Abstract
Background
Human Papillomavirus (HPV) infection is well established in oropharyngeal squamous cell carcinoma (OPSCC) and cervical cancer (CC). However, the association between both HPV related cancers remains unclear. The purpose of this study was to investigate the association between HPV related cancers of the oropharynx and cervix.
Methods
A provincial cancer registry was used to retrospectively identify all patients diagnosed with OPSCC from 1997-2015. The standardized incidence ratio (SIR) of CC history in women with p16+/-OPSCC was measured.
Results
From 372 women with OPSCC included, the SIR of CC was significantly higher across all ages compared to the general population in Alberta, Canada (p < 0.0001).
Conclusions
Women with HPV/p16+ OPSCC have a significantly higher risk of CC compared to the general population.
Keywords: Cervical cancer, Human papillomavirus, Oropharyngeal cancer, p16
1. Background
A wide variety of human papillomavirus (HPV) related malignancies affect men and women. Among women across the world, cervical cancer (CC) is the third most common cancer, with nearly 569,847 cases of CC diagnosed and 311,365 CC fatalities reported yearly [1]. Almost all cases of CC are caused by high-risk HPV infection, mainly HPV 16 that is found in around 50% of cases. Other subtypes include HPV 18 seen in 20% of cases [2], and HPV 31, 33, 45, 52, and 58 in 19% [3]. Other HPV related malignancies in women include vulvar and vaginal cancer, which are infrequent worldwide. In the United States, CC incidence is declining while the incidence of oropharyngeal squamous cell carcinomas (OPSCCs) is rising [4].
In North America and Europe up to 70–80% of OPSCCs are attributable to HPV as a prevalent risk factor [4]. There has been a notable increase in prevalence over the years, from 20.9% in the pre-1990s, to 51.4% between 1990-1999, to over 70% in the recent decade [5,6]. High-risk HPV infections have been attributed most particularly to HPV 16, although other subtypes including HPV 18, 31 and 33 were also implicated but to a lesser degree [7].
HPV+ve OPSCC strikingly differs from HPV-ve OPSCC from clinical, pathologic and molecular perspectives [[8], [9], [10], [11]]. Its implications affect staging, prognosis and treatment modalities [12,13]. HPV+ve OPSCC patients are significantly younger than HPV–ve OPSCCs at the time of diagnosis, with a significant preponderance of Caucasian males [12]. Whereas classically head and neck cancer (HNC) was attributed to risk factors such as tobacco and alcohol use, individuals with HPV+ve OPSCCs have a different risk factor profile, notably less smoking and alcohol use, more marijuana use, certain sexual behaviors and immunodeficiency [12].
Oncogenic HPV infection has been well established in OPSCC and CC, with HPV16 being the most commonly identified in both [14]. The primary risk factor for CC and HPV +ve OPSCC is sexual behavior. Smoking and immunocompromised status are also thought to play a role [15].
Several studies have looked at the association between OPSCC and CC [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Most of these studies included women with CC as their cohorts, they lacked p16 data and therefore included HNC sites that are non-HPV related [[16], [17], [18], [19], [20], [21], [22]]. This lead to unclear associations.
Due to similar risk factors thought to cause coinfection of HPV related cancers of the cervix and oropharynx, our primary objective was to determine the association between oropharyngeal and cervical oncogenic HPV in patients with confirmed HPV related OPSCC. In achieving this objective, we aimed to determine the standardized incidence ratio (SIR) of CC history in women with p16+/-OPSCC. Based on the shared risk factors, we hypothesized a strong association between HPV/p16+ve OPSCC and CC.
2. Methods
We performed a population-based study, which is primarily observational. Ethics approval was obtained from the University of Alberta Health Research Ethics Board (Pro00062302) prior to study commencement.
Data was collected from a provincial cancer registry between 1997 and 2015. The study subjects included retrospectively were women diagnosed with OPSCC. From 1997-2009, p16 staining was completed and validated using tissue microarray as described previously [27]. From 2010-2015, p16 immunohistochemistry was obtained from patients’ medical records, as this became the standard of practice for all patients with OPSCC.
Variables and factors were collected prospectively. This included: surname and first name, age at diagnosis, health care number, date of birth, primary tumor site and subsite, American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) staging (7th Edition) [28], tumor behavior, date of diagnosis, date of death, cell type of malignancy, initial treatment date, treatment modality, vital status, p16 status, smoking history, CC history and date of diagnosis, positive papanicolaou (PAP) history and date of last PAP performed. CC was defined as either invasive CC or carcinoma insitu. Other premalingnancies or different grades of dysplasia were not included in the analysis.
2.1. Statistical analysis
Basic descriptive and comparative statistics were used to analyze the patients’ cohort. Non-parametric Mann Whitney and t tests were used to compare means. Chi-square was used for categorical variables. SPSS version 25 (IBM, Chicago, IL) was used for the analysis. The SIR was calculated for CC history in women with p16+/-OPSCC. This was calculated based on the observed incidence of CC in women with OPSCC and the expected incidence of CC according to the year and region rates in the province of Alberta as per the Canadian Cancer Statistics [29] as previously reported [30]. SIRs were age-adjusted, with comparisons to expected incidence of CC for women of that age. A p-value below 0.05 was considered statistically significant.
3. Results
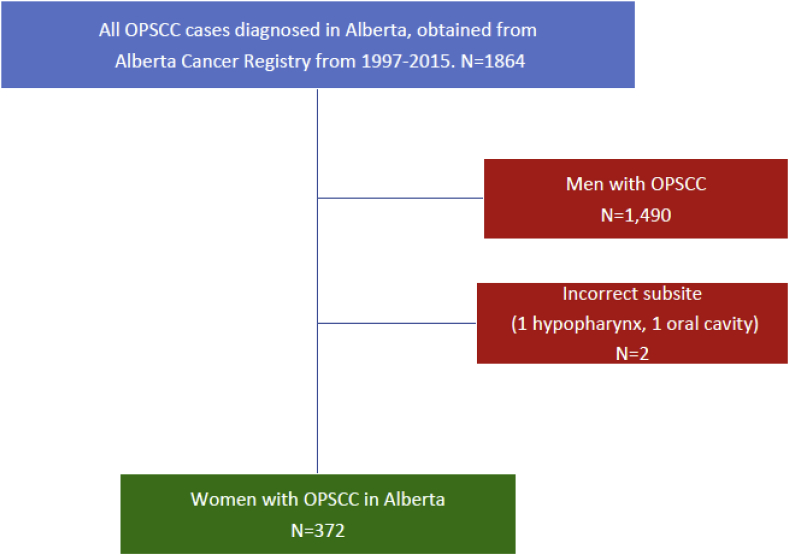
According to our retrospective data and based on the Alberta Cancer Registry between 1997-2015, 1864 patients were diagnosed with OPSCC. 1,490 male OPSCC patients were excluded. Two additional patients were excluded due to incorrect cancer sites labeling. A cohort of 372 women were included in the analysis (Fig. 1).
Fig. 1.
Inclusion of patients included for retrospective analysis in this study.
Demographics, clinical and pathologic characteristics of the patient cohort are described in Table 1. The data was stratified based on the presence or absence of CC history. Of all women with OPSCC, 33 were identified as having as ever having a history of CC. Patients with CC history presented with OPSCC at a significantly younger age (p = 0.002). The mean age that women presented with CC in p16+ OPSCC patients was 39.3 yrs compared to 44.4 yrs (p = 0.47) in patients without p16+OPSCC. The mean pack year in smoking was similar between groups (∼24%).
Table 1.
Clinical and pathologic characteristics of women with oropharyngeal cancer in this study.
| Characteristic | All (N = 372) | No history of Cervical Cancer (N = 339) |
History of Cervical Cancer (N = 33) |
P-valuea |
|---|---|---|---|---|
| Age (mean) | 63.3 | 63.9 | 56.8 | 0.002 |
| Mean pack years | 24.1 | 24.1 | 24.3 | 0.96 |
| T-stage (%) | ||||
| T1 | 22.1 | 22.0 | 23.8 | |
| T2 | 37.7 | 37.2 | 42.9 | |
| T3 | 16.7 | 16.1 | 23.8 | |
| T4 | 23.3 | 24.5 | 9.5 | 0.43 |
| N-stage (%) | ||||
| N0 | 22.0 | 22.2 | 20.0 | |
| N1 | 17.7 | 17.9 | 15.0 | |
| N2 | 54.7 | 54.2 | 60.0 | |
| N3 | 5.5 | 5.6 | 5.0 | 0.97 |
| Tumor subsite (%) | ||||
| Tonsil | 52.9 | 51.9 | 63.6 | 0.34 |
| BOT | 31.9 | 32.4 | 27.2 | |
| Soft palate | 8.6 | 8.6 | 9.1 | |
| PPP | 6.4 | 7.1 | 0 | |
| P16 positive (%) | 56.8 | 54.7 | 76.1 | 0.04 |
Denotes statistical significance between patients with or without a history of cervical cancer. BOT, base of tongue; PPP, posterior pharyngeal wall.
Tumor characteristics were also similar in both groups. OPSCC women with and without CC presented with comparable tumor stages, with (66.7 vs 59.2%) early- and (33.3 vs 40.6%) advanced-stage disease.
Tumor subsite distribution was also comparable. Women with OPSCC and no history of CC most commonly presented with tumors in the tonsil (51.9%), followed by the base of the tongue (BOT) (32.4%), soft palate (8.6%), and posterior pharyngeal wall (PPP) (7.1%). Similarly, women with OPSCC and CC most commonly presented with tonsillar tumors (63.6%), followed by the BOT (27.2%) and soft palate (9.1%). None had tumors arising from the PPP. Most importantly, women with history of CC had a significantly higher p16 positivity (p = 0.04). It was identified in (54.7%) of women with OPSCC, and a significantly higher percentage in those with positive history of CC (76.1%).
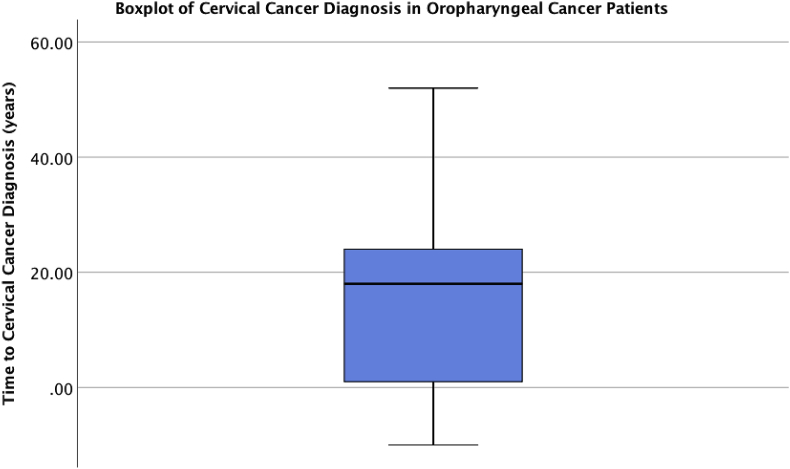
The mean time from diagnosis of CC to OPSCC was 15.3 (SD 16.1) years (Fig. 2). Most patients had a diagnosis of CC several years prior to the development of OPSCC (84.8%). One patient was found to have CC within the same year of OPSCC diagnosis.
Fig. 2.
Temporal relationship between time of cervical cancer and oropharyngeal cancer diagnosis. Dot plot demonstrates cases of oropharyngeal cancer in women relative to previous diagnosis of cervical cancer. Cases above 0 denote OPSCC cases diagnosed after cervical cancer. Mean time from diagnosis of cervical cancer to OPSCC is 15.3 (SD 16.1) years. Mean time to diagnosis according to subsite was as follows: 14.5 years for tonsil, 18.0 years for BOT and 13.7 years for soft palate (p = 0.61 between tonsil and BOT, p = 0.92 between tonsil and soft palate).
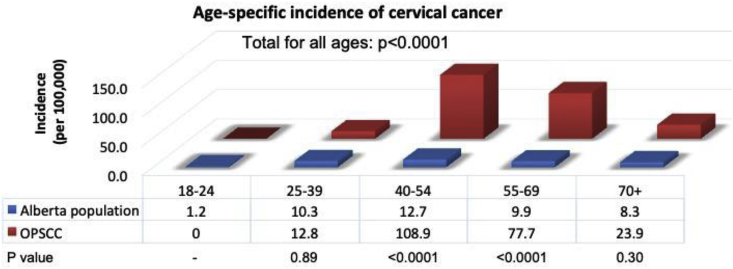
When calculating the age-specific incidence (per 100,000) for CC in patients with OPSCC compared to the general population in Alberta, women with OPSCC who were above the age of 40 and below the age of 70 had a significantly higher SIR of CC in comparison to the general population (p < 0.0001). Across all ages, women with OPSCC had a significantly higher SIR of CC in comparison to the general population (p < 0.0001). The age-specific incidence was low and similar in patients below the age of 40 (Fig. 3).
Fig. 3.
Age-specific incidence of cervical cancer in patients with oropharyngeal cancer. Age-specific incidence is shown (per 100,000) for cervical cancer in patients with OPSCC compared to the general population in Alberta. Statistics for the population in Alberta was obtained from the 2018 Canadian Cancer Statistics Report.
4. Discussion
Our study is consistent with several previous studies, which identified that women with a history of CC and/or premalignancies (specifically cervical intraepithelial neoplasia (CIN) and/or adenocarcinoma in situ) are at increased risk of OPSCC [[16], [17], [18], [19], [20], [21], [22]]. Most of these studies were limited by the heterogeneity of patient populations, which included HNC sites other than the oropharynx such as the oral cavity and larynx (not HPV-related). Although Svahn et al. [17] divided their HNC groups based on the predicted level of association with HPV, they all lacked p16 staining.
Some studies investigated smaller retrospective cohorts of women with OPSCC to identify their risk of CC [[22], [23], [24], [25]]. However, some lacked p16 status [22,25], while others stratified their data based on p16 status when available [23,24]. Skinner et al. [22] studied a mixed small cohort of men and women (n = 143) with multiple potentially HPV-related cancers at different sites. Despite the lack of p16 information, inability to identify the different sites and subsites of HNC, only half of their diagnosed HNC patients were from the oropharynx.
A recent Danish study [23] found no difference in the association between HPV/p16+ve OPSCC and CC. However, they identified 1.35 greater odds of developing HPV/p16+ve OPSCC in women with high grade squamous cell abnormalities or CC. They attribute the lack of statistical significance to the different oncogenic HPV subtypes available. We identified a p16 +ve status in 76.1%, which is more than 2/3 of the patients with history of CC (n = 33). In contrast, only 1.2% of females in their patient cohort had a history of CC (3 patients among the HPV+ve group and in 2 patients among the HPV-ve group) [23]. This may also be due to the shorter duration of their study that hindered the ability to identify more patients diagnosed with CC or dysplasia.
Rietbergen et al. [24] studied a smaller cohort (n = 308) of patients and showed that female patients with HPV+ve OPSCC had a significantly higher history of suspicious PAP results compared to the HPV-ve patients (16.1% vs. 4.2%), which is in agreement with our results. However, they did not define what a suspicious PAP meant, whether CC or dysplasia.
As with previous studies, our data suggests that CC is most often identified prior to OPSCC in cases patients who develop both cancers, however, this does not demonstrate causality [17,[22], [23], [24]]. The association between OPSCC and CC is not surprising due to the mutual shared risk factors, biologic characteristics and pathogenesis in both HPV related cancers [15,31,32]. Multiple theories contribute to carcinogenesis within the oropharynx, predominantly within the palatine tonsils and BOT (lingual tonsils). Squamous epithelium from the head and neck as with the cervix is derived embryologically from endoderm and is predisposed to metaplasia. Other theories include factors such as macroanatomy, microanatomy, microabrasion, M-cells and cytokines [31,32].
Our results show that the SIR of developing CC in p16+OPSCC patients in Alberta is considerably higher. This is in support of an older study from our institution that analyzed a smaller cohort of patients (n = 248), however p16 status was not available [25]. The results revealed an amplified risk of developing CC in OPSCC patients of at least 25 times greater in comparison to the general population. Multiple studies calculated the SIR in CC or CIN patients for developing a second primary [16,21,33], [18]. However, comparing our SIR with these studies was not particularly useful; as they used non specific terms to describe the different HNC sites [16,18,21,33].
One of the limitations of the study is related to the lack of identifiable risk factors such as human immunodeficiency virus status, immune status and sexual behavior that have been reported to play a role in both OPSCC and CC. The strengths of our study were the ability to obtain high-quality, individual-level data from a comprehensive provincially mandated cancer registry over a long time frame, which reduced the risk of selection bias and recall bias. Our study not only looked at the oropharynx site specifically, but it also used p16 as a surrogate marker for oncogenic HPV. Our results clarify previously unclear and conflicting literature regarding the association of CC in HPV+ve OPSCC patients. The results increase our awareness to have women with OPSCC counseled and screened for CC and vice versa to identify suspicious lesions early and offer the necessary treatment in a timely fashion.
Future prospective studies may help address the limitations of the retrospective nature of the current study.
5. Conclusion
Women with HPV/p16+ OPSCC have a significantly higher risk of CC compared to the general population. Based upon the known increased risk of a subsequent HPV related cancer in this patient population, patients should be counseled appropriately when seen in the outpatient setting.
Declaration of Competing interest
The authors declare that they have no competing interests.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.100188.
Contributor Information
Malak Jamal Gazzaz, Email: mgazzaz@ualberta.ca.
Caroline Jeffery, Email: caroline.jeffery@albertahealthservices.ca.
Daniel O'Connell, Email: dan.oconnell@ualberta.ca.
Jeffery Harris, Email: jeffrey.harris@albertahealthservices.ca.
Hadi Seikaly, Email: hadi.seikaly@albertahealthservices.ca.
Vincent Biron, Email: vbiron@ualberta.ca.
Funding sources
Funding for this study was obtained from the, Department of surgery, the Alberta Cancer Foundation (ACF), Edmonton Civic Employees’ Charitable Assistance Fund (ECECA) and Alberta Head and Neck Centre for Oncology and Reconstruction (AHNCOR) Foundation. Malak Jamal Gazzaz acknowledges her residency scholarship through Umm Al-Qura University, Makkah, Saudi Arabia.
Ethics approval and consent to participate
Ethics approval was obtained from the University of Alberta Health Research Ethics Board (Pro00062302) prior to study commencement.
Consent for publication
Not applicable.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MJG performed the literature review, followed by data collection, data analysis and interpretation of results. She was the primary contributor of this manuscript. CJ contributed to the study design, and edited the final version of the manuscript. DO contributed to the study design, and edited and approved the final version of the manuscript. JH contributed to study design, and edited final version of the manuscript. HS contributed to the study design and edited the final version of the manuscript. VB was responsible for the conception, design, overall execution of the study and oversaw all aspects of the research. He made substantial contributions and edits to the manuscript. All authors approved the final version of the manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bruni L., Albero G., Serrano B., Mena M., Gomez D., Munoz J. 2019. Human Papilloma Virus and Related Diseases in the World- Summary Report.https://hpvcentre.net/statistics/reports/XWX.pdf January:1–316. [Google Scholar]
- 2.de Sanjose S., Quint W.G.V., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Serrano B., Alemany L., Tous S., Bruni L., Clifford G.M., Weiss T. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect. Agents Cancer. 2012;7:1–13. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haeggblom L., Attoff T., Yu J., Holzhauser S., Vlastos A., Mirzae L. Changes in incidence and prevalence of human papillomavirus in tonsillar and base of tongue cancer during 2000-2016 in the Stockholm region and Sweden. Head Neck. 2018;December:7–9. doi: 10.1002/hed.25585. [DOI] [PubMed] [Google Scholar]
- 6.Stein A.P., Saha S., Kraninger J.L., Swick A.D., Yu M., Lambert P.F. Prevalence of human papillomavirus in oropharyngeal cancer. Cancer J. 2015;21:138–146. doi: 10.1097/PPO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N A.C., D S.S., P D.A., B J., C C., D S. Does HPV type affect outcome in oropharyngeal cancer? J. Otolaryngol. Head Neck Surg. 2013;42 doi: 10.1186/1916-0216-42-9. FEB:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivarajah S., Kostiuk M., Lindsay C., Puttagunta L., O'Connell D.A., Harris J. EGFR as a biomarker of smoking status and survival in oropharyngeal squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2019;48:9–11. doi: 10.1186/s40463-018-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziai H., Alenazi A., Hearn M., O'Connell D.A., Puttagunta L., Barber B. The association of Bcl-xL and p53 expression with survival outcomes in oropharyngeal cancer. Cancer Biomark. 2019;24:141–151. doi: 10.3233/CBM-182106. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay C.D., Kostiuk M.A., Harris J., O'Connell D.A., Seikaly H., Biron V.L. Efficacy of EZH2 inhibitory drugs in human papillomavirus-positive and human papillomavirus-negative oropharyngeal squamous cell carcinomas. Clin. Epigenet. 2017;9:1–13. doi: 10.1186/s13148-017-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark J.M., Holmes E.M., O'Connell D.A., Harris J., Seikaly H., Biron V.L. Long-term survival and swallowing outcomes in advanced stage oropharyngeal squamous cell carcinomas. Papillomavirus Res. 2019;7:1–10. doi: 10.1016/j.pvr.2018.09.002. March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Sullivan B., Huang S.H., Su J., Garden A.S., Sturgis E.M., Dahlstrom K. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 13.Lydiatt W.M., Patel S.G., O'Sullivan B., Brandwein M.S., Ridge J.A., Migliacci J.C. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 14.Kreimer A.R., Bhatia R.K., Messeguer A.L., González P., Herrero R., Giuliano A.R. Oral Human papillomavirus in healthy individuals: a systematic review of the literature. Sex. Transm. Dis. 2010;37:386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 15.Berman T.A., Schiller J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123:2219–2229. doi: 10.1002/cncr.30588. [DOI] [PubMed] [Google Scholar]
- 16.Evans H.S., Newnham A., Hodgson S.V., Møller H. Second primary cancers after cervical intraepithelial neoplasia III and invasive cervical cancer in Southeast England. Gynecol. Oncol. 2003;90:131–136. doi: 10.1016/s0090-8258(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 17.Svahn M.F., Munk C., Jensen S.M., Von Buchwald C., Frederiksen K., Kjaer S.K. Risk of head-and-neck cancer following a diagnosis of severe cervical intraepithelial neoplasia: a nationwide population-based cohort study in Denmark. Gynecol. Oncol. 2016;142:128–132. doi: 10.1016/j.ygyno.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Gaudet M., Hamm J., Aquino-Parsons C. Incidence of ano-genital and head and neck malignancies in women with a previous diagnosis of cervical intraepithelial neoplasia. Gynecol. Oncol. 2014;134:523–526. doi: 10.1016/j.ygyno.2014.07.088. [DOI] [PubMed] [Google Scholar]
- 19.E R.M.F., R D.W.E., I J., G W.J., M L.F., M A.G., B J. Long-lasting increased risk of human papillomavirus–related carcinomas and premalignancies after cervical intraepithelial neoplasia grade 3: a population-based cohort study. J. Clin. Oncol. 2017;35:2542–2550. doi: 10.1200/JCO.2016.71.4543. [DOI] [PubMed] [Google Scholar]
- 20.Rose Ragin C.C., Taioli E. Second primary head and neck tumor risk in patients with cervical cancer—SEER data analysis. Head Neck. 2008;30:58–66. doi: 10.1002/hed.20663. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi A.K., Engels E.A., Gilbert E.S., Chen B.E., Storm H., Lynch C.F. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J. Natl. Cancer Inst. 2007;99:1634–1643. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 22.Skinner H.D., Sturgis E.M., Klopp A.H., Ang K.-K., Rosenthal D.I., Garden A.S. Clinical characteristics of patients with multiple potentially human papillomavirus-related malignancies. Head Neck. 2014;36:819–825. doi: 10.1002/hed.23379. [DOI] [PubMed] [Google Scholar]
- 23.Christensen J.T., Grønhøj C., Zamani M., Brask J., Kjær E.K.R., Lajer H. Association between oropharyngeal cancers with known HPV and p16 status and cervical intraepithelial neoplasia: a Danish population-based study. Acta Oncol. 2018;58:267–272. doi: 10.1080/0284186X.2018.1546059. [DOI] [PubMed] [Google Scholar]
- 24.Rietbergen M.M., van Bokhoven A.A.J.D., Lissenberg-Witte B.I., Heideman D.A.M., Leemans C.R., Brakenhoff R.H. Epidemiologic associations of HPV-positive oropharyngeal cancer and (pre)cancerous cervical lesions. Int. J. Cancer. 2018;143:283–288. doi: 10.1002/ijc.31315. [DOI] [PubMed] [Google Scholar]
- 25.Biron V.L., Cote D.W.J., Seikaly H. Oropharyngeal squamous cell carcinoma and human papillomavirus - associated cancers in women: epidemiologic evaluation of association. J. Otolaryngol. Head Neck Surg. 2011;40 http://journals.bcdecker.com/pubs/JOT/volume 40, 2011/issue S1, February/JOT_2011_100093/JOT_2011_100093.pdf http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L361603311%5Cn SUPPL. 1:S65–9. [PubMed] [Google Scholar]
- 26.Lozza V., Pieralli A., Corioni S., Longinotti M., Bianchi C., Moncini D. HPV-related cervical disease and oropharyngeal cancer. Arch. Gynecol. Obstet. 2014;290:375–379. doi: 10.1007/s00404-014-3187-7. [DOI] [PubMed] [Google Scholar]
- 27.Seikaly H., Biron V.L., Zhang H., O'Connell D.A., Cote D.W.J., Ansari K. Role of primary surgery in the treatment of advanced oropharyngeal cancer. Head Neck. 2016;38(Suppl 1):E571–E579. doi: 10.1002/hed.24042. [DOI] [PubMed] [Google Scholar]
- 28.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 29.Smith L., Bryan S., De P., Rahal R., Shaw A., Turner D. Canadian Cancer Statistics; 2018. Canadian Cancer Statistics Advisory Committee. Stroke. 2018. doi:2018. [Google Scholar]
- 30.Biron V.L., Côté D.W., Seikaly H. vol. 40. 2011. (Oropharyngeal Squamous Cell Carcinoma and Human Papillomavirus-Associated Cancers in Women : Epidemiologie Evaluation of Association). [PubMed] [Google Scholar]
- 31.Heffernan C.B., O'Neill J.P., Timon C. Oncogenic impact of human papilloma virus in head and neck cancer. J. Laryngol. Otol. 2010;124:941–944. doi: 10.1017/S0022215110001179. [DOI] [PubMed] [Google Scholar]
- 32.Woods R.S., O'Regan E.M., Kennedy S., Martin C., O'Leary J.J., Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: a review. World J. Clin. Cases. 2014;2:172–193. doi: 10.12998/wjcc.v2.i6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balamurugan A., Ahmed F., Saraiya M., Kosary C., Schwenn M., Cokkinides V. Potential role of human papillomavirus in the development of subsequent primary in situ and invasive cancers among cervical cancer survivors. Cancer. 2008;113(10 SUPPL):2919–2925. doi: 10.1002/cncr.23746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.