Abstract
During embryonic development multiple waves of hematopoietic progenitors with distinct lineage potential are differentially regulated in time and space. Consistent with this view, some specialized lymphocytes emerge during a limited time-window in embryogenesis and migrate to the tissues where they contribute to organogenesis and to tissue homeostasis. These cells are not constantly produced by bone marrow derived hematopoietic stem cells but are maintained in tissues and self-renew throughout life. These particular cell subsets are produced from lymphoid restricted progenitors only found in the first days of fetal liver hematopoietic activity. Growing evidence of the heterogeneity and layered organization of the hematopoietic system is leading to a common view that some lymphocyte subsets are functionally different because they follow distinct developmental programs and emerge from distinct waves of lymphoid progenitors. However, understanding the influence of developmental origin and the relative contribution of local microenvironment on the development of these specialized lymphocyte subsets needs further analysis. In this review, we discuss how different pathways followed by developing B cells during ontogeny may contribute to the diverse functions.
Keywords: Lymphopoiesis, B cell, Embryo, Yolk sac, Hematopoietic stem cells
Circulating blood cells are continuously produced from bone marrow (BM)-derived hematopoietic stem cells (HSCs), however, recent studies have challenged this view by establishing an exclusive embryonic origin of some tissue resident immune cells that persist throughout life [1], [2], [3], [4]. These cells that comprise B-1 B, some subsets of γδ T and lymphoid tissue inducer (LTi) cells are capable of self-renewing and respond to changes in tissue environment [4]. In addition, they contribute to tissue homoeostasis and represent the first line of defense against pathogens [2], [4], [5]. The layered immune system hypothesis was first introduced by Herzenberg and Herzenberg [6] to highlight the unique fetal origin of some hematopoietic cell populations correlated with a more primitive phylogenetic origin. Recent advances in lineage tracing and cellular barcoding of hematopoietic progenitors contributed to reinforce the idea of a multi-layered immune system. Growing evidence is linking immune cell heterogeneity in homeostasis to their developmental origin during ontogeny, although the relative contribution of origin or of environmental signals to function is still under investigation. One clear example of embryonic origin and HSC-independency is the microglia and other tissue resident macrophages [7] shown by Perdiguero et al. to be derived from yolk sac (YS) erythro-myeloid progenitors (EMP). Whether the origin rather than the immediate location of tissue resident macrophages modulate their diverse functions and what are the differences in gene expression inherent to YS-derived macrophages are issues that remain to be resolved. In line with the view that the immune system is composed of cells that are generated in successive waves, some specialized lymphocytes produced during embryogenesis are tissue resident cells and share innate-like properties such as rapid response to antigen stimulation and contribution to tissue homeostasis [2]. The origin of these lymphocytes remain controversial as they have been suggested to emerge before definitive HSCs are detected [8], [9]. So, similar to tissue resident macrophages, innate-like B-1a cells, Vγ5+ or Vδ1+ IL-17+ γδ T cells or LTi could derive from hematopoietic progenitors independent from HSC [10]. Here we discuss how developmental origins and local tissue signals may contribute to the multi-layered developmental programs of lymphocyte subsets during ontogeny.
HSC-independent lymphopoiesis
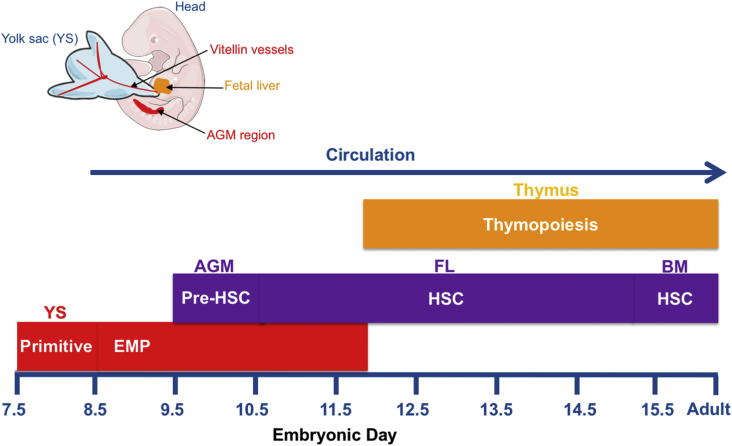
Fetal hematopoiesis occurs in distinct waves starting with a primitive wave from the YS which gives rise to nucleated erythrocytes and megakaryocytes [11]. Around embryonic day 8 (E8) the blood circulation is established [12] and the YS harbors multipotent erythro-myeloid progenitors (EMPs), which lack long term reconstitution capacity and showed recently to be the origin of tissue resident macrophages and mast cells [Fig. 1] [7], [13], [14], [15]. One day later, around E9.5, the pre-HSCs emerge in the major arteries [16], [17] through an endothelial to hematopoietic transition mechanism (EHT) [18], [19], [20], show multipotency in vitro but do not reconstitute hematopoiesis in vivo [17]. Only after maturation in an organ culture system can they behave like their adult BM counterparts. Consistent with these observations, fate mapping studies showed that pre-HSCs labeled between E9.5 and E10.5 are the main source of adult HSC in the BM [21], [22], [23] and de novo production of HSC is no longer detected after E10.5. Thus at E10.5 the fetal liver (FL), the main hematopoietic organ during embryonic development, harbors highly proliferative multipotent progenitors of dual origin derived from both pre-HSCs generated in the major arterial vessels and from EMP generated in the YS [24]. This tight sequence of events and the dual source of blood cell generation raise the possibility that distinct lymphoid progenitors may exist before the emergence of HSC. Using Ncx1−/− mice, which lack heartbeat, Yoshimoto et al. showed that E9.5 YS harbors T-cell, B-1 cell and MZ B-cell progenitors suggesting that they originate in situ. However, while Ncx1−/− mice die at E11 both YS-derived and HSC-derived progenitors emerge normally [25]. Considering that pre-HSCs emerge from the dorsal aorta but also from the vitellin and umbilical arteries [26], [27] that connect the embryo proper to the YS arterial vessels, it is likely that the lymphoid progenitors found in the YS correspond to emerging pre or immature HSC. In a different approach, Kobayashi et al. used HSC-deficient embryos to probe the origin of B and T cells in the mouse embryo [28]. Cbfβ −/−mice are not viable after E13 and have severe defects in EMPs and HSCs because the function of all Runx genes is compromised [29]. When endothelial-specific receptor tyrosine kinase (Tek) regulatory regions were used to drive Cbfβ expression, EMP cells were restored but not HSC. However, low numbers of LSK cells were detected possibly accounting for the B and T found in these mice although long-term reconstitution was severely compromised.
Fig. 1.
Emergence of waves of hematopoietic progenitors in the mouse embryo. Abbreviations: YS: yolk sac; EMP: erythromyeloid progenitors; AGM: aorta, gonads and mesonephros; HSC: hematopoietic stem cells; BM: bone marrow.
Combining reporter mouse lines with lineage tracing, Boiers et al. identified a population of progenitors contributing to fetal lymphocytes and myeloid cells (though not tissue resident macrophages), with no contribution to erythrocytes or megakaryocytes [8]. This lymphomyeloid (LMPs) restricted progenitors were further shown to co-express lymphoid and myeloid-associated genes (Il7r, Rag1, Rag2, Flk2, Csf1r, Csf2r and Csf3r). That they preceded long-term reconstitution ability was used as argument to their HSC independence and potential YS origin. However, the lineage potential of these progenitors at early developmental stages has not been thoroughly addressed and, as we previously mentioned, although long term reconstitution is detected at low levels before E12.5 the immature or pre-HSC emerge between E9.5 and E10.5, are highly proliferative and can progress to differentiate before acquiring adult reconstitution capacity.
In conclusion it is now established that the B cell compartment is composed of cells that develop in successive waves differentially regulated in time and space, the contribution of the YS-derived progenitors to lymphopoiesis remains controversial and further studies are required to show to what extent, if at all, HSC-independent progenitors contribute to the lymphoid compartment.
Early B cell differentiation and commitment
Lymphocyte homeostasis is dependent on the constant production of B cells from HSC-derived progenitors in the bone marrow. However, whereas follicular B cells are profoundly affected when BM B cell production is interrupted, marginal zone and B-1 B cells are minimally affected. These results suggest that B-1 B cells and marginal zone B cells are preferentially produced early in life.
B-1a cell development during embryogenesis
Unlike adult HSCs, fetal HSCs are highly proliferative in FL and are unique in their capacity to generate innate-like lymphocyte subsets [30], [31]. Why are innate-like lymphocytes only produced by fetal HSCs? And to what extent are developmental timing and signals from the microenvironment orchestrating this process? Tissue resident B-1a cells are found in the peritoneal and pleural cavities, they are the main producers of natural IgM antibodies and respond to bacterial products in a T-independent manner [32]. B-1a cell generation decreases after birth and the B-1a cell compartment is sustained by self-renewal independent of adult HSCs input, unlike B-2 B cells that need continuous replenishment from adult HSCs [33]. Although it was shown that B-1a cells can also be produced from BM HSCs, it is consensual that FL hematopoietic progenitors are more efficient than their BM counterparts in the generation of this cell subset [34]. In order to identify which cells in FL are capable of generating B-1a cells, Ghosn et al. purified E15 FL LSK [35] that failed to generate B-1a cells. These data suggest that B-1a could be a distinct lineage independent of fetal HSCs, although, differences in the gating strategy for identifying HSCs might result in differences in the cellular output (reviewed in Beaudin et al., 2016) [36]. Moreover fetal HSCs are CD11b+, and some might have been eliminated due to the presence of anti-CD11b antibody in the lineage cocktail [31], [36].
Following similar line of research data from the Forsberg laboratory [37] used a Flk-Switch mouse model to define two distinct populations of fetal HSCs. The authors identified a developmentally restricted Flk2 dependent HSCs population with apparent long-term reconstituting capacity particularly efficient in generating B-1 cells that ceased to persist after birth [38]. These cells are different from adult HSCs in their bias to generate lymphoid cells particularly innate-like B-1a and Vγ5+ T cells and express lymphoid-associated genes (Il7r, Rag1, Flk2, Rag2 and Ccr9). This work raised the possibility of the existence of distinct fetal HSC lineages and again providing evidence that B-1a cells might be derived from progenitors distinct from those generating other B cell subsets. In this study however, the transient nature of these progenitors and their expression of lymphoid specific transcripts do not coincide with the generally accepted definition of HSC [39]. Therefore, further investigations are required to clarify the extent to which these cells differ from the lympho-myeloid primed progenitors (LMPP).
In line with these data, a recent report from Dorshkind laboratory supported that B-1a cells emerge from multiple waves of fetal progenitors [40]. The analysis of a PU.1 hypomorphic mouse model (UREΔ/Δ) showed that both B-1 and B-2 B cells are differentially sensitive to different levels of PU.1 expression depending on whether they were generated from early or later embryonic stages, further reinforcing the idea that fetal and adult B cells follow different developmental programs.
Following a different strategy, cellular barcoding was used to label fetal HSCs and evaluate heterogeneity in fetal B cell production [41]. It was shown that unlike some previously mentioned studies fetal HSC generate upon transfer both B-1 and B-2 cells and the same barcodes are shared between B-1, B-2 and HSCs indicating their common origin. Interestingly, secondary transplantation of sorted LSK cells from a recipient of barcoded fetal LSK exhibited a lower B-1a potential compared to primary recipients. These data indicated that fetal HSC progressively lose the capacity to generate B-1 cells upon transfer and this coincided with the downregulation of Lin28b, a negative regulator of the let-7 family of microRNA expressed in fetal but not adult HSCs [42]. Consistent with this observation, Lin28b overexpression in BM HSCs restored the capacity to generate fetal lymphopoiesis (though not Vγ5+ T cells) including B-1a cells providing evidence that Lin28b is a key modulator of B-1 cell production. In line with this study, in vivo barcoding experiments in the absence of transplantation found similar barcodes expressed in adult B-1 and B-2 cells indicting that HSC do generate both B cell compartments [23]. Furthermore, work from the Rajewsky laboratory showed that switching specificities of mature B cells from a B-2 to a B-1 B-cell receptor is sufficient to induce cell proliferation and acquisition of the B-1 phenotype and function. Altogether these latter experiments argue against a distinct HSC-independent origin of B-1 lymphocytes [43]. The role of Lin28b in the process of B-1 cell selection remains to be identified, as the B-2 to B-1 lineage transition induced by B cell receptor switch appears to be Lin28b independent.
Key transcriptional regulators of B-cell lineage
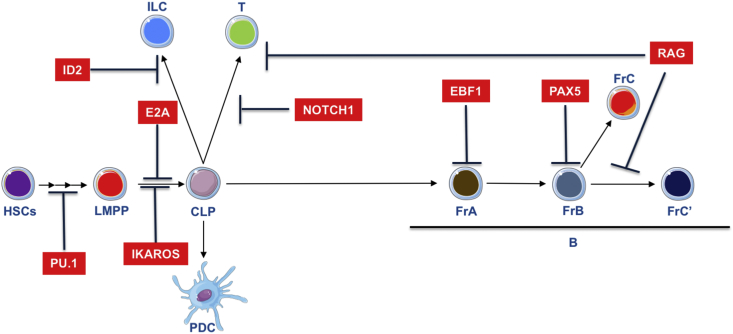
Lineage priming and commitment during hematopoiesis is a stepwise process starting in HSCs. During lymphoid specification, CLPs derived from HSC express the IL7Rα which is a hallmark of lymphoid commitment [44]. This lineage commitment process is achieved through differential expression of lineage specific transcription factors such as Pu.1, Ikaros, E2a, Ebf1 and Pax5. We will review the role of these key transcriptional factors in the establishment of B-cell identity [Fig. 2].
Fig. 2.
Major transcriptional requirements for lymphocyte development.
PU.1
Pu.1 is an ETS-domain transcription factor encoded by the Spi-1 gene that is exclusively expressed in hematopoietic cells during ontogeny [45], [46]. Pu.1 acts by binding to a purine-rich sequence (PU-box) near the promoter region of target genes. PU.1 deficient mice die around embryonic day E18 and lack both B cells and myeloid cells in the FL [46]. Of note, HSC [47], lympho-myeloid progenitors (AA4.1+, Lin−) [48], as well as CLPs and early B cell precursors (IL7R+Kit+) are dramatically reduced in PU.1 deficient embryos. In vitro cultures of any of these progenitors showed reduced capacity to differentiate into B or myeloid cells, indicating that Pu-1 acts at different stages of hematopoietic differentiation. Conditional deletion of Pu.1 in CD19-expressing B cells resulted in an increased compartment of cells resembling B-1 cells while B-2 cells were compromised. This imbalanced of B-1/B-2 cell development indicated a role of Pu.1 in B-2/B-1 cell reprograming [49] and is consistent with its role in the development of different B cell subsets [40].
Ikaros
Ikaros belongs to the family of zinc finger transcription factors, widely expressed in hematopoietic cells and one of the key regulators of hematopoiesis. In lymphoid lineage development, Ikaros deficient mice showed impaired B, T and NK cell production, while the myeloid and erythroid lineages were not significantly affected [50]. Similar deficiency of B, T, and NK compartments was observed in an Ikaros null mouse model in which the DNA binding site was deleted [51].
Ikaros regulates different aspects of B cell specification, development, maturation and response. Ikaros null LMPPs failed to express the lymphoid-associated transcripts Il7rα and Flt3, that are critical for early B lineage specification from lymphoid progenitors [51]. Ikaros also induced Ebf1 expression, which in turn promoted B cell differentiation by activating the B cell transcriptional program, and over expression of Ebf1 but not of Il7rα or Flt3 in Ikaros−/− LSK cells rescued B cell potential [52]. However, these rescued B lineage cells failed to recombine Igh locus demonstrating that Ikaros also activates Rag gene expression and controls VH gene accessibility [52].
During B cell responses in the periphery, Ikaros regulates B cell activation threshold [53], and antibody isotype selection via Ig class switch recombination (CSR). Ikaros deficient B cells showed higher sensitivity to anti-IgM stimulation and Ikaros regulates class switch recombination (CSR) by activating epigenetic marks and transcription at constant region gene promoters. Ikaros mutant mice exhibit reduction in serum IgG3 and IgG1, and increased in IgG2β and IgG2 production [53]. Ikaros binds the 3’ enhancer and S region promoters of Igh locus to increase AID accessibility to Sγ2β and Sγ2α to induce AID dependent CSR to IgG2β and IgG2α. Thus, Ikaros is a crucial regulator of CSR through modulating transcriptional competition between S regions.
E2A
The transcription factor E2a regulates gene expression by binding to the E box domain of DNA [54] and belongs to the helix-loop-helix (HLH) protein family. E2a is required for lymphopoiesis in regulating both B and T cell development. Further differentiation from CLP to B lineage is dependent on the successive expression of the transcriptional factors E2a, Ebf1, and Pax5 [55].
B cell development in E2A-deficient mice is arrested at the very early Pre-proB cell stage [56], [57], [58]. In the absence of E2a, lymphoid progenitors failed to express B lineage-associated genes, such as Rag1, Pax5, CD19, CD79a (mb1) and λ5, in addition, Ebf1 expression was suppressed. Moreover, induced expression of E2a in E2A-deficient progenitors can restore Ebf1 expression by binding and activating the promoter of Ebf1 [59], [60], [61]. Forced retroviral expression of Ebf1 in E2A-deficient progenitors restores their capacity to generate proB cells in vitro cultures, with normal V(D)J recombination at the IgH locus and normal expression of B-lymphoid-associated genes [62]. Altogether, these data indicate that E2a acts upstream of Ebf1 and Pax5 in the initiation of the B lineage differentiation program [56], [63].
Id2 that blocks E box protein function inhibits the T and B developmental programs restricting Id2 expressing CLP to the innate lymphoid differentiation pathway.
Ebf1
Ebf1 (Early B cell factor 1) is exclusively expressed in B lineage cells and has a regulatory role in the earliest stages of B cell specification and development [64]. Mice deficient in Ebf1 fail to express B lineage specific genes, such as Pax5, mb-1, surrogate light chain λ5 and VpreB [45], [64], [65]. B cell development in Ebf1−/− mice was blocked at Pre-proB stage and no heavy chain rearrangements were detected. This phenotype is similar to that found in E2A deficient mice, suggesting these two factors orchestrate B cell development. However, Ebf1 inactivation exclusively affects B cell development. This observation indicates that the transcription factors PU.1, Ikaros, and E2A, are expressed prior to Ebf1.
Ebf1 overexpression can launch the B cell program in progenitors deficient in upstream transcription factors, including PU.1 and E2A. Ectopic Ebf1 expression in E2A−/− [62], PU.1−/− [66], IL-7−/− [67], [68] mice can to some extend restore B cell development. Ebf1 is expressed in CLPs and coincides with B lineage restriction in Ly6D+Il7R+ CLPs [69], a stage at which most B cell specific genes are not expressed. Expression of Ebf1 in these cells is sufficient to activate a B lineage transcriptional network, comprising Pax5 [70] and silencing genes involved in T and NK cell development. Conditional deletion of Ebf1 in committed pro-B cells generated innate lymphoid cell (ILCs) and T cells after transfer into alymphoid mice. However, the T cells derived from Ebf1-deficient pro-B cells had rearrangements of loci encoding both B cell and T cell antigen receptors [71]. Thus, Ebf1 is required for B lineage commitment by blocking alternative cell fates.
Conditional deletion of Ebf1 at later B cell developmental stages did not result in major differences in the expression of a number of Ebf1 target genes, such as Pax5, mb-1 and CD79b [72].
Pax5
Pax5, also called B cell lineage specific activator protein (BSAP), is a member of a paired box family 5. Pax5 is exclusively expressed in B cells, starting at the pro-B cell stage [73]. One of the important roles of Pax5 is the regulation of CD19 expression. Deficiency of Pax5 arrests B cell development at a stage before pro-B stage in both fetal and adult cells [74].
Ebf1−/− deficient B cell progenitors cannot be rescued by Pax5 over-expression [66], suggesting that Pax5 acts downstream of E2a and Ebf1 in the transcriptional network of early B cell development [74]. In vitro cultures of Pax5−/− progenitors in the presence of IL-7 generated proB-like cells that further differentiated into macrophages, granulocytes, dendritic cells, or natural killer cells when IL-7 was withdrawn. In vivo in Rag−/− mice and in vitro co-culture with OP9-DL1 stromal Pax5−/− pro-B progenitor generated T cells [74]. These studies showed that Pax5 is critical to restrict the differentiation to B lineage pathway and inhibit alternative pathways.
B-cell lineage commitment: timing for lineage choice
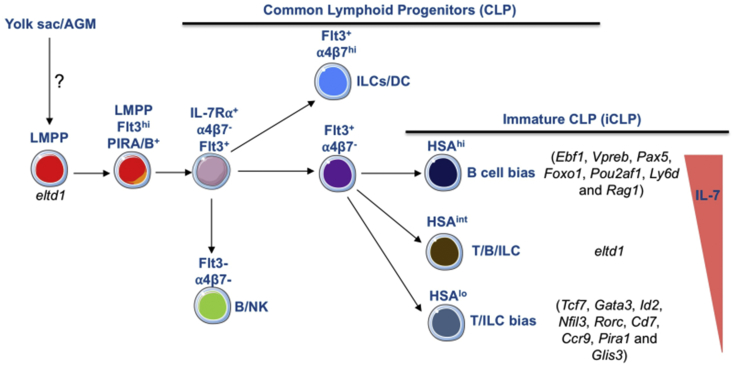
B cell development is one of the best studied differentiation processes orchestrated by known transcription factors that are activated early in B cell development and sustain their activity throughout the life of a B-cell [45]. Immune cells are produced from multipotent and self-renewing HSCs that give rise to multipotent progenitors (MPP). In the bone marrow, B cells develop from HSCs through a series of stepwise restriction towards the B cell lineage. Long-term (LT) HSCs give rise to short-term (ST) HSCs that expand and produce MPPs [75]. The MPPs are capable of generating all blood lineages but lack self-renewing capacity in transplantation experiments and give rise to LMPPs, which are the earliest progenitor cell to express lymphoid-associated genes (Il7r, Rag1, Rag2 and Flk2) and maintain myeloid potential but lack megakaryocyte-erythroid potential [21], [39], [76], [77]. LMPPs differentiate into common lymphoid progenitors (CLPs) that are Lin-c-KitloSca1loIL-7R+ [44]. These cells are different from LMPPs as they lost their myeloid potential and give rise to plasmacytoid dendritic cells (pDC), ILC, B and T cells [2], [45]. Of note, B cell developmental program depends on the successive upregulation of E2A, EBF1 and Pax5. In order to understand the initial stages of B lineage priming, we and others analyzed the CLP compartment in the FL. In the FL, CLPs [78] are identified as Lin-c-Kit+Sca1loIL-7R+ and they represent a heterogenous compartment capable of generating all lymphoid lineages including CD3- LTi [2]. Flk2 surface expression separates fetal CLPs into Flk2- population with restricted progenitors to B and ILC and a Flk2+ population representing immature progenitors capable of differentiating into B, T and ILC [Fig. 3] [79], [80]. Moreover, the integrin α4β7 further separate progenitors that lost B cell potential and retain T and ILC potential [81], [82]. The loss of T cell potential and the restriction to ILC is concomitant to the loss of Flk2 and the upregulation of C-X-C motif chemokine receptor 6 (CXCR6) [82]. Therefore, Lin−IL-7Rα+c-Kit+Sca-1loFlk2+α4β7- FL CLPs are the most immature CLPs (iCLPs) that generate all lymphoid lineages.
Fig. 3.
Lymphoid lineage choice during early stages of development in fetal liver. Abbreviations: LMPP: lympho-myeloid progenitors; HSA: heat stable antigen; ILC: innate lymphoid cells; DC: dendritic cells.
In order to further understand the molecular cues that control lineage choice before commitment, Berthault et al. used a combination of markers (Flk2, α4β7, HSA (also known as CD24) and PIRA/B) to address this question [83]. Based on the expression of HSA, the immature (i) CLPs (Flk2+ α4β7- IL-7Rα+) were separated into three different populations with different transcriptional signature and lymphocyte potential [Fig. 3]. The majority of iCLPs expressed intermediate levels of HSA (HSAint) and retained a robust B, T and ILC potential. The two other populations were either expressing high levels of HSA (HSAhi) or low level of HSA (HSAlo). While HSAlo iCLPs were biased toward T and ILC potential and had low B cell potential, HSAhi iCLPs were biased to generate B cells with low T and ILC potential. Single-cell multiplex transcriptional analysis showed that the B cell and T cell-bias signature were initiated at different stages of differentiation. While the transcriptional signature that marked cells biased towards the T/ILC lineage was detected in LMPPs, the B cell-bias signature was concomitant with the upregulation of IL-7rα and appeared only in CLPs. HSAint iCLPs were shown to derive from LMPPs that further differentiated into HSAhi iCLPs and HSAlo iCLPs. While HSAhi iCLPs expressed B-lineage associated genes (Ebf1, Vpreb, Pax5, Foxo1, Pou2af1, Ly6d and Rag1), HSAlo iCLPs expressed ILC-lineage associated genes (Tcf7, Gata3, Id2, Nfil3, Rorc, Cd7, Ccr9, Pira1 and Glis3) and some transcripts found in adult ETP (IMMGEN data base). However, they did not express known Notch1 signaling targets that are essential for T cell differentiation. Therefore, HSAlo iCLP although having partially lost B cell potential do not appear to be engaged in the T cell pathway of differentiation, only when they reach the thymus do we find signs of Notch activation (Hes1, Dtx) [83]. This sequence of events leads to the conclusion that the establishment of B versus T cell identity occurred at sequential stages of differentiation and it is not a binary choice, and, importantly, the loss of B cell lineage potential may take place before colonizing the thymus (for further discussion on this topic, also refer to Cumano et al., 2019 [84]).
Fetal hematopoietic microenvironment and lymphoid commitment
The FL provides the unique hematopoietic environment where in the mouse hematopoietic progenitors expand and differentiate. Once in the FL, lymphoid progenitors initiate differentiation and major differences between fetal and adult hematopoiesis have been detected. For example, IL-7 is an important cytokine for B cell development, in mice although not in humans. Il-7-defecient mice showed a complete absence of adult B cell development in BM and adult CLPs were reduced in numbers and in their B cell differentiation capacity [85]. However, fetal B cell development, although affected, was not absent and the numbers of CLPs were normal in the FL. These data indicated that fetal lymphopoiesis is partially independent of IL-7. Only when Il-7 and Flt3 deficiencies were combined, fetal lymphopoiesis were totally absent [86]. Because the analysis of FL CLP indicated that the B cell transcriptional program was only initiated after the expression of the IL-7Rα chain we investigated the role of IL-7 in B cell lineage priming and commitment in FL. In Il-7-defecient mice, the numbers of HSAlo iCLPs (that had lost B cell differentiation potential) were significantly increased while the numbers of HSAhi iCLPs (B biased) were reduced. In addition although the numbers of HSAin multipotent CLP were normal the numbers of cells that exhibited a B cell transcriptional priming was reduced compared to WT whereas the numbers of cells with T/ILC priming were increased [83]. These experiments indicated that IL-7 plays a role in determining the numbers of B and T/ILC biased progenitors during fetal development.
Between E12 and E15 the thymus is colonized by a first wave of fetal thymic settling progenitors (TSPs) similar in phenotype and function to HSAlo iCLPs and to circulating CLPs (CRLPs). At later stages the thymus is colonized by a second wave of LMPPs-like progenitors and FL does no longer support the production of HSAlo iCLPs [87]. A time course analysis showed that HSAlo iCLPs are abundant in FL between E11 and E15 and after E16 they were no longer detected. In contrast, HSAhi iCLPs were barely detected before E13 and gradually increased at later stages of development. This progression of events coincides with an upregulation in IL-7 production as development progresses in the FL suggesting a link between the presence of HSAlo iCLP and IL-7 availability. Consistent with this at E11 where availability of IL-7 is low the B lineage transcriptional signature is undetectable in IL-7Rα+ CLP. Taken together this data indicates that HSAlo iCLPs are a transient population only found at early stages of development and their presence is dependent on the absence of IL-7. At later stages IL-7 secures the expression of the B lineage transcriptional program and the thymus is colonized thereafter by multipotent progenitors that no longer can generate innate like lymphocytes and LTi. Therefore after the initial stages of FL hematopoiesis the CLP is devoted to B and ILC development and although maintaining T cell potential in vitro these progenitors show decreased efficiency in repopulating the T cell compartment, in vivo [51]. These data indicate that developmental timing and microenvironmental signals are associated with lineage choice and explain why only during early stages unique subsets of the immune system emerge.
Perspectives
In this review we discussed the origin(s) of lymphoid precursors and how the developmental timing and microenvironmental signals may contribute to the diversity of the immune system. It is clear that B cell development occurs in a multi-layered unique developmental program through successive waves of B cell progenitors. However, the origin of the first wave of B cell progenitors and tissue resident innate-like B-1a cells remains controversial. To determine the relative contribution of developmental timing on B cell output, efficient and faithful lineage tracing models will have to be developed. Lineage tracing or reporter mouse models that might be reliable and predictable during adult lymphopoiesis might not be so during FL lymphoid development that as we discussed above have a particular features.
The heterogeneity of fetal HSCs with respect to fetal lymphocyte production compared to their adult counterpart needs further investigation. Future work aimed at the functional characterization of cells derived from distinct origins will improve our understanding on how this HSC heterogeneity impacts on the establishment of the B cell compartment. Moreover, the transcriptional regulatory networks that govern B cell development are different in fetal and adult lymphopoiesis. How this molecular regulation is related to their cellular origin? And to what extent microenvironmental signals contribute to a lineage choice? Addressing these questions represent a major challenge in B cell development and understanding lymphoid cell ontogeny in general. Advances in lineage tracing and the growing field of high-throughput single cell transcriptional and epigenetic analysis might help in providing new insights into how successive layers during ontogeny contribute to different lineage output and deciphering their functional heterogeneity.
Conflicts of interest
The authors declare they have no conflict of interests.
Acknowledgments
We thank to members of the A.C. laboratory for many fruitful discussions.
This work was financed by the Institut Pasteur, INSERM, ANR (grant Twothyme) to A.C., REVIVE Future Investment Program to A.C. and R.E. and Pasteur-Weizmann Foundation through grants to A.C. J.Y. is part of the Pasteur – Paris University (PPU) International PhD program. This project has received funding from the CNBG Company, China.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Cumano A., Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 2.De Obaldia M.E., Bhandoola A. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu Rev Immunol. 2015;33:607–642. doi: 10.1146/annurev-immunol-032414-112032. [DOI] [PubMed] [Google Scholar]
- 3.Kristiansen T.A., Vanhee S., Yuan J. The influence of developmental timing on B cell diversity. Curr Opin Immunol. 2018;51:7–13. doi: 10.1016/j.coi.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Perdiguero E.G., Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen M.M., Witherden D.A., Havran W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzenberg L.A., Herzenberg L.A. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 7.Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C.A., Azzoni E. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto M., Montecino-Rodriguez E., Ferkowicz M.J., Porayette P., Shelley W.C., Conway S.J. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grönwall C., Vas J., Silverman G.J. Protective roles of natural IgM antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palis J., Robertson S., Kennedy M., Wall C., Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Dev Camb Engl. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 12.McGrath K.E., Koniski A.D., Malik J., Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1675. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand J.Y. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 14.Gentek R., Ghigo C., Hoeffel G., Bulle M.J., Msallam R., Gautier G. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 2018;48:1160–1171. doi: 10.1016/j.immuni.2018.04.025. e5. [DOI] [PubMed] [Google Scholar]
- 15.Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumano A., Dieterlen-Lievre F., Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 17.Medvinsky A., Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 18.Kissa K., Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y.R., Traver D. Hematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boisset J.-C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 21.Busch K., Klapproth K., Barile M., Flossdorf M., Holland-Letz T., Schlenner S.M. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 22.Samokhvalov I.M., Samokhvalova N.I., Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 23.Pei W., Feyerabend T.B., Rössler J., Wang X., Postrach D., Busch K. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature. 2017;548:456–460. doi: 10.1038/nature23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath K.E., Frame J.M., Fegan K.H., Bowen J.R., Conway S.J., Catherman S.C. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes K.E., Gekas C., Wang Y., Lux C.T., Francis C.S., Chan D.N. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bruijn M.F.T.R., Speck N.A., Peeters M.C.E., Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon-Keylock S., Sobiesiak M., Rybtsov S., Moore K., Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122:2338–2345. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M., Shelley W.C., Seo W., Vemula S., Lin Y., Liu Y. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbf for their development. Proc Natl Acad Sci. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bruijn M., Dzierzak E. Runx transcription factors in the development and function of the definitive hematopoietic system. Blood. 2017;129:2061–2069. doi: 10.1182/blood-2016-12-689109. [DOI] [PubMed] [Google Scholar]
- 30.Hadland B., Yoshimoto M. Many layers of embryonic hematopoiesis: new insights into B-cell ontogeny and the origin of hematopoietic stem cells. Exp Hematol. 2018;60:1–9. doi: 10.1016/j.exphem.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison S.J., Hemmati H.D., Wandycz A.M., Weissman I.L. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzenberg L.A. Layered evolution in the immune system: a view from history: layered evolution in the immune system. Ann N Y Acad Sci. 2015;1362:1–5. doi: 10.1111/nyas.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzenberg L.A., Kantor A.B., Herzenberg L.A. Layered evolution in the immune system: a model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 34.Duber S., Hafner M., Krey M., Lienenklaus S., Roy B., Hobeika E. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 35.Ghosn E.E.B., Waters J., Phillips M., Yamamoto R., Long B.R., Yang Y. Fetal hematopoietic stem cell transplantation fails to fully regenerate the B-lymphocyte compartment. Stem Cell Rep. 2016;6:137–149. doi: 10.1016/j.stemcr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaudin A.E., Forsberg E.C. To B-1a or not to B-1a: do hematopoietic stem cells contribute to tissue-resident immune cells? Blood. 2016;128:2765–2769. doi: 10.1182/blood-2016-10-697813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyer S.W., Schroeder A.V., Smith-Berdan S., Forsberg E.C. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaudin A.E., Boyer S.W., Perez-Cunningham J., Hernandez G.E., Derderian S.C., Jujjavarapu C. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19:768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adolfsson J., Månsson R., Buza-Vidas N., Hultquist A., Liuba K., Jensen C.T. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Montecino-Rodriguez E., Fice M., Casero D., Berent-Maoz B., Barber C.L., Dorshkind K. Distinct genetic networks orchestrate the emergence of specific waves of fetal and adult B-1 and B-2 development. Immunity. 2016;45:527–539. doi: 10.1016/j.immuni.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kristiansen T.A., Jaensson Gyllenbäck E., Zriwil A., Björklund T., Daniel J.A., Sitnicka E. Cellular barcoding links B-1a B cell potential to a fetal hematopoietic stem cell state at the single-cell level. Immunity. 2016;45:346–357. doi: 10.1016/j.immuni.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Yuan J., Nguyen C.K., Liu X., Kanellopoulou C., Muljo S.A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graf R., Seagal J., Otipoby K.L., Lam K.-P., Ayoub S., Zhang B. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. doi: 10.1126/science.aau8475. [DOI] [PubMed] [Google Scholar]
- 44.Kondo M., Weissman I.L., Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 45.Rothenberg E.V. Transcriptional control of early T and B cell developmental choices. Annu Rev Immunol. 2014;32:283–321. doi: 10.1146/annurev-immunol-032712-100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKercher S.R., Torbett B.E., Anderson K.L., Henkel G.W., Vestal D.J., Baribault H. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H.-G. The ETS family transcription factor PU.1 is necessary for the maintenance of fetal liver hematopoietic stem cells. Blood. 2004;104:3894–3900. doi: 10.1182/blood-2002-08-2425. [DOI] [PubMed] [Google Scholar]
- 48.Scott E.W., Fisher R.C., Olson M.C., Kehrli E.W., Simon M.C., Singh H. PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid–myeloid progenitors. Immunity. 1997;6:437–447. doi: 10.1016/s1074-7613(00)80287-3. [DOI] [PubMed] [Google Scholar]
- 49.Ye M., Ermakova O., Graf T. PU.1 is not strictly required for B cell development and its absence induces a B-2 to B-1 cell switch. J Exp Med. 2005;202:1411–1422. doi: 10.1084/jem.20051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgopoulos K., Moore D., Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 51.Allman D., Sambandam A., Kim S., Miller J.P., Pagan A., Well D. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 52.Reynaud D., Demarco I.A., Reddy K.L., Schjerven H., Bertolino E., Chen Z. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirstetter P., Thomas M., Dierich A., Kastner P., Chan S. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32:720–730. doi: 10.1002/1521-4141(200203)32:3<720::AID-IMMU720>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 54.Lin H., Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 55.Sigvardsson M., Clark D.R., Fitzsimmons D., Doyle M., Akerblad P., Breslin T. Early B-cell factor, E2A, and pax-5 cooperate to activate the early B cell-specific mb-1 promoter. Mol Cell Biol. 2002;22:8539–8551. doi: 10.1128/MCB.22.24.8539-8551.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seet C.S., Brumbaugh R.L., Kee B.L. Early B cell factor promotes B lymphopoiesis with reduced interleukin 7 responsiveness in the absence of E2A. J Exp Med. 2004;199:1689–1700. doi: 10.1084/jem.20032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina K.L., Pongubala J.M.R., Reddy K.L., Lancki D.W., DeKoter R., Kieslinger M. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Dias S., Silva H., Cumano A., Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kikuchi K., Lai A.Y., Hsu C.-L., Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inlay M.A., Bhattacharya D., Sahoo D., Serwold T., Seita J., Karsunky H. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansson R., Zandi S., Welinder E., Tsapogas P., Sakaguchi N., Bryder D. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 62.Nechanitzky R., Akbas D., Scherer S., Györy I., Hoyler T., Ramamoorthy S. Transcription factor EBF1 is essential for the maintenance of B cell identity and prevention of alternative fates in committed cells. Nat Immunol. 2013;14:867–875. doi: 10.1038/ni.2641. [DOI] [PubMed] [Google Scholar]
- 63.Hagman J., Travis A., Grosschedl R. A novel lineage-specific nuclear factor regulates mb-1 gene transcription at the early stages of B cell differentiation. EMBO J. 1991;10:3409–3417. doi: 10.1002/j.1460-2075.1991.tb04905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuxa M., Busslinger M. Reporter gene insertions reveal a strictly B lymphoid-specific expression pattern of Pax5 in support of its B cell identity function. J Immunol. 2007;178:3031–3037. doi: 10.4049/jimmunol.178.5.3031. [DOI] [PubMed] [Google Scholar]
- 65.Nutt S.L., Heavey B., Rolink A.G., Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 66.Massari M.E., Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nutt S.L., Kee B.L. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Robanus EC, Izon DJ, Amsen D, Weintraub C, Krop I, Schlissel MS, et al. E2A proteins are required for proper B cell development and initiation of lmmunoglobulin gene rearrangements. Cell 1994;79:885-892. [DOI] [PubMed]
- 69.Borghesi L., Aites J., Nelson S., Lefterov P., James P., Gerstein R. E47 is required for V(D)J recombinase activity in common lymphoid progenitors. J Exp Med. 2005;202:1669–1677. doi: 10.1084/jem.20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhuang Y. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 71.Smith E.M.K., Gisler R., Sigvardsson M. Cloning and characterization of a promoter flanking the early B cell factor (EBF) gene indicates roles for E-proteins and autoregulation in the control of EBF expression. J Immunol. 2002;169:261–270. doi: 10.4049/jimmunol.169.1.261. [DOI] [PubMed] [Google Scholar]
- 72.Roessler S., Gyory I., Imhof S., Spivakov M., Williams R.R., Busslinger M. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ikawa T., Masuda K., Lu M., Minato N., Katsura Y., Kawamoto H. Identification of the earliest prethymic T-cell progenitors in murine fetal blood. Blood. 2004;103:530–537. doi: 10.1182/blood-2003-06-1797. [DOI] [PubMed] [Google Scholar]
- 74.Bain G., Maandag E.C.R., te Riele H.P.J., Feeney A.J., Sheehy A., Schlissel M. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 75.Höfer T., Busch K., Klapproth K., Rodewald H.-R. Fate mapping and quantitation of hematopoiesis in vivo. Annu Rev Immunol. 2016;34:449–478. doi: 10.1146/annurev-immunol-032414-112019. [DOI] [PubMed] [Google Scholar]
- 76.Månsson R., Hultquist A., Luc S., Yang L., Anderson K., Kharazi S. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Sawai C.M., Babovic S., Upadhaya S., Knapp D.J.H.F., Lavin Y., Lau C.M. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mebius R.E., Miyamoto T., Christensen J., Domen J., Cupedo T., Weissman I.L. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3- cells, as well As macrophages. J Immunol. 2001;166:6593–6601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 79.Karsunky H., Inlay M.A., Serwold T., Bhattacharya D., Weissman I.L. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pereira de Sousa A., Berthault C., Granato A., Dias S., Ramond C., Kee B.L. Inhibitors of DNA binding proteins restrict T cell potential by repressing Notch1 expression in flt3-negative common lymphoid progenitors. J Immunol. 2012;189:3822–3830. doi: 10.4049/jimmunol.1103723. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida H., Kawamoto H., Santee S.M., Hashi H., Honda K., Nishikawa S. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511–2521. doi: 10.4049/jimmunol.167.5.2511. [DOI] [PubMed] [Google Scholar]
- 82.Possot C., Schmutz S., Chea S., Boucontet L., Louise A., Cumano A. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 83.Berthault C., Ramond C., Burlen-Defranoux O., Soubigou G., Chea S., Golub R. Asynchronous lineage priming determines commitment to T cell and B cell lineages in fetal liver. Nat Immunol. 2017;18:1139–1149. doi: 10.1038/ni.3820. [DOI] [PubMed] [Google Scholar]
- 84.Cumano A., Berthault C., Ramond C., Petit M., Golub R., Bandeira A. New molecular insights into immune cell development. Annu Rev Immunol. 2019;37:497–519. doi: 10.1146/annurev-immunol-042718-041319. [DOI] [PubMed] [Google Scholar]
- 85.Carvalho T.L., Mota-Santos T., Cumano A., Demengeot J., Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/-) mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vosshenrich C.A.J., Cumano A., Muller W., Di Santo J.P., Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci. 2004;101:11070–11075. doi: 10.1073/pnas.0402919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramond C., Berthault C., Burlen-Defranoux O., de Sousa A.P., Guy-Grand D., Vieira P. Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat Immunol. 2013;15:27–35. doi: 10.1038/ni.2782. [DOI] [PubMed] [Google Scholar]