Alopecia areata (AA) is a nonscarring form of hair loss characterized by well-defined patches of alopecia, typically involving the scalp, and less commonly by near-complete or complete scalp and body hair loss.1 In a murine model of AA, up-regulation of interleukin-15 in hair follicles leads to recruitment and activation of natural killer gene 2D–expressing CD8 T cells, which, in turn, produce interferon-γ, further activating the hair follicle epithelial cells.2 Cell signaling via interferon-γ and interleukin-15 occurs via the Janus kinase (JAK) family of enzymes, and JAK inhibitors have been found to reverse disease. In particular, the JAK1/3 inhibitor, tofacitinib, and the JAK1/2 inhibitor, ruxolitinib, have been found in larger series of patients to be effective for severe disease.3, 4, 5 There is a report of a patient with AA and chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome, whose AA was successfully treated during treatment of CANDLE syndrome with high-dose baricitinib, 7 to 11 mg daily, in addition to prednisone.6 Baricitinib is a relatively new JAK1/2 inhibitor that was recently approved for the treatment of rheumatoid arthritis in Europe and Japan at doses of 2 and 4 mg daily and in the United States at 2 mg daily. Here we describe a case of a woman with severe AA, with complete scalp and near-complete body hair loss, who experienced complete hair regrowth with baricitinib 4 mg daily.
Report of a case
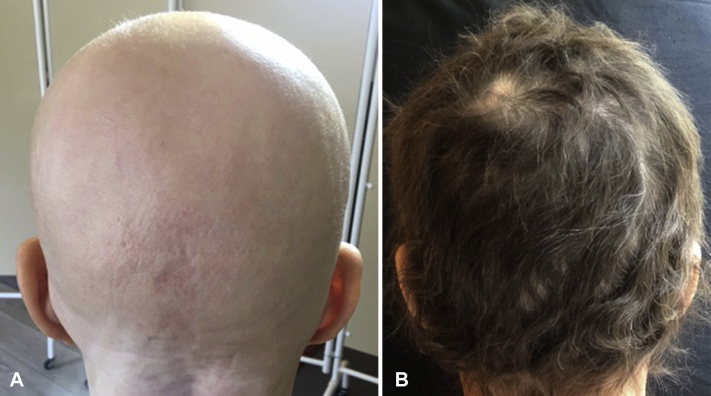
A woman in her 60s with a medical history of psoriasis and melanoma in situ (treated 10 years prior with surgical excision) presented with a 9-month history of AA. Her hair loss began as 2 patches involving the scalp and progressed over 4 months to complete scalp (Fig 1, A) and body hair loss in spite of intralesional triamcinolone, platelet-rich plasma, and tofacitinib 1% solution.
Fig 1.
Scalp before and during treatment with baricitinib. A, There is no scalp hair before treatment. B, After 8 months of baricitinib 4 mg daily, there is near-complete scalp hair regrowth.
After a discussion of the potential risks of JAK inhibitors, including cancer, which, in light of the patient's remote history of melanoma in situ, was particularly pertinent, the patient started treatment with the JAK1/2 inhibitor, baricitinib, 2 mg daily. After 4 months, there was no terminal hair regrowth, so the dose was increased to 4 mg daily. After 8 months, she experienced 97% scalp hair regrowth (Fig 1, B) as well as regrowth of eyebrows and eyelashes. She continues on treatment, and, after 13 months, there have been no adverse effects.
Discussion
To our knowledge, this is the first report of a patient with AA successfully treated with baricitinib monotherapy. While the potential risks of JAK inhibition must be discussed with every patient, they are especially important to consider in a patient with a history of cancer. Like the tumor necrosis factor-α inhibitors (eg, adalimumab), tofacitinib and baricitinib carry a black box warning regarding infection and malignancy risk. In phase 2, phase 3, and long-term extension studies of baricitinib and tofacitinib treatment of rheumatoid arthritis, the rate of all malignancies (excluding nonmelanoma skin cancer) was 0.8 per 100 patient years with baricitinib (95% confidence interval, 0.6 to 1.0) and 0.85 per 100 patient years with tofacitinib (95% confidence interval, 0.7 to 1.02), similar to what is observed for rheumatoid arthritis, in general.7, 8 It is interesting that during the controlled clinical trials of tofacitinib in ulcerative colitis (1220 patients, up to 52 weeks of treatment), there were no cases of solid organ malignancy or lymphoma.9 As with the tumor necrosis factor-α inhibitors, it may be that there are different risks of JAK inhibitor treatment in different disease states. The results of large clinical trials using different JAK inhibitors in AA will begin to answer this and other questions, although many thousands of treated patients and long surveillance periods will be necessary to fully assess malignancy risk, especially for cancers that have long latency periods.
AA is a common disorder that is often associated with poor quality of life, depression, and anxiety. As with atopic dermatitis and psoriasis, the treatment of AA with systemic medications is merited, and it has been shown that quality of life in AA can be restored with successful treatment.10 Although effective therapy for patients with severe disease has historically been elusive, the emergence of JAK inhibitors is changing this. This report of successful treatment of a patient with severe AA using the new JAK inhibitor, baricitinib, adds to the growing body of evidence showing efficacy not only of this class of medication as a whole, but also of JAK inhibitors of different specificities (eg, JAK1/2 vs JAK1/3) for AA. We eagerly await the results of ongoing clinical trials using baricitinib (NCT#03570749) and other JAK inhibitors (NCT#03732807, 03594227, 03811912).
Footnotes
Funding sources: Dr King received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
Conflicts of interest: Dr King has served on advisory boards and/or is a consultant for Aclaris Therapeutics, Inc; Concert Pharmaceuticals, Inc; Dermavant Sciences, Inc; Eli Lilly and Company; and Pfizer, Inc; he is a clinical trial investigator for Concert Pharmaceuticals, Inc; Eli Lilly and Company; and Pfizer, Inc; he is on the speakers bureau for Pfizer, Inc and Regeneron and Sanofi Genzyme. Ms Olamiju and Dr Friedmann have no conflicts to disclose.
References
- 1.Strazzulla L.C., Wang E.H.C., Avila L. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1–12. doi: 10.1016/j.jaad.2017.04.1141. [DOI] [PubMed] [Google Scholar]
- 2.Xing L., Dai Z., Jabbari A. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy Crispin M., Ko J.M., Craiglow B.G. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi: 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackay-Wiggan J., Jabbari A., Nguyen N. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi: 10.1172/jci.insight.89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L.Y., Craiglow B.G., Dai F. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22–28. doi: 10.1016/j.jaad.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Jabbari A., Dai Z., Xing L. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2:351–355. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis J.R., Lee E.B., Kaplan I.V. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–841. doi: 10.1136/annrheumdis-2014-205847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen J., Genovese M., Winthrop K. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46(1):7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn W., Panes J., D'Haens G. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2018 doi: 10.1016/j.cgh.2018.11.035. S1542-3565(18)31278-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu L.Y., King B.A., Craiglow B.G. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806–812.e3. doi: 10.1016/j.jaad.2016.04.035. [DOI] [PubMed] [Google Scholar]