Summary
Many clinical and preclinical studies report an increased prevalence and severity of chronic pain among females. Here, we identify a sex-hormone-controlled target and mechanism that regulates dimorphic pain responses. Prolactin (PRL), which is involved in many physiologic functions, induces female-specific hyperalgesia. A PRL receptor (Prlr) antagonist in the hind paw or spinal cord substantially reduced hyperalgesia in inflammatory models. This effect was mimicked by sensory neuronal ablation of Prlr. Although Prlr mRNA is expressed equally in female and male peptidergic nociceptors and central terminals, Prlr protein was found only in females and PRL-induced excitability was detected only in female DRG neurons. PRL-induced excitability was reproduced in male Prlr+ neurons after prolonged treatment with estradiol but was prevented with addition of a translation inhibitor. We propose a novel mechanism for female-selective regulation of pain responses, which is mediated by Prlr signaling in sensory neurons via sex-dependent control of Prlr mRNA translation.
Subject Areas: Sensory Neuroscience, Female Reproductive Endocrinology
Graphical Abstract

Highlights
-
•
Local or spinal PRL injection induces hyperalgesia in a female-selective manner
-
•
Sensory neuron Prlr regulates tissue injury-induced pain only in females
-
•
PRL regulates excitability in Prlr+ neurons depending on sex and estrogen
-
•
Regulation of Prlr translation defines female-selective neuronal excitability
Sensory Neuroscience; Female Reproductive Endocrinology
Introduction
In recent years a renewed focus on sexual dimorphic mechanisms of pain has emerged. It is now widely recognized that many key mechanisms driving persistent pain differ between males and females in both animals and humans (Martin et al., 2019, Mogil et al., 2011, North et al., 2019, Sorge et al., 2011). Although time course and magnitudes of nociceptive hypersensitivity for a variety of pain conditions are often similar in females and males, the mechanisms responsible for this hypersensitivity and degree of chronicity are sex dependent (Martin et al., 2019, Mogil et al., 2011, Rosen et al., 2017, Sorge et al., 2011, Sorge et al., 2015). Gonadal hormones, for instance, are known to be key contributors to sex differences in a variety of physiological and pathophysiological processes (Karp et al., 2017, Morselli et al., 2017). Human and animal studies of pain symptoms and severity have established correlations with the menstrual cycle, menopause, and alterations in gonadal hormone concentrations (Aloisi and Sorda, 2011, Houghton et al., 2002, LeResche et al., 2003, Slade et al., 2011, Traub and Ji, 2013).
Recent findings on sexual dimorphisms have demonstrated a role for spinal microglia in male-specific pain mechanisms (Sorge et al., 2011) and a T cell selective contribution to nociceptive transmission in females (Rosen et al., 2019, Sorge et al., 2015), although other investigators have described T cells to be involved in protection and resolution of pain (Krukowski et al., 2016, Laumet et al., 2019). It is possible that a neuron-specific, sexually dimorphic pain mechanism also could be involved and mediated by gonadal hormone controlled signaling. A prime candidate for this potential mechanism is prolactin (PRL) and its receptor (Prlr), since responsiveness to PRL in a variety of cell types depends on sex, menstrual cycle phase, pregnancy status, and lactation (Belugin et al., 2013, Childs et al., 1999, Diogenes et al., 2006, Pi and Voogt, 2002). PRL is involved in female-specific regulation of transient receptor potential (TRP) and other ligand-gated channels in sensory neurons (Diogenes et al., 2006, Liu et al., 2016, Patil et al., 2013b). Global ablation of PRL and Prlr leads to a substantial and female-selective reduction in postoperative and inflammatory heat hypersensitivity (Patil et al., 2013a, Patil et al., 2013b) and mechanical hypersensitivity, but the latter effect is observed in male and female mice (Patil et al., 2013a, Patil et al., 2013b). These studies demonstrate a clear role for PRL-Prlr signaling in pain hypersensitivity after injury, but the cells mediating these effects and the mechanisms generating female-specific nociceptive responses remain unknown.
The central goals of the work described here were to gain insight into whether Prlr expression in sensory neurons drives female-specific nociceptive responses to PRL and to understand how these female-specific effects emerge. We show that PRL signaling to Prlr expressed in sensory neurons at the level of peripheral and central terminals regulates female-specific hyperalgesia in several pain models. We also elucidate mechanisms responsible for PRL's female-selective actions in the regulation of pain. Gonadal hormones regulate cellular phenotypes via classic genomic and transient non-genomic signaling pathways (Amandusson and Blomqvist, 2013, Kelly et al., 1976, Revankar et al., 2005). However, surprisingly, our work points to a novel mechanism for sex-specific regulation of nociceptor plasticity that is dependent on selective and estrogen-dependent translation of Prlr mRNA in female DRG neurons. Overall, our work establishes sensory neuron participation of a major neuroendocrine hormone PRL in female-selective regulation of pain as well as a novel paradigm connecting sex- and gonadal hormone-dependent translational control that could be critical to understanding sexual dimorphism in many biological processes.
Results
Exogenous PRL Induces Thermal and Mechanical Hypersensitivity in Females but Not in Males
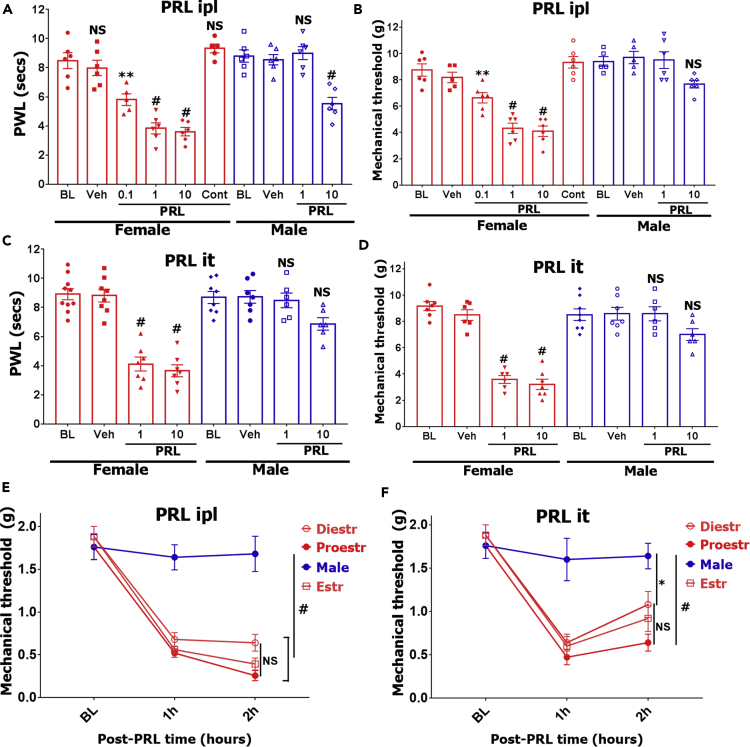
Exogenous fully processed and non-modified human PRL (PRL) generated in an expression system sensitizes a subset of mouse female sensory neurons (Belugin et al., 2013, Patil et al., 2013b). Statistically significant sensitization in male sensory neurons is achieved with approximately a 40-fold higher concentration of PRL (Patil et al., 2013b). To establish if this major difference is also found in vivo, we evaluated whether PRL produces hyperalgesia in female and/or male mice. PRL injected into the hind paw (ipl) generated profound heat (Figure 1A) and mechanical hyperalgesia (Figure 1B) in a dose-dependent manner in estrus female mice (two-way ANOVA; heat - F (3, 40) = 13.4; P < 0.0001; mechanical - F (3, 37) = 10.9; P < 0.0001). As low as 0.1 μg PRL generated thermal and mechanical hyperalgesia in females, whereas 1 μg PRL injected in the contralateral paw did not produce an effect ipsilaterally (bars “Cont” on Figures 1A and 1B). This indicates that PRL-induced hyperalgesia involves peripheral (i.e., local) mechanisms. In contrast, for male mice, higher amounts of PRL (10 μg) produced heat (two-way ANOVA; P < 0.0001; Figure 1A) but not mechanical hypersensitivity P = 0.1; Figure 1B).
Figure 1.
Exogenous PRL-Induced Hypersensitivity in Female and Male Mice
(A–D) PRL-induced heat (A and C) and mechanical (B and D) hypersensitivity was assessed at 1 h post-PRL-administration time point in male and estrous female mice. PRL was administrated into the hind paw (ipl; A and B) or intrathecal space of spinal cord (SC; C and D). PRL dosages (0.1, 1, or 10 μg) and sex of mice are indicated. Mechanical threshold was measured with the Dynamic Plantar Aesthesiometer. “Cont” indicates contralateral injection of 1 μg PRL and measurements of hyperalgesia in the ipsilateral hind paw.
(E and F) PRL (1 μg) was injected ipl (intra-plantar; panel E) or it (intrathecal; panel F), and mechanical hypersensitivity was measured in males and females at different estrous phases (diestrus [Diestr], estrous [Estr], and proestrus [Proestr]). BL is baseline reading before PRL administration.
Data are represented as mean ± SEM. Statistical test is regular two-way ANOVA with Tukey's post hoc test (n = 5–10; NS, non-significant; *p < 0.05; **p < 0.01; #p < 0.0001). See also Figure S1.
Administration of PRL into the spinal cord via intrathecal injection (it) also produced substantial heat (Figure 1C) and mechanical hypersensitivity (Figure 1D) predominantly in females (two-way ANOVA; heat - F (3, 51) = 14; P < 0.0001; mechanical - F (3, 45) = 20.6; P < 0.0001). Spinal PRL-induced hypersensitivity was not significant in male mice (two-way ANOVA; heat P = 0.1; mechanical P = 0.2; Figures 1C and 1D). Unbound PRL protein undergoes relatively fast degradation (Freeman et al., 2000). Consistent with this pharmacokinetic property, PRL (1 and 10 μg) injected in the hind paw (Figures S1A and S1B) or spinal cord (Figures S1C and S1D) of female mice produced significant heat and mechanical hypersensitivity for up to ~4 h (especially for high doses) and peaked at 1–2 h post administration.
Prolonged withdrawal of estrogen and progesterone in ovariectomized (OVX) females totally ablates PRL responsiveness in rats (Diogenes et al., 2006). We evaluated whether PRL responsiveness depends on the estrous phase of mice. Intraplantar (ipl) or spinal cord (it) injection of PRL (1 μg) induced mechanical hypersensitivity in females but not in males (Figures 1E and 1F two-way ANOVA; peripheral - F (6, 48) = 9.7; P < 0.0001; n = 5; Figure 1E; and spinal - F (6, 48) = 4.5; P = 0.0011; n = 5). PRL sensitivity was not affected by female estrous phases (Figure 1F). These results show that exogenous PRL delivered locally into the hind paw or spinal cord triggers 4-h-long-lasting pain hypersensitivity in a female-selective manner, but independent of female estrous phases.
Female-Selective Suppression of Postoperative Pain by Prlr Antagonist
Incision surgery and inflammation up-regulates PRL in a sex-dependent fashion in the hind paw and especially spinal cord, where the larger magnitude of upregulation is found (Patil et al., 2013a, Scotland et al., 2011). We used the specific Prlr antagonist, Δ1-9-G129R-hPRL (ΔPRL) (Rouet et al., 2010), which is a modified PRL that binds to and blocks the function of Prlr in rat, mouse, and human (Bernichtein et al., 2003), to evaluate the role of Prlr in the regulation of postoperative pain in female and male mice and rats. In estrus female mice at 1 day post incision, ΔPRL (5 μg) applied into the spinal cord by intrathecal injection (it) significantly reversed heat (two-way ANOVA; F (6, 44) = 8.2; P < 0.0001; n = 4–5; Figure S2A) and mechanical hypersensitivity (P = 0.014 at 60 min; P = 0.03 at 120 min; n = 5–6; Figure S2B). In contrast, ΔPRL (5 μg) did not show antagonism of incision-induced heat and mechanical hypersensitivity in male mice (Figures S2C and S2D). We did not escalate the dosage of ΔPRL, since at dosages >25 μg, it could show agonistic properties (Scotland et al., 2011). However, 5 μg of ΔPRL did not exhibit agonistic or antagonistic properties on mice that underwent sham procedures (Figures S2A–S2D).
Since peak effects were observed at 60 min post ΔPRL, we recorded vehicle and ΔPRL actions at 60 min post injection. Male and estrous females were injected with vehicle or ΔPRL into hind paws (ipl) at 1 day post incision. Heat hypersensitivity in females, but not in males, was significantly reversed with ΔPRL (two-way ANOVA; F (4, 31) = 9.4; P < 0.0001; n = 4–5; Figure 2A). Mechanical hyperalgesia in males as well as females was not significantly affected by hind paw administration of ΔPRL (two-way ANOVA; F (4, 50) = 0.2; P = 0.9; Figure 2B). Spinal injection of ΔPRL substantially reversed both heat (two-way ANOVA; F (4, 41) = 12.6; P < 0.0001; n = 4–6; Figure 2C) and mechanical hypersensitivity (F (4, 40) = 18.2; P < 0.0001; n = 5–6; Figure 2D) in a female-selective fashion.
Figure 2.
Suppression of Postoperative Pain by Prlr Antagonist in Female and Male Mice
Vehicle (Veh) or Prlr antagonist (5 μg; ΔPRL) was injected into hind paw (ipl) of male and estrous female mice at 1 day post incision (Inc) or sham procedures. Heat (A) and mechanical (B) hypersensitivity was assessed at 1 h post Veh/ΔPRL injection. Vehicle or ΔPRL (5 μg) was injected intrathecally (it) into spinal cord of male and estrous female mice at 1 day post incision or sham procedures. Heat (C) and mechanical (D) hypersensitivity was assessed at 1 h post Veh/ΔPRL injection. BL are baseline values before incision procedures. Procedures and animal sex are indicated below the x axis.
Data are represented as mean ± SEM. Statistical test is regular two-way ANOVA with Tukey's post hoc test (NS, p > 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; n = 5–7). See also Figure S2.
PRL actions could vary between species and PRL release from the pituitary depends on the estrous phase (Freeman et al., 2000), but exogenous PRL responsiveness at periphery and spinal cord of naive female mice is not dependent on the estrous phase (Figures 1E and 1F). Accordingly, we evaluated whether ΔPRL effects differ in diestrus (diestrus 1 and 2 combined) versus estrus female rats. Spinal (it) injection of ΔPRL (5 μg) at 1 day post incision significantly reversed postoperative mechanical hypersensitivity in diestrus and estrus female (two-way ANOVA; F (8, 60) = 6.5; P < 0.0001; n = 5) but not in male rats (Figure 3A). The inhibition of mechanical hypersensitivity was more pronounced in estrus compared with diestrus female rats (two-way ANOVA; P = 0.011; Figure 3A). Our findings indicate that blockage of Prlr leads to female-selective inhibition of postoperative hypersensitivity, which depends on the site of Prlr antagonist action (Figures 1B versus 1D) and partially on the estrous phase (Figure 3A) but not the rodent species (Figures 2 and 3A).
Figure 3.
Effects of Prlr Antagonist and Agonist in Different Inflammatory Pain Models in Female and Male Rats and Mice
(A) Vehicle (Veh) or ΔPRL (5 μg) was injected into spinal cord of male, diestrus female (D-female), or estrous female (E-female) rats at 1 day post incision surgery (POP) or sham procedures. Mechanical hyperalgesia was assessed with Dynamic Plantar Aesthesiometer at 1 h post Veh/ΔPRL injection. BL are baseline values. Statistical test is two-way ANOVA with Tukey's post hoc test (NS, p > 0.05; *p < 0.05; ****p < 0.0001; n = 5–6).
(B) IL-6 (1 ng) was injected into hind paw, and vehicle or ΔPRL (5 μg) was injected approximately simultaneously into hind paw (paw) or spinal cord (it) of estrous female mice. Mechanical hyperalgesia was assessed at 1 h post IL-6/Veh or IL-6/ΔPRL co-injections. BL is baseline value. Data are represented as mean ± SEM. Statistical test is regular two-way ANOVA Bonferroni's post hoc test (NS, p > 0.05; ****p < 0.0001; n = 6).
(C and D) In the model of hyperalgesic priming, 0.5 ng PRL produces mechanical hypersensitivity in IL-6 (0.1 ng)-primed females (panel C) but not males (panel D). RB is baseline. Statistical test is regular two-way ANOVA Bonferroni's post hoc test ***p < 0.001; # p < 0.0001; n = 5).
(E) IL-6 (1 ng) and vehicle or PRL (1 μg) were co-injected into the paw in estrous-phase female mice. Mechanical hyperalgesia was assessed at indicated time points. BL is baseline value. Data are represented as mean ± SEM. Statistical test is regular two-way ANOVA Bonferroni's post hoc test (**p < 0.01; ***p < 0.001; #p < 0.0001; n = 6).
Prlr Modulates Inflammatory Hypersensitivity in Female Mice
Inflammatory hypersensitivity was induced by hind paw injection of interleukin (IL)-6 (Melemedjian et al., 2010). Co-injection of IL-6 (1 ng; into the hind paw, ipl) and ΔPRL (5 μg; into the spinal cord, it) effectively inhibited mechanical hypersensitivity in female mice, whereas IL-6 and vehicle or ΔPRL co-administration into the hind paw did not produce anti-mechanical hypersensitivity effects (two-way ANOVA; F (2, 28) = 13.3; P < 0.0001; n = 5–6; Figure 3B).
The concentration of endogenous PRL in serum of rodents could be increased up to 100 ng/mL during inflammatory conditions (Patil et al., 2013a, Scotland et al., 2011). It could be presumed that inflammation will sensitize Prlr signaling. To evaluate this possibility, we used the hyperalgesic priming model (Melemedjian et al., 2010) and examined whether hind paw injection of PRL could precipitate hyperalgesic priming in mice primed with IL-6. IL-6 (0.1 ng) was injected into the hind paw and following hypersensitivity resolution (5 days post IL-6), PRL was injected into the same hind paw. Priming with IL-6 dramatically (>100-fold) sensitized Prlr signaling wherein estrus female, but not male mice, showed hypersensitivity to as low as 0.5 ng PRL (two-way ANOVA; for females; F (3, 24) = 7; P = 0.0015; n = 5; Figure 3C and for males; F (3, 20) = 2.5; P = 0.085; n = 5; Figure 3D).
It is well documented that many inflammatory and idiopathic chronic pain conditions have 2- to 6-fold greater prevalence, chronicity, and symptom severity in women as compared with men (Berkley, 1997, Fillingim et al., 2009, Traub and Ji, 2013, Unruh, 1996). Hence, we examined whether addition of exogenous PRL to IL-6 could alter chronicity and/or severity of mechanical hypersensitivity. Single co-administration of PRL (1 μg) and IL-6 (1 ng) into the hind paw resulted in a substantial increase in the duration of mechanical hypersensitivity compared with co-injection of vehicle and IL-6 into females (two-way ANOVA; F (14, 136) = 4.6; P < 0.0001; n = 5–6; Figure 3E). However, PRL did not increase the severity (i.e., magnitude) of inflammatory hypersensitivity (Figure 3E). In summary, these results indicate that peripheral and spinal Prlr signaling is involved in modulation of inflammatory pain in females.
Sensory Neuronal Prlr Regulates Inflammatory Pain in a Female-Selective Fashion
Prlr is expressed not only in sensory neurons but also in DRG fibroblasts and satellite glial cells, some immune cells, and possibly by intrinsic spinal cord neurons (Ben-Jonathan et al., 2008, Haring et al., 2018, Patil et al., 2014, Patil et al., 2019). Here, we evaluated whether sensory neuronal Prlr is essential in female-selective regulation of chemical-induced, inflammatory, and neuropathic pain. To do so, we ablated the Prlr gene in the Nav1.8+ subset of sensory neurons (Prlr CKO). The Prlrfl/fl line was generated by insertion of inverse lox sites around exon 5 (Brown et al., 2016). Hence, cre-recombination ablates the gene and activates GFP in Nav1.8+ neurons (Figures S3A and S3B). Cre-recombination was verified by GFP mRNA expression that can be amplified from DRG RNA of Prlr CKO but not Prlrfl/fl female mice (Figure S3C). To show conditional ablation of Prlr protein in sensory neurons, we performed immunohistochemistry (IHC) on spinal cord sections with CGRP and Prlr antibodies. Figure S3D shows that Prlr protein is eliminated in central terminals of the dorsal horn of spinal cord but not in other Prlr+ cells of Nav1.8cre/-/Prlrfl/fl female mice. IHC was performed only in female mice, since Prlr antibodies do not reliably label DRG sensory neurons and central terminals in spinal cord of male mice, probably owing to low Prlr expression in males and/or low sensitivity of Prlr antibodies. As an additional test of the validity of our conditional deletion approach, we also tested sensitization of TRPV1 by exogenous PRL in female mice and found that 1 μg/mL PRL sensitizes capsaicin (CAP)-evoked CGRP release in Prlrfl/fl but not Nav1.8cre/-/Prlrfl/fl (Prlr CKO) spinal cord slices (Figure S3E).
Ablation of Prlr gene in sensory neurons substantially and female-selectively reduced postoperative heat (two-way ANOVA; F (3, 40) = 5.2; P = 0.004; n = 5–8; Figure 4A) and mechanical hypersensitivity (F (3, 48) = 3.5; P = 0.021; n = 5–8; Figure 4B) at the 1-day post-incision time point. In Prlr CKO animals, IL-6-induced mechanical hypersensitivity was also significantly reversed in females (two-way ANOVA; F (3, 28) = 13.3; P < 0.0001; n = 5) but in not males (P = 0.99; n = 5) at 3 h post-IL6 time point (Figure 4C). Examination of the time course of IL-6 hypersensitivity development showed that IL-6-induced heat (two-way ANOVA; F (4, 40) = 0.74; P = 0.57; n = 5) and mechanical hypersensitivity (F (4, 40) = 0.09; P = 0.99; n = 5) were equally well developed in Prlrfl/fl and Prlr CKO male mice (Figures 4D and 4E). In contrast, IL-6-induced heat (two-way ANOVA; F (4, 30) = 3.8; P = 0.012; n = 5) and mechanical hypersensitivity (P = 0.011 at 1 h post IL-6; P = 0.004 at 3 h post IL-6; n = 5) were substantially lesser in Prlr CKO compared with Prlrfl/fl females at all time points (Figures 4F and 4G).
Figure 4.
Hypersensitivity in Inflammatory Pain Models in Sensory Neuronal Prlr CKO Male and Female Mice
(A) Postoperative (POP) heat hypersensitivity was measured 1 day post incision in Prlrfl/fl (lox; control) and Nav1.8cre/-/Prlrfl/fl (CKO) female and male mice.
(B) POP mechanical hypersensitivity was measured 1 day post incision in lox and CKO female and male mice.
(C) IL-6 (1 ng)-induced mechanical hyperalgesia was measured 3 h post IL-6 (ipl) in lox and CKO female and male mice.
For (A)–(C), Lox BL and CKO BL are baseline measurements in indicated mouse lines. For (A)–(C), data are represented as mean ± SEM and the statistical test is regular two-way ANOVA with Tukey's post hoc test (NS, p > 0.05; *p < 0.05; **p < 0.01; ****p < 0.0001; n = 5–7).
(D and E) Development of IL-6-induced heat (D) and mechanical (E) hyperalgesia in Prlr LOX (control) and Prlr CKO male mice.
(F and G) Development of IL-6-induced heat (F) and mechanical (G) hyperalgesia in Prlr LOX and Prlr CKO female mice. BL are baseline measurements in indicated mouse lines.
For (D)–(G), data are represented as mean ± SEM and the statistical test is regular two-way ANOVA with Bonferroni's post hoc test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; n = 5–6). See also Figures S3 and S4.
In a neuropathic model of chronic constriction injury (CCI), heat and mechanical hyperalgesia were similarly developed in Prlrfl/fl and Prlr CKO males (Figures S4A and S4B). CCI-induced hypersensitivity was slightly less pronounced in Prlr CKO compared with Prlrfl/fl females (Figures S4C and S4D). In a test of chemical nociception, mustard oil-induced hypersensitivity was quickly resolved (within 30 min) and identical in Prlrfl/fl and Prlr CKO females (Figures S4E and S4F). Overall, these data show that sensory neuronal Prlr contributes to female-selective regulation of hypersensitivity in inflammatory pain models but may play a lesser role in neuropathic and chemical-induced pain models.
Prlr Isoform mRNA Expression by DRG Sensory Neuronal Subtypes in Females and Males
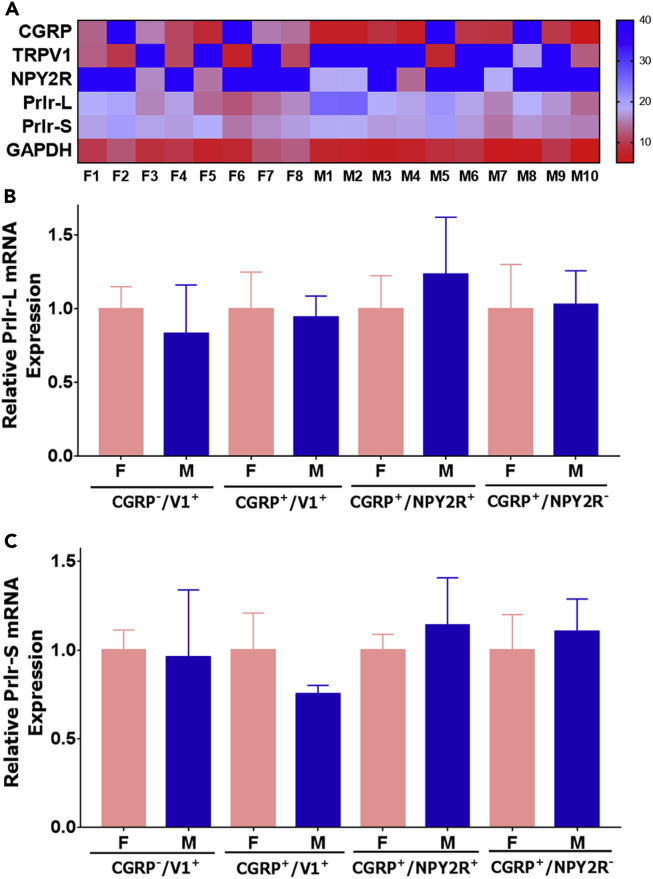
Sensory neuronal Prlr signaling appears to be female-selective for in vivo (Figure 4) and in vitro models (Diogenes et al., 2006, Patil et al., 2013b). This suggests that Prlr mRNA should have predominant expression in female compared with male sensory neurons. Prlr mRNA is mainly expressed in a subset of medium- and small-sized peptidergic and CGRP−/trpV1+ sensory neurons of female and male mice (Patil et al., 2019). Prlr+ medium-sized peptidergic neurons can be divided into two subpopulations: NPY2R+ and NPY2R− (Patil et al., 2019). Separate analysis of single-cell sequencing for female versus male data shows Prlr expression is at similar levels in Prlr+ sensory neuronal groups (Patil et al., 2019, Usoskin et al., 2015). However, data on sex-dependent expressions of Prlr long (Prlr-L) and short (Prlr-S) isoforms in sensory neurons, which have distinct functions (Belugin et al., 2013, Ben-Jonathan et al., 2008, Freeman et al., 2000), are not available. Accordingly, we examined Prlr-L and Prlr-S mRNA expression in sensory neurons using single-cell quantitative PCR (qPCR). We randomly collected single small or medium-sized Prlr-cre+ DRG neurons from female and male Prlrcre/+/Rosa26LSL-tDTomato/+ mice and performed real-time qPCR with Prlr-L, Prlr-S, as well as sensory neuronal marker CGRP, TRPV1, and NPY2R primer sets. Expression of GAPDH was used as a loading and normalization control. All visualized and collected Prlr-cre+ neurons contained Prlr-L and Prlr-S mRNA (Figure 5A). Statistical analysis showed that Prlr-L and Prlr-S mRNA were expressed at approximately similar levels in female and male neuron subtypes: CGRP+/TrpV1+, CGRP−/TrpV1+, CGRP+/NPY2R−, and CGRP+/NPY2R+ (for Prlr-L t = 0.1 df = 34; P = 0.92 and for Prlr-S t = 0.51 df = 36; P = 0.62; Figures 5B and 5C). These findings lead to the unexpected conclusion that there are not clear differences for Prlr-L and Prlr-S mRNA expression levels between sexes in a variety of Prlr-cre+ sensory neuronal subtypes.
Figure 5.
Real-time Single Cell Quantitative PCR for Prlr-L and Prlr-S from Prlr-cre+ Female and Male DRG Neurons
(A) Representative heatmap showing Ct values generated by single-cell RT-PCR from Prlr-cre+ female (F) and male (M) DRG neurons. Y axis shows amplified set of genes. X axis marks randomly picked cells for PCR. Values of ≥38 on the heatmap is considered as no amplification. Normalized mRNA expression levels of Prlr-L (B) and Prlr-S (C) isoforms in sensory neuronal groups. Groups for single Prlr-cre+ neurons from female and male mouse DRG are indicated on the x axis. Data are represented as mean ± SEM. Statistical test is unpaired t test (non-significant p > 0.05; n = 6–32 depending on Prlr-cre+ neuronal group) for each sensory neuronal group.
Sex- and Estrogen-Dependent Regulation of Neuronal Excitability in Prlr-cre+ Nociceptors
We have shown that exogenous, unmodified 23 kDa-PRL is able to produce hypersensitivity in a female-selective manner (Figures 1 and S1) and enhance excitability in female DRG neurons (Patil et al., 2014). Hence, regulation of excitability is a valid, dependable measure for Prlr activity in DRG neurons. We investigated whether PRL could regulate neuronal excitability in Prlr-cre+ sensory neurons isolated from Prlrcre/+/Rosa26LSL-tDTomato/+ female and male mouse DRG and whether this regulation is reliant on estrogen (E2). For these experiments, only small-sized Prlr-cre+ neurons belonging to CGRP+/TrpV1+ or CGRP−/TrpV1+ groups were selected (Patil et al., 2019), since it is challenging to reliably produce an action potential (AP) train in medium- to large-diameter DRG neurons using whole-cell current-clamp patch recordings. Initially, a single depolarizing pulse was applied to identify the Prlr-cre+ neuronal group on the basis of AP parameters that clearly distinguish CGRP−/TrpV1+ from CGRP+/TrpV1+ as well as from medium-sized CGRP+/TrpV1-/NPY2R− and CGRP+/TrpV1-/NPY2R+ neurons (Patil et al., 2018, Patil et al., 2019). CGRP+/TrpV1+ neurons could be further sub-grouped (Patil et al., 2018, Usoskin et al., 2015). However, data from different CGRP+/TrpV1+ sub-types were grouped together, because additional recording are required to discriminate these CGRP+/TrpV1+ neuronal sub-types. As soon as the Prlr-cre+ neuronal subtype was defined, a ramp protocol was applied to evaluate how PRL (0.2 μg/mL) treatment (2–3 min) regulates excitability in Prlr-cre+ neurons.
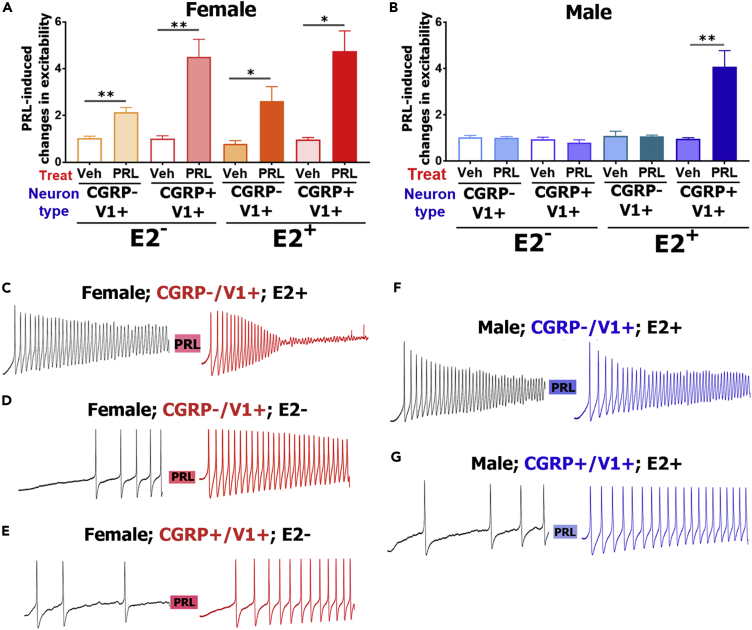
PRL triggered an increase in excitability in vehicle-treated CGRP+/TrpV1+ and CGRP−/TrpV1+ Prlr-cre+ estrus female (one-way ANOVA; F (3, 26) = 7.9; P = 0.0007; Figures 6A, 6D, and 6E) but not male DRG neurons (one-way ANOVA; F (3, 16) = 1.3; P = 0.3; Figure 6B). Mean value of PRL-induced excitability was larger in Prlr-cre+ CGRP+/TrpV1+ compared with CGRP−/TrpV1+ DRG female neurons (Figure 6A). Some female CGRP−/TrpV1+ Prlr-cre+ neurons had a higher firing frequency upon first ramp application and showed only a short AP train “burst” after PRL treatment (Figure 6C). We did not analyze these neurons. This burst firing pattern is typical for sensitized C-nociceptors (or naive A-LTMR neurons) (Koltzenburg et al., 1997). These data indicate that despite equal Prlr-L and Prlr-S mRNA expression in female and male DRG neurons, Prlr produces female-selective regulation of excitability in small-sized Prlr-cre+ DRG nociceptors. Moreover, these data provide strong independent support for the behavioral findings that sensory neuronal Prlr signaling contributes to mediation of pain in a female-specific manner (Figures 1 and 4).
Figure 6.
Sex and Estrogen-dependent Regulation of PRL-induced Excitability of Prlr-cre+ DRG Neurons
Exogenous PRL induces regulation of excitability in female (A) and male (B) DRG neurons. DRG neurons in culture were treated with vehicle (E2-) or 17β-estradiol (E2+; 1 μg/mL) for 6–36 h in culture. Y axis is change in action potential (AP) frequency (i.e., excitability) after treatment with vehicle or PRL. Data are represented as mean ± SEM. The statistical test is one-way ANOVA with Tukey's post hoc test separately for females or males and for vehicle- or E2-treated groups (*p < 0.05; **p < 0.01; n = 4–12). Examples of AP trains before and after treatment with PRL for 2–3 min are shown in female CGRP−/trpV1+ E2-treated DRG neurons (C); female CGRP−/trpV1+ vehicle-treated DRG neurons (D); female CGRP+/trpV1+ vehicle-treated DRG neurons (E); male CGRP−/trpV1+ E2-treated DRG neurons (F); and male CGRP+/trpV1+ E2-treated DRG neurons (G).
OVX females and males have very low PRL sensitivity in sensory neurons (Diogenes et al., 2006, Patil et al., 2013b). OVX females and males have substantially lower E2 serum levels than cycling females. Hence, we asked whether E2 treatment could enhance PRL sensitivity in female neurons and generate PRL responsiveness in male neurons. To test this possibility, DRG neurons in culture were maintained in the presence of 17β-estradiol (E2; 1 μg/mL) for 6–36 h. In such conditions (i.e., E2+), PRL (0.2 μg/mL) increased the excitability of male CGRP+/TrpV1+, Prlr-cre+ neurons (one-way ANOVA; F (3, 18) = 10.3; P = 0.0004; Figures 6B and 6G). However, E2 treatment did not change PRL sensitivity of male CGRP−/TrpV1+, Prlr-cre+ neurons (Figures 6B and 6F). Interestingly, according to single-cell sequencing data, estrogen receptor mRNA is absent in CGRP−/TrpV1+ (i.e., NP-3 group) male neurons (Usoskin et al., 2015). E2 treatment for less than 6 h did not have any effect on male DRG neurons (Veh 1 ± 0.1 versus PRL 0.9 ± 0.2, n = 6, P = 0.9). E2 treatment of female DRG neurons did not substantially enhance the already existing PRL sensitivity of CGRP+/TrpV1+ and CGRP−/TrpV1+ Prlr-cre+ neurons (two-way ANOVA; variables are sensory neuronal groups and treatment with E2; F (3, 41) = 0.09; P = 0.99 [for CGRP+/TrpV1+]; P = 0.98 [for CGRP−/TrpV1+]; Figure 6A). These data show that Prlr activity in female DRG neurons does not undergo additional sensitization by E2 treatment and may explain the independence of PRL responsiveness from the estrous phase (Figures 1E and 1F). In summary, PRL increases excitability only in female DRG neurons and prolonged (≥6 h) E2 treatment establishes PRL responsiveness in male CGRP+/TrpV1+ but not CGRP−/TrpV1+ Prlr-cre+ neurons.
PRL Responsiveness in Prlr-cre+ Neurons Are Regulated by a Prlr mRNA Translation Mechanism
Generation of PRL sensitivity in male DRG neurons by E2 treatment implies that E2 is responsible for the production of functional Prlr. In the classical pathway for nuclear receptors, E2 would increase male Prlr mRNA and in doing so generate functional Prlr in sensory neurons. To evaluate regulation of Prlr mRNA expression by E2, we performed in vivo E2 treatment/replacement of male as well as female mice according to a standard protocol (Diogenes et al., 2006, Patil et al., 2014). Then, Prlr-cre+ neurons were counted in single-cell suspension or Prlr-L and Prlr-S mRNA expressions were quantified. E2 treatment did not significantly alter the percentage of Prlr-cre+ male DRG neurons (9.71 ± 0.34% for vehicle (E2-) versus 10.15 ± 0.68% for E2+, n = 3 independent mice for single-cell suspensions) suggesting that E2 has little impact on Prlr transcription. Similarly, E2 did not affect the percentage of Prlr-cre+ female neurons (10.2 ± 0.4% for E2- versus 12.0 ± 0.5% for E2+, n = 3). Quantitative RT-PCR on DRG tissue from wild-type males showed that neither Prlr-L nor Prlr-S mRNA expression in DRG was substantially altered by in vivo E2 treatment (unpaired t test for Prlr-S: t = 0.1; df = 6; P = 0.9; for Prlr-L: t = 0.8; df = 6; P = 0.4; Figure 7A). Female DRG Prlr-L and Prlr-S mRNA expression levels were also not affected by E2 treatment (unpaired t test for Prlr-S: t = 1.4 df = 4; P = 0.2; for Prlr-L: t = 1.2 df = 4; P = 0.3; Figure 7B). Therefore, we find no evidence for E2 control of transcription of either Prlr isoform.
Figure 7.
E2-controlled Prlr mRNA Transcription and Translation in Male and Female DRG Neurons
(A and B) Expression of Prlr-L and Prlr-S mRNA in male (A) and female (B) DRG tissues that was isolated from mice in vivo treated with vehicle (E2-) or E2 (E2+) for 7 days. mRNA levels were assessed by quantitative RT-PCR. Data were analyzed by one-way ANOVA (n = 3–4).
(C) Inhibition of PRL (1 μg)-induced mechanical hypersensitivity in female mice by spinal treatment for 1–72 h with translation inhibitor 4EGI-1 (10 μg). BL is baseline read before PRL and 4EGI-1 treatment. “None” is no treatment with 4EGI-1. Data were analyzed by one-way ANOVA with Tukey’s post hoc test (NS, p > 0.05; **p < 0.01; n = 5).
(D) PRL (0.2 μg/mL)-induced increase in excitability in female Prlr-cre+ cultured DRG neurons pre-treated for 16–20 h with 4EGI-1 (1 μg/mL). Data are represented as mean ± SEM. Statistical test is regular two-way ANOVA with Tukey's post hoc test (variables are treatments with Veh/PRL and Media/4EGI-1; ***p < 0.001; n = 4–8).
(E) PRL (0.2 μg/mL)-induced excitability of male Prlr-cre+ DRG neurons pre-treated for 16–20 h with mixtures of indicated drugs. Data are represented as mean ± SEM. Statistical test is one-way ANOVA with Tukey's post hoc test (**p < 0.01; n = 6–13).
See also Figure S5.
To this point our results reveal that: (1) sensory neuronal expressed Prlr regulates inflammatory pain in a female-selective manner, (2) prolonged (>6 h) E2 treatment is required to confer PRL sensitivity in male DRG neurons without affecting Prlr mRNA expression levels, (3) Prlr mRNA level is similar between Prlr-cre+ neuronal groups in male and female mice, and (4) E2 treatment does not induce changes in Prlr-L or Prlr-S mRNA expression. These results lead us to conclude that female-selective regulation of inflammatory pain via Prlr signaling pathway and female-selective and E2-mediated control of PRL sensitivity are not due to transcriptional or post-translational regulation of Prlr function in DRG neurons. Accordingly, we examined whether translation regulation of Prlr mRNA could be the mechanism underlying female-selective and E2-controlled PRL responsiveness. To test this, we first estimated the half-life for Prlr protein function. Cap-dependent translation in the DRG and spinal cord was blocked by intrathecal injection of 4EGI-1 (10 μg). Intrathecal PRL (1 μg)-induced mechanical hypersensitivity was measured at 1–72 h post 4EGI-1 time points in female mice. PRL-induced hypersensitivity was assessed 1 h post PRL. PRL-triggered mechanical hypersensitivity became insignificant at >24 h post 4EGI-1 (one-way ANOVA; F (6, 28) = 5; P = 0.0013; Figure 7C). From this we conclude that the half-life for Prlr functional protein is likely 16–20 h and that new functional Prlr protein is not readily produced when cap-dependent translation is blocked (Figure 7C).
To test this finding in an independent system, we evaluated the effects of 4EGI-1 on PRL sensitivity of female DRG neurons in culture. To do so, we first evaluated whether 12- to 24-h-long 4EGI-1 treatment affects AP properties and/or excitability. CGRP-cre+/TRPV1-GFP+ DRG neurons, which represent the CGRP+/TRPV1+ group, were selected from female reporter mice. Since transient effects of PRL in sensory neurons are mediated via PKCε-dependent mechanisms (Belugin et al., 2013), cultured DRG neurons were treated with 4EGI-1 for the indicated time (Figure S5A) and then the increase in excitability by PMA (a PKC activator) was evaluated. 4EGI-1 had no adverse effect on AP properties and did not block PMA-induced hyperexcitability over a 16- to 20-h-long 4EGI-1 treatment (one-way ANOVA; F (3, 19) = 7.5; P = 0.0017; Figures S5A and S5B). Longer treatment led to an inhibition of PMA-induced excitability and distortion in AP shapes (Figure S5A). The findings in Figures 7C and S5 indicate that PRL-induced increase in Prlr-cre+ neuron excitability should be evaluated only during the 16- to 20-h pre-treatment window with 4EGI-1.
Figure 7D shows that 16- to 20-h-long 4EGI-1 pre-treatment resulted in a substantial inhibition of PRL responsiveness in female Prlr-cre+ neurons (two-way ANOVA; F (3, 19) = 13.4; P < 0.0001). E2 conferred PRL sensitivity in male DRG neurons (Figures 6A and 7E). We examined whether this E2 action depends on translation mechanisms in male neurons. A 16- to 20-h-long pre-treatment of male Prlr-cre+ DRG neurons with a mix of E2 and 4EGI-1 (1 μg/mL each) totally blocked E2-dependent establishment of PRL sensitivity in these neurons (Figure 7E). These findings support the hypothesis that sex dimorphism in Prlr functional expression and consequently PRL responsiveness involve translation control mechanisms in DRG neurons wherein Prlr mRNA in male neurons is not effectively translated into functional protein owing to E2-driven control of a translational machinery.
To provide additional in vivo evidence for this hypothesis, we examined the relative presence of Prlr mRNA and protein in DRG neuron central terminals of males and females. We focused on central terminals because our behavioral findings had the largest magnitudes with intrathecally delivered treatments. IHC was conducted on spinal cord sections from male and female Prlrcre/+/Rosa26LSL-tDTomato/+ mice (Figure 8A). Prlr mRNA reporter expression in Prlr-cre+ fibers (red) was very similar in females and males (un-paired t test; t = 1.2 df = 4; P = 0.3; n = 3; background is subtracted and data normalized per measured area; Figure 8B), whereas Prlr protein detected by polyclonal anti-Prlr antibodies was substantially greater in females than in males in the dorsal horn (one-way ANOVA; F (2, 12) = 14.9; P = 0.0006; n = 3; Figure 8C). Similarly, female-predominant expression of Prlr protein was observed in spinal cord of rats using mouse monoclonal anti-Prlr (U5) antibodies (un-paired t test; t = 3 df = 4; P = 0.042; n = 3; Figures 8D and S6). Taken together, these findings support the conclusion that the female-selective regulation of inflammatory pain by sensory neuronal Prlr and PRL sensitivity found in the DRG is due to translational regulation of Prlr function in sensory neurons.
Figure 8.
Prlr mRNA Reporter Expression and Prlr Protein Localization in Female and Male Mouse Spinal Cord
(A) IHC with Prlr antibodies (polyclonal) and CD68 (rat monoclonal) on spinal cord sections from Prlrcre/+/Rosa26LSL-tDTomato/+ female and male mice.
(B) Intensity of TdTomato (Prlr-cre) labeling in spinal cord of female and male Prlrcre/-/TdTomato mice.
(C) Intensity of Prlr protein (Prlr-ab) labeling in spinal cord of female and male Prlrcre/-/TdTomato mice. Bgr is normalized intensity of background.
(D) Intensity of Prlr protein (Prlr-ab) labeling in spinal cord of female and male rats. A representative scale bar of 50 μm is shown.
Data are represented as mean ± SEM. Statistical test is unpaired t test (B and D) or one-way ANOVA with Tukey's post hoc test (panel C) (NS, p > 0.05; *p < 0.05; **p < 0.01; n = 3). See also Figure S6.
Discussion
Studies in animals and humans demonstrate clear sex dimorphisms in mechanisms that control development of chronic pain (Dance, 2019), and the existing paradigm is that these dimorphisms are directly or indirectly regulated by gonadal hormones (Traub and Ji, 2013). A growing body of research suggests that an important mechanistic difference between development of chronic pain in male and female mice is that distinct immune cells are critical drivers, microglia in males (Paige et al., 2018, Sorge et al., 2011, Sorge et al., 2015, Taves et al., 2016) and T cells in females (Sorge et al., 2015). The male-specific microglia effects are conserved for mice and rats (Mapplebeck et al., 2018) and can be conferred to females with testosterone treatment (Sorge et al., 2015). On the other hand, the T cell findings are controversial because T cells also play a critical role in pain resolution in some pain models (Krukowski et al., 2016, Laumet et al., 2019). Several recent studies have also found sex differences in mRNA expression using RNA sequencing in whole human DRG and tibial nerve (North et al., 2019, Ray et al., 2019). The DRG transcriptomic work suggests that of the monocyte lineage in DRG and nerves may play a critical role in promoting neuropathic pain in males but to a lesser extent in females (North et al., 2019). Sex differences in the tibial nerve transcriptomes, although not directly related to chronic pain since the tissues were from organ donors and not patients, found a clear signature for gonadal hormones in regulating transcriptomes in this tissue across the lifespan in females (Ray et al., 2019). Collectively, these findings in rodents and humans support the classical viewpoint that gene regulation via gonadal hormone nuclear receptor-mediated transcription control mechanisms is a cornerstone of sex-dependent processes (Ormandy and Sutherland, 1993) including sex dimorphisms in pain (Rosen et al., 2017). Our findings suggest a new twist on this paradigm wherein gonadal hormones could also regulate sensory neuron excitability via regulation of translation machinery or transcription of proteins belonging to the translation complex. As a key example, we demonstrated this mechanism for female-selective in vitro and in vivo PRL responsiveness in sensory neurons.
Previous studies showed gonadal hormones-dependent regulation of Prlr in non-neuronal cells (Furigo et al., 2014, Hu et al., 1996, Hu et al., 1998, Tanaka et al., 2005), but this is the first demonstration of such an effect in sensory neurons. Since Prlr does not have classical gonadal hormone-response element (Ormandy and Sutherland, 1993), Prlr mRNA expression is thought to be regulated by E2 utilizing alternative transcription binding sites, such as C/EBP, Sp3, and/or Sp1A (Dong et al., 2006, Goldhar et al., 2011). Based on the literature, it could be extrapolated that Prlr mRNA should be predominantly expressed in female DRG neurons in an estrogen-dependent fashion. However, surprisingly, our data, which were generated by multiple, independent methods, show that Prlr-L and Prlr-S mRNA expression in sensory neuronal subtypes are not sex- or E2-dependent. Post-translational (i.e., phosphorylation, glycosylation) regulation is also unlikely, since prolonged E2 treatment (>6 h) is required for establishing PRL sensitivity in male neurons despite expression of Prlr mRNA. Our data support the conclusion that translation regulation of Prlr function is critical for the observed dimorphisms in nociceptive processes during inflammatory pathological pain conditions. Accordingly, inhibition of cap-dependent translation almost entirely ablated PRL responsiveness in Prlr-cre+ female neurons and blocked the behavioral response to PRL in vivo. In further support of this model, we showed that blockage cap-dependent translation eliminated E2-established PRL responsiveness in male DRG neurons. Moreover, we also observed robust expression of Prlr-cre+ sensory neurons and fibers in both male and female mice but found substantially higher Prlr protein expression in female rodent (rat and mice) spinal cord.
Based on these findings, we propose that sex- and E2-dependent translational regulation could be a novel mechanism for sexual dimorphism observed in many pain conditions (Fillingim et al., 2009). This is especially relevant considering that translation control mechanisms are already known to strongly contribute to modulation and sensitization of nociceptors but suggests that therapeutic targeting of these mechanisms may have additional benefits in females (Khoutorsky and Price, 2018, Megat and Price, 2018). Gonadal hormone-controlled translational regulation has been a subject of speculation but not studied in detail (Bronson et al., 2010, Ochnik et al., 2016). This mechanism could be due to an increase of efficiency of translation by gonadal hormones and/or gonadal hormone-controlled additional mRNA transcription of proteins involved in translational machinery. Thus, translation regulation factors encoded by Eif2s3y and Eif2s3x genes exhibit strong sex dependency in mRNA expression in many types of neurons (Armoskus et al., 2014, Ray et al., 2019). E2-driven translational control over the suppressor of cytokine signaling (SOCS) protein family has been proposed (Arbocco et al., 2016, Matthews et al., 2005) where E2 can affect the translational machinery via mTOR phosphorylation (Augusto et al., 2010) and regulation of Rheb signaling (Pochynyuk et al., 2006). Translation can also be controlled by factors binding to mRNA. One of such factors is Musashi (Msi-1 and 2 genes), which binds specific sequences in the 3′ untranslated region (UTR) of mRNA and controls translation of proteins. It was shown that leptin can control translation of proteins by regulating Msi-1 expression (Odle et al., 2018). These signaling pathways also play key roles in pain sensitization whereby they control the on-demand synthesis of new proteins that alter the excitability of nociceptors (Khoutorsky and Price, 2018, Moy et al., 2017).
A corollary of our work is that molecules involved in sex-dependent regulation of nociceptive pathways could be (1) induced by injury, (2) controlled by gonadal hormones, and (3) capable of regulating many other genes. PRL and its receptor Prlr fit these requirements. First, Prlr-mediated PRL effects are sex dependent in many tissues and cell types (Belugin et al., 2013, Ben-Jonathan et al., 2008, Patil et al., 2013a, Torner et al., 2001). It is well documented that PRL responsiveness is closely controlled by E2 and to a lesser extent progesterone. Many clinical and preclinical studies also show that stress related to injury and inflammatory conditions trigger PRL release not only from the pituitary (Chernow et al., 1987, Noreng et al., 1987, Yardeni et al., 2007), but also from extra-pituitary tissues, such as cells in skin and in the spinal cord (BenJonathan et al., 1996, Patil et al., 2013a, Scotland et al., 2011). Finally, Prlr activation could lead to epigenetic changes and transcription regulation of many genes via the STAT5 pathway (Ben-Jonathan et al., 2008, Bole-Feysot et al., 1998). This downstream transcriptional control may lead to additional diversification of nociceptor responses to injury since this pathway would not be induced in male neurons, which have very low levels of Prlr functional protein in most sensory neuronal types. The preponderance of data on sex-specific mechanisms of chronic pain has focused on male rodents. To our knowledge, this is one of the first demonstrations of a female-specific inflammatory pain mechanism acting directly on the sensory neuron. Interestingly, the discovery of a far greater potency of calcitonin gene related peptide (CGRP) in producing migraine pain-like behaviors in female mice also involves a peptide with intimate connections to nociceptor biology (Avona et al., 2019).
In conclusion, our data demonstrate that sensory neuronal Prlr contributes to female-selective regulation of hypersensitivity in inflammatory pain models via local and spinal mechanisms. Sex dependency of PRL responsiveness and the generation of hypersensitivity by this mechanism in sensory neurons is likely controlled via translation mechanisms. We favor the hypothesis that sex-specific regulation of sensory neuronal excitability by PRL is governed via E2-controlled translation regulation of Prlr mRNA, and this in turn explains female-selective mechanisms for regulation of PRL-induced hypersensitivity in non-injured animals and inflammatory pain by sensory neuronal Prlr. These results add a new layer to our understanding of sex dimorphisms in pain signaling and further substantiate the critical role that translation regulation plays in setting nociceptor excitability in response to a broad variety of important physiological stimuli.
Limitations of Study
PRL responsiveness in humans is influenced by the menstrual phases (Freeman et al., 2000). Unfortunately, data obtained in different estrous phases of rodent females (Figures 1E, 1F, and 3A) do not translate well to human menstrual phases. There are several reasons for this lack of direct translation. First, the full estrous cycle in rodents is only 4–5 days. Therefore, stable proteins, such as Prlr (Figure 7C), synthesized in the previous estrous phase may still be present in the subsequent phase. Thus, in the diestrus phase, some of Prlr synthesized in estrous is still functional. Second, changes in patterns and levels of estrogen and progesterone from one phase to another are different for menstrual versus estrous cycles. This could influence Prlr synthesis. Another limitation of our study is the lack of currently available tools to separately study effects mediated by Prlr-L or Prlr-S isoforms. There are no reliable and validated antibodies labeling Prlr-L or Prlr-S, and mouse lines targeting only Prlr-S do not exist. Moreover, there are several Prlr-S isoforms (Freeman et al., 2000). Therefore, our work is unable to address questions related to short- versus long-term effects of Prlr signaling that are mediated by these different isoforms.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Dr. Dustin Green for advice on the experimental strategy, Dr. Michael Henry for guidance on IHC, Dr. Shivani Ruparel for guidance in analyzing quantitative PCR data, Dr. Florence Boutillon for producing recombinant PRL, and Dr. Pao-Tien Chuang (UC San Francisco, San Francisco, CA) for kindly providing the Calcacre/+−ER mouse line. Single-cell gene expression analysis was performed by Bioanalytic and Single-cell core (BASiC) at UT Health at San Antonio. The BASiC core is supported by the Cancer Prevention Research Institute of Texas (CPRIT; RP150600) and the Office of Vice President of Research of UT Health at San Antonio. This work was supported by NINDS/NIH NS102161 (T.J.P and A.N.A.), NIH/NIGMS GM112747 (A.N.A.), NINDS/NIH NS065926 (T.J.P.), and UT BRAIN Pilot Program ID: 1503083 (G.D. and A.N.A.).
Author Contributions
M.P. conducted a majority of experiments and contributed to manuscript writing and revision; S.B., J.M., C.P., P.A.B., and J.T.B performed certain experiments and analysis; A.W. performed certain experiments and analyzed PCR data and prepared corresponding figures and graphical depictions; V.G. generated human PRL and contributed to experimental design, manuscript writing, and revision; D.G. and U.B. generated Prlr-cre and Prlrfl/fl mouse lines and contributed to experimental design and manuscript revision; G.D. helped with electrophysiology experiments and experimental design and contributed to the manuscript preparation; T.J.P. and A.N.A designed and directed the project, helped with experiments, wrote the first draft of the manuscript, and prepared the final version of the manuscript.
Declaration of Interests
All authors declare that they have no competing interests.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.09.039.
Contributor Information
Theodore J. Price, Email: theodore.price@utdallas.edu.
Armen N. Akopian, Email: akopian@uthscsa.edu.
Supplemental Information
References
- Aloisi A.M., Sorda G. Relationship of female sex hormones with pain perception: focus on estrogens. Pain Manag. 2011;1:229–238. doi: 10.2217/pmt.11.13. [DOI] [PubMed] [Google Scholar]
- Amandusson A., Blomqvist A. Estrogenic influences in pain processing. Front. Neuroendocrinol. 2013;34:329–349. doi: 10.1016/j.yfrne.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Arbocco F.C.V., Sasso C.V., Actis E.A., Caron R.W., Hapon M.B., Jahn G.A. Hypothyroidism advances mammary involution in lactating rats through inhibition of PRL signaling and induction of LIF/STAT3 mRNAs. Mol. Cell Endocrinol. 2016;419:18–28. doi: 10.1016/j.mce.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Armoskus C., Moreira D., Bollinger K., Jimenez O., Taniguchi S., Tsai H.W. Identification of sexually dimorphic genes in the neonatal mouse cortex and hippocampus. Brain Res. 2014;1562:23–38. doi: 10.1016/j.brainres.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto T.M., Bruni-Cardoso A., Damas-Souza D.M., Zambuzzi W.F., Kuhne F., Lourenco L.B., Ferreira C.V., Carvalho H.F. Oestrogen imprinting causes nuclear changes in epithelial cells and overall inhibition of gene transcription and protein synthesis in rat ventral prostate. Int. J. Androl. 2010;33:675–685. doi: 10.1111/j.1365-2605.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- Avona A., Burgos-Vega C., Burton M.D., Akopian A., Price T.J., Dussor G. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J. Neurosci. 2019;39:4323–4331. doi: 10.1523/JNEUROSCI.0364-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belugin S., Diogenes A.R., Patil M.J., Ginsburg E., Henry M.A., Akopian A.N. Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J. Biol. Chem. 2013;288:34943–34955. doi: 10.1074/jbc.M113.486571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N., LaPensee C.R., LaPensee E.W. What can we learn from rodents about prolactin in humans? Endocr. Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BenJonathan N., Mershon J.L., Allen D.L., Steinmetz R.W. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr. Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- Berkley K.J. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Bernichtein S., Kayser C., Dillner K., Moulin S., Kopchick J.J., Martial J.A., Norstedt G., Isaksson O., Kelly P.A., Goffin V. Development of pure prolactin receptor antagonists. J. Biol. Chem. 2003;278:35988–35999. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C., Goffin V., Edery M., Binart N., Kelly P.A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Bronson M.W., Hillenmeyer S., Park R.W., Brodsky A.S. Estrogen coordinates translation and transcription, revealing a role for NRSF in human breast cancer cells. Mol. Endocrinol. 2010;24:1120–1135. doi: 10.1210/me.2009-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.S.E., Kokay I.C., Phillipps H.R., Yip S.H., Gustafson P., Wyatt A., Larsen C.M., Knowles P., Ladyman S.R., LeTissier P. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2016;36:9173–9185. doi: 10.1523/JNEUROSCI.1471-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernow B., Alexander H.R., Smallridge R.C., Thompson W.R., Cook D., Beardsley D., Fink M.P., Lake C.R., Fletcher J.R. Hormonal responses to graded surgical stress. Arch. Intern. Med. 1987;147:1273–1278. [PubMed] [Google Scholar]
- Childs G.V., Unabia G., Miller B.T., Collins T.J. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J. Endocrinol. 1999;162:177–187. doi: 10.1677/joe.0.1620177. [DOI] [PubMed] [Google Scholar]
- Dance A. Why the sexes don't feel pain the same way. Nature. 2019;567:448–450. doi: 10.1038/d41586-019-00895-3. [DOI] [PubMed] [Google Scholar]
- Diogenes A., Patwardhan A.M., Jeske N.A., Ruparel N.B., Goffin V., Akopian A.N., Hargreaves K.M. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J. Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Tsai-Morris C.H., Dufau M.L. A novel estradiol/estrogen receptor alpha-dependent transcriptional mechanism controls expression of the human prolactin receptor. J. Biol. Chem. 2006;281:18825–18836. doi: 10.1074/jbc.M512826200. [DOI] [PubMed] [Google Scholar]
- Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., Rahim-Williams B., Riley J.L. Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.E., Kanyicska B., Lerant A., Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Furigo I.C., Kim K.W., Nagaishi V.S., Ramos-Lobo A.M., de Alencar A., Pedroso J.A., Metzger M., Donato J., Jr. Prolactin-sensitive neurons express estrogen receptor-alpha and depend on sex hormones for normal responsiveness to prolactin. Brain Res. 2014;1566:47–59. doi: 10.1016/j.brainres.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Goldhar A.S., Duan R.Q., Ginsburg E., Vonderhaar B.K. Progesterone induces expression of the prolactin receptor gene through cooperative action of Sp1 and C/EBP. Mol. Cell Endocrinol. 2011;335:148–157. doi: 10.1016/j.mce.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M., Zeisel A., Hochgerner H., Rinwa P., Jakobsson J.E.T., Lonnerberg P., La Manno G., Sharma N., Borgius L., Kiehn O. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- Houghton L.A., Lea R., Jackson N., Whorwell P.J. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.Z., Zhuang L., Dufau M.L. Multiple and tissue-specific promoter control of gonadal and non-gonadal prolactin receptor gene expression. J. Biol. Chem. 1996;271:10242–10246. doi: 10.1074/jbc.271.17.10242. [DOI] [PubMed] [Google Scholar]
- Hu Z.Z., Zhuang L., Dufau M.L. Prolactin receptor gene diversity: structure and regulation. Trends Endocrinol. Metab. 1998;9:94–102. doi: 10.1016/s1043-2760(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Karp N.A., Mason J., Beaudet A.L., Benjamini Y., Bower L., Braun R.E., Brown S.D.M., Chesler E.J., Dickinson M.E., Flenniken A.M. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat. Commun. 2017;8:15475. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M.J., Moss R.L., Dudley C.A. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Khoutorsky A., Price T.J. Translational control mechanisms in persistent pain. Trends Neurosci. 2018;41:100–114. doi: 10.1016/j.tins.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M., Stucky C.L., Lewin G.R. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Krukowski K., Eijkelkamp N., Laumet G., Hack C.E., Li Y., Dougherty P.M., Heijnen C.J., Kavelaars A. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J. Neurosci. 2016;36:11074–11083. doi: 10.1523/JNEUROSCI.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G., Edralin J.D., Dantzer R., Heijnen C.J., Kavelaars A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain. 2019;160:1459–1468. doi: 10.1097/j.pain.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L., Mancl L., Sherman J.J., Gandara B., Dworkin S.F. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Qu Z.W., Ren C., Gan X., Qiu C.Y., Hu W.P. Prolactin potentiates the activity of acid-sensing ion channels in female rat primary sensory neurons. Neuropharmacology. 2016;103:174–182. doi: 10.1016/j.neuropharm.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Mapplebeck J.C.S., Dalgarno R., Tu Y., Moriarty O., Beggs S., Kwok C.H.T., Halievski K., Assi S., Mogil J.S., Trang T. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159:1752–1763. doi: 10.1097/j.pain.0000000000001265. [DOI] [PubMed] [Google Scholar]
- Martin L.J., Acland E.L., Cho C., Gandhi W., Chen D., Corley E., Kadoura B., Levy T., Mirali S., Tohyama S. Male-specific conditioned pain hypersensitivity in mice and humans. Curr. Biol. 2019;29:192–201.e4. doi: 10.1016/j.cub.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Matthews J., Almlof T., Kietz S., Leers J., Gustafsson J.A. Estrogen receptor-alpha regulates SOCS-3 expression in human breast cancer cells. Biochem. Biophys. Res. Commun. 2005;335:168–174. doi: 10.1016/j.bbrc.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Megat S., Price T.J. Therapeutic opportunities for pain medicines via targeting of specific translation signaling mechanisms. Neurobiol. Pain. 2018;4:8–19. doi: 10.1016/j.ynpai.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian O.K., Asiedu M.N., Tillu D.V., Peebles K.A., Yan J., Ertz N., Dussor G.O., Price T.J. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J. Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S., Sorge R.E., LaCroix-Fralish M.L., Smith S.B., Fortin A., Sotocinal S.G., Ritchie J., Austin J.S., Schorscher-Petcu A., Melmed K. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat. Neurosci. 2011;14:1569–1573. doi: 10.1038/nn.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E., Santos R.S., Criollo A., Nelson M.D., Palmer B.F., Clegg D.J. The effects of oestrogens and their receptors on cardiometabolic health. Nat. Rev. Endocrinol. 2017;13:352–364. doi: 10.1038/nrendo.2017.12. [DOI] [PubMed] [Google Scholar]
- Moy J.K., Khoutorsky A., Asiedu M.N., Black B.J., Kuhn J.L., Barragan-Iglesias P., Megat S., Burton M.D., Burgos-Vega C.C., Melemedjian O.K. The MNK-eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. J. Neurosci. 2017;37:7481–7499. doi: 10.1523/JNEUROSCI.0220-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreng M.F., Jensen P., Tjellden N.U. Per- and postoperative changes in the concentration of serum thyreotropin under general anaesthesia, compared to general anaesthesia with epidural analgesia. Acta Anaesthesiol. Scand. 1987;31:292–294. doi: 10.1111/j.1399-6576.1987.tb02569.x. [DOI] [PubMed] [Google Scholar]
- North R., Yan L., Ray P., Rhines L., Tatsui C., Rao G., Johansson C., Kim Y.H., Zhang B., Dussor G. Electrophysiologic and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain. 2019;142:1215–1226. doi: 10.1093/brain/awz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochnik A.M., Peterson M.S., Avdulov S.V., Oh A.S., Bitterman P.B., Yee D. Amplified in breast cancer regulates transcription and translation in breast cancer cells. Neoplasia. 2016;18:100–110. doi: 10.1016/j.neo.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odle A.K., Benes H., Melgar Castillo A., Akhter N., Syed M., Haney A., Allensworth-James M., Hardy L., Winter B., Manoharan R. Association of Gnrhr mRNA with the stem cell determinant musashi: a mechanism for leptin-mediated modulation of GnRHR expression. Endocrinology. 2018;159:883–894. doi: 10.1210/en.2017-00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy C.J., Sutherland R.L. Mechanisms of prolactin receptor regulation in mammary-gland. Mol. Cell. Endocrinol. 1993;91:C1–C6. doi: 10.1016/0303-7207(93)90247-h. [DOI] [PubMed] [Google Scholar]
- Paige C., Maruthy G.B., Mejia G., Dussor G., Price T. Spinal inhibition of P2XR or p38 signaling disrupts hyperalgesic priming in male, but not female, mice. Neuroscience. 2018;385:133–142. doi: 10.1016/j.neuroscience.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M., Hovhannisyan A.H., Wangzhou A., Mecklenburg J., Koek W., Goffin V., Grattan D., Boehm U., Dussor G., Price T.J. Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J. Neuroendocrinol. 2019;31:e12759. doi: 10.1111/jne.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.J., Green D.P., Henry M.A., Akopian A.N. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience. 2013;253:132–141. doi: 10.1016/j.neuroscience.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.J., Henry M.A., Akopian A.N. Prolactin receptor in regulation of neuronal excitability and channels. Channels (Austin) 2014;8:193–202. doi: 10.4161/chan.28946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.J., Hovhannisyan A.H., Akopian A.N. Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines. PLoS One. 2018;13:e0198601. doi: 10.1371/journal.pone.0198601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.J., Ruparel S.B., Henry M.A., Akopian A.N. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1154–E1164. doi: 10.1152/ajpendo.00187.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi X., Voogt J.L. Sex difference and estrous cycle: expression of prolactin receptor mRNA in rat brain. Brain Res. Mol. Brain Res. 2002;103:130–139. doi: 10.1016/s0169-328x(02)00194-8. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O., Medina J., Gamper N., Genth H., Stockand J.D., Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J. Biol. Chem. 2006;281:26520–26527. doi: 10.1074/jbc.M603716200. [DOI] [PubMed] [Google Scholar]
- Ray P.R., Khan J., Wangzhou A., Tavares-Ferreira D., Akopian A.N., Dussor G., Price T.J. Transcriptome analysis of the human tibial nerve identifies sexually dimorphic expression of genes involved in pain, inflammation, and neuro-immunity. Front. Mol. Neurosci. 2019;12:37. doi: 10.3389/fnmol.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rosen S., Ham B., Mogil J.S. Sex differences in neuroimmunity and pain. J. Neurosci. Res. 2017;95:500–508. doi: 10.1002/jnr.23831. [DOI] [PubMed] [Google Scholar]
- Rosen S.F., Ham B., Haichin M., Walters I.C., Tohyama S., Sotocinal S.G., Mogil J.S. Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain. 2019;160:358–366. doi: 10.1097/j.pain.0000000000001420. [DOI] [PubMed] [Google Scholar]
- Rouet V., Bogorad R.L., Kayser C., Kessal K., Genestie C., Bardier A., Grattan D.R., Kelder B., Kopchick J.J., Kelly P.A. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc. Natl. Acad. Sci. U S A. 2010;107:15199–15204. doi: 10.1073/pnas.0911651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland P.E., Patil M., Belugin S., Henry M.A., Goffin V., Hargreaves K.M., Akopian A.N. Endogenous prolactin generated during peripheral inflammation contributes to thermal hyperalgesia. Eur. J. Neurosci. 2011;34:745–754. doi: 10.1111/j.1460-9568.2011.07788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade G.D., Bair E., By K., Mulkey F., Baraian C., Rothwell R., Reynolds M., Miller V., Gonzalez Y., Gordon S. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J. Pain. 2011;12:T12–T26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., LaCroix-Fralish M.L., Tuttle A.H., Sotocinal S.G., Austin J.S., Ritchie J., Chanda M.L., Graham A.C., Topham L., Beggs S. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Mapplebeck J.C., Rosen S., Beggs S., Taves S., Alexander J.K., Martin L.J., Austin J.S., Sotocinal S.G., Chen D. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Suzuki M., Kawana T., Segawa M., Yoshikawa M., Mori M., Kobayashi M., Nakai N., Saito T.R. Differential effects of sex steroid hormones on the expression of multiple first exons including a novel first exon of prolactin receptor gene in the rat liver. J. Mol. Endocrinol. 2005;34:667–673. doi: 10.1677/jme.1.01702. [DOI] [PubMed] [Google Scholar]
- Taves S., Berta T., Liu D.L., Gan S., Chen G., Kim Y.H., Van de Ven T., Laufer S., Ji R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L., Toschi N., Pohlinger A., Landgraf R., Neumann I.D. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J. Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R.J., Ji Y. Sex differences and hormonal modulation of deep tissue pain. Front. Neuroendocrinol. 2013;34:350–366. doi: 10.1016/j.yfrne.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh A.M. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Usoskin D., Furlan A., Islam S., Abdo H., Lonnerberg P., Lou D., Hjerling-Leffler J., Haeggstrom J., Kharchenko O., Kharchenko P.V. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Yardeni I.Z., Shavit Y., Bessler H., Mayburd E., Grinevich G., Beilin B. Comparison of postoperative pain management techniques on endocrine response to surgery: a randomised controlled trial. Int. J. Surg. 2007;5:239–243. doi: 10.1016/j.ijsu.2006.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.