Introduction
Dupilumab, a fully human monoclonal antibody that inhibits interleukin (IL) 4 and IL-13 by blocking the shared IL-4 receptor α and thereby suppressing the T helper type 2–mediated inflammatory response (Th2), is the first biological treatment for moderate to severe atopic dermatitis (AD). We present 2 cases of dupilumab facial redness (DFR), which was not reported in phase 3 clinical trials investigating the efficacy and safety of dupilumab. However, DFR is found to affect approximately 10% of patients treated with dupilumab in daily practice.1 In both of our cases, DFR was considered to be caused by hypersensitivity to Malassezia species. The differential diagnosis included allergic contact dermatitis (ACD) and rosacea.
Case reports
Case 1
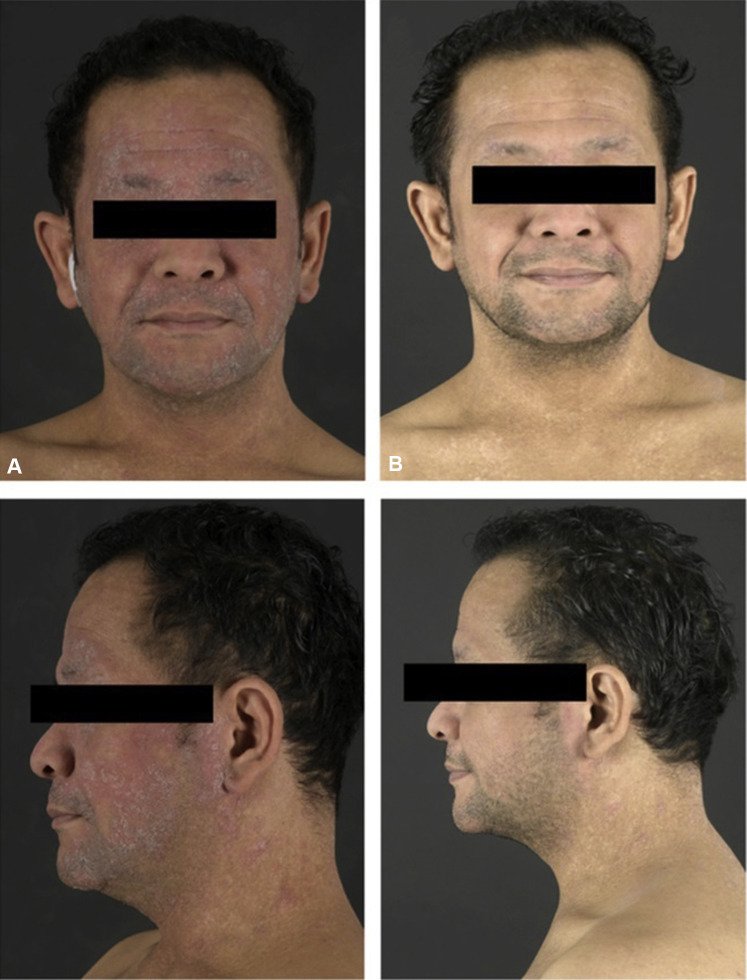
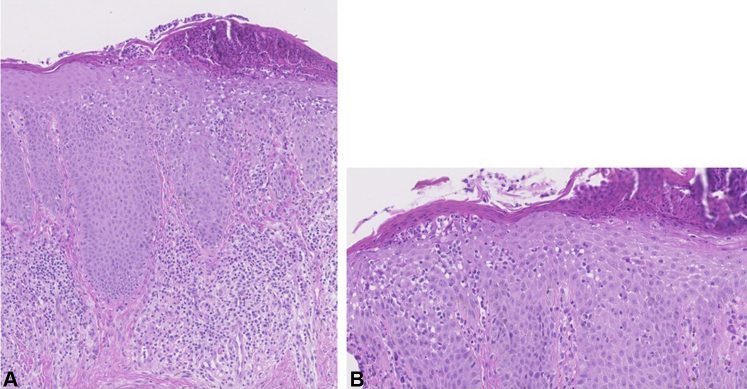
A 39-year-old man with severe AD since childhood was treated with dupilumab 300 mg every 2 weeks after a loading dose of 600 mg subcutaneously at our outpatient clinic. Initially, significant improvement of AD was observed. However, after 11 weeks of dupilumab treatment, the patient developed worsening of redness and scaling of the face, accompanied by itch and pain, that did not respond to treatment with topical corticosteroids. Physical examination showed erythematous and scaly plaques exclusively affecting the head and neck (Fig 1, A), raising clinically suspicion of head-neck dermatitis (HND). Because of the painful appearance, atypical rosacea was also considered. Histopathologic examination showed remarkable parakeratosis with numerous neutrophilic granulocytes, acanthosis, and spongiosis. In the upper dermis, a dense infiltrate of lymphocytes, neutrophils, and eosinophils was observed (Fig 2). The histopathologic findings did not correspond with rosacea. Additionally, an elevated serum level of Malassezia-specific immunoglobulin E (48.50 kU/L; reference value, 0.0-0.34 kU/L) was found. Consequently, treatment with oral itraconazole 200 mg once daily was started, and treatment with dupilumab was continued. After 1 week of itraconazole treatment, the patient reported significant improvement of signs and symptoms during telephonic evaluation. During re-evaluation at our outpatient clinic after 3 weeks of itraconazole treatment, signs and symptoms were completely cleared (Fig 1, B). Treatment with oral itraconazole 200 mg once daily was continued for a total period of 1 month.
Fig 1.
A, Before treatment with oral itraconazole. B, During treatment with oral itraconazole.
Fig 2.
Histopathology case 1. A, Parakeratosis with numerous neutrophilic granulocytes, acanthosis, and spongiosis. In the upper dermis, there is a dense infiltrate of lymphocytes, neutrophils, and eosinophils. B, Neutrophilic granulocyte migration through the epithelium.
Case 2
A 29-year-old man with severe AD since childhood was treated with dupilumab 300 mg every 2 weeks after a loading dose of 600 mg subcutaneously at our outpatient clinic. Initially, significant improvement of AD was observed. However, after 6 months of dupilumab treatment, the patient developed erythematous and scaly plaques in the face, accompanied by itch and pain, that did not respond to treatment with topical corticosteroids. Because of clinical suspicion for rosacea, treatment with topical ivermectin was initiated without success. Patch testing was performed to exclude ACD but did not result in clinically relevant positive reactions. Histopathology showed an identical pattern as presented in case 1. The clinical diagnosis of HND was suspected, and treatment with oral itraconazole 200 mg once daily led to significant improvement of signs and symptoms while dupilumab was continued.
Discussion
To our knowledge, this is the first case report suggesting Malassezia hypersensitivity as a possible cause for DFR. An elevated Malassezia-specific immunoglobulin E level was found in 1 patient, and in both cases, HND was clinically suspected and improved after itraconazole treatment.
HND is a clinical diagnosis that can be observed in patients with AD from adolescence. The skin disease is characterized by erythematous and scaling plaques affecting the head and neck, accompanied by itch, and mostly inadequately responding to topical corticosteroids. Malassezia furfur, a yeast belonging to normal skin flora and mostly located on skin rich in sebaceous glands (head and neck), probably plays a role in the pathophysiology. Hypothetically, because of disturbed skin barrier function in patients with AD, Malassezia furfur can easily penetrate the skin and locally impair and activate keratinocytes, consequently enhancing inflammation. In response to Malassezia antigen load, T cells further activate B cells to produce Malassezia-specific immunoglobulin E.2
In the first case, a high serum level of Malassezia-specific immunoglobulin E was found, which strengthens the Malassezia hypersensitivity theory. Elevated serum levels of Malassezia-specific immunoglobulin E have been previously described in AD and HND patients.2, 3, 4 In a Dutch study investigating Malassezia-specific immunoglobulin E in patients with AD with and without HND, all patients with AD and HND had elevated serum levels of Malassezia-specific immunoglobulin E (100%), in contrast to patients with AD but without HND (13.6%).3 Elevated serum levels of Malassezia-specific immunoglobulin E have been shown to be specific for patients with HND in contrast to patients with seborrheic dermatitis or pityriasis versicolor.4
To our knowledge, this is the first publication to include evaluation of skin biopsy samples in DFR. The histopathologic characteristics of both DFR and HND have not been clarified yet. In both of our cases, a heterogeneous histology was observed with characteristics of eczema underlying a (reactive) neutrophilic dermatosis, probably resulting from the Malassezia yeast. There was no histopathologic evidence of rosacea, such as dilated capillaries in the upper dermis and perivascular and/or perifollicular mononuclear cell infiltrates. Seborrheic dermatitis was not likely due to numerous neutrophilic granulocytes.
The positive response to oral itraconazole in our patients supports the Malassezia hypersensitivity theory. This finding is in line with randomized, placebo-controlled trials describing significant clinical improvement after treatment with systemic antimycotics in patients with AD with suspected HND.2, 5, 6, 7 Both daily use of 200 mg itraconazole and 200 mg ketoconazole are recommended for a treatment duration of 1 to 2 months, followed by long-term twice weekly treatment if necessary. Itraconazole is preferred because of the smaller risk of hepatotoxicity.
In patients presenting with DFR and not responding to oral itraconazole, patch testing is reasonable; some previous published case reports described DFR as a result of paradoxical worsening of ACD.1, 8, 9
Different hypotheses have been suggested for the development of DFR, including triggering of Th1-mediated skin diseases such as psoriasis, ACD, and rosacea by blocking the Th2 pathway.8, 9, 10 DFR due to Malassezia hypersensitivity, a more Th2-driven condition, cannot be explained by this theory.
In conclusion, for patients with AD presenting with DFR, Malassezia hypersensitivity should be considered, with rosacea and ACD as differential diagnoses. Malassezia-specific immunoglobulin E and histologic examination may further clarify the diagnosis. In addition, positive treatment response to itraconazole supports the diagnosis. In the case of significant clinical improvement, treatment with oral itraconazole once daily should be continued for 1 to 2 months, followed by long-term twice-weekly treatment if necessary.
Footnotes
Funding sources: None.
Disclosure: Dr de Bruin-Weller is a consultant/advisory board member for Regeneron Pharmaceuticals, Sanofi Genzyme, AbbVie, Eli Lilly, UCB, and Pfizer and principal investigator for AbbVie, Regeneron Pharmaceuticals, Pfizer, Sanofi Genzyme. Drs de Beer, Bakker, Haeck, Ariens, van der Schaft, and van Dijk have no conflicts of interest to declare.
References
- 1.Waldman RA, Dewane M, Sloan SB, Grant-Kels JM. Characterizing dupilumab facial redness: a multi-institution retrospective chart review. J Am Acad Dermatol. doi: 10.1016/j.jaad.2019.07.031. In press. [DOI] [PubMed]
- 2.Darabi K., Hostetler S.G., Bechtel M.A., Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009;60(1):125–136. doi: 10.1016/j.jaad.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Devos S.A., van der Valk P.G. The relevance of skin prick tests for Pityrosporum ovale in patients with head and neck dermatitis. Allergy. 2000;55(11):1056–1058. doi: 10.1034/j.1398-9995.2000.00782.x. [DOI] [PubMed] [Google Scholar]
- 4.Nordvall S.L., Lindgren L., Johansson S.G., Johansson S., Petrini B. IgE antibodies to Pityrosporum orbiculare and Staphylococcus aureus in patients with very high serum total IgE. Clin Exp Allergy. 1992;22(8):756–761. doi: 10.1111/j.1365-2222.1992.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 5.Lintu P., Savolainen J., Kortekangas-Savolainen O., Kalimo K. Systemic ketoconazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy. 2001;56(6):512–517. doi: 10.1034/j.1398-9995.2001.056006512.x. [DOI] [PubMed] [Google Scholar]
- 6.Svejgaard E., Larsen P.O., Deleuran M., Ternowitz T., Roed-Petersen J., Nilsson J. Treatment of head and neck dermatitis comparing itraconazole 200 mg and 400 mg daily for 1 week with placebo. J Eur Acad Dermatol Venereol. 2004;18(4):445–449. doi: 10.1111/j.1468-3083.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaffenberger B.H., Mathis J., Zirwas M.J. A retrospective descriptive study of oral azole antifungal agents in patients with patch test-negative head and neck predominant atopic dermatitis. J Am Acad Dermatol. 2014;71(3):480–483. doi: 10.1016/j.jaad.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 8.Zhu G.A., Chen J.K., Chiou A., Ko J., Honari G. Assessment of the development of new regional dermatoses in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155(7):850–852. doi: 10.1001/jamadermatol.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suresh R., Murase J.E. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4(9):899–904. doi: 10.1016/j.jdcr.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey E.H., Elston C., Feasel P., Piliang M., Michael M., Vij A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep. 2018;4(7):708–710. doi: 10.1016/j.jdcr.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]