Practice Gap
Asthma is the most common chronic respiratory disease of childhood, a leading cause of emergency department visits, and 1 of the top 3 indications for hospitalization in children. Despite advances in the management of pediatric asthma, significant disparities in care and outcomes persist. To bridge these gaps, we must embrace the concept of asthma care across the continuum and extend our reach beyond the immediate patient-provider visit.
Objectives
After completing this article, readers should be able to:
Understand trends in prevalence, outcomes, and health disparities in pediatric asthma.
Describe advances in understanding the pathophysiology of asthma.
Translate an understanding of asthma to a differential diagnosis in a child with wheeze.
Practice guideline-based management with accurate assessment of asthma severity and control.
Apply recent evidence-based emerging trends in the treatment of asthma.
Identify community-based strategies and quality improvement projects in your practice setting to ensure asthma care across the continuum for vulnerable children.
Introduction
Asthma is defined by episodic and reversible airway constriction and inflammation in response to infection, environmental allergens, and irritants. It is a complex, multifactorial, and immune-mediated process that presents with various clinical phenotypes.
Despite novel treatments and guideline-based care, asthma remains a significant public health problem. Medical care, missed school, and missed work related to asthma continue to burden our communities, costing more than $80 billion each year. (1) Furthermore, asthma disproportionately affects minorities and socioeconomically disadvantaged children. (2) Black children have the highest asthma morbidity and mortality rates of any US group, with twice the emergency department (ED) visit/hospitalization rate and 9 times the mortality rate as white children. (3)(4)(5)
In 2012, the President’s Task Force on Environmental Health Risks and Safety Risks to Children commissioned a cabinet-level, multiagency group to address overall asthma morbidity and its racial and ethnic disparities. (6) The group chose 4 integrated strategies.
Remove barriers to guideline-based care.
Improve the identification of at-risk children.
Build capacity for integrated, community-based care.
Support efforts to prevent asthma.
Epidemiology and Prevalence
Asthma affects 1 in 12 US children aged 0 through 17 years. (3) After decades of increases, the prevalence of asthma in this group plateaued between 2010 and 2012, (2) decreased in 2013 from 9.3% in 2012 to 8.3%, and remained stable through 2016. (2)(3) In contrast, pediatric asthma prevalence in black children increased between 2001 and 2009, leveling off by 2013. (2) In 2016, asthma prevalence in black children rose sharply to 15.7% (a 2.3% increase from 2014 and 2015), twice that of white children. (3) This rate surpassed that of Puerto Rican children, who previously had the highest prevalence of asthma of all US children. (3) The prevalence of asthma in children in poverty did not decrease between 2001 and 2013 and remained high in 2016 (10.5%). (2)(3)
Outcomes
Asthma exacerbation is a leading cause of (ED) visits (7) and 1 of the top 3 indications for hospitalization in children. (8) Comparing asthma outcomes between subgroups, an at-risk rate (ARR) is preferred over the traditional population-based rate (PBR). (9) An ARR is the number of children with the outcome (eg, asthma hospitalization or death) divided by the total number of children at risk for that outcome (those with asthma). Conversely, a PBR is the number of children with the asthma outcome divided by the total population. PBRs measure the overall burden of disease in a particular population or subgroup, whereas an ARR accounts for differences in prevalence between subgroups to describe disparities.(4) Between 2001 and 2010, ARRs for black and white children revealed a decrease in racial disparities for ED visit and hospitalization rates and no change in death rate due to asthma. (4) It is important to note, however, that ARRs do not account for differences in underlying asthma severity. A 2013 analysis of hospitalizations for chronic conditions in the Pediatric Health Information System database found that black children had the highest asthma readmission rate (21.4%) of any race or ethnicity. (10) Finally, because ARRs rely on prevalence estimates, any undercounting of "current asthma" in disadvantaged groups could result in an overestimation of risk.
Disparities in asthma outcomes and prevalence also exist based on geographic location. (11)(12) In Washington, DC, for example, 1 in 9 children, compared with 1 in 12 nationally, had asthma in 2014. (13) In addition, children in DC with the highest rates of ED visits and hospitalizations for asthma live in socioeconomically disadvantaged neighborhoods. Geographic variation in DC is likely due to a variety of factors, including but not limited to race and socioeconomic status. Population health using geospatial mapping, (2) disease-specific registries based on local electronic health records, and higher-level data analytics (machine learning, artificial intelligence, etc) (14) are promising tools to identify and decrease disparities in asthma outcomes. Collaborative efforts should target hot spots to address the causes of disparate outcomes. (15) Examples include the following:
Map local asthma prevalence using geographic information systems software to identify hot spots of poor asthma control (ED visits, hospitalizations, readmissions, ICU admission, or death). Investigate neighborhood-specific factors (access to routine primary care, [16] housing conditions) through focus groups or needs assessments and initiate quality improvement projects to improve asthma control.
Create an asthma registry using data from a health system’s electronic health records to identify an at-risk subpopulation. Initiate a systemwide collaborative effort to address disparities in care (initiation of inhaled corticosteroid [ICS], [17] ensuring follow-up after hospitalization and emergency care, [18] management of comorbid conditions). Consider education or electronic decision support as interventions for a quality improvement project to reduce disparities in care.
Use machine learning to analyze large data sets (eg, all ED visits, [19] asthma registry) to identify associations between risk factors and poor asthma outcomes previously not considered. Collaborate with local resources to mitigate risk (legal aid to assist with poor housing, care coordination to ensure medications prescribed are covered and there is subspecialty follow-up).
Pathophysiology
Asthma is a highly complex, immune-mediated, inflammatory disease, with intermittent and reversible lower airway obstruction due to smooth muscle constriction and airway narrowing in response to an environmental trigger, often in association with a viral upper airway infection. Numerous inflammatory pathways to airway swelling result in the many clinical phenotypes of pediatric asthma.
Longitudinal studies attempting to further understand the natural history of asthma have identified 3 distinct phenotypes: transient early wheezing, nonatopic wheezing, and atopy-associated wheezing. (20) Transient early wheezing is characterized by resolution of wheeze by 3 years of life, a lack of association with family history of asthma or early allergic sensitization as well as a positive association with prematurity, exposure to other children at child care, maternal smoking during pregnancy, and environmental tobacco smoke exposure during infancy. (20) Nonatopic wheezing is characterized by viral-induced wheeze during first the 2 to 3 years of life, with respiratory syncytial virus infection being associated with persistent wheeze later in life. (20) In contrast, atopic wheezing is characterized by a family history of atopy, early allergen sensitization, and a genetic predisposition for sensitization. (20) With any of these phenotypes, severe illness early in life is associated with progressive and persistent wheeze in later life. (20)
Five distinct clusters of asthma phenotypes (characterized by asthma and allergy severity, pulmonary physiology, allergy sensitization, total serum immunoglobulin E [IgE], and allergic inflammation) were recently described in a large urban longitudinal cohort from 9 cities in disadvantaged and largely minority children aged 6 to 17 years. (21) These phenotypes were distinguishable by the intensity of the allergic response. Similarly, a birth cohort study compared multiple groups with documented IgE antibody responses and found early exposure to multiple allergens predictive of asthma later in life. (22)
The significance of variation in early exposures to allergens versus microbes in children with asthma has been an area of speculation for some time. Ecologic data are suggestive of the "hygiene hypothesis" whereby countries with higher rates of early microbial exposure have decreased prevalence of asthma, and, conversely, countries with less exposure during childhood have higher rates of asthma. (23) Current understanding of cellular and molecular immunology suggests that exposures very early in life shape the development of a child’s immune system to favor either a Th1 (nonallergic) or Th2 (allergic) lymphocyte predominance. (24) Theoretically, if a child is exposed to various microbes early in life, the immune system will favor a nonallergic, infection-fighting, ie, healthy, response. On the other hand, if a child is not exposed to many microbes and is instead exposed to certain allergens (eg, dust, mold, animal dander) the immune system will favor an allergic, inflammatory, ie, unhealthy, response. Studies support that children exposed to microbes early in life (living on farms, with older siblings or pets) have a lower risk of developing persistent wheeze later in life. Thus, the microbiome may be the intermediary step between the genetic risk of the host and clinical phenotype in asthma, and perhaps the key to primary prevention of asthma. (25)
Furthermore, allergen exposure and viral infections act synergistically in the development of asthma. (26) Children with asthma, or at risk for asthma, sensitized and exposed to perennial allergens (ie, dust, mold, mouse, rat, or cockroach), often experience airway inflammation when infected with rhinovirus. (27) Rhinovirus (subtype C) has specifically been implicated in connection with development of asthma and asthma exacerbation. (27)(28)
Cytokine-mediated inflammation in allergic asthma is the most important recent discovery in asthma pathogenesis. The airways of a sensitized child with asthma release mediators, damaging the epithelium. Th2 lymphocytes trigger the release of cytokines (interleukin [IL]-4, IL-5, IL-9, IL-13, among others), which upregulate or downregulate other cytokine receptors. (29)(30) Cytokines stimulate IgE production, mast cells, basophils, and eosinophils, which mediate inflammation via histamine, prostaglandins, and leukotrienes. (30) Newer therapies interrupt the inflammatory pathway by inhibiting the actions of specific mediators (IgE and IL-5).
Mechanistic analysis at an individual’s cellular level could help guide targeted therapy. A precision medicine approach could reduce morbidity in disproportionately affected subgroups. (31) A recent study identified pro-inflammatory monocytes and type 2 cytokine activity to be more commonly associated with poorly controlled asthma in socioeconomically disadvantaged children than children with a higher socioeconomic status after controlling for potential confounders (age, sex, racial group, BMI, asthma severity, and medication use). These children also had reduced expression of genes that coded for interferon. (32) Lower levels of interferon have previously been associated with increased viral-induced exacerbations. (33)(34)(35)
Triggers
Respiratory viral infections are the most common triggers of asthma exacerbation in children. (36)(37) Annual fall increases in asthma exacerbation, or the September epidemic, occur when many children returning to school have asthma exacerbations triggered by rhinovirus infection. (38)(39) Specific viral infections have also been found to be associated with increased exacerbation severity (rhinovirus [subtype C] [27] and influenza A [H1N1] [40]) and treatment failure (respiratory syncytial virus, influenza, and parainfluenza). (41) A study of influenza-related pediatric deaths between 2000 and 2016 found that 12% had asthma. (42) Annual influenza vaccination for children with asthma is considered best practice and is recommended for patients with asthma. (43) The 2018-2019 recommendations from the Advisory Committee on Immunization Practices recommend against use of the live attenuated influenza vaccine (LAIV) in children aged 2 through 4 years who have a diagnosis of asthma or have had a wheezing illness in the preceding 12 months due to a concern that the LAIV could induce exacerbation. (44) Although the 2018-2019 LAIV guidelines have not been changed, new evidence from a large trial showed improved protection and no increased risk of exacerbation with the LAIV in children with asthma. (45) Of note, the 2017-2018 and 2018-2019 American Academy of Pediatrics guidelines did not recommend the LAIV as the primary choice of influenza vaccination for all children 6 months and older owing to concerns of decreased effectiveness of the LAIV against influenza A/H1N1 in past seasons and uncertainty regarding its effectiveness this season. (46) Recently published 2019-2020 American Academy of Pediatrics guidelines, consistent with Centers for Disease Control and Prevention (CDC) recommendations, advise patients to get the LAIV. To be clear, however, details regarding recommendations for patients with asthma have not been released.
Other triggers can be organized into several categories. First, irritants bother any airway and can induce bronchospasm when a child with asthma is exposed. These include environmental tobacco smoke (47)(48)(49) and other burnt substances (candles, incense, marijuana, and electronic cigarettes); air pollution, particulate matter (fractions of particles with an aerodynamic diameter smaller than 2.5 and 10 μm), and ozone (50); chemicals in certain cleaning products; particles in the air (construction sites, perfumes, paints, soaps); and wood or charcoal fires. Allergens are specific to the child and trigger asthma in children with atopy, sometimes referred to as allergic asthma. These are divided into perennial allergens (mold spores, cockroaches, rats, mice, dust mites, and pet dander) and seasonal allergens (tree, weed, and grass pollens).
Weather (rapid changes or extreme cold/hot air) and strong emotions (laughter, crying, or anger) are other common triggers of bronchospasm in children with asthma. Also, medications such as β-blockers, aspirin, and nonsteroidal anti-inflammatory drugs are associated with inducing asthma exacerbations in a subset of the population.
Last, exercise and other strenuous activity are frequent triggers. Exercise-induced symptoms are more common with poorly controlled asthma. (43) Preexercise preventive short-acting inhaled β2-agonist (SABA) with or without a step-up in asthma control is standard management. (43) Exercise-induced asthma is a rare, often overdiagnosed phenotype, where a child wheezes or coughs only after a short period of vigorous activity and symptoms improve 20 to 30 minutes after cessation of activity. Other comorbid conditions, such as severe uncontrolled allergic rhinitis, obesity, and vocal cord dysfunction, should be considered when evaluating exercised-induced symptoms. Vocal cord dysfunction, or paradoxical vocal cord motion on inspiration, typically presents with the following distinguishing features: rapid onset and offset, usually minutes to seconds; dyspnea reported on inspiration (or inspiration and expiration); patient localization of the throat and not the chest as the source of discomfort; SABA or rescue inhaler usually reported as not helping; symptoms induced by stress, anxiety, aerobic exercise, or irritants; and a flow-volume loop with a classic appearance, ie, a truncated inspiratory flow loop with a normal expiratory loop.
Another entity often confused with asthma is exercise-induced laryngeal obstruction (EILO). Any concern for vocal cord dysfunction and EILO should prompt referral to otolaryngology for further testing. Continuous laryngoscopy during exercise is the gold standard for diagnosis of EILO.
Families should be counseled on trigger identification and avoidance at routine visits for asthma care. Efforts should be made to mitigate exposure to perennial allergens (mold spores, dust mites, animal dander, cockroaches, rats, and mice) for patients with asthma with evidence by clinical history (symptoms with exposure) and allergen sensitization on skin prick testing or from serum specific IgE measurements. The following is a list of recommendations by allergen:
Dust mites—provide mattress and pillow encasements, (51) vacuum twice a week, consider removal of carpet in the bedroom if there are significant nighttime symptoms, remove any stuffed animals from the child’s bed or recommend machine washing of stuffed animals in hot water
Mold spores—avoid humidifier use; remediate any water damage/mold in the home
Pests—keep food out of the bedroom and in closed trash containers, exterminate if needed (preference of traps over sprays)
Animal dander—consider removal of animal if that is an option or recommend special hypoallergenic breeds
Environmental triggers are a major contributor to health disparities in pediatric asthma. (6) Removal of allergens in homes of children younger than 6 years with associated asthma could reduce asthma risk by 40%. (52) In addition, poor mental health is associated with poor asthma control. (53)(54) A thorough approach to potential exposures includes care coordination of appropriate referrals (allergist, nutritionist, gastroenterologist, otolaryngologist, mental health consultant), connection to community partners (local organizations that conduct home visits, provide vacuums or mattress and pillow encasements; local housing authority; 1-800-QUITLINE or other smoking cessation assistance), legal advocacy (legal aid to assist the family in advocating for housing remediation if the landlord is not being compliant with housing regulations, local law schools may also have free legal services), social work, and close follow-up for at-risk children. Tailored, comprehensive interventions reduce asthma morbidity rates in at-risk children. (15)(18)(55)(56)
Clinical Aspects
Diagnosis
Asthma in children remains largely a clinical diagnosis. A child with a family history of asthma presenting with episodic and recurring chest tightness, cough, difficulty breathing, or wheeze in response to common triggers who also demonstrates improvement with a SABA likely has asthma. In older children, demonstration of reversible bronchospasm on pulmonary function testing (PFT) is diagnostic for asthma.
The diagnosis of asthma in younger children remains challenging. Children younger than 5 years are unable to provide the effort required to reliably obtain PFT results. The Asthma Predictive Index is a clinical tool sometimes applied to children 3 years and younger with wheezing to assist with treatment and prediction of asthma. A combination of major criteria (doctor-diagnosed parental asthma or eczema) and minor criteria (doctor-diagnosed allergic rhinitis, wheezing without viral upper respiratory tract infections, or peripheral eosinophilia ≥4%) help determine risk. (57) In the original cohort, a child with wheezing and either 1 major or 2 minor criteria was 2 to 5 times more likely to have active asthma at age 6 to 13 years and 4 to 9 times more likely if they had both 1 minor and 2 major criteria. (57) A recent study developed a new clinical tool, the Pediatric Asthma Risk Score, which performed better than the Asthma Predictive Index to predict mild to moderate asthma. (58) The tool is available online for clinicians to use at https://pars.research.cchmc.org/.
In addition, measuring airway inflammation using fractional excretion of nitric oxide (FeNO) may help evaluate asthma in children who have eosinophil-mediated lung inflammation. (59) However, FeNO testing is not always covered by insurance, the equipment is seldom available in the primary care office, and guidelines do not recommend routine use. (43)(60) The 2018 Global Initiative for Asthma guidelines did report on benefits in certain situations, (60) and the update for the next National Asthma Education and Prevention Program (NAEPP) guidelines has listed FeNO as 1 of 5 areas for intensive future review. (61)
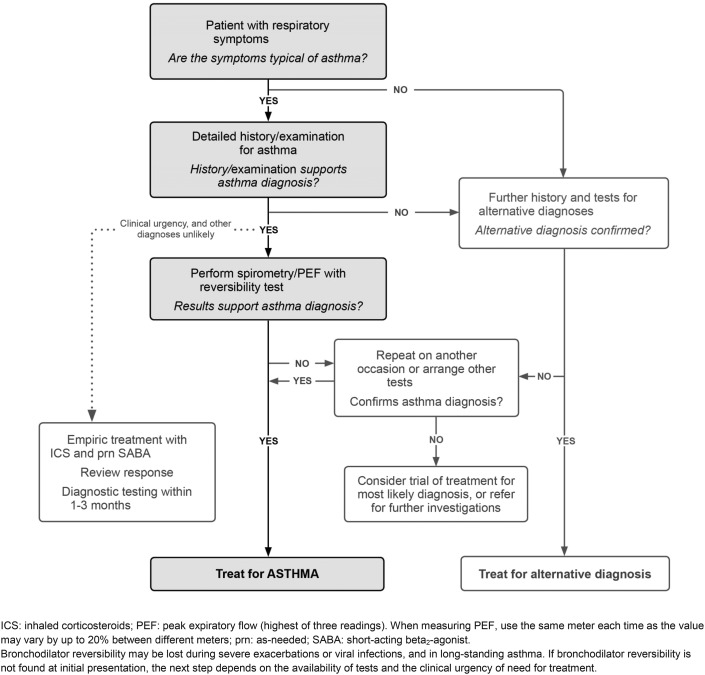
Wheezing is the characteristic finding on lung auscultation in a child experiencing asthma exacerbation, but it is not specific to asthma. Wheezing results from airflow obstruction in the lower airways and is present in many other conditions. Broad differential diagnoses of wheezing should be considered for any child with an initial episode of wheeze or difficult-to-control asthma or if there is report of little or no relief of symptoms with SABA rescue. When considering an alternative diagnosis, it is important to remember that a child could still have concomitant asthma. Table 1 lists alternative diagnoses in a child with wheeze, starting with more common entities, by age, typical findings, and testing recommendations. Another approach would be to consider alternative diagnoses by system (infectious, cardiac, pulmonary, hematologic) or mechanical obstruction causing wheeze. The latter approach is helpful when eliciting a review of systems and completing a focused physical examination in a child with first-time wheeze or difficult-to-control previously diagnosed asthma. Finally, several key historical features are important to elicit to assess severity: family history of asthma/atopy or other illness, age at onset of symptoms, episodic versus persistent disease, potential triggers or exposures, responsiveness to SABA, positionality of symptoms, and systemic signs of illness (poor weight gain, fevers, fatigue, or pallor) (Fig 1). Occasionally, a dry chronic cough is the only presenting symptom of asthma in children. These children warrant a detailed history and examination, spirometry, and a trial of bronchodilators if asthma is suspected. However, cough variant asthma is rare in children, and failure to improve with bronchodilator use should prompt further investigation.
Table 1.
Differential Diagnosis of Asthma by Age and Characteristic Findings
| DIAGNOSIS | AGE | FINDINGS THAT DIFFERENTIATE FROM ASTHMA | DIAGNOSTIC TESTS |
|---|---|---|---|
| Bronchiolitis | <24 mo | No atopy, typically not responsive to SABA, context of fever and viral respiratory tract infection | Clinical diagnosis |
| Viral-induced wheeze | Any age (<4 y more common) | Wheezing only with viral respiratory tract infections, less atopy; usually resolves by age 4 y (62) | PFT if frequent or severe |
| Bronchopulmonary dysplasia | <24 mo | Symptoms since birth in premature infant | Clinical diagnosis |
| Chronic aspiration/GERD | Any | Chronic cough especially after meals, gastrointestinal symptoms | Gastroenterology referral, endoscopy, pH probe |
| Tracheomalacia/bronchomalacia | <24 mo | Symptoms since birth, constant (as opposed to intermittent or triggered); stridor present with tracheomalacia | Laryngoscopy |
| Bronchiectasis | Any | Symptoms since birth, wet chronic cough, recurrent wheeze with focal lung findings, recurrent pneumonia in same location on CXR, sometimes with hemoptysis | Chest CT, bronchoscopy |
| Food allergy | Any | First-time wheeze in context of new food exposure and other signs of food allergy (lip swelling, vomiting, hives, hypotension) | Allergy testing, PFT |
| Anxiety/panic attack | School age or older | No wheezing, no improvement with SABA | Clinical diagnosis, PFT results normal |
| Vocal cord dysfunction | Adolescent + | Acute onset of symptoms within minutes of exercise or exposure to an irritant, symptoms quickly resolve and do not respond to SABA, sensation of airway closing, throat tightness, no symptoms during sleep; occasionally will hear inspiratory stridor on examination; hoarse voice | PFT shows classic pattern of dynamic airflow obstruction: laryngoscopy |
| Heart failure | Any | Fever (viral myocarditis), missed congenital heart disease, failure to thrive, symptoms worsen with feeding in young infant or with laying down; murmur, poor central pulses, hepatomegaly, crackles | Echocardiography, electrocardiography, CXR |
| Foreign body aspiration | <4 y (could be any age) | Acute onset of symptoms, unilateral findings, history of choking spell | Anteroposterior/posteroanterior or bilateral decubitus CXR; bronchoscopy |
| Mass effect (from vascular anomaly or tumor) | Any | Unilateral wheeze or stridor (may be positional, if due to a mass, ring, or sling around upper airways), symptoms worse with laying down; weight loss and other systemic signs if oncologic | Chest CT, bronchoscopy |
| Underlying immunodeficiency | Any | Recurrent bacterial pneumonia | CXR, immunology evaluation |
| Allergic bronchopulmonary aspergillosis | Any | Difficult-to-control asthma symptoms, chronic cough and intermittent fevers | Sputum, Aspergillus IgE and IgG, CXR |
| Cystic fibrosis | Any | Failure to thrive (may still have a normal newborn screen) | Sweat chloride testing, genetic testing |
CT=computed tomography, CXR=chest radiography, GERD=gastroesophageal reflux disease, IgE=immunoglobulin E, IgG=immunoglobulin G, PFT=pulmonary function testing. SABA=short-acting inhaled β2-agonist.
Figure 1.
Diagnosis of asthma: basic approach to a child with respiratory symptoms consistent with asthma. ©2019 Global Initiative for Asthma, available from www.ginasthma.org [ginasthma.org], reprinted with permission.
Children with poor access to primary care often use urgent or emergency care for wheezing episodes; the chronic and recurrent nature of symptoms may lead to a delayed or missed asthma diagnosis. (63)
Diagnostic Testing/Adjuncts in Asthma
Peak Flows.
Measurement of peak expiratory flow (PEF) is portable and relatively inexpensive and provides families of children older than 5 years with objective data to initiate rescue medications. A child should determine his or her personal best (the best of 3 measurements performed daily for 2 weeks) and use this number to guide acute management. (43) If the PEF falls below 80% of the child’s personal best, the child or caregiver should initiate rescue medications per the asthma action plan. Generally, 70% to 80% of personal best is equivalent to yellow zone management of asthma, and 40% to 70% is equivalent to red zone management (Tables 2 and 3, Fig 2). (43) In practice, specialists who have access to in-office spirometry sometimes correlate a patient's PFT to PEF and use that to guide the determination of a child’s personal best. If a child does not know his or her personal best, charts based on age and height can be used to provide general ranges. Symptoms (cough, shortness of breath, exercise intolerance) correlate with PEF and should be used to guide acute management, especially in younger children who are not able to reliably measure their PEF. When teaching patients how to use a peak flow meter, it is important to demonstrate and ask that a tight seal is made with the lips around the mouthpiece. After forcefully inhaling in, the child forcefully exhales with 1 quick breath to measure his or her PEF. Limitations of PEF include poor reliability between devices, high dependence on patient effort, and inability to detect small airway obstruction. Therefore, patients should use the same peak flow meter each time, and technique should be reviewed frequently.
Table 2.
Asthma Medications (Quick Relief) (43)
| MEDICATION | DOSING RECOMMENDATION | NOTES |
|---|---|---|
| INH Short-Acting β2-Agonists (to relax the smooth muscles; activates β2 adrenergic receptors in the lungs, resulting in bronchodilation) | ||
| Albuterol MDI (90 μg/puff; 200 puffs/canister) | Preexercise dosing: 2 puffs INH 15 min before exercise | Indicated for quick relief of bronchospasm or to prevent bronchospasm related to exercise |
| For single dose (yellow zone management on AAP): 2–4 puffs INH every 4–6 h as needed for symptoms | Adverse effects include tachycardia and tachypnea | |
| For acute exacerbation (red zone management on AAP): 4–8 puffs every 20 min × 3 doses: | ||
| ≥5 to <10 kg: 4 puffs INH every 20 min for 3 doses | ||
| ≥10 to <30 kg: 6 puffs INH every 20 min for 3 doses | ||
| ≥30 kg: 8 puffs INH every 20 min for 3 doses | ||
| Albuterol inhalation solution (0.63 mg/3 mL, 1.25 mg/3 mL, 2.5 mg/3 mL, or 5 mg/mL) | ≥5 to <30 kg: 2.5-mg neb INH for single dose OR 7.5-mg neb INH for 1 h | |
| ≥30 kg: 5-mg neb INH for single dose OR 15-mg neb INH for 1 h | ||
| If still wheezing and in distress after 2 h of short-acting β2-agonist, consider continuous neb treatment (0.6 mg/kg per hour): | ||
| ≥5 to <10 kg: 5-mg/h neb INH every 1 h | ||
| ≥10 to <20 kg: 10-mg/h neb INH every 1 h | ||
| ≥20 to <30 kg: 15-mg/h neb INH every 1 h | ||
| ≥30 kg: 20-mg/h neb INH every 1 h | ||
| Anticholinergics (antimuscarinic agent [blocks action of acetylcholine], which results in decreased contractility of smooth muscle resulting in bronchodilation) | ||
| Ipratropium bromide inhalation solution (0.25 mg/mL) | 0.25–0.5 mg neb INH every 20 min for 3 doses | Indicated in the ED setting for moderate to severe exacerbation |
| ≥5 to <30 kg: 0.5 mg neb INH once | Can cause transient dilation of the pupil(s) and blurry vision if neb formulation is blown into the eyes for a prolonged period | |
| 30 kg: 1 mg neb INH once | ||
| Ipatropium bromide MDI (18 μg/puff) | 4–8 puffs every 20 min INH for 3 doses | |
| Systemic Corticosteroids (anti-inflammatory; reverses B2-receptor downregulation) | ||
| Dexamethasone | Short course (burst): | Indicated for treatment of moderate to severe exacerbations |
| ≥7 to <10 kg: 6 mg PO once with repeat dose in 24 h | ||
| ≥10 to <20 kg: 10 mg PO once with repeat dose in 24 h | ||
| ≥30 kg: 16 mg PO once with repeat dose in 24 h | ||
| OR 0.6 mg/kg IV or IM (max, 16 mg) in children not tolerating oral medications | ||
| Prednisone (1-, 2.5-, 5-, 10-, 20-, and 50-mg tablets) | Short course (burst): 1–2 mg/kg per day PO (max, 60 mg) for 3–10 d divided twice daily | Consider comorbidities, eg, studies show patients with sickle cell disease and asthma have rebound acute chest syndrome with systemic corticosteroids and, hence, should be avoided (64) |
| Prednisolone (5 mg/5 mL or 15 mg/5 mL) | Short course (burst): 1–2 mg/kg per day PO (max, 60 mg) for 3–10 d divided twice daily | Will raise serum glucose so use with caution in diabetes |
| Methylprednisolone | Short course (burst): 1–2 mg/kg per day IV (max, 60 mg) for 3–10 d divided every 6–12 h | |
| Adjunct acute asthma medications | ||
| Magnesium sulfate | 50 mg/kg per dose (max, 2,000 mg/dose) IV once over 20 min | Consider after 1 h of short-acting β2-agonists and after systemic corticosteroids if still with respiratory distress and wheezing |
| Epinephrine (1:1,000) (1 mg/mL) | 0.01 mg/kg per dose (max, 0.5 mg/dose) IM once | Consider in severe, life-threatening asthma |
AAP=asthma action plan, ED=emergency department, h=hour, INH=inhaled, IM=intramuscular, IV=intravenous, max=maximum, MDI=metered-dose inhaler, neb=nebulized, PO=‘per os’ or by mouth.
Table 3.
Asthma Medications (Maintenance or Controller Medications) (65)
| MEDICATION | DAILY DOSE, BY AGE | ADVERSE EFFECTS | |||
|---|---|---|---|---|---|
| 0–4 y | 5–11 y | ≥12 y | |||
| Leukotriene modifiers | |||||
| Montelukast (leukotriene receptor antagonist) | (1–5 y): 4 mg by mouth once before bedtime | ||||
| 4- or 5-mg chewable tablet | (6–14 y): 5 mg by mouth once before bedtime | ||||
| 4-mg granules or 10-mg tablet | (>14 y): 10 mg by mouth once before bedtime | ||||
| Immunomodulators | |||||
| Omalizumab (anti-IgE) SC injection, 150 mg/1.2 mL, after reconstitution with 1.4 mL of sterile saline | NA | ≥6 y: 150–375 mg SC every 2–4 wk (depends on bodyweight and pretreatment serum IgE levels) | 150–375 mg SC every 2–4 wk (depends on bodyweight and pretreatment serum IgE levels) | Pain and bruising at injection sites | |
| Risk of anaphylaxis (0.2%) | |||||
| Long-acting muscarinic antagonist | |||||
| Tiotropium bromide*2.5 μg (2 actuations; 1.25 μg/actuation) | NA | ≥6 y: 2 actuations inhaled daily | 2 Actuations inhaled daily | Caution with use if creatinine clearance <60 ml/min | |
| Inhaled corticosteroids | |||||
| Beclomethasone HFA: 40 or 80 μg/puff | NA | (L): 80–160 μg | (L): 80–240 μg | Must rinse mouth after each use to prevent oral thrush and systemic absorption | |
| (M): >160–320 μg | (M): >240–480 μg | ||||
| (H): >320 μg | (H): >480 μg | ||||
| Budesonide DPI: 90 or 180 μg/inhalation | NA | (L): 180–360 μg | (L): 180–540 μg | ||
| (M): 360–720 μg | (M): 540–1080 μg | ||||
| (H): >720 μg | (H): >1,080 μg | ||||
| Budesonide nebules: 0.25, 0.5, or 1 mg | (L): 0.25–0.5 mg | (L): 0.5 mg | NA | ||
| (M): >0.5–1 mg | (M): 1 mg | ||||
| (H): >1 mg | (H): 2 mg | ||||
| Flunisolide HFA: 80 μg/puff | NA | (L): 160 μg | (L): 320 μg | ||
| (M): 320–480 mg | (M): >320–640 μg | ||||
| (H): ≥480 μg | (H): >640 μg | ||||
| Fluticasone HFA/MDI: 44, 110, 220 μg/puff | (L): 176 μg | (L): 88–176 μg | (L): 88–264 μg | ||
| (M): >176–352 μg | (M): >176–352 μg | (M): >264–440 μg | |||
| (H): >352 μg | (H): >352 μg | (H): >440 μg | |||
| Fluticasone DPI: 50, 100, or 250 μg/inhalation | NA | (L): 100–200 μg | (L): 100–300 μg | ||
| (M): >200–400 μg | (M): >300–500 μg | ||||
| (H): >400 μg | (H): >500 μg | ||||
| Mometasone | NA | (L): 110 μg | (L): 100–220 μg | ||
| DPI: 110 μg, 220 μg/inhalation | (M):>220–440 μg | (M): >200–440 μg | |||
| HFA: 100 μg, 200 μg/puff | (H): >440 μg | (H): >440 μg | |||
| Inhaled corticosteroid + long-acting B2-agonist (combination medications) | |||||
| Fluticasone/salmeterol | NA | 1 Inhalation BID | Must rinse mouth after each use to prevent oral thrush | ||
| DPI: 100 mcg/50 μg, 250 mcg/50 μg, or 500 mcg/50 μg | Not well controlled and on low- to medium-dose ICS: 100/50 DPI or 45/21 HFA | ||||
| HFA: 45 mcg/21 μg, 115 mcg/21 μg, 230 mcg/21 μg | Not well controlled and on medium- to high-dose ICS: 250/50 DPI or 115/21 HFA | ||||
| Budesonide/formoterol HFA MDI: 80 mcg/4.5 μg, 160 mcg/4.5 μg | NA | Not well controlled and on low- to medium-dose ICS: 80/4.5 | |||
| Not well controlled and on medium- to high-dose ICS: 160/4.5 | |||||
| Mometasone/formoterol MDI: 100 μg/5 mcg | NA | NA | 2 Inhalations BID (depends on asthma severity) | ||
Doses are per National Asthma Education and Prevention Program update in September 2012 except for (*). Source: National Heart, Lung, and Blood Institute; National Institutes of Health; US Department of Health and Human Services. BID=twice a day, DPI=dry powder inhaler, (H)=high daily dose, HFA=hydrofluoroalkane, ICS=inhaled corticosteroid, IgE=immunoglobulin E, (L)=low daily dose, (M)=medium daily dose, MDI=metered-dose inhaler, NA=not available (not approved, no data available, or safety and efficacy not established in this age group), SC=subcutaneous.
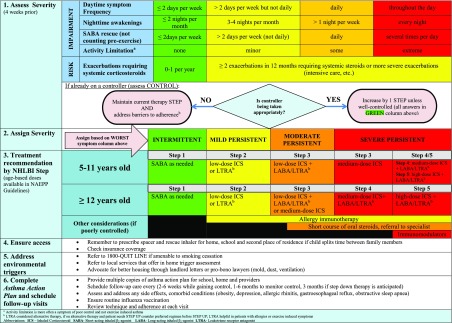
Figure 2.
Asthma control, severity classification, and treatment flow diagram for children 5 years and older. (43)(69)
PFT/Spirometry.
PFT is recommended in children older than 5 years for routine management of asthma and when the diagnosis is in question. PFT is more accurate than PEF. The 2 readings important in the diagnosis and evaluation of asthma are forced expiratory volume in 1 second (FEV1) and the ratio of FEV1 to forced vital capacity or FEV1%. FEV1% is the proportion of forced vital capacity a child is able to expire in 1 second. The goal of PFT in asthma is to document expiratory flow limitation or obstruction with reversibility. An FEV1% of less than 0.80 is diagnostic for airflow obstruction, and reversibility with administration of a bronchodilator is diagnostic for asthma. If a child is able to fully cooperate, an FEV1% greater than 0.8 is normal. The classic flow-volume curve for asthma shows a dampening and scooping out of the expiratory curve. Periodic spirometry measurements can also be helpful to measure lung function because a primary goal of asthma is to maintain healthy lung function.
Methacholine Challenge.
Bronchoprovocation tests, such as the methacholine challenge, are not a common practice in pediatrics and are usually performed only by specialists. In addition, methacholine challenge testing has high negative predictive power and, hence, performs well in establishing the lack of asthma, or ruling out disease. (66)
Impulse Oscillometry.
Impulse oscillometry is a newer PFT that allows for passive measurement of lung function. It is useful for children as young as 3 years and other children who have difficulty with traditional PFT because minimal effort is required by the child. (67) However, impulse oscillometry is only available in a pulmonologist’s office.
Chest Radiography.
Chest radiographs are low yield in the acute setting and rarely change management. However, obtaining a radiograph should be considered when a child presents with hypoxia and high fever and is not improving on albuterol and systemic corticosteroids. (68)
Furthermore, a chest radiograph may be indicated at the first presentation of wheeze or when considering alternative diagnoses other than asthma (Table 1).
Management
The overall goal in managing asthma in children is to reduce impairment (maintaining a good quality of life, allowing for normal activities without limitation, and reducing missed school days) and to reduce risk (keeping children off systemic corticosteroids, out of the hospital and ED, and maintaining healthy lung function to prevent airway remodeling that is associated with chronic inflammation).
Severity Classification
The NAEPP guidelines, last revised in 2007, set age-based criteria to standardize the classification and management of asthma. The guidelines for classification are based on the magnitude of a child’s risk (occurrence of acute severe exacerbations in the past 12 months) and impairment (day-to-day symptoms over the past 4 weeks). Based on risk and impairment, asthma is classified into intermittent, mild persistent, moderate persistent, and severe persistent. Control, using similar assessments of risk and impairment, should be assessed every 3 months at routine asthma visits and is classified as well controlled, not well controlled, or very poorly controlled. Figure 2 details the current approach to initial assessment of disease severity and ongoing assessment of control. Classification and control designation of asthma guides should be made at each visit per NAEPP guidelines.
Longitudinal Care
Once asthma severity and level of control have been determined, the guidelines specify initial therapy or change of therapy in a stepwise manner. (43) The mainstay of asthma therapy consists of bronchodilators (ie, SABA) for rescue therapy to relax the smooth muscles surrounding the airway (bronchodilation) and corticosteroids to help reduce inflammation inside the airway. A routine asthma visit should be scheduled every 3 to 6 months. Barriers to asthma control, adherence, and demonstration of proper medication administration should be assessed and addressed at each visit. Treatment decisions should be based on recommended step level only after adherence has been assessed and triggers have been identified in conjunction with a plan for trigger modification/avoidance (Fig 2). (69)
Management of chronic asthma can be confusing for children and families. Education should be provided using lay terminology. Visual aids to illustrate narrowed airways and explain which medications reverse bronchospasm and offer quick relief (SABAs) versus reduce and control inflammation (corticosteroids or leukotriene receptor antagonists [LTRAs]) are helpful. A misunderstanding in the difference between quick-relief medications and daily controller medications is a common barrier to adherence (Tables 2 and 3). The instructions should be clear, communicated verbally and in writing (with a detailed asthma action plan), and reinforced at each visit. A typical asthma action plan is divided into 3 sections. The green zone provides instructions on routine medications (ICSs, LTRAs, preexercise SABAs, allergy medications). The yellow zone provides instructions about home management of acute, mild exacerbations with escalation to red zone management while seeking emergency medical care. Children with persistent asthma should be seen every 3 months to assess ongoing control and to adjust controller medications. Providing tools (smartphone alarms, mobile applications, or simply placing the daily inhaler and spacer next to the toothbrush) may improve adherence. (70)
Adherence to medication is also affected by improper delivery. Spacer use with a demonstration of appropriate technique should be assessed at each visit. All patients, regardless of age, should use a spacer when using a metered-dose inhaler rescue or controller medication. The spacer ensures delivery of the medication to the lungs and reduces medication that deposits inside of the mouth with subsequent systemic absorption. Both mask and mouthpiece spacer should be appropriate, and the choice is mostly based on age and ability to demonstrate proper technique. Exceptions for spacer use include new devices that either have a built-in spacer and devices with specific instructions to not use a spacer (this should be included in the package insert). All children taking controller ICSs should rinse their mouth after use to prevent oral thrush and to minimize systemic absorption. A video demonstrating proper spacer technique is available online (https://vimeo.com/channels/impactdc).
Frequent introductions of new delivery systems for asthma medications are common with changes in insurance formularies and the availability of new devices on the market. Providers must be vigilant to ensure insurance coverage of prescribed medications and should check manufacturer websites for online videos to familiarize themselves with proper technique of new devices to teach their patients.
Finally, it is crucial to assess parent and adolescent "buy-in" to chronic medication use. For a parent, it helps to explain the process inside the lungs in the context of therapeutic effect and potential for airway remodeling (71) if chronic uncontrolled asthma persists. For an adolescent, the consequences have to be more immediate, ie, the ability to perform better at their specific sport or extracurricular activity with regular use of controller medications.
Persistent asthma requires a thorough and ongoing assessment of potential comorbid conditions. Addressing potentially treatable comorbidities commonly seen with childhood asthma (obesity, [72] obstructive sleep apnea, allergic rhinitis, gastroesophageal reflux, and stress or depression) could avoid unnecessary escalation of asthma therapy. A complete review of symptoms should reveal these diagnoses.
Finally, when prescribing controller medications, the provider should ensure patient access to medications. In addition to assessing insurance coverage of the ICS brand, a family’s ability to pay or afford the co-pay should be considered. Privately insured families with limited additional funds may qualify for coupons available on the manufacturer’s website. Also, if possible, one should obtain prior authorizations for additional spacers and rescue inhalers for school and multiple residences because a child may live in multiple households. Routine follow-up appointments should be scheduled at the time of visits, and the importance of these visits with plans for step-up or step-down therapy should be communicated to families. Addressing barriers, (73) coordinating care, (15) and ensuring adherence to guideline-based care (74) are imperative for reducing disparities in asthma care.
Allergy testing for indoor allergens (certain molds, animal dander, house dust mites, and cockroach) is recommended for patients with persistent asthma and reported exposure to allergens. (43)(60) Guidelines recommend targeted skin prick testing because it is low cost and accurate, and targeted serum specific IgE if a child is uncooperative. (60) In practice, it is important to obtain a clinical history of allergen exposure because any report of asthma symptoms in reference to an exposure supersedes allergy testing results, and allergen avoidance should continue to be recommended.
In addition, children with severe allergic rhinitis may benefit from subspecialist referral to an allergist for consideration of specific and targeted immunotherapy. Current evidence supports in-office subcutaneous immunotherapy for single allergens in children with persistent asthma and a clear relationship between symptoms and exposure to a specific allergen to which the patient has documented sensitivity. Guidelines support immunotherapy for 3 specific allergens: dust mites, animal dander, and pollen. (43) Patients who receive immunotherapy are at risk for anaphylaxis and should be closely monitored. The update for the 2007 NAEEP guidelines lists immunotherapy as 1 of the 5 specific areas of future in-depth review. (61)
Severe Asthma
Immunomodulatory medications, or biological agents, are emerging treatments in children with moderate to severe asthma. If a child is taking medium- to high-dose ICS and long-acting inhaled β2-agonist and demonstrates poor control despite proper technique and good adherence (frequent exacerbations requiring systemic corticosteroids, intensive care, or intubation), (43)(60) he or she should be referred to a specialist for treatment of severe asthma. The specialist should evaluate for other diagnoses that mimic asthma (Table 1) before consideration of asthma treatment with biological agents (anti-IgE, anti–IL-5, or anti–IL-5α). (43)(60) Omalizumab, a monoclonal antibody that binds to IgE, is recommended for difficult-to-control moderate to severe persistent asthma in children 6 years and older with elevated IgE counts. (75) In a cohort of inner-city youth aged 6 to 20 years with uncontrolled persistent asthma and elevated IgE levels, omalizumab significantly decreased fall exacerbations and improved overall control of asthma. (76) Mepolizumab (anti–IL-5), (77) benralizumab (anti–IL-5α), (78)(79) and dupilumab (anti–IL-4α) (80) are options for children with eosinophilia (eosinophil count >150 and 300/μL [0.15 and 0.30×109/L], respectively) who are 12 years and older. Early initiation of biological agents in poorly controlled moderate to severe asthma is associated with reduced hospitalizations and ED visits for asthma. (81) Newer biological agents that target different mediators of the inflammatory pathway and other emerging trends are discussed in Table 4.
Table 4.
Recent Evidence for Emerging Trends in the Treatment of Asthma
| PRACTICE CONSIDERATION | EVIDENCE | RECOMMENDATION | |||
|---|---|---|---|---|---|
| POPULATION, No. | DESIGN | FINDINGS (LIMITATIONS) | |||
| School-based asthma therapy | |||||
| Halterman et al (2011) (82) | Children aged 3–10 y with asthma and Medicaid insurance (n = 530) | Prospective RCT comparing home versus school administration of ICS | School administration of ICS effectively improves adherence (performed in conjunction with dose adjustment as needed and tobacco smoke reduction program) (single site) | Recommend partnership with local school to see whether school-based asthma therapy is a feasible alternative to home-based therapy, especially for those patients with poor adherence. Consider direct delivery of medications to the school. | |
| Fall montelukast | |||||
| Johnston et al (2007) (83) | Children aged 2–14 y with asthma (n = 194) | RCT, double-blind, placebo-controlled trial comparing a group started on age-appropriate nightly dose of montelukast from September 1 for 45 d | 53% Decrease in “worse asthma symptoms” and 78% reduction in unscheduled asthma visits; boys 2–5 y and girls 10–14 y with most benefit (no minimum asthma severity for inclusion; pragmatic trial with poor adherence to ICS in study population) | Mixed results; could consider Fall montelukast in a persistent asthma population with poor ICS adherence (especially males aged 2–5 y and females aged 10–14 y) | |
| Weiss et al (2010) (84) | Children aged 6–14 y with asthma (n = 1162) | RCT, multicenter, double blind, placebo controlled, comparing 5-mg montelukast night before day 1 of school for 8 wk; evaluated percent days with worse asthma | No difference between placebo and montelukast groups (no minimum asthma severity for inclusion) | ||
| Preseasonal treatment with omalizumab | |||||
| Teach et al (2015) (85) | Inner-city asthmatic children aged 6–17 y with ≥1 recent exacerbations (n = 727) | 3-arm RCT, double blind, double placebo controlled, multicenter | Compared to placebo, omalizumab had a significantly lower rate of fall exacerbations, and omalizumab boosted interferon levels with a decrease in rhinovirus infection in the omalizumab group | Consider the addition of omalizumab to guidelines-based therapy for inner city children, aged 6-17 y, before the fall season, especially if they have a recent history of exacerbation | |
| Intermittent, extreme high-dose ICS dose for acute asthma | |||||
| Jackson et al (2018) (86) | Children aged 5–11 y with mild to moderate persistent asthma, with 1 course of systemic corticosteroids in previous year (n = 254) | Double-blind RCT comparing low-dose ICS with quintupled-dose ICS for 7 d at early signs of loss of asthma control | No difference between the groups in degree of asthma control | Good evidence to recommend against intermittent escalation in ICS for yellow zone management in children; however, seasonal increase may be supported in an urban setting (87)(88) | |
| McKeever et al (2018) (89) | ≥16 y on any dose of ICS with ≥1 exacerbation in previous 12 mo requiring systemic corticosteroids (n = 1,922) | Pragmatic, randomized, unblinded trial comparing self-increase in ICS to 4 times the dose with those that did not increase baseline ICS dose for yellow zone management × 14 d or peak flow normal | Fewer severe exacerbations in high-dose ICS group (no children in low-dose ICS group; subject to bias and found only 19% reduction in increased ICS group) (87)(88) | * This contradicts current GINA recommendations. (60) | |
| Decision support for guideline-based care | |||||
| Bell et al (2010) (17) | Urban primary care clinics (n = 12) | Cluster RCT trial of clinical decision support in the electronic health record | Improved primary care provider compliance with NAEPP guideline–based care (17) | Strong recommendation to consider implementation if feasible | |
| Oral prednisolone in preschoolers with wheeze | |||||
| Panickar et al (2009) (90) | Children aged 10–24 mo hospitalized for viral-induced wheeze (n = 700) | RCT, double-blind, placebo-controlled, 5-d course of oral prednisolone | No difference in LOS of hospitalization | Good evidence to suggest against use of OCS in viral-induced wheeze | |
| Foster et al (2018) (91) | Children aged 24–72 mo presenting to ED with viral-induced wheeze (n = 605) | RCT, double-blind, placebo-controlled, noninferiority trial, single dose of oral prednisolone to reduce ED LOS | Placebo group with longer LOS (540 min) versus prednisone group (370 min), single center, baseline very long LOS, unclear whether generalizable to different settings, unclear whether meaningful outcome studied) | ||
| Dupilumab (anti–interleukin-4 receptor α monoclonal antibody) for moderate to severe uncontrolled asthma | |||||
| Castro et al (2018) (92) | Children aged ≥12 y, uncontrolled asthma (n = 1902) | Randomized to 4 arms to receive add on dupilumab every 2 weeks versus placebo for 1 y at 2:2:1:1 ratio | Lower rates of severe exacerbation and better lung function; results better in children with higher baseline eosinophilia | Promising results, good evidence for use of dupilumab in severe uncontrolled asthma (93) | |
| Rabe et al (2018) (80) | Children age ≥12 y; OCS-dependent severe asthma (n = 210) | Random assignment of add-on dupilumab every 2 wk versus placebo for 24 wk in an attempt to reduce OCS dose | Improved lung function, decreased OCS dose, and fewer exacerbations in treatment group (small study) | ||
ED=emergency department, GINA=Global Initiative for Asthma, ICS=inhaled corticosteroid, LOS=length of stay, NAEPP=National Asthma Education and Prevention Program, OCS=oral corticosteroid, RCT=randomized controlled trial.
Acute Asthma
The management of acute asthma includes early and repeated administration of SABA per the individual asthma action plan (usually 2–4 puffs inhaled with spacer every 4 hours in the yellow zone) at the first symptoms of bronchospasm for mild exacerbations. For moderate to severe exacerbations (red zone on the asthma action plan), the typical asthma action plan usually recommends 4 to 8 puffs inhaled with spacer every 15 minutes for 3 doses while on the way to the ED. High-dose ICS for yellow zone management of acute asthma is not effective and should not generally be recommended. (86)(89) Specific guidelines for prescribing a home supply of emergency oral corticosteroids with specific instructions for use with or without a telephone consultation with a physician do not exist. However, a small study did show decreased ED use in children with moderate to severe asthma who had a home supply of emergency oral corticosteroids. (94)
ED management of acute asthma includes prompt systemic corticosteroids and higher doses of SABA mixed with 2 to 3 doses of ipratropium bromide for moderate to severe exacerbations. It is important to assess response to therapy after 1 hour of treatment and if there is no significant improvement to consider intravenous magnesium sulfate because this has been shown to reduce risk of hospitalization. (95) SABA should be continued until there is clinical evidence of reversal of obstruction (decreased respiratory effort and rate, improved oxygenation and ventilation) (Tables 2 and 3). In addition to reversing bronchoconstriction, supportive therapies, including supplemental oxygen, intravenous fluids, or ventilatory support, should be initiated as needed. A lethargic or agitated child with asthma exacerbation may have acidosis from hypercarbia secondary to poor ventilation and would benefit from noninvasive positive pressure support or intubation. In addition, transient hypoxia secondary to ventilation-perfusion mismatch is not uncommon after treatment with bronchodilator therapy.
ED management of asthma often relies on clinical pathways using asthma severity scores to expedite initiation of treatment. (96) Multiple pediatric asthma severity scores exist, with wide variation in rigor of validation and ability to predict response to treatment. (97)(98)(99) Evidence-based management of acute asthma is discussed in Table 5.
Table 5.
Evidence-Based Management of Acute Asthma
| PRACTICE | RECOMMENDATION |
|---|---|
| Systemic corticosteroids | Supports early administration of systemic corticosteroids in moderate to severe asthma exacerbations with reduction in need for hospitalization if given within 1 h of ED presentation. (100) Oral route is preferred route, and effects are considered equivalent. (43) |
| Short course (1–2 d) of dexamethasone equivalent to 3- to 5-d burst of prednisolone in acute asthma exacerbation. (101) Equivocal data on single versus 2 doses of dexamethasone. (102) | |
| Bronchodilator administration | MDI with spacer equally effective as nebulized bronchodilator therapy in the ED. (103)(104) Additional benefits include cost-savings (105) and decreased ED length of stay. (106) Equivocal studies on continuous versus intermittent bronchodilator nebulization for severe asthma, GINA guidelines recommend initial continuous therapy with spacing to intermittent in severe asthma. (60) |
| Inhaled ipratropium bromide | Supports use in moderate to severe exacerbations with SABA in preventing need for hospitalization, (107) no additional benefit during hospitalization. (108) Not routinely recommended in mild exacerbations. (43)(60) |
| Intravenous magnesium sulfate | No clear support for routine use due to paucity of data; however, administration of intravenous magnesium sulfate in moderate to severe asthma exacerbations if not improving after 1 h of bronchodilator and systemic corticosteroid treatment may reduce need for admission. (43)(109) A recent trial showed potential benefit for patients with severe asthma and pulse oximetry <92%. (95) More data needed. |
| Epinephrine | Insufficient evidence; however, guidelines support administration for children with very poor effort unable to adequately inhale nebulized bronchodilators or possibility of anaphylaxis and in life-threatening situations. Although no significant detrimental effects either. (43)(60) |
| Noninvasive respiratory support | Bilevel positive airway pressure has been studied more than HFNC in the management of severe asthma exacerbation, however still with limited evidence to support or recommend against its use. (110) Insufficient data in support of HFNC in setting of asthma to recommend use. Small pilot study using HFNC for severe asthma compared it with nasal cannula oxygen with promising results. (111) Often used in ICU settings to avoid intubation. |
| Heliox | Consensus-based recommendation for severe exacerbations in conjunction with standard therapy, but caution to not delay intubation if needed. (43)(60) Maximum oxygen content of heliox is 30% FiO2 and, therefore, is not recommended in patients requiring higher % FiO2. |
| Terbutaline | Insufficient evidence to support use. (112) Sometimes given to children with very poor effort unable to adequately inhale nebulized bronchodilators, similar to epinephrine indicated in life-threatening situations; however, has more adverse effects than epinephrine. (43)(60) |
| Ketamine | Insufficient evidence for ventilated or nonventilated patients. (113) |
| Intravenous aminophylline | Evidence recommends against use due to poor safety profile in children. (43)(60)(112) |
| Volatile anesthetics | Used in ICU settings in ventilated patients, not mentioned in guidelines, with insufficient evidence for routine use; no difference in outcomes in a large pediatric retrospective review. (114) |
| Chest radiography | Low yield in the ED and rarely changes management, consider with hypoxia and high fever if not improving on albuterol and systemic corticosteroids. (68) |
| ICS prescription at time of discharge from ED or admission | ICS should be initiated before ED discharge. (115) Regular use of low-dose ICS is associated with decreased risk of death from asthma. (116) |
ED=emergency department, FiO2=fraction of inspired oxygen, GINA=Global Initiative for Asthma, HFNC=high-flow nasal cannula, ICS=inhaled corticosteroid, MDI= metered-dose inhaler, SABA=short-acting inhaled β2-agonist.
Pharmacogenetics and Race/Ethnicity.
As mentioned previously, Puerto Rican children had the highest asthma prevalence of any group until 2016, when the prevalence in black children increased to 15.7%. (3) Although it is likely multifactorial, there is some evidence of impaired response to SABAs in Puerto Rican children secondary to factors involving the β2-adrenergic receptor genes. (117) In addition, a small cross-sectional study of children with persistent asthma compared response to SABA in Mexican American and Puerto Rican children and found improved responsiveness in Puerto Rican children when an LTRA was added. (118) Further investigation of asthma pharmacogenetics comparing children from different ethnic groups may help address health disparities in asthma.
Conclusion
Management of many other chronic diseases has benefited from a coordinated approach extending beyond the clinic and into the community. Quality improvement efforts should focus on improving outcomes for vulnerable children by ensuring adherence to guideline-based therapy, correct assessment of severity and control, and a coordinated approach to care. Population health approaches and novel therapies showing effectiveness in specific phenotypes present an opportunity to identify at-risk children with asthma and to begin to decrease the health disparities in childhood asthma.
Summary.
Based on observation, health disparities in pediatric asthma exist. (4) Based on expert opinion, providers should design and implement quality improvement projects to help bridge the gap. (15)
Based on observation, environmental triggers are a major contributor to health disparities in pediatric asthma. Working to reduce exposure to individual triggers will improve asthma control. (52)(55)
Based on observation, complex interactions between a child’s genetic risk, environment (allergen sensitization, exposure to viral infections), (26) and changes to the airway at the cellular level help predict disease and target therapy. (31)
Based on expert opinion, always consider alternative or concomitant diagnoses in a child with new wheeze or difficult-to-control asthma. (43)(60)
Based on moderate recommendation, guideline-based care is associated with improved outcomes in asthma. (74)
Based on moderate recommendation, children with moderate to severe asthma often benefit from treatment with seasonal escalation of inhaled corticosteroid dose (although not higher than recommended by the National Asthma Education and Prevention Program guidelines), (43)(85) leukotriene receptor antagonist (83) or novel immunomodulatory medications (85) to reduce fall exacerbations. There seems to be no benefit from high-dose inhaled corticosteroid use for yellow zone management of acute asthma. (86)(89)
Glossary
- ARR
at-risk rate
- CDC
Centers for Disease Control and Prevention
- ED
emergency department
- EILO
exercise-induced laryngeal obstruction
- FeNO
fractional excretion of nitric oxide
- FEV1
forced expiratory volume in 1 second
- ICS
inhaled corticosteroid
- IgE
immunoglobulin E
- IL
interleukin
- LAIV
live attenuated influenza vaccine
- LTRA
leukotriene receptor antagonist
- NAEPP
National Asthma Education and Prevention Program
- PBR
population-based rate
- PEF
peak expiratory flow
- PFT
pulmonary function testing
- SABA
short-acting inhaled β2-agonist
Footnotes
AUTHOR DISCLOSURE
Dr Patel has disclosed that she receives research support from National Heart, Lung, and Blood Institute awards for ORBEX (Oral Bacterial Extract) study and for the ED-SAMS (ED-Intiated School-based Asthma Medication Supervision) study. Dr Teach has disclosed that he receives research support from a National Institute of Child Health and Human Development K12 career development award and as part of ECHO (Environmental Influences on Child Health Outcomes), from a National Institute of Allergy and Infectious Diseases R38 career development award and as part of the Inner City Asthma Consortium, from a National Heart, Lung, and Blood Institute R38 career development award and for the ORBEX (Oral Bacterial Extract) Study, and from the Patient-Centered Outcomes Research Institute for investigator-initiated research. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
References
- 1.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356 [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1):e20152354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children—United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001-2010. J Allergy Clin Immunol. 2014;134(3):547–553.e5 [DOI] [PMC free article] [PubMed]
- 5. Centers for Disease Control and Prevention. Asthma as the underlying cause of death. Available at: https://www.cdc.gov/asthma/asthma_stats/asthma_underlying_death.html. Accessed August 19, 2018.
- 6. President’s Task Force on Environmental Health Risks and Safety Risks to Children. Coordinated federal action plan to reduce racial and ethnic asthma disparities. Available at: https://www.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf. Published May 2012. Accessed August 15, 2018.
- 7.McDermott KW, Stocks CS, Freeman WJ. Overview of pediatric emergency department visits, 2015: Statistical Brief #242. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb242-Pediatric-ED-Visits-2015.pdf. Published August 2018. Accessed August 20, 2018 [PubMed]
- 8.Healthcare Cost and Utilization Project. HCUP fast stats—most common diagnoses for inpatient stays. Available at: https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Accessed August 19, 2018
- 9.Measures to identify and track racial disparities in childhood asthma: Asthma Disparities Workgroup subcommittee recommendations. Centers for Disease Control and Prevention website. Available at: https://www.cdc.gov/asthma/asthma_disparities/default.htm. Published April 2016. Accessed August 19, 2018
- 10.Parikh K, Berry J, Hall M, et al. Racial and ethnic differences in pediatric readmissions for common chronic conditions. J Pediatr. 2017;186:158-164.e1 [DOI] [PubMed] [Google Scholar]
- 11.Malhotra K, Baltrus P, Zhang S, McRoy L, Immergluck LC, Rust G. Geographic and racial variation in asthma prevalence and emergency department use among Medicaid-enrolled children in 14 southern states. J Asthma. 2014;51(9):913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia E, Serban N, Swann J, Fitzpatrick A. The effect of geographic access on severe health outcomes for pediatric asthma. J Allergy Clin Immunol. 2015;136(3):610–618 [DOI] [PubMed] [Google Scholar]
- 13.American Lung Association. Estimated prevalence and incidence of lung disease. Available at: http://www.lung.org/assets/documents/research/estimated-prevalence.pdf. Published May 2014. Accessed September 12, 2018
- 14.Children’s Health Foundation Pediatric Asthma Registry. Children’s Health Foundation website. Available at: http://www.ch-foundation.org/improving-pediatric-practices/quality-improvement/pediatric-asthma-care-management/pediatric-asthma-registry. Accessed August 30, 2018
- 15.Volerman A, Chin MH, Press VG. Solutions for asthma disparities. Pediatrics. 2017;139(3):e20162546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teach SJ, Guagliardo MF, Crain EF, et al. Spatial accessibility of primary care pediatric services in an urban environment: association with asthma management and outcome. Pediatrics. 2006;117(4)(pt 2):S78–S85 [DOI] [PubMed] [Google Scholar]
- 17.Bell LM, Grundmeier R, Localio R, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4):e770–e777 [DOI] [PubMed] [Google Scholar]
- 18.Teach SJ, Crain EF, Quint DM, Hylan ML, Joseph JG. Improved asthma outcomes in a high-morbidity pediatric population: results of an emergency department-based randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(5):535–541 [DOI] [PubMed] [Google Scholar]
- 19.Patel SJ, Chamberlain DB, Chamberlain JM. A machine learning approach to predicting need for hospitalization for pediatric asthma exacerbation at the time of emergency department triage. Acad Emerg Med. 2018;25(12):1463–1470 [DOI] [PubMed] [Google Scholar]
- 20.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(2)(suppl):362–367 [PubMed] [Google Scholar]
- 21.Zoratti EM, Krouse RZ, Babineau DC, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138(4):1016–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181(11):1200–1206 [DOI] [PubMed] [Google Scholar]
- 23.Ege MJ, Mayer M, Normand AC, et al. ; GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709 [DOI] [PubMed] [Google Scholar]
- 24.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135(1):25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gergen PJ, Teach SJ, Togias A, Busse WW. Reducing exacerbations in the inner city: lessons from the Inner-City Asthma Consortium (ICAC). J Allergy Clin Immunol Pract. 2016;4(1):22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bizzintino J, Lee WM, Laing IA, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37(5):1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304 [DOI] [PubMed] [Google Scholar]
- 30.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2)(suppl 2):S73–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung KF. Precision medicine in asthma: linking phenotypes to targeted treatments. Curr Opin Pulm Med. 2018;24(1):4–10 [DOI] [PubMed] [Google Scholar]
- 32.Miller GE, Chen E, Shalowitz MU, et al. Divergent transcriptional profiles in pediatric asthma patients of low and high socioeconomic status. Pediatr Pulmonol. 2018;53(6):710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026 [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57(4):328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston NW, Johnston SL, Duncan JM, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115(1):132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117(3):557–562 [DOI] [PubMed] [Google Scholar]
- 40.Sposato B, Croci L, Canneti E, et al. Influenza A H1N1 and severe asthma exacerbation. Eur Rev Med Pharmacol Sci. 2010;14(5):487–490 [PubMed] [Google Scholar]
- 41.Merckx J, Ducharme FM, Martineau C, et al. ; Pediatric Emergency Research Canada (PERC) DOORWAY team. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics. 2018;142(1):e20174105. [DOI] [PubMed] [Google Scholar]
- 42.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics. 2018;141(4):e20172918. [DOI] [PubMed] [Google Scholar]
- 43.Guidelines for the diagnosis and management of asthma (EPR-3). National Heart, Lung, and Blood Institute website. Available at: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma. Accessed August 18, 2018
- 44.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67(3):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray GT, Lewis N, Goddard K, et al. Asthma exacerbations among asthmatic children receiving live attenuated versus inactivated influenza vaccines. Vaccine. 2017;35(20):2668–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2018-2019. Pediatrics. 2018;142(4):e20182367. [DOI] [PubMed] [Google Scholar]
- 47.Akinbami LJ, Kit BK, Simon AE. Impact of environmental tobacco smoke on children with asthma, United States, 2003-2010. Acad Pediatr. 2013;13(6):508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kit BK, Simon AE, Brody DJ, Akinbami LJ. US prevalence and trends in tobacco smoke exposure among children and adolescents with asthma. Pediatrics. 2013;131(3):407–414 [DOI] [PubMed] [Google Scholar]
- 49.Quinto KB, Kit BK, Lukacs SL, Akinbami LJ. Environmental tobacco smoke exposure in children aged 3-19 years with and without asthma in the United States, 1999-2010. NCHS Data Brief. 2013;(126):1–8 [PubMed] [Google Scholar]
- 50.Pollock J, Shi L, Gimbel RW. Outdoor environment and pediatric asthma: an update on the evidence from North America. Can Respir J. 2017;2017:8921917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: a randomized trial of mite-impermeable bedcovers. Am J Respir Crit Care Med. 2017;196(2):150–158 [DOI] [PubMed] [Google Scholar]
- 52.Lanphear BP, Aligne CA, Auinger P, Weitzman M, Byrd RS. Residential exposures associated with asthma in US children. Pediatrics. 2001;107(3):505–511 [DOI] [PubMed] [Google Scholar]
- 53.Sullivan PW, Ghushchyan V, Kavati A, Navaratnam P, Friedman HS, Ortiz B. Trends in asthma control, treatment, health care utilization, and expenditures among children in the United States by place of residence: 2003-2014. J Allergy Clin Immunol Pract. 2019;7(6):1835–1842.e2 [DOI] [PubMed] [Google Scholar]
- 54.Sullivan PW, Ghushchyan V, Navaratnam P, et al. Indicators of poorly controlled asthma and health-related quality of life among school-age children in the United States. Allergy Asthma Proc. 2017;38(6):431–439 [DOI] [PubMed] [Google Scholar]
- 55.Morgan WJ, Crain EF, Gruchalla RS, et al. ; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080 [DOI] [PubMed] [Google Scholar]
- 56.Shah AY, Dooley D, Shelef DQ, Patel SJ. Improving asthma outcomes in children: from the emergency department and into the community. Clin Pediatr Emerg Med. 2018;19(1):92–100 [Google Scholar]
- 57.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4)(pt 1):1403–1406 [DOI] [PubMed] [Google Scholar]
- 58.Biagini Myers JM, Schauberger E, He H, et al. A pediatric asthma risk score to better predict asthma development in young children. J Allergy Clin Immunol. 2019;143(5):1803–1810.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caudri D, Wijga AH, Hoekstra MO, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax. 2010;65(9):801–807 [DOI] [PubMed] [Google Scholar]
- 60.Global Initiative for Asthma. Global strategy for asthma management and prevention. Available at: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf. Updated 2018. Accessed August 28, 2018
- 61.Mensah GA, Kiley JP, Gibbons GH. Generating evidence to inform an update of asthma clinical practice guidelines: perspectives from the National Heart, Lung, and Blood Institute. J Allergy Clin Immunol. 2018;142(3):744–748 [DOI] [PubMed] [Google Scholar]
- 62.Bush A. Practice imperfect—treatment for wheezing in preschoolers. N Engl J Med. 2009;360(4):409–410 [DOI] [PubMed] [Google Scholar]
- 63.Bruzzese J, Kingston S, Bruzelis E, Poghosyan L. Individual and neighborhood factors associated with undiagnosed asthma in a large cohort of urban adolescents. In: Proceedings from the American Thoracic Society International Conference; May 18-23, 2018; San Diego, CA [DOI] [PMC free article] [PubMed]
- 64.Sobota A, Graham DA, Heeney MM, Neufeld EJ. Corticosteroids for acute chest syndrome in children with sickle cell disease: variation in use and association with length of stay and readmission. Am J Hematol. 2010;85(1):24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Heart, Lung, and Blood Institute. Asthma Care Quick Reference Guide: Diagnosing and Managing Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2012. NIH Publication No. 12-5075
- 66.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999: this official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–329 [DOI] [PubMed] [Google Scholar]
- 67.Desiraju K, Agrawal A. Impulse oscillometry: the state-of-art for lung function testing. Lung India. 2016;33(4):410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allie EH, Dingle HE, Johnson WN, et al. ED chest radiography for children with asthma exacerbation is infrequently associated with change of management. Am J Emerg Med. 2018;36(5):769–773 [DOI] [PubMed] [Google Scholar]
- 69.Hogan AH, Rastogi D, Rinke ML. A quality improvement intervention to improve inpatient pediatric asthma controller accuracy. Hosp Pediatr. 2018;8(3):127–134 [DOI] [PubMed] [Google Scholar]
- 70.Wu AC, Carpenter JF, Himes BE. Mobile health applications for asthma. J Allergy Clin Immunol Pract. 2015;3(3):446–448.e1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364(21):2006–2015 [DOI] [PubMed] [Google Scholar]
- 72.Akinbami LJ, Rossen LM, Fakhouri THI, Simon AE, Kit BK. Contribution of weight status to asthma prevalence racial disparities, 2-19 year olds, 1988-2014. Ann Epidemiol. 2017;27(8):472–478.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klok T, Lubbers S, Kaptein AA, Brand PL. Every parent tells a story: why non-adherence may persist in children receiving guideline-based comprehensive asthma care. J Asthma. 2014;51(1):106–112 [DOI] [PubMed] [Google Scholar]
- 74.Szefler SJ, Gergen PJ, Mitchell H, Morgan W. Achieving asthma control in the inner city: do the National Institutes of Health asthma guidelines really work? J Allergy Clin Immunol. 2010;125(3):521–526, quiz 527–528 [DOI] [PubMed] [Google Scholar]
- 75.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659 [DOI] [PubMed] [Google Scholar]
- 78.Bleecker ER, FitzGerald JM, Chanez P, et al. ; SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127 [DOI] [PubMed] [Google Scholar]
- 79.FitzGerald JM, Bleecker ER, Nair P, et al. ; CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141 [DOI] [PubMed] [Google Scholar]
- 80.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485 [DOI] [PubMed] [Google Scholar]
- 81.Patel TR, Sur S. IgE and eosinophils as therapeutic targets in asthma. Curr Opin Allergy Clin Immunol. 2017;17(1):42–49 [DOI] [PubMed] [Google Scholar]
- 82.Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnston NW, Mandhane PJ, Dai J, et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120(3):e702–e712 [DOI] [PubMed] [Google Scholar]
- 84.Weiss KB, Gern JE, Johnston NW, et al. The Back to School asthma study: the effect of montelukast on asthma burden when initiated prophylactically at the start of the school year. Ann Allergy Asthma Immunol. 2010;105(2):174–181 [DOI] [PubMed] [Google Scholar]
- 85.Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson DJ, Bacharier LB, Mauger DT, et al. ; National Heart, Lung, and Blood Institute AsthmaNet. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378(10):891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bardin PG. Escalating inhaled glucocorticoids to prevent asthma exacerbations. N Engl J Med. 2018;378(10):950–952 [DOI] [PubMed] [Google Scholar]
- 88.Bardin PG. Inhaled glucocorticoids in asthma. N Engl J Med. 2018;378(21):2052. [DOI] [PubMed] [Google Scholar]
- 89.McKeever T, Mortimer K, Wilson A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med. 2018;378(10):902–910 [DOI] [PubMed] [Google Scholar]
- 90.Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329–338 [DOI] [PubMed] [Google Scholar]
- 91.Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97–106 [DOI] [PubMed] [Google Scholar]
- 92.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496 [DOI] [PubMed] [Google Scholar]
- 93.Drazen JM, Harrington D. New biologics for asthma. N Engl J Med. 2018;378(26):2533–2534 [DOI] [PubMed] [Google Scholar]
- 94.Sarzynski LM, Turner T, Stukus DR, Allen E. Home supply of emergency oral steroids and reduction in asthma healthcare utilization. Pediatr Pulmonol. 2017;52(12):1546–1549 [DOI] [PubMed] [Google Scholar]
- 95.Powell C, Kolamunnage-Dona R, Lowe J, et al. ; MAGNETIC Study Group. Magnesium sulphate in acute severe asthma in children (MAGNETIC): a randomised, placebo-controlled trial. Lancet Respir Med. 2013;1(4):301–308 [DOI] [PubMed] [Google Scholar]
- 96.Iqbal SF, Brown KM. Improving timeliness and reducing variability in asthma care through the use of clinical pathways. Clin Pediatr Emerg Med. 2018;19(1):52–54 [Google Scholar]
- 97.Arnold DH, Sills MR, Walsh CG. The asthma prediction rule to decrease hospitalizations for children with asthma. Curr Opin Allergy Clin Immunol. 2016;16(3):201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel SJ, Arnold DH, Topoz MD, Sills MR. Literature review: prediction modeling of emergency department disposition decisions for children with acute asthma exacerbations. Clin Pediatr Emerg Med. 2018;19(1):76–87 [Google Scholar]
- 99.Bekhof J, Reimink R, Brand PL. Systematic review: insufficient validation of clinical scores for the assessment of acute dyspnoea in wheezing children. Paediatr Respir Rev. 2014;15(1):98–112 [DOI] [PubMed] [Google Scholar]
- 100.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. [DOI] [PubMed] [Google Scholar]
- 101.Cronin JJ, McCoy S, Kennedy U, et al. A randomized trial of single-dose oral dexamethasone versus multidose prednisolone for acute exacerbations of asthma in children who attend the emergency department. Ann Emerg Med. 2016;67(5):593–601.e3 [DOI] [PubMed] [Google Scholar]
- 102.Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133(3):493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157(1):76–80 [DOI] [PubMed] [Google Scholar]
- 104.Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;(9):CD000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doan Q, Shefrin A, Johnson D. Cost-effectiveness of metered-dose inhalers for asthma exacerbations in the pediatric emergency department. Pediatrics. 2011;127(5):e1105–e1111 [DOI] [PubMed] [Google Scholar]
- 106.Staggs L, Peek M, Southard G, et al. Evaluating the length of stay and value of time in a pediatric emergency department with two models by comparing two different albuterol delivery systems. J Med Econ. 2012;15(4):704–711 [DOI] [PubMed] [Google Scholar]
- 107.Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013;(8):CD000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vézina K, Chauhan BF, Ducharme FM. Inhaled anticholinergics and short-acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database Syst Rev. 2014;(7):CD010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4:CD011050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korang SK, Feinberg J, Wetterslev J, Jakobsen JC. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database Syst Rev. 2016;9:CD012067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ballestero Y, De Pedro J, Portillo N, Martinez-Mugica O, Arana-Arri E, Benito J. Pilot clinical trial of high-flow oxygen therapy in children with asthma in the emergency service. J Pediatr. 2018;194:204–210.e3 [DOI] [PubMed] [Google Scholar]
- 112.Travers AH, Jones AP, Camargo CA, Jr, Milan SJ, Rowe BH. Intravenous beta(2)-agonists versus intravenous aminophylline for acute asthma. Cochrane Database Syst Rev. 2012;12:CD010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jat KR, Chawla D. Ketamine for management of acute exacerbations of asthma in children. Cochrane Database Syst Rev. 2012;11:CD009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Char DS, Ibsen LM, Ramamoorthy C, Bratton SL. Volatile anesthetic rescue therapy in children with acute asthma: innovative but costly or just costly? Pediatr Crit Care Med. 2013;14(4):343–350 [DOI] [PubMed] [Google Scholar]
- 115.Self TH, Twilla JD, Rogers ML, Rumbak MJ. Inhaled corticosteroids should be initiated before discharge from the emergency department in patients with persistent asthma. J Asthma. 2009;46(10):974–979 [DOI] [PubMed] [Google Scholar]
- 116.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343(5):332–336 [DOI] [PubMed] [Google Scholar]
- 117.Burchard EG, Avila PC, Nazario S, et al. ; Genetics of Asthma in Latino Americans (GALA) Study. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. Am J Respir Crit Care Med. 2004;169(3):386–392 [DOI] [PubMed] [Google Scholar]
- 118.Tcheurekdjian H, Thyne SM, Williams LK, et al. Augmentation of bronchodilator responsiveness by leukotriene modifiers in Puerto Rican and Mexican children. Ann Allergy Asthma Immunol. 2009;102(6):510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]