Abstract
Purpose
Discoveries of oncogenic driver alterations in non–small-cell lung cancer (NSCLC) have been accompanied by the development of effective targeted therapies. The frequencies of these mutations vary between populations but are less well characterized in the Vietnamese population. In this study, we analyzed the frequencies of lung cancer driver oncogenic alterations in Vietnamese patients compared with Vietnamese patients treated in the United States.
Methods
We collected data on tumor and disease characteristics of Vietnamese patients with NSCLC treated at Stanford. In addition, we collected NSCLC tumor specimens from patients with NSCLC diagnosed in Hue, Vietnam, and performed next-generation–based genotyping on these samples. The molecular and clinical characteristics of these groups were compared.
Results
Fifty-nine Vietnamese patients were identified at Stanford. Of the 44 patients with molecular testing results, there were 21 (47.7%) with EGFR alterations, six (13.6%) with ALK alterations, two (4.5%) with KRAS alterations, one (2.3%) with BRAF alterations, and no ROS1 or RET alterations. Across all stages, the median overall survival for patients with a tumor having a targetable genomic alteration driver mutation was 42.4 months, compared with 27.1 months for patients without such alterations. In the 45 genotyped samples from Vietnam, there were 26 (57.8%) with EGFR, 11 (24.4%) with KRAS, and one each (2.2%) with ALK, ROS1, and RET.
Conclusion
The majority of tumors from both Stanford and Vietnam had targetable oncogenic alterations. This suggests that routine implementation of molecular testing may have a significant, positive impact on the treatment of Vietnamese patients with NSCLC, but affordability of testing and treatments remains a barrier to adoption.
INTRODUCTION
Discoveries of oncogenic mutations in non–small-cell lung cancer (NSCLC) over the past decade have led to significant treatment advances with orally available targeted therapies. Tumors driven by activating EGFR mutations, commonly including exon 19 deletions or the L858R point mutation on exon 21, have been shown in multiple randomized controlled trials to be more sensitive to EGFR tyrosine kinase inhibitors (TKIs) than to cytotoxic chemotherapy in the first-line setting.1-3 Similarly, tumors with ALK gene rearrangements are also highly sensitive to ALK TKIs, such as crizotinib, ceritinib, and alectinib.4-6 Another druggable target in NSCLC is the ROS1 gene rearrangement, and ROS1-positive tumors are also highly sensitive to crizotinib.7 Unfortunately, despite being more prevalent than other genomic alterations, KRAS-mutant tumors seem to have little sensitivity to targeted therapies.
The frequencies of these oncogenic alterations in NSCLC seem to be widely different among geographic populations for unclear reasons. EGFR mutations are known to occur in approximately 15% of NSCLCs in the Western population, yet closer to 50% in the East Asian population.1,2,8,9 ALK gene rearrangements are consistently observed in approximately 4% of NSCLC tumors in Western and East Asian populations10-17 and ROS1 gene rearrangements in approximately 1% to 2% of NSCLC in white and Chinese populations.18-20 Because most of the clinical trials on targeted therapies for NSCLC have been conducted in the United States, Europe, China, Japan, South Korea, and Taiwan, less is known about the frequencies of driver mutations in NSCLC in developing East Asian countries. In particular, molecular profiles of NSCLC remain relatively uncharacterized in Vietnam, a World Bank–designated lower-middle–income country with more than 90 million people. Vietnam is struggling to contain a rapidly growing cancer burden, where lung cancer ranks second only to liver cancer in incidence and mortality, with more than 20,000 patients reported per year. Molecular analysis is rarely performed in Vietnam because of high costs and limited availability. This article describes the molecular profiles of NSCLC in Vietnamese patients by comparing two cohorts of Vietnamese patients with lung cancer: those diagnosed in Stanford, CA, and those diagnosed in Hue, Vietnam, using modern diagnostic assays.
METHODS
Study Design
This was a retrospective study of oncogenic alterations in patients of Vietnamese origin with NSCLC of all histologies. Under an institutional review board–approved protocol, the Stanford Cancer Institute Research Database was used to identify patients with NSCLC of Vietnamese origin on the basis of self-reported ethnicity, language, or country of origin. Patients who were seen for an initial visit to the Stanford Cancer Center thoracic oncology clinic between April 2009 and December 2013 were included. We collected data on sex, smoking status, smoking pack-years, date of pathologic diagnosis, date of first clinic visit, date of last follow-up, date of death, tumor histology, disease stage, molecular data, and treatment data (including modality of treatment, start date, and date of progression). The data cutoff date was May 1, 2014. Another 46 samples of NSCLC tumors were obtained from patients who underwent surgical resection of tumors between December 2012 and February 2014 at Hue Central Hospital, Hue, Vietnam, and were collected under an institutionally approved protocol that also allowed research testing of clinical specimens outside the country. The majority of these patients had the associated clinical variables: sex, smoking status, tumor histology, and disease stage. Treatment and clinical outcome data were not available.
Molecular testing was performed as part of routine clinical care of the Stanford patients as follows: ALK status was determined using the standard break-apart ALK fluorescent in situ hybridization assay13; ROS1 status was determined with break-apart fluorescent in situ hybridization18; and EGFR, KRAS, and other cancer-related genes were determined using DNA sequencing (2007 to 2011) or SNaPshot (2011 to 2013).21 Molecular testing was performed in the Hue specimens using a research version of the next-generation sequencing–based Stanford Actionable Mutation Panel for solid tumors, which, subsequent to this study, became the routine next-generation sequencing–based testing platform for clinical use at Stanford.22
Statistical Analyses
Descriptive statistics were performed for oncogenic alteration frequencies, sex, smoking status, histology, and disease stage. Frequencies of oncogenic alterations were compared among different subgroups with χ2 or Fisher's exact test. Survival analyses for the Stanford population were performed using the Kaplan-Meier method. All statistical analyses were programmed using SAS 9.2 statistical software (SAS Institute, Cary, NC). Statistical significance was assumed for a two-tailed P value less than .05.
RESULTS
Patient Characteristics
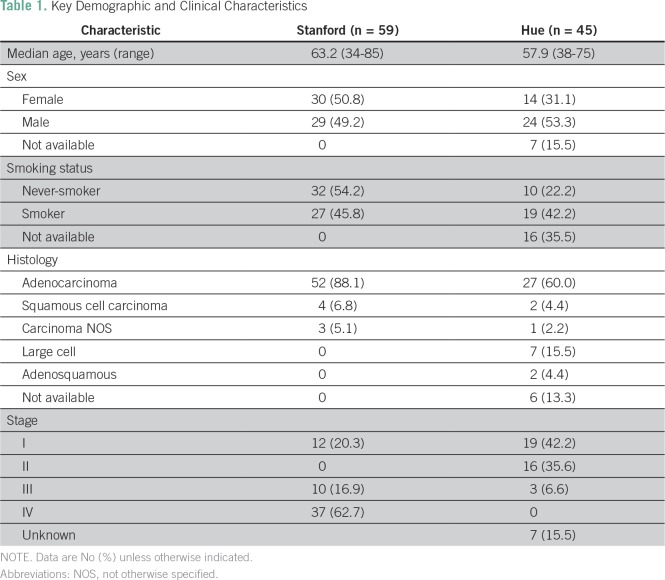
In the Stanford cohort, 59 patients of Vietnamese origin were identified, including 29 men (49.2%) and 30 women (50.8%), with a mean age at diagnosis of 63.2 years, ranging from 34 to 85 years. In the Hue cohort, 24 patients (52.2%) were male, 15 (32.6%) were female, and no sex information was available for seven patients (15.2%); the mean age was 57.9 years, ranging from 38 to 75 years (Table 1).
Table 1.
Key Demographic and Clinical Characteristics
In the Stanford group, 32 (54.2%) were never-smokers, 26 (44.1%) were former smokers, and only one (1.7%) was a current smoker. Twenty-eight of the 30 women (93.3%) were never-smokers, whereas only four of the 29 men (13.8%) were never-smokers. The median number of cigarette pack-years for smokers was 24. In the Hue cohort, 10 patients (21.8%) were never-smokers, 19 (41.3%) were smokers, and 17 (37%) patients had no information collected on smoking status; pack-year information was not collected.
Tumor Characteristics and Molecular Profiles
The majority of the tumors from the Stanford cohort, 52 of 59 (88.1%), were adenocarcinomas, with only four (6.8%) squamous cell carcinomas and three (5.1%) carcinomas not otherwise specified. Interestingly, the histologies from Hue were different, with 27 (58.7%) adenocarcinomas, two (6.3%) squamous cell carcinomas, one (2.2%) carcinoma not otherwise specified, seven (15.2%) large cell carcinomas, and two (6.3%) adenosquamous carcinomas. In seven patients (15.2%), no specific histology was given (Table 1).
At Stanford, the majority of patients presented with an advanced stage: 12 patients (20.3%) at stage I, none at stage II, 10 (16.9%) at stage III, and 37 (62.7%) at stage IV. In contrast, most of the samples collected at Hue were from patients presenting with a local stage: 39 (84.8)% at stage I, 19 (41.3%) at stage II, three (6.5%) at stage III, and no patients with stage IV.
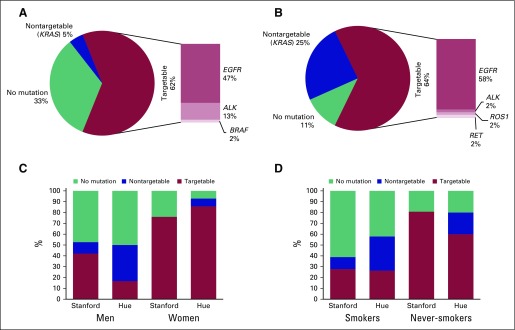
In the Stanford cohort, 44 of the 59 patients underwent molecular testing. Of the tumors that were tested, 47.7% had an EGFR-activating mutation, 13.6% had an ALK gene rearrangement, and 4.5% had a KRAS mutation. One third (33%) had no identified oncogenic alteration (Fig 1A). Of the 21 patients with EGFR-mutant NSCLC, 17 (81.0%) were never-smokers, and 16 (76.2%) were female. Of the six patients with an ALK gene rearrangement, three were female, and three never smoked. Interestingly, one of the ALK-positive tumors had a confirmed squamous cell carcinoma. No ROS1 gene rearrangements were found among 11 patients tested. One patient (2.3%) was found to have a BRAF mutation. Overall, 21 of 32 never-smokers (65.6%) and seven of 27 smokers (25.9%) had a targetable driver mutation (EGFR, ALK, BRAF). Of note, we excluded KRAS mutations from the list of targetable driver mutations because there was no standard targeted therapy for KRAS-mutant NSCLC.
Fig 1.
Oncogenic alterations in Vietnamese patients with non–small-cell lung cancer. Frequency of oncogenic alterations for (A) the overall Stanford cohort, (B) the overall Vietnam cohort, (C) each cohort subdivided by sex, and (D) each cohort by smoking status. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ROS1, repressor of silencing 1.
In the Hue cohort, all 45 patients had molecular testing. Twenty-six tumors (57.8%) had an EGFR-activating mutation, and 11 tumors (24.4%) had a KRAS mutation (Fig 1B). There was one patient (2.2%) each with ALK and ROS1 gene rearrangement and one patient (2.2%) with RET translocation.
When comparing the patients by site and gender, more female patients had driver oncogenic alterations than males (Fig 1C), and more never-smokers had driver alterations than smokers (Fig 1C). Also, within gender and smoking subgroups, Hue patients consistently had a higher frequency of driver alterations than Stanford patients.
Clinical Outcomes
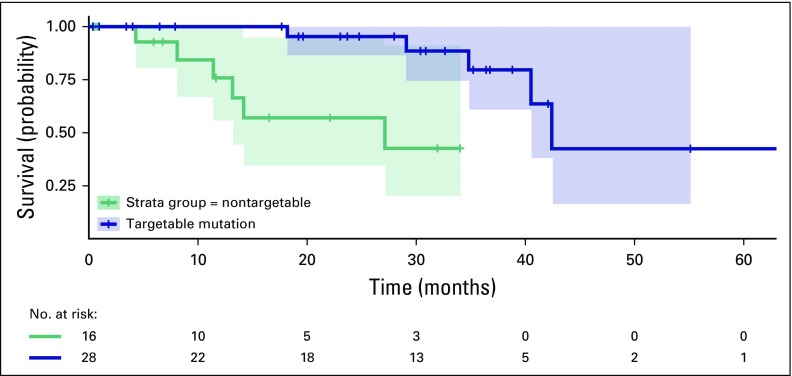
Treatment and clinical outcome data were only available for the Stanford patients. Of the 59 patients in this cohort, 12 had died at the time of data cutoff. The median overall survival (OS) for all patients was 40.5 months (95% CI, 29.1 to 68.1 months). The estimated median OS for patients who were tested and found to have no targetable driver mutation was 27.1 months (95% CI, 8.1 months to not reached), and that for patients with a targetable driver mutation was 42.4 months (95% CI, 34.8 to 68.1 months log rank P = .00154; Fig 2).
Fig 2.
Overall survival of Vietnamese patients with non–small-cell lung cancer treated at Stanford. Kaplan-Meier curve of overall survival of the Stanford cohort, divided by patients with a targetable mutation, versus patients with a nontargetable mutation or no identified mutation. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
DISCUSSION
Our study was an international comparison of the patterns of oncogenic alterations in patients with NSCLC of Vietnamese origin treated in the United States and in Vietnam. There is a paucity of research on lung cancer in the Vietnamese population living in Vietnam and overseas. A retrospective study of 1,124 Asian American patients with NSCLC from 2001 to 2005 in Southern California included 369 Vietnamese Americans, of whom 39.7% were never-smokers. The median age of diagnosis for Vietnamese never-smokers was 65 years, and that for smokers was 67 years. Men made up approximately 28% of the never-smokers and 85% of the smokers with lung cancer. Adenocarcinomas comprised approximately half of the Vietnamese NSCLC histologies, and more than half of the Vietnamese patients were diagnosed at stage IV. In this study, the authors found no statistically significant difference in OS among five Asian American subgroups.23 Another study of Asian American patients with cancer in California revealed that Vietnamese men and women had the highest incidence of lung cancer among the five Asian American groups: Chinese, Filipino, Japanese, Korean, and Vietnamese. Vietnamese patients were found to have the lowest income and education level among the Asian ethnic groups.24 There exist some published data comparing cancer incidence rates in the overseas Vietnamese population to Vietnamese living inside Vietnam. In one study, the lung cancer incidence rate was 29.8 per 100,000 in Hanoi, Vietnam, compared with 55.9 per 100,000 in Vietnamese patients from California and SEER databases.25 The authors argued that the difference might have been due to environmental differences or underdiagnosis and underascertainment in Vietnam.
We found that 63.6% of Vietnamese patients at Stanford and 64.4% of those in Hue, Vietnam, had an oncogenic alteration. We confirmed a clinical association between never-smoking status and the female sex with having an EGFR-activating mutation. The first published literature on the molecular profiles of Vietnamese patients with lung cancer was the PIONEER study, a prospective study examining EGFR mutations in Asian patients with advanced pulmonary adenocarcinoma.26 This study included 121 patients from a hospital in northern Vietnam. The frequency of EGFR-activating mutations was 64.2% in the Vietnamese cohort from this study. Another study from the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam, performed Sanger sequencing on 135 samples and found EGFR mutations in 40.7%.27 In August 2015, researchers from Bach Mai Hospital, Hanoi, Vietnam, presented an abstract at a national oncology conference showing that of 166 tested NSCLC samples at their hospital, 38.0% had EGFR-activation mutations. These studies together with our study showing that 57.8% of patients from Hue, Vietnam, had EGFR mutations and 47.7% of Vietnamese patients at Stanford had EGFR mutations, show that EGFR mutations are frequent in this population. There do not seem to be published data on the rate of other driver oncogenes in Vietnamese patients. Our Stanford population also shows a higher-than-expected ALK-positive frequency of 13.6%, but with a limited sample size, this may be due to statistical variation. The single instances of ALK, ROS1, and RET identified in the cohort from Vietnam are consistent with our expectation that these are low-frequency, but present, alterations in this population. For the patients treated at Stanford, OS was superior in those who had a targetable oncogenic alteration, as demonstrated in other populations.28
This study has some limitations. For both the Stanford and Hue cohorts, the sample size was relatively small. All patients in the study received care at academic centers; thus, the findings may not be generalizable to the broader Vietnamese population. At Stanford, there might have been patients of Vietnamese origin who were not included in the study if they did not have a Vietnamese name or if they declined to declare their ethnicity or language. In addition, the stages at presentation varied widely between the two cohorts. At Stanford, as expected, a majority of patients presented at stage IV. However, almost all of the samples obtained from Hue were from patients presenting at stage I or II, with only a few at stage III and none at stage IV, because the samples suitable for collection and sharing for research, including molecular analysis, were generally surgical samples, and patients at an advanced stage are unlikely to undergo surgery. However, there may be understaging in Vietnam, with limited access to the use of full-body positron emission tomography/computed tomography scanners and contrast-enhanced head magnetic resonance imaging; therefore, it is likely that a subgroup of patients in the Vietnamese cohort had more advanced disease. Furthermore, in Vietnam, advanced-stage lung cancer may not be pathologically diagnosed, because it is not uncommon for patients who present with radiographically presumed advanced-stage lung cancer to forgo a diagnostic biopsy and proceed directly to palliative treatment. Another weakness of the study results from not performing a single uniform molecular test on all patients. For example, of the 59 total patients at Stanford, only tumors from 44 patients had any molecular testing, and some patients only had EGFR testing, whereas others had a more comprehensive multiplex mutation panel. This is because of the improvements in clinical molecular testing over time in NSCLC, but samples did not remain on the majority of patients to permit performance of next-generation sequencing. Therefore, it is difficult to accurately assess the absolute frequencies of various oncogenic alterations in this cohort. Last, survival data are unavailable on the patients from Hue, preventing a comparison between the sites or assessment of survival by molecular alteration subgroups.
Despite these limitations, this study revealed that a significant majority of patients with lung cancer of Vietnamese origin have an oncogenic alteration that can be targeted therapeutically. This is consistent with reports from other East Asian countries in which the rates of EGFR mutations are quite high. Although additional studies are needed to confirm these interesting findings, this study underscores the need for increased access to affordable molecular testing and targeted therapies. Unfortunately, in a country where the per capita median income is US $1,130 per year, an EGFR mutation test costs between US $1,000 and $2,000 (Q.T. Khanh, personal communication, October 2012). Furthermore, the majority of patients cannot afford the approximate cost of US $1,000 per month or more for an EGFR TKI (N.V. Cau, personal communication, August 2015). The disparity in lung cancer treatment, and cancer care in general, is apparent for those of us who have cared for patients in both countries. Personalized lung cancer treatment with targeted therapies, which could only become widespread with lower-cost molecular testing and affordable oral TKIs, remains a worthwhile but elusive goal in Vietnam.
ACKNOWLEDGMENT
The Stanford Cancer Institute Research Database group assisted with cohort generation for this work. The Stanford Cancer Institute Research Database is a research and development project at Stanford Cancer Institute that developed and manages a modular informatics platform that integrates internal and external data sources and streamlines curation to support cancer research.
Footnotes
Supported by the Stanford Cancer Institute Cancer Center Support Grant of the National Institutes of Health under Award No. P30 CA124435. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presented at the 15th World Conference on Lung Cancer, Sydney, Australia, October 27-30, 2013.
AUTHOR CONTRIBUTIONS
Conception and design: Kim-Son H. Nguyen, Phan Canh Duy, Richard Thorp, Maximilian Diehn, Joel W. Neal
Financial support: Richard Thorp, Maximilian Diehn, Joel W. Neal
Provision of study materials or patients: Pham Nhu Hiep, Nguyen Van Cau, Phan Canh Duy, Richard Thorp, Heather A. Wakelee, Joel W. Neal
Collection and assembly of data: Kim-Son H. Nguyen, Henning Stehr, Li Zhou, Pham Nhu Hiep, Nguyen Van Cau, Phan Canh Duy, Richard Thorp, Heather A. Wakelee, Joel W. Neal
Data analysis and interpretation: Kim-Son H. Nguyen, Henning Stehr, Anh-Hoa Nguyen, Phan Canh Duy, Heather A. Wakelee, Maximilian Diehn, Joel W. Neal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kim-Son H. Nguyen
No relationship to disclose
Henning Stehr
No relationship to disclose
Li Zhou
Employment: GRAIL
Anh-Hoa Nguyen
No relationship to disclose
Pham Nhu Hiep
No relationship to disclose
Nguyen Van Cau
No relationship to disclose
Phan Canh Duy
No relationship to disclose
Richard Thorp
Employment: Global Cancer Initiative
Stock and Other Ownership Interests: ILSBio
Heather A. Wakelee
Honoraria: Novartis, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst), Eli Lilly (Inst), Celgene (Inst), AstraZeneca/MedImmune (Inst), Exelixis (Inst), Novartis (Inst), Clovis Oncology (Inst), Xcovery (Inst), Bristol-Myers Squibb (Inst), Gilead Sciences (Inst), Pharmacyclics (Inst), ACEA Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Maximilian Diehn
Stock and Other Ownership Interests: CiberMed
Consulting or Advisory Role: Roche
Research Funding: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection assigned to Stanford University (Inst), patent filings on tumor treatment resistance mechanisms assigned to Stanford University (Inst)
Travel, Accommodations, Expenses: Roche, Varian Medical Systems
Joel W. Neal
Consulting or Advisory Role: ARIAD/Takeda, AstraZeneca, Genentech, Eli Lilly
Research Funding: Genentech, (Inst), Merck (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Exelixis (Inst), ARIAD/Takeda (Inst), Nektar
REFERENCES
- 1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 5. Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Solomon BJ. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med. 2015;372:683–684. doi: 10.1056/NEJMc1415359. [DOI] [PubMed] [Google Scholar]
- 8. Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 9. D’Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non–small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 11. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boland JM, Erdogan S, Vasmatzis G, et al. Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non–small cell lung carcinomas. Hum Pathol. 2009;40:1152–1158. doi: 10.1016/j.humpath.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 13. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non–small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: A rare acquired event. Neoplasia. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 16. Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 17. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 18. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non–small cell lung cancer: Identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 20. Chin LP, Soo RA, Soong R, et al. Targeting ROS1 with anaplastic lymphoma kinase inhibitors: A promising therapeutic strategy for a newly defined molecular subset of non–small-cell lung cancer. J Thorac Oncol. 2012;7:1625–1630. doi: 10.1097/JTO.0b013e31826baf83. [DOI] [PubMed] [Google Scholar]
- 21. Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stanford Health Care Stanford solid tumor actionable mutation panel. https://www.stanfordlab.com/esoteric/test-stanford-solid-tumor-actionable-mutation-panel.html
- 23. Ou S-HI, Ziogas A, Zell JA. A comparison study of clinicopathologic characteristics of Southern California Asian American non–small cell lung cancer (NSCLC) patients by smoking status. J Thorac Oncol. 2010;5:158–168. doi: 10.1097/JTO.0b013e3181c8cc62. [DOI] [PubMed] [Google Scholar]
- 24. McCracken M, Olsen M, Chen MS, Jr, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 25. Le GM, Gomez SL, Clarke CA, et al. Cancer incidence patterns among Vietnamese in the United States and Ha Noi, Vietnam. Int J Cancer. 2002;102:412–417. doi: 10.1002/ijc.10725. [DOI] [PubMed] [Google Scholar]
- 26. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vu HA, Xinh PT, Ha HT, et al. Spectrum of EGFR gene mutations in Vietnamese patients with non–small cell lung cancer. Asia Pac J Clin Oncol. 2016;12:86–90. doi: 10.1111/ajco.12448. [DOI] [PubMed] [Google Scholar]
- 28. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]