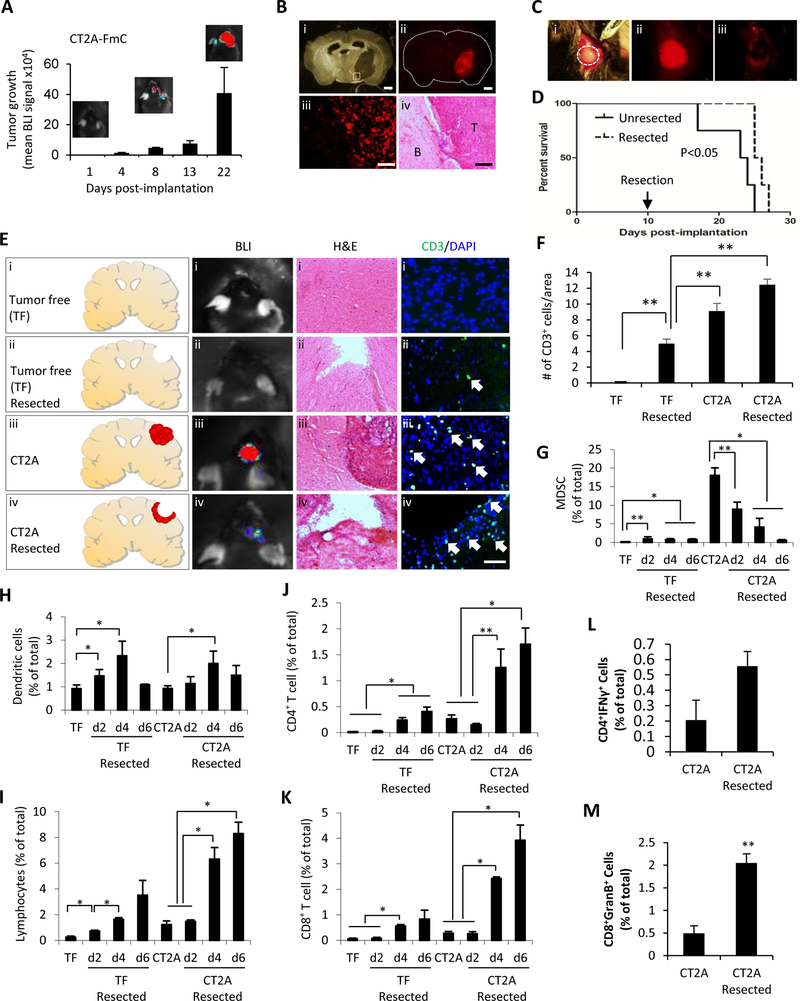
Figure 1. Surgical GBM tumor resection reduces MDSCs and enhances infiltration of DC and T cells:
(A) Tumor growth was monitored by bioluminescent imaging in mice bearing intracranial CT2A-FmC cells (n=4). (B) Photomicrographs of coronal brain sections bearing an established CT2A-FmC tumor; (i) light micrograph; (ii) fluorescence micrograph of tumor; (iii) high magnification of tumor of inlet in (i); (iv) hematoxylin and eosin (H&E) staining. T=tumor; B=brain. Scale bars, 400 μm (i, ii), 100 μm (iii, iv) (C) Photomicrograph of intracranial tumor in dashed circles (i); fluorescence micrographs of CT-2A-FmC tumor (red) before (ii), and after resection (iii). (D) Kaplan-Meier survival curve of mice with/without resected CT2A--FmC tumors. P < 0.05 (E) Schematic showing tumor free (TF) (i), TF resected (ii), CT2A (iii) and CT2A resected (iv) experimental groups. Representative bioluminescence images of mice, H&E staining and immunohistochemical CD3 (green) and DAPI staining (blue) (CD3/DAPI) of coronal brain sections harvested 2 days after resection (F) Plots showing of CD3+ cells in coronal brain sections shown in (E) (n=9/group). Scale bars, 50 μm (CD3/DAPI) (G-K) The mononuclear cells isolated from mouse brains of experimental groups (n=3/group). Brains were harvested 2, 4, 6 days post-resection in TF resected and CT2A resected groups. The mononuclear cells were stained with antibodies recognizing CD45, CD11b, Gr-1, CD11c, CD4 and CD8, and analyzed by flow cytometry. (G) The percentage of MDSC (CD45highCD11bhighGr-1high) (H) dendritic cells (DC) (CD11chighCD11blow), (I) lymphocytes (CD45highCD11blow), (J) CD4+ T cells and (K) CD8+ T of total isolated mononuclear cells were shown. (L-M) Comparison of effector CD4+ and CD8+ T cells isolated from brains 5 days post-tumor resection.
We next investigated the significance of surgical tumor resection on T cell immune response in CT2A mouse model, mice were divided into tumor free, tumor free resected, CT2A and CT2A resected groups (Fig. 1E). Bioluminescence imaging confirmed the presence of Fluc signal in CT2A-FmC-bearing mice and reduction of signal in the resected group (Fig. 1E). H & E staining on histological sections further confirmed the correlation of tumor size with Fluc signal (Fig. 1E). Immunohistochemistry with the T cell marker CD3 indicated that brains of resected and unresected CT2A bearing mice had significantly greater numbers of T cells as compared to tumor free and tumor free resected brains (Figs. 1E–F). To further analyze immune response post-tumor resection over time, immune cells were isolated as mononuclear cells from brains harvested 2, 4 and 6 days post-resection in tumor free resected and CT2A resected groups (Fig. S3A). The tumor bearing group of mice showed a gradual, time-dependent increase in the number of mononuclear cells up to day 6 post-tumor resection, compared to the tumor free resected group (Fig. S3B). Flow cytometry sub-analysis on mononuclear cells further indicated that significant numbers of MDSC resided within CT2A tumors, which were markedly reduced after tumor resection (Fig. 1G). Infiltration of dendritic cells peaked at day 4 post-tumor resection (Figs. 1H). Infiltration of lymphocytes including CD4+ and CD8+ T cells gradually increased post-resection in the tumor free resected group and the increase was further enhanced in CT2A resected group (Figs. 1I–K). Tumor resection enhanced recruitment of both CD4+ and CD8+ effector T cells into resected area at day 5 post-tumor resection (Figs 1L–M, S4). Together, these results demonstrate that surgical resection of CT2A tumors greatly decreases the number of tumor-associated MDSCs and simultaneously increases the number of effector T lymphocytes recruited into the remaining tumor area.
IFNβ boosts infiltration of CD8+ T cells post-tumor resection and has direct and indirect anti-tumor effects
The observed immune reaction post-tumor surgery raised the question whether immune response could be regulated via administration of immune modulators other than the currently used checkpoint inhibitors. We chose IFNβ since it is known to specifically enhance CD8+ cytotoxic T cell recruitment (14,29) and also directly induce apoptosis in cancer cells thereby possibly exposing tumor-related antigens and potentiating antitumor efficacy (9,10). Both mouse GBM lines (CT2A and GL261) expressed the Ifnar1 and Ifnar2 transcripts for IFNβ’s cognate receptors IFNAR1 and IFNAR2 (Fig. S5A) and displayed a reduced cell viability in the presence of recombinant mouse IFNβ (mIFNβ) in vitro (Figs. S5B–C) confirming the direct anti-proliferative effects of IFNβ (11–13). To validate the immunostimulatory effect of mIFNβ, syngeneic mice bearing CT2A tumors were resected and recombinant mIFNβ (60ng/mice) or PBS were instilled into the resection cavity. Presence of recombinant mIFNβ increased CD8+ T cell infiltration more than two fold, whilst the percentage of CD4+ helper T cells out of total mononuclear cells remained unchanged (Fig. 2A). M1 and M2 macrophages were gated based on CD38 and Egr2 (30). Tumor resection lead to an increase in M1/M2 macrophage ratio and mIFNβ treatment to the resection cavity shifted the macrophage polarization toward a higher M1/M2 ratio with a reduction in Egr2+ M2 macrophages (Fig. S6). This shift toward the anti-tumor macrophage is consistent with an increased frequency of IFNγ-producing CD4+ T cells (Fig. 1L), which are known to polarize macrophages and enhances their pro-inflammatory and anti-tumor properties (31)
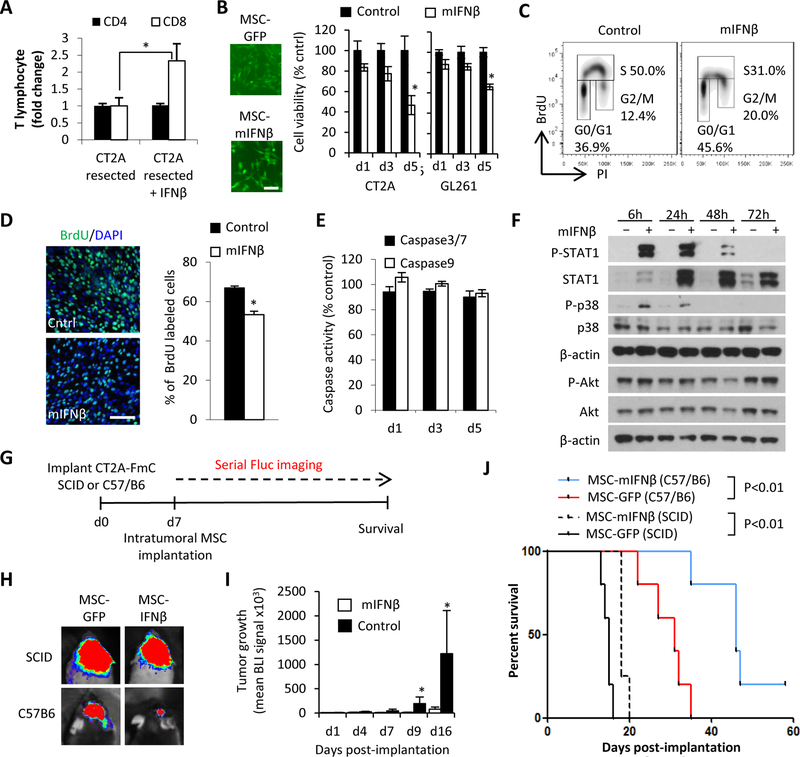
Figure 2. IFNβ boosts infiltration of CD8+ T cells post-tumor resection and has direct and indirect anti-tumor effects:
(A) CD4+ and CD8+ T cells recruitment at 5 days post-tumor resection in response to mouse IFNβ. (B) (left) Fluorescence images of MSC-GFP and MSC-mIFNβ. Scale bar, 100 μm (right) Viability of CT2A and GL261 cells treated for 1, 3 and 5 days with CM of MSC-mIFNβ and MSC-GFP. (C) Representative flow cytometry density plots of cell cycle analysis and (D) immunocytochemical quantification of CT2A cells treated with mIFNβ CM for 72h and followed by a 1h BrdU pulse. BrdU positive nuclei (green)/ DAPI (blue). Scale bar, 100 μm (E) Comparison of Caspase 3/7 and 9 activity in CT2A-FmC cells cultured with mIFNβ CM for 1, 3 and 5 days. (F) Western blot analysis on CT2A cell lysates collected 6, 24, 48 and 72hrs after treatment with mIFNβ showing phosphorylation status of STAT1 (Tyr701), p38 (T189/Y182), and Akt (S473). (G) Scheme for testing efficacy of intratumoral MSC-mIFNβ or MSC-GFP in intact CT2A-FmC tumors in SCID (n=5 for MSC-GFP, n=4 for MSCmIFNβ) and C57/B6 mice (n=5 in each group). (H) Representative visible light plus superimposed bioluminescence images (BLI) of mice from each treatment group 16 days post-MSC implantation. (I) Plot of mean tumor growth following intratumoral MSC injections in C57/B6 mice. (J) Kaplan-Meier survival curves of C57/B6 or SCID mice bearing CT2A-FmC tumors intratumorally injected with MSC-mIFNβ or MSC-GFP.
Since prolonged systemic treatment of therapeutic dosages of IFNβ has been shown to result in considerable side-effects (32), we next explored the feasibility of on-site delivery of IFNβ from within the tumor resection cavity. We generated lenti- and retro-viral vectors bearing a highly secretable variant of human and mouse IFNβ by swapping the original signal sequence of mouse IFNβ with that of the Flt3 ligand signal sequence (herein referred to as hIFNβ and mIFNβ respectively) in order to maximize secretion of IFNβs (Figs. S7A–C). Utilizing these vectors, mouse mesenchymal stem cells (MSC) were engineered to express mIFNβ (MSC-mIFNβ) (Fig. 2B). Treatment with conditioned medium (CM) from MSC-mIFNβ reduced the cell viability of both CT2A-FmC and GL261-FmC lines in a time dependent manner similar to recombinant mIFNβ (Figs. 2B, S5B–C). To test which cellular mechanisms are involved in cell viability reduction, CT2A-FmC cells were treated with mIFNβ CM and consecutively pulsed with BrdU. Cell cycle analysis revealed that CT2A GBM displayed a reduction in the proportion of cells in S phase, with a concomitant increase in G0/G1 and G2/M phases (Figs. 2C–D). However, no evidence of apoptosis-induction was observed in response to mIFNβ CM treatment (Figs. 2C, E, S7D), contrary to the previously reported effect of IFNβ on human GBMs (33). mIFNβ further resulted in the phosphorylation of STAT1 and p38 indicating a conserved response to this classical JAK/STAT signaling pathway and alternative signaling pathways respectively (Fig. 2F). CT2A did not show the change of phosphorylation status in Akt (S473) in response to mIFNβ (Fig. 2F). In conclusion, these data indicate that mIFNβ can directly suppress proliferation of mouse GBM via induction of cell cycle arrest.
In order to dissect out the immunomodulatory effects from the direct anti-GBM response of mIFNβ in vivo, mouse MSC expressing mIFNβ or GFP (MSC-mIFNβ/-GFP) were injected into established CT2A-FmC tumors in immunocompromised (SCID) or immunocompetent (C57/B6) mice (n=5/group) (Figs. 2G). In SCID mice, intratumoral injection of MSC-mIFNβ did not demonstrate significant inhibition of tumor growth but produced a small increase in survival compared to MSC-GFP-treated counterparts (18 days versus 15 days of median survival; P<0.01) (Figs 2H, J). However, in the context of an intact immune system, MSC-mIFNβ greatly suppressed tumor growth and significantly prolonged survival (46 days versus 31 days of median survival; P<0.01), with one mouse showing long term survival exceeding 80 days (Figs. 2H–J). These results indicate that therapeutic MSC-mIFNβ can act directly on mouse GBM in vivo, and that anti-tumor efficacy is enhanced when acting in conjunction with a fully functional immune system.
MSC-mIFNβ have anti-tumor efficacy in resected GBM, leading to increase survival of mice and can be eliminated post-therapy
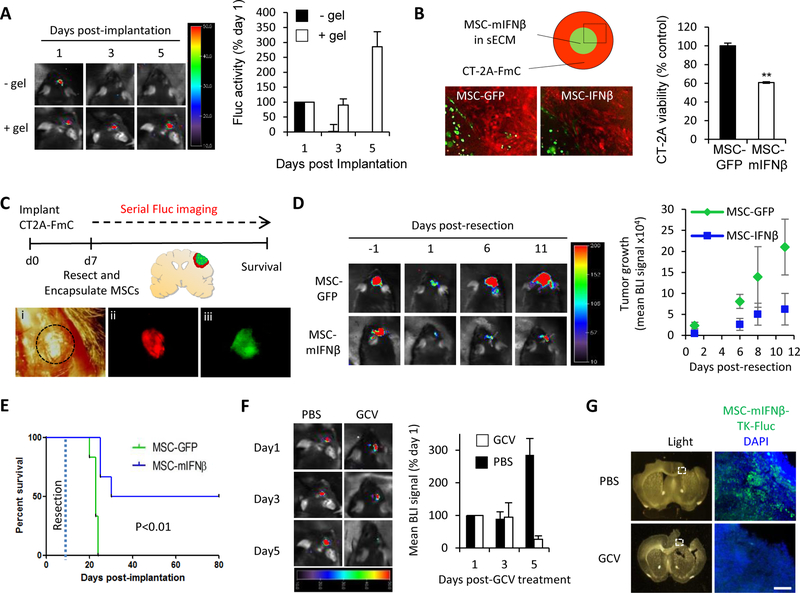
In light of the immune cell infiltration caused by surgical resection, we tested if the therapeutic efficacy of MSC-mIFNβ post-tumor resection results in an additional survival benefit by potentiating IFNβ’s immunostimulatory response. Encapsulating MSCs in a synthetic extracellular matrix (sECM) was necessary for their effective retention in the brain (Fig. 3A). In culture, sECM-encapsulated MSC-mIFNβ were shown to reduce CT2A-Fmc cell viability (Fig. 3B). In vivo, syngeneic mice bearing CT2A-FmC tumors were resected, and the cavity was filled with sECM-encapsulated MSC-mIFNβ/-GFP (Figs. 3C). The growth of residual tumors was suppressed in mice implanted with MSC-mIFNβ, compared to the increasing tumor volumes in mice implanted with MSC-GFP (Fig. 3D). This translated into significantly prolonged survival of MSC-mIFNβ-treated mice compared to MSC-GFP controls, with half of the mice surviving beyond 80 days (versus 23 days of median survival of control group; P=0.0008) (Fig. 3E). Nevertheless, one concern with using therapeutic stem cells in the brain is that they could aberrantly proliferate and/or differentiate. To circumvent this concern, our MSC-mIFNβ were additionally engineered to express the suicide gene, herpes simplex virus thymidine kinase (HSV-TK) and its imageable variant HSV-TK-GFP-Fluc, enabling their selective eradication by treatment with the prodrug ganciclovir (GCV). In culture, MSC-mIFNβ-TK were readily killed in response to GCV in monolayers and encapsulated in sECM (Fig. S8). In vivo, when MSC-mIFNβ-TK-Fluc were encapsulated and placed in the resection cavity, systemic treatment with GCV also effectively reduced their numbers, compared to PBS-treated controls in which MSC number increased (Fig. 3F). Indeed, sectioned brains confirmed a large reduction in GFP-positive MSC-TK-mIFNβ upon treatment with GCV (Fig. 3G). In conclusion, these data indicate that the immune reaction following surgical tumor resection can be regulated by placing sECM-encapsulated MSC-mIFNβ into the resection cavity and that prolonged local delivery of IFNβ results in a significant survival benefit. Furthermore, our suicide gene strategy to eliminate MSC-mIFNβ from the brain post-treatment provides a solution regarding the concerns of using therapeutic stem cells in clinical settings of cancer therapy.
Figure 3. MSC-mIFNβ show antitumor efficacy in resected GBM, leading to increased survival of mice and can be eliminated post-therapy:
(A) Representative BLIs and plot of mean Fluc signal intensity of mice bearing intracranial MSC-GFP-Fluc cells with or without sECM encapsulation (1 × 106 cells/mouse, n=4/group) (B) Schematic and fluorescence images showing MSC-GFP or MSC-mIFNβ cells encapsulated in sECM (1 × 104 cells/drop), surrounded by CT2A-FmC cells. Plot showing CT2A cell viability at day5 (C) Scheme for testing efficacy of sECM-encapsulated MSCs expressing GFP or mIFNβ on resected CT2A-FmC tumors (n=6/group): (i) Light image of a craniotomy above tumor, delineated by dashed black circle. (ii, iii) Fluorescence images of before-resected CT2A-FmC tumor (red) and encapsulated MSC-mIFNβ cells in the resection cavity (green). (D) Representative BLIs of mice from MSC-GFP and -mIFNβ groups pre- and post-resection, plot of mean tumor growth and (E) survival curves. (F-G) Resected mice were implanted with sECM-encapsulated MSC-mIFNβ-TK-Fluc (n=6) and treated with GCV (50 mg/kg) or PBS daily for 5 days. (F) Representative BLIs of mice at 1, 3 and 5 days post-GCV treatment and plot of mean tumor growth (G) Light and fluorescence micrographs of coronal brain sections containing encapsulated MSC-mIFNβ-TK-Fluc (green) from brains harvested 7 days post-GCV treatment and DAPI counterstained (blue). White dashed box indicates region of interest. Scale bar 100 μm.
hIFNβ suppresses tumor growth through Chk1 mediated S phase arrest followed by apoptosis
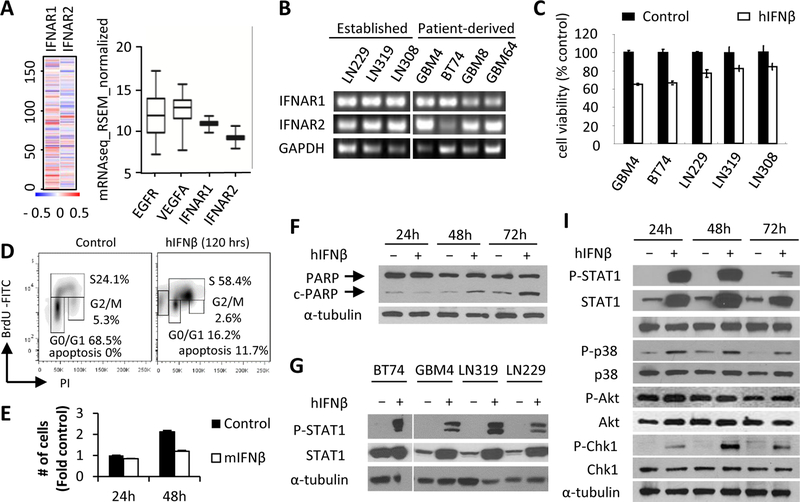
In order to translate our findings into clinical settings of GBM therapy, we next set out to test hIFNβ’s direct anti-cancer activity in human GBM lines. We first confirmed that IFNAR1/2 are universally expressed in transcriptional level with relatively low range of variations in human GBM patients in TCGA database (n=166) indicating IFNβ’s broader applicability as anti-cancer agent for GBM patients compared to target EGFR and VEGFA, two well-known therapeutic targets for GBM treatment (Fig. 4A). Consistently, transcriptional expression of IFNAR1/2 were confirmed in all of the human GBM lines (Fig. 4B). A significant reduction in cell viability in two patient-derived primary GBM lines (GBM4 and BT74) was seen following 72h incubation with CM (100ng/ml hIFNβ: obtained from 293T expressing a secretable variant of hIFNβ, Figs. S7B–C) (P < 0.01 versus non-treated for all lines) (Figs. 4C, S9). GBM4 cells showed significant increase of S phase cells in cell cycle and the emergence of an apoptotic population was coincident with reduction of total cell number in S phase following hIFNβ exposure as compared to controls (Figs. 4D, S9). The increase of cells in S phase in response to hIFNβ was not due to greater rate of cell proliferation (Fig. 4E). Cleaved poly-ADP-ribose polymerase (c-PARP) was observed starting 48h post-hIFNβ treatment in GBM4 cells (Figs. 4F). These data suggest that hIFNβ can suppress cell viability of GBMs by inducing prolonged cell cycle arrest followed by apoptosis.
Figure 4. hIFNβ suppresses tumor growth through Chk1 mediated S phase arrest followed by apoptosis:
(A) Heat map and box and whisker plot showing relative transcriptional expressional level of IFNAR1 and IFNAR2 compared to EGFR and VEGFA analyzed from TCGA-GBM RNA-Seq database (n=166 GBM patients). (B) RT-PCR analysis of IFNAR1/2 in cDNA extracts from GBM lines (C) Cell viability of GBM lines 72h post-treatment with hIFNβ (100ng/ml). (D) Flow cytometry analysis of GBM4 cells treated with hIFNβ for 120h, followed by a 1h BrdU pulse. (E) The comparison of the number of GBM4 cells 24h and 48h post-treatment of hIFNβ (100ng/ml). (F) Western blot analysis of PARP and cleaved-PARP (c-PARP) from GBM4 cells in response to hIFNβ (100ng/ml) treatment. (G) Western blot analysis of GBM lysates to detect phosphorylated STAT1 (Tyr701) and STAT1 overexpression induced 24h post-hIFNβ (100ng/ml) treatment. (I) Western blot analysis of lysates from GBM4 cells treated with hIFNβ (100ng/ml) for 24, 48, and 72hrs showing phosphorylation status of STAT1 (Tyr701), p38 (T189/Y182), Chk1 (S345), and Akt (S473).
To further characterize the direct hIFNβ-mediated anti-tumor effects on human GBM, IFNβ-induced signaling pathways were explored in more detail. A panel of GBM lines showed STAT1 activation after hIFNβ treatment (Fig. 4G) which lasted up to 72h in GBM4 cells (Fig. 4I). hIFNβ treatment also induced activation of the MAPK p38 tumor suppressor and the DNA checkpoint protein Chk1, but not Akt in GBM4 cells (Fig. 4I). Chk1 phosphorylation was not observed in CT2A mouse GBM cells in response to mIFNβ (Figure S10). These data are consistent with the result showing an increase of GBM cells in S phase arrest in response to hIFNβ (Figs. 4D, S11) and suggest that p38 and Chk1 are both involved in the IFNβ signaling that leads to cell cycle arrest in S phase and prolonged cell cycle arrest may induce apoptosis in GBM4 cells.
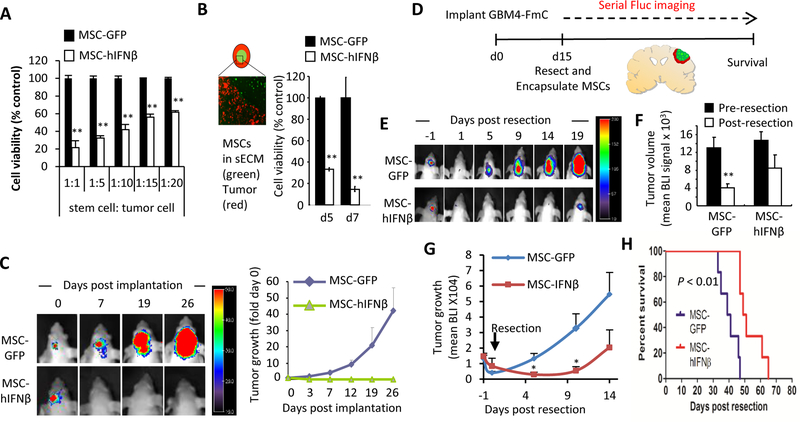
MSC-hIFNβ suppresses tumor growth and extends survival of mice bearing surgically resected intracranial GBM
Next, we wanted to test the direct anti-tumor effect of MSC secreted hIFNβ on patient-derived GBM cells in an orthotopic xenograft mouse model of GBM resection. Co-culture of MSC-hIFNβ with GBM4-FmC cells demonstrated a significant reduction of cell viability, confirming functionality of the therapeutic stem cells expressing hIFNβ (Fig. 5A). Furthermore, in vitro anti-tumor efficacy was retained after sECM-encapsulation of MSC-hIFNβ indicating that hIFNβ can readily diffuse out of the sECM and effect surrounding tumor cells in a time-dependent manner (Fig. 5B). In vivo, the patient-derived GBM4-FmC line admixed with MSC-hIFNβ and intracranially implanted into immunodeficient SCID mice did not grow in contrast to its continuous growth when admixed with MSC-GFP control (Fig. 5C). Having confirmed the anti-tumor effect of MSC-hIFNβ we then resected fully established GBM4-FmC (Figs. 5D–H), followed by implantation of sECM-encapsulated MSC-hIFNβ or MSC-GFP into the tumor resection cavity. MSC-hIFNβ completely suppressed GBM4 growth up to 10 days post-treatment in contrast to immediate tumor progression observed in the control group (Figs. 5E, G). However, despite initial suppression of tumor re-growth in MSC-hIFNβ-treated mice, tumors started to re-establish their growth pattern after 10 days, overall resulting in a modest but significant survival benefit compared to the control group (50 days vs 40 days of median survival; P<0.01) (Fig. 5H). These results reveal that human IFNβ has a greater direct anti-tumor activity than mouse IFNβ due to combined effects of cell cycle arrest and apoptosis. Given the lack of immune response in this xenograft model and the intact immune systems in human GBM patients, the use of MSC-hIFNβ offers a great potential for future clinical translation.
Figure 5. MSC-hIFNβ suppress tumor growth and extend survival of mice bearing resected intracranial GBM:
(A) GBM4-FmC cells were co-cultured with MSC-GFP and MSC-hIFNβ at the indicated ratio for 3 days. Cell viability was measured by Firefly luciferase (Fluc) activity. (B) MSC-GFP/-hIFNβ were encapsulated in sECM as shown in schematic and fluorescence micrograph. GBM4-FmC cells were plated 1:1 with MSCs. GBM4 Cell viability was measured by Fluc activity at day5 and day7. (C) Mix of GBM4-Fluc-mCherry and MSC-GFP/-hIFNβ (1:1 ratio, 5×105 cells/mouse) were intracranially implanted into nude mice (n=5 in each group). Representative visible light plus superimposed BLI of tumor bearing mice for each group are shown (left). Fold change in tumor volumes was calculated by normalizing the mean Fluc intensity at the indicated time point with that of day 0 (right). (D) Scheme for testing efficacy of sECM-encapsulated MSC-GFP or MSC-hIFNβ in mice with resected GBM4-FmC tumors (n=6 in each group). (E) Representative BLIs of mice pre- and post-resection, (F) The Change of mean tumor volume was estimated based on Fluc signal pre- and post-tumor resection from mice bearing intracranial GBM4-FmC. (G) plot of mean tumor growth and (H) Kaplan-Meier survival curves.
Discussion
In this study, we developed and characterized syngeneic orthotopic mouse models of GBM resection and show for the first time that surgical debulking of orthotopic GBM tumor evokes a characteristic, time-dependent peritumoral immune reaction in tumor microenvironment which in combination with local delivery of immunomodulatory therapeutics can be utilized to significantly boost the hosts’ anti-cancer immune response. Our syngeneic mouse tumor models of GBM resection have great value for the study of immune response to immunomodulatory agents, and provide new insights that may help guide clinicians in the difficult transition from preclinical to clinical anti-GBM immunotherapies.
One of the cornerstones of clinical care for GBM patients is surgical tumor debulking (2), yet nearly all pre-clinical models focus on treating established solid tumors, often in sub-cutaneous, non-orthotopic settings. Previously, we have shown the feasibility of resecting GBMs developed from human patient derived cell lines in orthotopic xenograft animal models (22). In an effort to understand the changes in immune response post-tumor resection, we developed a syngeneic mouse GBM model by integrating fluorescent and bioluminescent markers and extensive optical imaging to simultaneously confirm the presence of established tumors, visualize the extent of tumor debulking and serially monitor tumor re-growth post-resection. Since tissue damage is known to result in activation of both innate and adaptive immune response (8), our tumor resection model may enhance three key elements crucial for successful anti-cancer immunotherapy: (i) removal of immune suppressive cells such as MDSCs from the tumor microenvironment (34); (ii) enhancement of effector CD4/CD8 T cell infiltration into tumor microenvironment (35) and (iii) reduction of tumor size (36). Although temporal reduction of the number of MDSCs in spleen after surgical resection of tumor was reported in the 4T1 murine mammary tumor model (37), our study showed that surgical tumor debulking of GBM significantly reduces MDSC in the tumor microenvironment. As such, our findings serve as an excellent preclinical immuno-oncology model to study how current and/or developing immunotherapeutic agents alter different immune-related effector elements in the clinical context of post-resection tumor microenvironments.
The inherent risk associated with systemic immunomodulatory therapies to induce autoimmune reactions against healthy tissues or organs motivated us to seek out an alternative that would allow safe and efficient modulation of the observed post-resection immune response. Strategically, a cytokine-induced establishment of favorable conditions for the host immune system post-resection would be an ideal solution to allow tumor elimination via activation of a variety of stimulated immune effectors. However, short half-lives and toxicity associated with systemic cytokine treatments so far have prevented clinical translation of such approaches (38). IFNβ is an immunostimulatory molecule, which is known to indirectly provoke an antitumor response via various modulations of the immune system. These include potentiating MHC class I expression, initiating specific T cell responses against tumor cells, activating the innate immune system and inhibiting angiogenesis (14–16,39). Our results also showed that tumor resection and IFNβ treatment shift macrophage polarization toward a higher M2/M2 ratio. It has been shown that M1 macrophages have anti-tumor function (40) further supporting therapeutic potential of our IFNβ therapy in a tumor resection model. Although, a number of pre-clinical studies have shown that IFNβ has potent direct anti-tumor activity in addition to its well-known immunomodulatory effects (11–13), clinical trials utilizing systemic administration of IFNβ have had only very limited success, mostly due to its short half-life of approximately 5h and systemic toxicity (17,18). In the case of GBM, clinical trials have generally been disappointing. A study in which patients with recurrent GBM received intravenously administered recombinant IFNβ, documented no evidence of treatment efficacy (41). In a separate Phase I study, patients with recurrent GBM were administered IFNβ through a reservoir connected via a catheter to the tumor cavity (Ommaya/Rickham reservoir). In this study side effects from IFNβ therapy as well as problems with the reservoir itself (blocking and infection) resulted in many study dropouts; however 25% (3/12 patients) showed a partial response (42). These studies indicate that despite IFNβ demonstrating promising efficacy in preclinical cancer models, its successful clinical translation requires further investigation, especially in regard to better delivery methods. In an effort to circumvent the above issues, we therefore genetically engineered MSC to release a highly secretable variant of IFNβ and subsequently utilized these engineered MSCs as a vehicle for prolonged on-site delivery to intracranial GBMs.
Despite extensive pre-clinical evidence demonstrating the potential of cell-based therapies for GBM, only very few studies have explored methods to introduce therapeutically “armed” stem cells in GBM resection cavities. Hereby, stem cell “wash-out” and rapid diffusion from the resection cavity by cerebrospinal fluid are potential threats to treatment success. Based on our previous studies (21,22,24), we therefore employed biodegradable sECM encapsulation as an attractive approach for retention of MSC-IFNβ within the resection cavity. The importance of sECM has been intensively examined in general cell therapy especially in regenerative medicine in which the nature of sECM as porous hydrogels promotes nutrient input and waste outflow and enhances cell seeding, retention and migration (43). In this study we observed superior survival of sECM encapsulated mMSC-IFNβ in resection cavities in comparison to non-sECM encapsulated mMSC and our study further reveals that sECM encapsulated MSC are effective in increasing local drug concentrations and exposure times. Together these properties resulted in enhanced tumor cell killing and significantly increased survival of sECM-MSC-IFNβ treated mice.
As discussed above, in addition to its immunomodulatory function, IFNβ has direct cytotoxic effects on tumor cells. However, the detailed mechanisms leading to cell death after IFNβ treatment were not fully understood. A recent study in human neuroblastoma cells demonstrated that IFNβ induces tumor cell killing by p38 mediated reactive oxygen species (ROS) production (44). IFNβ further promotes senescence in human fibroblasts mediated by ROS production and activation of the ATM/Chk2 DNA checkpoint leading to activation of p53 and RB (45). Exploring the species-specific therapeutic effects of IFNβ in more detail we demonstrate that IFNβ has direct anti-proliferative activity in both human and mouse GBM types, however with noticeable differences in their response. STAT1 activation followed by p38 phosphorylation lasted much less in CT2A cells than in human GBM4 in response to IFNβ treatment (Figs. 2F, 5G–I). The wild type status of p53 in CT2A may further favor accumulation of CT2A cells in the G0/G1 and G2 cell cycle phases (46). In contrast, we show that in human GBM4 cells, hIFNβ induces prolonged JAK/STAT activation independent of PI3K-Akt, activates p38 tumor suppressor and DNA check point via Chk1 which ultimately leads to cell cycle arrest in the S phase. Homozygous deletion of CDKN2A&B (p15, CDK4 inhibitor) in GBM4 may also contribute to bypass cell cycle arrest in G1/S transition in response to IFNβ (47). In addition, mutations either in p53 or its downstream p14/p16 CDK inhibitors in most of the established GBM cell lines indicates that IFNβ may function similarly in other patient-derived GBM lines as shown in our studies with GBM4 (48).
The biggest safety concern with stem cell-based therapies for cancer is the survival and potential proliferation of remaining therapeutic stem cells post-tumor therapy. In this study, we solved the safety issue with the prodrug converting system HSV-TK/GCV. Moreover, stem cells expressing HSV-TK provide additional advantages when translated into clinical GBM therapies, particularly the ability of the prodrug GCV to cross the blood brain barrier and its dual function as a PET (Positron emission tomography) imaging agent as well as the bystander effect on surrounding tumor cells associated with GCV-induced stem cell elimination. Positron detection is the most sensitive imaging modality. PET ligands such as 18F-FHBG, 18F-FEAU and 124I-FAIU have been used to provide 3D images of the concentration of cells in different locations within a tumor (49,50). Since the HSV-TK expression can be repetitively evaluated over time by PET scan and does not fade with cell division, the HSV-TK/GCV system is an ideal strategy for the monitoring and safe termination of stem cell based therapies in clinical settings.
In conclusion, our study shows the feasibility of using clinically relevant GBM tumor resection models to assess immunotherapeutic effects in the context of additional treatments with locally delivered immunomodulatory agents. Given that human IFNβ shows greater direct effect on human GBM cells than their mouse counterparts, this study has a great potential to be translated into GBM patients. We envision that after the neurosurgical removal of the main tumor mass, the patient’s own reprogrammed cells or HLA (human leukocyte antigen)-compatible MSCs engineered to express IFNβ will be encapsulated in sECM and implanted into the resection cavity after tumor debulking. These cells would then result in simultaneous tumor cell killing as well as targeted immune response, which could be further potentiated via the systemic administration of immune checkpoint inhibitors, with the ultimate goal being improved patient outcome.
Supplementary Material
Translational Relevance.
To our knowledge, this is the first preclinical investigation on the potential of tumor debulking in combination with immune therapy in the setting of syngeneic orthotopic mouse model of GBM. In this study we developed syngeneic orthotopic mouse models of GBM resection and show that surgical tumor debulking evokes a characteristic, time-dependent peritumoral immune response in tumor-microenvironment. This immune response significantly enhanced the anti-tumor efficacy of encapsulated-mouse mesenchymal stem cells (MSC) engineered to release a highly secretable variant of a dual-functioning anti-proliferative and immunomodulatory-cytokine, interferon (IFN) β in the tumor-resection cavity. Given that ~75% of GBM patients undergo tumor-resection and systemically delivered drugs post-resection have been marginally successful in GBM patients, we envision a therapeutic modality in which at the time of primary GBM surgery, the main tumor mass will be removed and encapsulated stem-cells expressing IFNβ will be introduced in the cavity and allowed to target the remaining GBM cells.
Acknowledgments
We thank G. Prestwich (University of Utah) and T. Zarembinski (Biotime Inc.) for providing us with synthetic extracellular matrix gels and Ravi Mylvaganam and Christina Luo for their help with flow cytometry.
Grant Support
This work was supported by US National Institutes of Health grants R01CA138922 (K.S.), R01CA173077 (K.S.) and 1S10RR023440-01A1 (MGH Pathology CNY Flow Cytometry Core and sorting shared instrumentation grant).
Footnotes
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 2009;18(8):1061–83. [DOI] [PubMed] [Google Scholar]
- 2.Asthagiri AR, Pouratian N, Sherman J, Ahmed G, Shaffrey ME. Advances in brain tumor surgery. Neurol Clin 2007;25(4):975–1003, viii-ix. [DOI] [PubMed] [Google Scholar]
- 3.Margolin K. The Promise of Molecularly Targeted and Immunotherapy for Advanced Melanoma. Curr Treat Options Oncol 2016;17(9):48. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri I, Cattarino S, Giantulli S, Nazzari C, Collalti G, Sciarra A. A Perspective of Immunotherapy for Prostate Cancer. Cancers (Basel) 2016;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 6.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake CG. Combined Immune Checkpoint Blockade. Semin Oncol 2015;42(4):656–62. [DOI] [PubMed] [Google Scholar]
- 8.Valparaiso AP, Vicente DA, Bograd BA, Elster EA, Davis TA. Modeling acute traumatic injury. J Surg Res 2015;194(1):220–32. [DOI] [PubMed] [Google Scholar]
- 9.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature reviews Immunology 2005;5(5):375–86. [DOI] [PubMed] [Google Scholar]
- 10.Pokrovskaja K, Panaretakis T, Grander D. Alternative signaling pathways regulating type I interferon-induced apoptosis. J Interferon Cytokine Res 2005;25(12):799–810. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Greene G, Pettaway C, Dinney CP, Eue I, Lu W, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-beta. Cancer research 1999;59(4):872–9. [PubMed] [Google Scholar]
- 12.Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A, et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 1998;95(24):14411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lykhova AA, Kudryavets YI, Strokovska LI, Bezdenezhnykh NA, Semesiuk NI, Adamenko IN, et al. Suppression of proliferation, tumorigenicity and metastasis of lung cancer cells after their transduction by interferon-beta gene in baculovirus vector. Cytokine 2014;71(2):318–26. [DOI] [PubMed] [Google Scholar]
- 14.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. The Journal of experimental medicine 2011;208(10):2005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of clinical investigation 2010;120(4):1151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 1996;272(5270):1947–50. [DOI] [PubMed] [Google Scholar]
- 17.Fierlbeck G, Ulmer A, Schreiner T, Stroebel W, Schiebel U, Brzoska J. Pharmacodynamics of recombinant IFN-beta during long-term treatment of malignant melanoma. J Interferon Cytokine Res 1996;16(10):777–81. [DOI] [PubMed] [Google Scholar]
- 18.Salmon P, Le Cotonnec JY, Galazka A, Abdul-Ahad A, Darragh A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res 1996;16(10):759–64. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Type I interferon: friend or foe? The Journal of experimental medicine 2010;207(10):2053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SH, Tamura K, Khajuria RK, Bhere D, Nesterenko I, Lawler J, et al. Antiangiogenic Variant of TSP-1 Targets Tumor Cells in Glioblastomas. Mol Ther 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duebgen M, Martinez-Quintanilla J, Tamura K, Hingtgen S, Redjal N, Wakimoto H, et al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J Natl Cancer Inst 2014;106(6):dju090. [DOI] [PubMed] [Google Scholar]
- 22.Kauer TM, Figueiredo JL, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci 2012;15(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proceedings of the National Academy of Sciences of the United States of America 2009;106(12):4822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuckey DW, Hingtgen SD, Karakas N, Rich BE, Shah K. Engineering toxin-resistant therapeutic stem cells to treat brain tumors. Stem Cells 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer 2014;14(10):683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Quintanilla J, Bhere D, Heidari P, He D, Mahmood U, Shah K. Therapeutic efficacy and fate of bimodal engineered stem cells in malignant brain tumors. Stem Cells 2013;31(8):1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods 2004;122(2):131–9. [DOI] [PubMed] [Google Scholar]
- 28.Pino PA, Cardona AE. Isolation of brain and spinal cord mononuclear cells using percoll gradients. Journal of visualized experiments : JoVE 2011(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natsume A, Tsujimura K, Mizuno M, Takahashi T, Yoshida J. IFN-beta gene therapy induces systemic antitumor immunity against malignant glioma. J Neurooncol 2000;47(2):117–24. [DOI] [PubMed] [Google Scholar]
- 30.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, et al. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS One 2015;10(12):e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci 2015;72(21):4111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yung WK, Prados M, Levin VA, Fetell MR, Bennett J, Mahaley MS, et al. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol 1991;9(11):1945–9. [DOI] [PubMed] [Google Scholar]
- 33.Saito R, Mizuno M, Hatano M, Kumabe T, Yoshimoto T, Yoshida J. Two different mechanisms of apoptosis resistance observed in interferon-beta induced apoptosis of human glioma cells. J Neurooncol 2004;67(3):273–80. [DOI] [PubMed] [Google Scholar]
- 34.Hou W, Sampath P, Rojas JJ, Thorne SH. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell 2016;30(1):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, et al. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade . Cancer Cell 2016;29(3):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reardon DA, Gokhale PC, Klein SR, Ligon KL, Rodig SJ, Ramkissoon SH, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res 2016;4(2):124–35. [DOI] [PubMed] [Google Scholar]
- 37.Ghochikyan A, Davtyan A, Hovakimyan A, Davtyan H, Poghosyan A, Bagaev A, et al. Primary 4T1 tumor resection provides critical “window of opportunity” for immunotherapy. Clin Exp Metastasis 2014;31(2):185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3(4):3856–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research 2011;71(7):2488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie C, Liu C, Wu B, Lin Y, Ma T, Xiong H, et al. Effects of IRF1 and IFN-beta interaction on the M1 polarization of macrophages and its antitumor function. Int J Mol Med 2016;38(1):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahaley MS Jr, Dropcho EJ, Bertsch L, Tirey T, Gillespie GY. Systemic beta-interferon therapy for recurrent gliomas: a brief report. Journal of neurosurgery 1989;71(5 Pt 1):639–41. [DOI] [PubMed] [Google Scholar]
- 42.Fetell MR, Housepian EM, Oster MW, Cote DN, Sisti MB, Marcus SG, et al. Intratumor administration of beta-interferon in recurrent malignant gliomas. A phase I clinical and laboratory study. Cancer 1990;65(1):78–83. [DOI] [PubMed] [Google Scholar]
- 43.Prestwich GD, Healy KE. Why regenerative medicine needs an extracellular matrix. Expert Opin Biol Ther 2015;15(1):3–7. [DOI] [PubMed] [Google Scholar]
- 44.Dedoni S, Olianas MC, Onali P. Interferon-beta counter-regulates its own pro-apoptotic action by activating p38 MAPK signalling in human SH-SY5Y neuroblastoma cells. Apoptosis 2014;19(10):1509–26. [DOI] [PubMed] [Google Scholar]
- 45.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol Biol Cell 2006;17(4):1583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binello E, Qadeer ZA, Kothari HP, Emdad L, Germano IM. Stemness of the CT-2A Immunocompetent Mouse Brain Tumor Model: Characterization In Vitro. J Cancer 2012;3:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakimoto H, Mohapatra G, Kanai R, Curry WT Jr, Yip S, Nitta M, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol 2012;14(2):132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 1999;9(3):469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gambhir SS, Bauer E, Black ME, Liang Q, Kokoris MS, Barrio JR, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America 2000;97(6):2785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res 2005;11(21):7749–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.