Abstract
Maturation of B cells in Germinal Centers (GC) is a hallmark in adaptive immunity and the basis of successful vaccines that protect us against lethal infections. Nonetheless, vaccination efficacy is very much reduced in aged population and against highly mutagenic viruses. Therefore, it is key to understand how B cell selection takes place in GC in order to develop new and fully protective vaccines. The cellular mechanisms that control selection of GC B cells are performed by different T cell populations. On one side, cognate entanglement of B cells with T follicular helper (Tfh) cells through cytokines and co-stimulatory signals promotes survival, proliferation, mutagenesis and terminal differentiation of GC B cells. On the other hand, regulatory T cells have also been reported within GC and interfere with T cell help for antibody production. These cells have been classified as a distinct T cell sub-population called T Follicular regulatory cells (Tfr). In this review, we investigate the phenotype, function and differentiation of these two cell populations. In addition, based on the different functions of these cell subsets, we highlight the open questions surrounding their heterogeneity.
Keywords: T lymphocytes, Immune regulation, Germinal center, Antibody response
Heightened reactivity to antigen (Ag) recall is the central defining characteristic of adaptive immunity. Protein vaccination co-opts this process to generate memory cells endowed with enhanced or novel functional capacities capable of inducing an amplified and faster immune response to subsequent pathogen exposure. As such, immune memory can confer long-term protection of the host against microbial infections.
In the course of Antibody (Ab) responses to T cell-dependent Ag, Ag-activated B cells require cooperation with T cells at the border of the B and T cell zones in secondary lymphoid organs (SLO). This cell interaction is followed by the migration of some of the interacting T and B cells into the adjacent B cell follicles where they initiate the germinal center (GC) reaction. GC B cells then acquire somatic mutations in their Ag receptors (BCR) and activate class-switch recombination through the action of AID (Activation-Induced cytidine Deaminase). This process is responsible for the affinity maturation of the Ab response. GC B cells generate two types of progeny: long-lived PC secreting high-affinity Ab and memory B cells, which upon Ag re-exposure, can mount an amplified and accelerated Ab response of higher affinity than the primary Ab response.
Tight regulation of mutagenesis and B cell selection is essential in GC. The cellular mechanisms that control positive selection of GC B cells rely on a specialized spectrum of functions that are delivered by T cells. More precisely, interaction of B cells with T follicular helper cells (Tfh) is at the centre of this process. Tfh cells provide positive help to the selected GC B cells bearing high-affinity Ab. Tfh-B interactions involve TCR-pMHCII, co-stimulatory molecules and cytokines that regulate commitment to Ab isotype switch, survival, proliferation and terminal differentiation of B cells into PC and memory B cells. Notably, the type of pathogen and the inflammatory context imprint the cytokines produced by Tfh cells and, eventually, direct the Ab isotype switch. Until recently, the processes of B cell selection were only assigned to Tfh cells. However, it was demonstrated in the past years that other T cells, the follicular Foxp3+ regulatory T cells (Tfr), were also major actors of GC reactions. Their main function was coined to the regulation of the GC magnitude by controlling GC B cell and GC T cell extent. However, recent evidences show that the Tfr cell compartment is not homogeneous and that Tfr cells also regulate affinity maturation and class switch.
This review discusses the established phenotypes and functions of Tfh and Tfr cells, emphasizing the complementary role of these two cell populations in the regulation of B cell responses in the GC.
T follicular helper cells (Tfh): the cognate regulators of B cell responses
-
•
Phenotype of Tfh cells
By the mid 1960s, studies of neonatal thymectomy identified an essential role of the thymus for efficient adaptive immunity and transfers of bone marrow (BM) and thymus cell mixtures suggested these cells worked together. Subsequent studies of the hapten-carrier effect began to probe the mechanisms of T-B cell collaboration with the idea of an Ag bridge. It is now well described that inflammation induces dendritic cell (DC) maturation and their migration to local draining lymphoid tissues. In SLO, Ag-experienced DC prime naïve T helper cells. These events result in the development of effector Th cells with a variety of phenotypes and functions. Among them, Tfh cells are considered as the cognate regulators of the B cell response. Tfh cell differentiation is a multistep process that involves continuous interaction with Ag-presenting cells, but also co-stimulatory signals and an appropriate cytokine milieu.
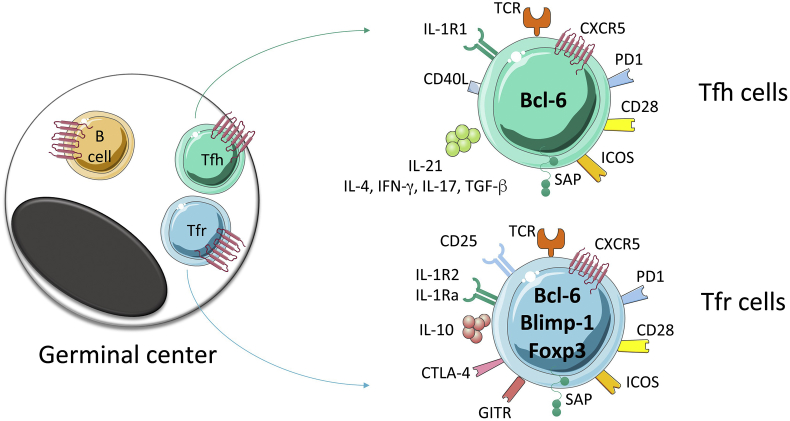
One of the main features of Tfh cells is their localization into B cell area in lymphoid follicles. Tfh cells express the chemokine receptor CXCR5, receptor for chemokine ligand 13 (CXCL13), which in association with CCR7 down-regulation allows their re-positioning in B follicles [1] [Fig. 1]. Specific deletion of CXCR5 in T cells using mixed BM chimera clearly demonstrated that, indeed, CXCR5+ Th cells are essential to support B cell responses [2]. The expression of co-stimulatory molecules can also be specifically associated to Tfh cell phenotypes and functions. CD28 and OX40 engagements at the surface of activated Th cells induce CXCR5 expression and promote GC formation [3]. CD40L-CD40 interactions promote B cell survival and BCR isotype switch [4]. ICOS-deficient mice challenged with T-cell-dependent Ag showed impaired primary antibody (Ab) responses [5] and ICOS-L-ICOS interactions have been shown to be critical for Tfh cell differentiation [6]. SLAM-associated protein (SAP) deficiency specifically in T cells interferes with GC formation since SAP is important to stabilize prolonged T-B interactions [7], [8]. Finally, Tfh cells also highly express the inhibitory receptor PD-1, whose ligands PD-L1 and PD-L2 are expressed by GC B cells, and their interactions suppress Tfh cell recruitment and survival [9], [10]. Despite this negative effect, PD-1 was also reported to be required for optimal GC localization of Tfh cells and for setting the threshold for GC B cell selection [11]. Overall, the strength and quality of Tfh-B cell interaction is a central factor for GC reaction and the more interaction with Tfh cells is stable, the more GC B cells become PC versus recycling GC B cells [12]. While Tfh cells control B cell responses, the reciprocal is also true and Tfh-B cell interactions shape the Tfh compartment. Indeed, B/Tfh cell interaction stabilizes expression of Bcl-6 in Tfh cells as well as CXCR5 expression [13]. Further, B cell expression of ICOS-L is required for Tfh cell differentiation and GC reaction [14]. Finally, naïve B cells receiving high intensity signal through their BCR are more prone to extra-follicular response whereas low BCR signal induces GC [15], [16], a phenomenon relying on the correlation between BCR affinity and ICOS-L expression, which ultimately modulates Tfh cell development and survival [17].
-
•
Differentiation of Tfh cells
Fig. 1.
Some of the basic molecules expressed by Tfh and Tfr cells. Tfh and Tfr cells act within follicles and light zones of germinal centers (left) and localize there, together with germinal center B cells, through the attraction of CXCL13, which binds to CXCR5 at the surface of Tfh cells, Tfr cells and B cells. Functions of Tfh cells and Tfr cells involve expression of various molecules and secretion of soluble factors (right). Not all of these are exclusive to Tfh and Tfr cells. For example, ICOS is also highly expressed by other effector Th cells and cytokines such as IL-4 and IFN-γ are also produced and secreted by Th2 and Th1 cells.
The relationship of Tfh cells to other effector Th cell subsets and the mechanisms that control Tfh cell differentiation are now well described [Fig. 2, left]. Gene expression analyses in Tfh cells showed that these cells are distinct from other Th cell subsets [18]. Tfh cells were shown to express specifically the transcription repressor Bcl-6, and since then Tfh cells have been coined as an independent Th cell subset exhibiting a unique CXCR5hiCCR7loPD-1+ICOS + surface phenotype [19], [20], [21]. After Ag priming, Bcl-6 expression in Th cells is inhibited by high concentrations of IL-2 [22], [23] and supported by IL-6 and IL-21 produced locally that, ultimately, promote Tfh cell development [14], [24]. While human and mouse Tfh cells do not differ phenotypically nor functionally, the cytokine TGF-β acts with IL-12 and IL-23 to promote the differentiation of Tfh cells in Human by inducing CXCR5 and Bcl-6 expression [25]. In contrast, in mice, TGF-β signals suppress Tfh cell differentiation by blocking ICOS and Bcl-6 expression [26]. When DC primed naïve Th cells, strength of TCR-pMHCII recognition has been shown to control Th cell clonal selection [27]. Consequently, a single naïve Th cell can produce different types of effector cells. It was further shown that naive Th cells expressing high-affinity TCR are better instructed to become Tfh cells [28], [29] and that a correlation between TCR signal strength and IL-2 production stratifies the divergence between Tfh and non-Tfh cell fate [30]. Moreover, continuous interaction with Ag-presenting cells influence Tfh cell survival since Tfh cell frequencies are significantly higher with increased amounts of available Ag, after chronic antigenic stimulation through multiple immunization [31], [32].
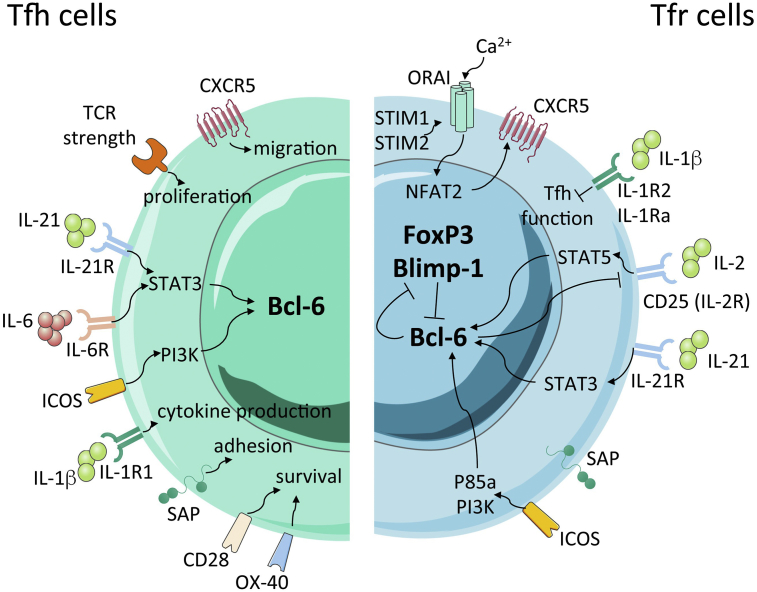
Fig. 2.
Signalling and transcriptional regulation of Tfh and Tfr cells. While the Tfh cell program is controlled by Bcl-6 (left), a balance between Bcl-6 and Blimp-1 is critical for the establishment of a full transcriptional profile of Tfr cells (right), which also express FoxP3. IL-21, through IL-21R/STAT3 signalling, promotes Bcl-6 expression while IL-2/STAT5 signalling inhibits Tfh cell differentiation and controls Bcl-6-dependent Tfr-cell program through Blimp-1 up-regulation. Additionally, IL-1β promotes cytokine production by Tfh cells such as IL-4 and IL-21 through its agonist receptor IL-1R1, while it induces Tfh cell inhibition by Tfr cells though its antagonistic receptors, IL-1Ra and IL-1R2. Finally, ICOS signalling through PI3K protects Bcl-6 from ubiquitin-dependent proteasome degradation.
The signals provided by Tfh cells to B cells also include cytokines. Among them, IL-21 is not only critical for Tfh cell development but is also a key regulator of GC, as in its absence, B cells show defects in affinity maturation and generation of long-lived PC [33], [34]. Tfh cells also produce cytokines shared by other Th cell subsets such as IFN-γ, IL-4, TGF-β and IL-17, which promote B cell isotype switch [35], [36], [37]. In addition, IL-9 produced by Tfh cells controls B cell progeny and is essential for optimal formation of memory B cells [38]. However, whether these cytokines are secreted by bona-fide Tfh cells or by effector Th cells that have acquired Tfh features is still unsolved. Indeed, while Tfh cells form a specific lineage of Th cells, it was shown that Th1 and Th2 are plastic and can become Tfh cells [39], this plasticity towards the Tfh lineage being in part dependent on the inflammatory context [40]. In contrast, it was also demonstrated that Tfh cells progressively differentiate during the GC reaction from IL-21 producing cells to IL-4 producing cells in order to regulate the GC response [41].
-
•
Circulating and memory Tfh cells
Tfh cells therefore compose a heterogeneous compartment of effector cells found within and in proximity to GC in SLO. Anyhow, Th cells expressing CXCR5 were reported outside SLO in human blood more than 20 years ago [42]. Since then, it was shown that these circulating Th cells actually include several populations with unique phenotypes and functions. Based on their chemokine receptor expression, they were subdivided in circulating Tfh cells that share properties with Th1 cells (CXCR3+CCR6-), Th2 cells (CXCR3-CCR6-) and Th17 cells (CXCR3-CCR6+) [43]. This circulating pool of Tfh cells was found to correlate with human Ab responses to vaccines, infections and auto-Ab responses [44], [45]. In addition, memory CXCR5+ Th cells were also discovered in draining lymphoid tissue in mice [46]. Using cell transfer experiment and reporter mice, it was demonstrated that memory Bcl-6+ CXCR5+ Th cells become effector Tfh cells upon reactivation [39]. The existence of memory Tfh cells was confirmed in both mice and humans [47], [48], [49], [50], [51] and their differentiation does not require participation in the GC reaction [52]. Interestingly, memory Tfh cells are not only found retained in SLO or circulating in the blood but can be present in the BM, where their function remains at issue [53], [54]. Notably, after Ag re-encounter, memory B cells induce rapid effector function by memory Tfh cells establishing the close relationship between these two cell compartments for initiation of secondary Ab responses [55]. Moreover, while local memory Tfh cells are found retained in the B follicle of SLO at proximity of memory B cells, circulating memory Tfh cells in non-draining lymphoid organs localize outside the B follicle. Interestingly, both local and circulating memory Tfh cell subsets sustain early B cell response after reactivation but exhibit different functions [56].
T follicular regulatory cells (Tfr): a multifunctional cell population
During GC reaction, a fine regulation is required to ensure the selection of the high-affinity B cell clones but also to prevent the emergence of auto-reactive B cells. For a long time, these attributes have been assigned to Tfh cells, through positive selection of the high-affinity B cell clones receiving survival signal following a cognate interaction. Anyhow, it is known that Foxp3+ regulatory T cells (Treg) play a pivotal role in maintaining immune self-tolerance and homeostasis. In 2004, a T cells with suppressive function in vitro had been visualized in human GC [57]. Few years after, three different groups explored the role of this Treg subset on the regulation of GC by using specific experimental mouse models of Tfr cell depletion [58], [59], [60]. It appeared from in vivo and in vitro data that Tfr cells are suppressor of the GC response; notwithstanding, the suppressive activity of Tfr cells has a critical impact on the quantity as well as the quality of the B cell response. Indeed, ablation of Tfr cells led to an increase in the magnitude of the Tfh and GC B cells. It also modified the affinity and isotype switch of Ab and promoted the generation of auto-reactive Ab. Overall, these observations suggest that Tfr cells have not only a passive suppressive function to maintain self-tolerance but they also participate to the process leading ultimately to effective B cells responses. Whether Tfr cells form a homogeneous multifunctional cell population that controls homeostasis, tolerance and B cell efficiency or whether these functions are achieved by distinct Tfr cell subsets still remain largely unknown.
-
•
Differentiation of Tfr cells
Similar to Tfh cells, Tfr cell differentiation is a multistep process that starts at initial DC priming [59] [Fig. 2, right]. We recently identified that Tfr cells can be specific for the immunizing Ag, irrespective of whether it is a self or a foreign Ag [61]. Interestingly, transient Treg cell depletion at the time of immunization after diphtheria toxin (DT) injection in mice expressing DT receptor under the control of Foxp3 promoter resulted in a smaller Tfr cell compartment [59] and a profound diminishment of Ag-specific Tfr cells [61]. We also showed that, in addition to developing from thymic derived Treg cells, Tfr cells could also arise from Foxp3neg precursors if the adjuvant used was one that supports T cell plasticity [61]. Anti-PD-L1 blocking Ab treatment during initial priming led to a significant decrease of the Tfr cell pool derived from naïve T cells showing that this phenomenon was PD-L1 dependent [61]. Anyhow, recent studies showed that the TCR repertoires of the Tfh and Tfr cell compartments are distinct, with Tfh cells responding to foreign Ag and Tfr cells expressing potentially autoreactive TCR, suggesting that a majority of Tfr cells derived from thymic Treg [62], [63]. In mice, Tfh cells are absolutely dependent on B cells for their formation and on the GC for their maintenance. Similarly, Tfr cells have co-opted the same follicular differentiation pathways and Tfr cells therefore require TCR stimulation, SAP [59] as well as engagement of ICOS [64] and CD28 [65]. Anyhow, treatment with B cell-depleting Ab in human showed no effects on the circulating Tfr cell (cTfr) populations [66]. Further studies demonstrated that, indeed, cTfr cells were generated by B cell independent mechanisms during T cell priming by DC either in human [67] or mouse settings [68]. Overall, it demonstrates the crucial contribution of DC in the Tfr cell differentiation.
Despite their regulatory ability, Tfr cells share several common proprieties with Tfh cells [Fig. 1]. Among them, Tfr cells have similar expression pattern of several surface molecules. They express the chemokine receptor CXCR5, responsible for their re-positioning into the follicular region of SLO, as well as the surface molecules PD-1 and ICOS. The expression of CXCR5 is initiated by NFAT2 [69] and is maintained by Bcl-6 [59], [60]. Upon TCR engagement, stromal interaction molecule 1 (STIM1) and STIM2 were shown to mediate Store-operated Ca2+ entry (SOCE) through Ca2+ release-activated Ca2+ (CRAC) channels and to promote Tfr cell differentiation through NFAT-mediated IRF4, BATF, and Bcl-6 transcription factors [70]. Transcriptomic studies also revealed that CRAC channels control the lineage identity and effector functions of Tfr cells [71]. In Humans, loss-of function mutations in STIM 1 and ORA1 genes that abolish CRAC channel function displayed impaired production of specific-Ab upon vaccination or infection, highlighting the importance of CRAC channels in humoral immunity [72], [73]. In T cells, PI3 kinase (PI3K) is strongly induced by ICOS signaling, which is connected to rapamycin complex 1 and 2, mTOR1 and mTOR2, respectively [74], [75]. The p85 regulatory subunit of the PI3K interacts with intracellular osteopontin (OPN-i) allowing the translocation of OPN-i to the nucleus and its interaction with Bcl-6, which prevents Bcl-6 from ubiquitin-dependent proteasome degradation. Therefore p85-OPN-i axis is essential to sustain Tfh and Tfr cell formation [64]. While both mTOR1 and mTOR2 are required for Tfh cell differentiation [76], [77], a recent study has shown that mTOR1 but not mTOR2 was essential for both the differentiation and functional ability of Tfr cells by activating a transcriptional axis consisting of Stat3-TCF-1-Bcl-6 during immunization or infection [78].
In contrast to Tfh cells, Tfr cells express both Blimp-1 and Bcl-6, which are known to suppress each other [20] [Fig. 1]. It was shown that Blimp-1 deficiency enhances Tfr cell differentiation but reduces their suppressive ability [79]. In addition, the helix-loop-helix protein inhibitor of DNA binding 2 (Id2) and Id3 display distinct functions during the differentiation of Tfr cells. While Id2 and Id3 depletion in Treg cells resulted in increased Tfr cell formation, a small Id3 expression at the initial step of Tfr cell differentiation was essential to induce the specific transcription signature of Tfr cells [80]. Co-inhibitory signals are also important for Tfr cell differentiation and PD-1 and CTLA-4 deficiency resulted in a large increase of Tfr cells [81], [82], [83]. Finally, the cytokine cues play also an important role in controlling Tfr cell differentiation. IL-2 prevents Tfr cell differentiation, which is counterbalanced by Bcl-6 expression that rescues the Tfr cell program through the reduction of CD25 expression and subsequently IL-2 responsiveness [84]. Anyhow, not all the Tfr cells express CD25. It was indeed shown that CD25 + and CD25neg Tfr cells co-exist and follow distinct developmental waves mostly regulated by the nature of the Ag and IL-2 availability [85], [86]. In the context of systemic lupus erythematosus (SLE), low-dose IL-2 therapy reduced Tfh and Th17 cell subsets and promoted Treg cells [87]. Thereby, IL-2/CD25 axis may promote the generation of Tfr cells at early time while disturbing Tfr cell maintenance in the effector phase. While Treg/Th are mainly regulated by the IL-2 axis, Tfh cells lack the expression of CD25 [84]. It was further demonstrated that Tfh cells express the IL-1R1 agonist receptor, whereas Tfr cells express the IL-1 decoy receptor IL-1R2 and the IL-1 receptor antagonist IL-1Ra. IL-1 treatment expanded Tfh cells in vivo but Tfr cells suppressed the IL-1-dependent activation of Tfh cells. Thus, this study revealed an IL-1 axis in the control of the Tfr/Tfh cell axis [84]. Tfr cells do not express the B cell helper molecules (IL-21, IL-4 and CD40L) that are distinct properties of Tfh cells. Moreover, in contrast to Tfh cells, IL-21 was shown to limit the number of Tfr cells by regulating Bcl-6 expression in Tfr cells [88]. Interestingly, loss-of-function mutations in the IL-21R in human were shown to cause a primary immunodeficiency syndrome [89]. Indeed, these patients displayed an increase in circulating Treg and Tfr cell pool [88] and had a defective memory B cell compartment [90]. Further, in a mouse model of spontaneous autoimmune disease, ablation of the IL-21 gene increased the Tfr cell pool and the Tfr cell suppressive activity, which reduced GC formation and production of auto-Ab [91]. Overall, these studies highlight the regulation of the Tfr cell compartment, quantitatively and qualitatively, through the IL-21/IL-21R axis.
-
•
Tfr cells in physiological and pathological contexts
During B cell response, Tfh and Tfr cells play a crucial role in the generation of high-affinity Ab that could play either protective function during infection but also deleterious function when they are directed against self-Ag. In 2011, the pioneer studies revealed that Tfr cells are able to interfere with several events occurring during the GC reaction such as the modulation of Ab affinity as well as functions inherited from non-follicular Treg such as maintaining self-tolerance and immune homeostasis [58], [59], [60]. In response to a foreign Ag, using different experimental models to deplete Tfr cells, Linterman and colleagues observed a decrease of the high-affinity Ab in absence of Tfr cells while Chung and colleagues observed the opposite [59], [60]. This discrepancy certainly accounts for the different experimental systems used to deplete Tfr cell population in these two studies. More recently, by using a Foxp3cre/Bcl-6floxed mouse model, in which the Tfr cell compartment is depleted and the Treg one remains intact, it was shown that Tfr cell deficiency has no impact on the Tfh cell and GC B cell extent but induced a decrease in avidity of Ag-specific Ab and a decrease of Ag-specific IgG [92]. Therefore, these results suggested that Tfr cells could have a helper role in the GC-dependent B cell maturation. Using the same animal model, it was further shown that Tfr cell deficiency promoted late-onset spontaneous autoimmunity as well as aggravated an inducible Sjögren's syndrome model [93].
Studies performed in human did not show an uniform correlation between disease progression and level of cTfr cells [Table 1]. Indeed, in the peripheral blood of patients with Hashimoto's thyroiditis, allergic rhinitis and Sjögren's syndrome, the frequency of cTfr cells was found to be increased as compared to healthy donors and this frequency was positively correlated with clinical pathological score [94], [95], [96]. In contrast, in patients with multiple sclerosis (MS), myasthenia gravis (MG), SLE and immune thrombocytopenia, the cTfr cells were reported to be decreased [97], [98], [99], [100]. Intriguingly, in rheumatoid arthritis (RA) patients, the Tfr and Tfh cells were present at similar numbers from those of healthy donors [101]. Anyhow, in two recent studies, conflicting observations were made concerning cTfr cells in RA patients. Thus, the role of Tfr cells in RA progression is still debated [102], [103]. Many interpretations could explain such differences. First of all, studied patients can vary in term of disease duration and treatment status. Moreover, during infection, the nature of the pathogen was shown to impact differently the Tfr cell pool. Indeed, Tfr cells expand after resolution of influenza infection [85]. In contrast, during lymphocytic choriomeningitis virus (LCMV) infection, Tfr cells promote GC response through the production of IL-10 and follow the magnitude of the B cell response [104]. Therefore, depending on the Ag, the Tfr cells could be either an indicator of the ongoing or resolving GC reaction. Moreover, defective Treg suppressive activity has been linked to the pathogenesis of many autoimmune diseases such as MS and MG. In the context of Tfr cells, these cells may also display dysfunction. Finally, one other reason concerning these conflicting results of cTfr cell studies might be due to the variability of strategies used to track cTfr cells since distinct markers to delineate Tfr cell subsets among follicular T cells are used, either Foxp3, CD127 or CD25.
Table 1.
Magnitude of the Tfr cell pool during autoimmune diseases and infections.
| Inflammatory context | Species | Magnitude of the Tfr cell pool |
Reference |
|---|---|---|---|
| Hashimoto's thyroiditis | Human | Increase of cTfr cells | [94] |
| Allergic rhinitis | Human | Increase of cTfr cells | [95] |
| Sjögren's syndrome | Human | Increase of cTfr cells | [96] |
| mouse | Tfr cell deficiency enhances pathological signs | [93] | |
| Multiple sclerosis | Human | Decrease of cTfr cells | [97] |
| Myasthenia gravis | Human | Decrease of cTfr cells | [98] |
| Systemic lupus erythematosus | Human | Decrease of cTfr cells | [99] |
| mouse | Tfr cell deficiency enhances pathological signs | [92] | |
| Immune thrombocytopenia | Human | Decrease of cTfr cells | [100] |
| Rheumatoid arthritis | Human | Still in debate | [101], [102], [103] |
| LCMV infection | mouse | Tfr cells support GC formation | [104] |
| Influenza infection | mouse | Tfr cells expand after resolution of infection | [85] |
| mouse | Tfr cell deficiency promotes protection | [93] |
Conclusions
An efficient B cell immunity requires a dynamic equilibrium between positive and negative signals. It is now largely admitted that these signals are provided by distinct populations of T cells, the Tfh and Tfr cells. The distinct functions performed by these cells range from the control of Ab affinity, the maintenance of self-tolerance and immune homeostasis. Current studies on Tfh and Tfr cells highlight the capacity of these cells to sense the environment cues such as the nature of the Ag and/or the inflammatory context, which ultimately modulate their development and function. Very recently, it was even shown that alternative vaccination strategy such as slow delivery allows modulation of Ag immunodominance and promotes neutralizing antibodies to HIV envelope protein in non-human primates [105]. It is therefore important in the future to better define how the environmental factors influence quantitative and qualitative Tfh and Tfr cell functions, which ultimately could reveal new depths in the regulation of GC reaction that could eventually result in better vaccine protocols.
Fundings
The authors of this review are funded by INSERM and by Institut National contre le Cancer (INCA-12642), ANR (ANR-16-CE15-0019-02 and ANR-16-CE15-0002-02) and Fondation ARSEP (Fondation pour l’aide à la Recherche sur la sclérose en plaques).
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Haynes N.M., Allen C.D., Lesley R., Ansel K.M., Killeen N., Cyster J.G. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 2.Arnold C.N., Campbell D.J., Lipp M., Butcher E.C. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007;37:100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 3.Walker L.S., Gulbranson-Judge A., Flynn S., Brocker T., Raykundalia C., Goodall M. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 5.Dong C., Juedes A.E., Temann U.A., Shresta S., Allison J.P., Ruddle N.H. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y.S., Kageyama R., Eto D., Escobar T.C., Johnston R.J., Monticelli L. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crotty S., Kersh E.N., Cannons J., Schwartzberg P.L., Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 8.Qi H., Cannons J.L., Klauschen F., Schwartzberg P.L., Germain R.N. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good-Jacobson K.L., Szumilas C.G., Chen L., Sharpe A.H., Tomayko M.M., Shlomchik M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuccarino-Catania G.V., Sadanand S., Weisel F.J., Tomayko M.M., Meng H., Kleinstein S.H. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J., Hou S., Fang Q., Liu X., Liu X., Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. 2018;49:264–274. doi: 10.1016/j.immuni.2018.06.012. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ise W., Fujii K., Shiroguchi K., Ito A., Kometani K., Takeda K. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. 2018;48:702–715.e4. doi: 10.1016/j.immuni.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Baumjohann D., Okada T., Ansel K.M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 14.Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paus D., Phan T.G., Chan T.D., Gardam S., Basten A., Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor J.J., Pape K.A., Steach H.R., Jenkins M.K. Apoptosis and antigen affinity limit effector cell differentiation of a single naive B cell. Science. 2015;347:784–787. doi: 10.1126/science.aaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacquin A., Gador M., Fazilleau N. The strength of BCR signaling shapes terminal development of follicular helper T cells in mice. Eur J Immunol. 2017;47:1295–1304. doi: 10.1002/eji.201746952. [DOI] [PubMed] [Google Scholar]
- 18.Chtanova T., Tangye S.G., Newton R., Frank N., Hodge M.R., Rolph M.S. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston R.J., Poholek A.C., Ditoro D., Yusuf I., Eto D., Barnett B. Bcl6 and blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Ballesteros-Tato A., Leon B., Graf B.A., Moquin A., Adams P.S., Lund F.E. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston R.J., Choi Y.S., Diamond J.A., Yang J.A., Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt N., Liu Y., Bentebibel S.E., Munagala I., Bourdery L., Venuprasad K. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt N., Liu Y., Bentebibel S.E., Munagala I., Bourdery L., Venuprasad K. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human T cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malherbe L., Hausl C., Teyton L., McHeyzer-Williams M.G. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Fazilleau N., McHeyzer-Williams L.J., Rosen H., McHeyzer-Williams M.G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tubo N.J., Pagan A.J., Taylor J.J., Nelson R.W., Linehan J.L., Ertelt J.M. Single naive CD4(+) T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiToro D., Winstead C.J., Pham D., Witte S., Andargachew R., Singer J.R. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science. 2018;361 doi: 10.1126/science.aao2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumjohann D., Preite S., Reboldi A., Ronchi F., Ansel K.M., Lanzavecchia A. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Tam H.H., Melo M.B., Kang M., Pelet J.M., Ruda V.M., Foley M.H. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A. 2016;113:E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zotos D., Coquet J.M., Zhang Y., Light A., D'Costa K., Kallies A. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu H.C., Yang P., Wang J., Wu Q., Myers R., Chen J. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt R.L., Liang H.E., Locksley R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Shi J., Yan J., Xiao Z., Hou X., Lu P. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol. 2017;18:921–930. doi: 10.1038/ni.3788. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Yan X., Zhong B., Nurieva R.I., Wang A., Wang X. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med. 2012;209:1841–1852. doi: 10.1084/jem.20120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakarov S., Fazilleau N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol Med. 2014;6:590–603. doi: 10.1002/emmm.201403841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein J.S., Herman E.I., Lainez B., Licona-Limon P., Esplugues E., Flavell R. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17:1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 43.Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crotty S.T. Follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno H., Banchereau J., Vinuesa C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazilleau N., McHeyzer-Williams L.J., McHeyzer-Williams M.G. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Weber J.P., Fuhrmann F., Hutloff A. T follicular helper cells survive as long-term memory cells. Eur J Immunol. 2012;42:1981–1988. doi: 10.1002/eji.201242540. [DOI] [PubMed] [Google Scholar]
- 48.Luthje K., Kallies A., Shimohakamada Y., Belz G.T., Light A., Tarlinton D.M. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 49.Hale J.S., Youngblood B., Latner D.R., Mohammed A.U., Ye L., Akondy R.S. Distinct memory CD4 T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chevalier N., Jarrossay D., Ho E., Avery D.T., Ma C.S., Yu D. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 51.Locci M., Havenar-Daughton C., Landais E., Wu J., Kroenke M.A., Arlehamn C.L. Human circulating PD-1CXCR3CXCR5 memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J., Tsai L.M., Leong Y.A., Hu X., Ma C.S., Chevalier N. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Tokoyoda K., Zehentmeier S., Hegazy A.N., Albrecht I., Grun J.R., Lohning M. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Shinoda K., Tokoyoda K., Hanazawa A., Hayashizaki K., Zehentmeier S., Hosokawa H. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci U S A. 2012;109:7409–7414. doi: 10.1073/pnas.1118539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ise W., Inoue T., McLachlan J.B., Kometani K., Kubo M., Okada T. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci U S A. 2014;111:11792–11797. doi: 10.1073/pnas.1404671111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asrir A., Aloulou M., Gador M., Perals C., Fazilleau N. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun. 2017;8:847. doi: 10.1038/s41467-017-00843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim H.W., Hillsamer P., Kim C.H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Investig. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wollenberg I., Agua-Doce A., Hernandez A., Almeida C., Oliveira V.G., Faro J. Regulation of the germinal center reaction by foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 59.Linterman M.A., Pierson W., Lee S.K., Kallies A., Kawamoto S., Rayner T.F. Foxp3(+) follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung Y., Tanaka S., Chu F., Nurieva R.I., Martinez G.J., Rawal S. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aloulou M., Carr E.J., Gador M., Bignon A., Liblau R.S., Fazilleau N. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun. 2016;7:10579. doi: 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritvo P.G., Saadawi A., Barennes P., Quiniou V., Chaara W., El Soufi K. High-resolution repertoire analysis reveals a major bystander activation of Tfh and Tfr cells. Proc Natl Acad Sci U S A. 2018;115:9604–9609. doi: 10.1073/pnas.1808594115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maceiras A.R., Almeida S.C.P., Mariotti-Ferrandiz E., Chaara W., Jebbawi F., Six A. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun. 2017;8:15067. doi: 10.1038/ncomms15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leavenworth J.W., Verbinnen B., Yin J., Huang H., Cantor H. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang R., Sage P.T., Finn K., Huynh A., Blazar B.R., Marangoni F. B cells drive autoimmunity in mice with CD28-deficient regulatory T cells. J Immunol. 2017;199:3972–3980. doi: 10.4049/jimmunol.1700409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallin E.F., Jolly E.C., Suchanek O., Bradley J.A., Espeli M., Jayne D.R. Human T follicular helper and T follicular regulatory cell maintenance is independent of germinal centers. Blood. 2014;124:2666–2674. doi: 10.1182/blood-2014-07-585976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fonseca V.R., Agua-Doce A., Maceiras A.R., Pierson W., Ribeiro F., Romao V.C. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol. 2017;2:eaan1487. doi: 10.1126/sciimmunol.aan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sage P.T., Alvarez D., Godec J., von Andrian U.H., Sharpe A.H. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Investig. 2014;124:5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaeth M., Muller G., Stauss D., Dietz L., Klein-Hessling S., Serfling E. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med. 2014;211:545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaeth M., Eckstein M., Shaw P.J., Kozhaya L., Yang J., Berberich-Siebelt F. Store-operated Ca(2+) entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity. 2016;44:1350–1364. doi: 10.1016/j.immuni.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaeth M., Wang Y.H., Eckstein M., Yang J., Silverman G.J., Lacruz R.S. Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat Commun. 2019;10:1183. doi: 10.1038/s41467-019-08959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuchs S., Rensing-Ehl A., Speckmann C., Bengsch B., Schmitt-Graeff A., Bondzio I. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol. 2012;188:1523–1533. doi: 10.4049/jimmunol.1102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Picard C., McCarl C.A., Papolos A., Khalil S., Luthy K., Hivroz C. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powell J.D., Pollizzi K.N., Heikamp E.B., Horton M.R. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapman N.M., Chi H. mTOR links environmental signals to T cell fate decisions. Front Immunol. 2014;5:686. doi: 10.3389/fimmu.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Lin X., Pan Y., Wang J., Chen P., Huang H. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. eLife. 2016;5 doi: 10.7554/eLife.17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng H., Cohen S., Guy C., Shrestha S., Neale G., Brown S.A. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L., Huang Q., Wang H., Hao Y., Bai Q., Hu J. The kinase mTORC1 promotes the generation and suppressive function of follicular regulatory T cells. Immunity. 2017;47:538–551.e5. doi: 10.1016/j.immuni.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Yang G., Yang X., Zhang J., Li G., Zheng D., Peng A. Transcriptional repressor Blimp1 regulates follicular regulatory T-cell homeostasis and function. Immunology. 2018;153:105–117. doi: 10.1111/imm.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyazaki M., Miyazaki K., Chen S., Itoi M., Miller M., Lu L.F. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat Immunol. 2014;15:767–776. doi: 10.1038/ni.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sage P.T., Francisco L.M., Carman C.V., Sharpe A.H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2012;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sage P.T., Paterson A.M., Lovitch S.B., Sharpe A.H. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wing J.B., Ise W., Kurosaki T., Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Ritvo P.G., Churlaud G., Quiniou V., Florez L., Brimaud F., Fourcade G. Tfr cells lack IL-2Ralpha but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Sci Immunol. 2017;2:eaan0368. doi: 10.1126/sciimmunol.aan0368. [DOI] [PubMed] [Google Scholar]
- 85.Botta D., Fuller M.J., Marquez-Lago T.T., Bachus H., Bradley J.E., Weinmann A.S. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol. 2017;18:1249–1260. doi: 10.1038/ni.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wing J.B., Kitagawa Y., Locci M., Hume H., Tay C., Morita T. A distinct subpopulation of CD25- T-follicular regulatory cells localizes in the germinal centers. Proc Natl Acad Sci U S A. 2017;114:E6400–E6409. doi: 10.1073/pnas.1705551114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He J., Zhang X., Wei Y., Sun X., Chen Y., Deng J. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 88.Jandl C., Liu S.M., Canete P.F., Warren J., Hughes W.E., Vogelzang A. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat Commun. 2017;8:14647. doi: 10.1038/ncomms14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kotlarz D., Zietara N., Uzel G., Weidemann T., Braun C.J., Diestelhorst J. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210:433–443. doi: 10.1084/jem.20111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stepensky P., Keller B., Abuzaitoun O., Shaag A., Yaacov B., Unger S. Extending the clinical and immunological phenotype of human interleukin-21 receptor deficiency. Haematologica. 2015;100:e72–e76. doi: 10.3324/haematol.2014.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding Y., Li J., Yang P., Luo B., Wu Q., Zajac A.J. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu H., Chen Y., Liu H., Xu L.L., Teuscher P., Wang S. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol. 2016;46:1152–1161. doi: 10.1002/eji.201546094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu W., Liu X., Lin X., Feng H., Sun L., Li S. Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med. 2018;215:815–825. doi: 10.1084/jem.20170901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao J., Chen Y., Xu Z., Yang W., Zhu Z., Song Y. Increased circulating follicular regulatory T cells in Hashimoto's thyroiditis. Autoimmunity. 2018;51:345–351. doi: 10.1080/08916934.2018.1516759. [DOI] [PubMed] [Google Scholar]
- 95.Yao Y., Wang Z.C., Wang N., Zhou P.C., Chen C.L., Song J. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144:118–128. doi: 10.1016/j.jaci.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Fonseca V.R., Romao V.C., Agua-Doce A., Santos M., Lopez-Presa D., Ferreira A.C. The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary sjogren's syndrome. Arthritis Rheumatol. 2018;70:774–784. doi: 10.1002/art.40424. [DOI] [PubMed] [Google Scholar]
- 97.Dhaeze T., Peelen E., Hombrouck A., Peeters L., Van Wijmeersch B., Lemkens N. Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol. 2015;195:832–840. doi: 10.4049/jimmunol.1500759. [DOI] [PubMed] [Google Scholar]
- 98.Wen Y., Yang B., Lu J., Zhang J., Yang H., Li J. Imbalance of circulating CD4(+)CXCR5(+)FOXP3(+) Tfr-like cells and CD4(+)CXCR5(+)FOXP3(-) Tfh-like cells in myasthenia gravis. Neurosci Lett. 2016;630:176–182. doi: 10.1016/j.neulet.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 99.Xu B., Wang S., Zhou M., Huang Y., Fu R., Guo C. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol. 2017;183:46–53. doi: 10.1016/j.clim.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui Y., Guan Y., Liu W., Li Y., Li H., Guo M. The changes of circulating follicular regulatory T cells and follicular T helper cells in children immune thrombocytopenia. Zhonghua Xue Ye Xue Za Zhi. 2014;35:980–984. doi: 10.3760/cma.j.issn.0253-2727.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Pandya J.M., Lundell A.C., Hallstrom M., Andersson K., Nordstrom I., Rudin A. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol. 2016;100:823–833. doi: 10.1189/jlb.5A0116-025R. [DOI] [PubMed] [Google Scholar]
- 102.Liu C., Wang D., Lu S., Xu Q., Zhao L., Zhao J. Increased circulating follicular Treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol. 2018;70:711–721. doi: 10.1002/art.40430. [DOI] [PubMed] [Google Scholar]
- 103.Romao V.C., Fonseca J.E., Agua-Doce A., Graca L. T Follicular Regulatory Cells Are Decreased in Patients With Established Treated Rheumatoid Arthritis With Active Disease: comment on the Article by Liu et al. Arthritis Rheumatol. 2018;70:1893–1895. doi: 10.1002/art.40586. [DOI] [PubMed] [Google Scholar]
- 104.Laidlaw B.J., Lu Y., Amezquita R.A., Weinstein J.S., Vander Heiden J.A., Gupta N.T. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol. 2017;2:eaan4767. doi: 10.1126/sciimmunol.aan4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cirelli K.M., Carnathan D.G., Nogal B., Martin J.T., Rodriguez O.L., Upadhyay A.A. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2019;177 doi: 10.1016/j.cell.2019.04.012. 1153–71.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]