Abstract
Purpose:
The purpose of this study is to identify dosimetric variables that best predict for acute esophagitis in patients treated for locally advanced non–small cell lung cancer in a prospectively accrued statewide consortium.
Methods and materials:
Patients receiving definitive radiation therapy for stage II-III non–small cell lung cancer within the Michigan Radiation Oncology Quality Consortium were included in the analysis. Dose-volume histogram data were analyzed to determine absolute volumes (cc) receiving doses from 10 to 60 Gy (V10, V20, V30, V40, V50, and V60), as well as maximum dose to 2 cc (D2cc), mean dose (MD), and generalized equivalent uniform dose (gEUD). Logistic regression models were used to characterize the risk of toxicity as a function of dose and other covariatcs. The ability of each variable to predict esophagitis, individually or in a multivariate model, was quantified by receiver operating characteristic analysis.
Results:
There were 533 patients who met study criteria and were included; 437 (81.9%) developed any grade of esophagitis. Significant variables on univariate analysis for grade ≥2 esophagitis were concurrent chemotherapy, V20, V30, V40, V50, V60, MD, D2cc, and gEUD. For grade ≥3 esophagitis, the predictive variables were: V30, V40, V50, V60, MD, D2cc, and gEUD. In multivariable modeling, gEUD was the most significant predictor of both grade ≥2 and grade ≥3 esophagitis. When gEUD was excluded from the model, D2cc was selected as the most predictive variable for grade ≥3 esophagitis. For an estimated risk of grade ≥3 esophagitis of 5%, the threshold values for gEUD and D2cc were 59.3 Gy and 68 Gy, respectively.
Conclusions:
In this study, we report the novel finding that gEUD and D2cc, rather than MD, were the most predictive dose metrics for severe esophagitis. To limit the estimated risk of grade ≥3 esophagitis to <5%, thresholds of 59.3 Gy and 68 Gy were identified for gEUD and D2cc, respectively.
Introduction
Concurrent chemoradiation therapy is the preferred definitive treatment for patients with locally advanced non–small cell lung cancer (NSCLC), given the survival benefit over sequential chemotherapy and radiation as demonstrated in Radiation Therapy Oncology Group (RTOG) 94-10.1 In this trial with radiation dose to 63 Gy, acute toxicity was higher in the concurrent arms, with the rate of grade ≥3 esophagitis reported as 23% in the once-daily chemoradiation therapy arm. In the recently reported RTOG 0617 trial, the rate of grade ≥3 esophagitis in the 60-Gy arm was reduced to 7%, suggesting that improvements in the planning and delivery of radiation therapy over the period separating these 2 studies have resulted in decreased rates of esophagitis.2 Despite these advances, acute esophagitis remains a significant clinical problem among patients receiving concurrent therapy, potentially resulting in hospitalization, treatment break, and the need for parenteral feeding tube placement in severe cases.
Current recommended dose limits to the esophagus are based on limited clinical data. Mean dose (MD) <34 Gy is a widely accepted and commonly used dose constraint based on multiple retrospective studies as outlined in the Quantitative Analysis of Normal Tissue Effects in the Clinic analysis.3 The volume of esophagus receiving higher doses, particularly the volume receiving at least 60 Gy (V60), has also been correlated with grade ≥3 esophagitis in numerous studies, most notably in a recent meta-analysis.4 There are a paucity of prospective data validating these constraints, however, and there are practically no data regarding toxicity rates for patients treated off-protocol in “real-world” settings. The purpose of this study is to analyze the outcomes of patients treated for lung cancer in a multi-institutional, prospective cohort to determine the dosimetric variables that predict for acute esophagitis.
Methods and materials
Patients who received definitive radiation therapy for stage II-III NSCLC from May 2012 through July 2015 were included in the analysis. Patient information was collected in a prospective manner from 21 academic and community sites participating in the Michigan Radiation Oncology Quality Consortium (MROQC). MROQC is a multicenter, prospective collaboration through which detailed clinical, sociodemographic, treatment, dosimetric, and outcomes data are collected for patients receiving definitive radiation therapy for NSCLC in the state of Michigan. It is funded by Blue Cross and Blue Shield of Michigan and Blue Care Network, but data are collected on all eligible patients within participating practices, regardless of insurance type. All data analyses were performed by the coordinating center independent of the funding entity. Collected information included patient demographics, tumor stage, location, histology, and treatment information including treatment plans, dose-volume histograms, and use of chemotherapy; prescription dose was reported separately from dose-volume histogram data by each treating institution.5 Patients were evaluated on a weekly basis and in follow-up by the treating radiation oncologist; toxicities were prospectively scored according to Common Terminology Criteria for Adverse Events, version 4.0, on standardized forms at each encounter. For inclusion in this analysis, patients must have had weekly encounter forms during radiation and either an end of treatment evaluation or 1-month follow-up data recorded. Patients for whom toxicity data was not recorded were omitted from the analysis. Toxicities were reported as maximum grade at any point in the follow-up period.
Esophageal dose-volume histogram data were analyzed to determine absolute volumes (cc) receiving doses from 10 to 60 Gy (V10, V20, V30, V40, V50, and V60), as well as maximum dose to 2 cc (D2cc), MD to the esophagus, and generalized equivalent uniform dose (gEUD). D2cc was selected as the parameter for maximum dose because it is a well-recognized surrogate for point dose and is a frequently used constraint for rectum and bladder toxicity.6,7 The gEUD is a single numeric summary of the nonuniform dose distribution to the esophagus and is a function of a single parameter “a.” If “a”= 1, then the gEUD is equal to the MD. If “a” is <1, then gEUD is somewhere between the mean and minimum dose, whereas if “a” is >1, then gEUD is between the mean and maximum dose. We estimated the “a” parameter using maximum likelihood. Dosimetric data were reported in absolute volumes to reduce the effect of esophageal contouring variability on the results. Proper contouring of the esophagus per RTOG guidelines was emphasized at triannual consortium meetings, and an atlas was available in an online knowledge base accessible by all practitioners in the consortium for review. Patients for whom the esophageal volume was more than 2 standard deviations below the mean were excluded on the assumption that these represented partial esophageal volumes rather than whole esophagus.
Logistic regression models were used to characterize the risk of toxicity as a function of dose and other covariates. Toxicity was modeled as either grade ≥2 versus grade ≤ 1 or as grade ≥3 versus grade ≤2. Parameters were estimated via maximum likelihood. From the fitted regression models, the risk of toxicity was calculated at each dose value, with associated 95% pointwise confidence intervals. Dose was included as a linear term in the logistic regression model. To check this assumption, we also fit models in which the dose effect was modeled nonparametrically via a kernel smoother. In addition, we calculated the empirical overall proportion of patients with toxicity in each dose quartile. A stepwise modeling procedure was used to attempt to build a multivariate model in which all predictors were jointly significant. The ability of each dose metric or other covariate, individually or in a multivariate model, to discriminate among patients who had toxicity compared with those who did not, was quantified as the area under the receiver operating characteristic (ROC) curve (AUC). These were estimated nonparametrically. The sensitivity and specificity of various dose thresholds were calculated. SAS software (version 9.3, Cary, NC) was used to perform all analyses.
Results
There were a total of 613 patients identified, of whom 533 patients met study criteria and were included in the analysis (Table 1). The median age was 67 years; 59% were men and 41% were women. Sixteen percent were stage II and 84% were stage III. Chemotherapy was delivered concurrently with radiation therapy to 432 (81.1%) patients. The mean esophageal volume for all patients was 37.6 cc; 64 patients of the initial 613 were excluded because of having esophageal contour volumes <20 cc. Mean volumes (cc) for each dose metric were 21.1, 17.0, 14.1, 11.3, 7.6, and 3.3 for V10, V20, V30, V40, V50, V60, respectively; mean gEUD, MD, and D2cc were 46.7 Gy, 23.7 Gy, and 53.4 Gy (Table 2).
Table 1.
Patient and treatment characteristics
| Characteristic | Total number (%) |
|---|---|
| Gender | |
| Male | 314 (58.9) |
| Female | 219 (41.1) |
| Age (mean) | 67.2 y |
| Race | |
| Caucasian | 407 (76.4) |
| African American | 101 (18.9) |
| Asian | 1 (0.2) |
| Other or not identified | 24 (4.5) |
| ECOG performance status | |
| 0–1 | 432 (86.4) |
| 2 | 28 (5.6) |
| 3–4 | 12 (2.4) |
| Unknown | 28 (5.6) |
| Smoking status | |
| Current/former smoker | 508 (95.3) |
| Never smoker | 25 (4.7) |
| Body mass index | |
| <20 | 66 (12.4) |
| 20 to <25 | 157 (29.5) |
| ≥25 | 310 (58.1) |
| AJCC stage | |
| II | 83 (15.5) |
| IIIA | 300 (56.3) |
| IIIB | 150 (28.1) |
| Chemotherapy | |
| Concurrent | 432 (81.1) |
| Sequential | 55 (10.3) |
| None | 46 (8.6) |
| Radiation therapy dose, Gy | |
| <60 | 61 (11.4) |
| 60–70 | 374 (70.2) |
| >70 | 71 (13.3) |
| Not reported | 27 (5.1) |
| Radiation therapy | |
| 3D-CRT | 240 (45.0) |
| IMRT | 228 (42.8) |
| Mixed plan | 62 (11.6) |
| Esophagitis grade | |
| 0 | 96 (18.1) |
| 1 | 148 (27.8) |
| 2 | 280 (52.5) |
| 3 | 9 (1.7) |
| 4–5 | 0 (0) |
AJCC, American Joint Committee on Cancer; 3D-CRT, 3-dimensional conformal radiation therapy; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity modulated radiation therapy.
Table 2.
Dosimetric variables
| Variable | Mean | SD | Range |
|---|---|---|---|
| Volumetric variables | |||
| V10 | 21.1 cc | 10.3 | (0.7–73.1) |
| V20 | 17.0 cc | 10.3 | (0–66.0) |
| V30 | 14.1 cc | 9.6 | (0–58.4) |
| V40 | 11.3 cc | 8.7 | (0–56.8) |
| V50 | 7.6 cc | 7.3 | (0–53.0) |
| V60 | 3.3 cc | 4.8 | (0–40.2) |
| Mean dose | 23.7 Gy | 10.1 | (0.3–58.5) |
| gEUD | 46.7 Gy | 11.1 | (0.6–70.0) |
| D2cc | 53.4 Gy | 14.1 | (0.6–79.1) |
D2cc, maximum dose of 2cc; gEUD, generalized equivalent uniform dose.
A total of 437 patients (81.9%) developed any grade of esophagitis during treatment or within 1 month of completing radiation therapy. One hundred and forty-eight (27.8%) were scored as grade 1, 280 (52.5%) grade 2, and 9 (1.7%) grade 3; no grade 4 or 5 esophagitis was reported.
On univariate analysis, the following variables were found to be predictive of grade ≥2 esophagitis: concurrent chemotherapy, V20, V30, V40, V50, V60, MD, D2cc, and gEUD (Table 3). For grade ≥3 esophagitis, the predictive variables on univariate analysis were: V30, V40, V50, V60, MD, D2cc, and gEUD (Table 4). In multivariable modeling, gEUD was selected as the most significant predictor of both grade ≥2 and grade ≥3 esophagitis. When gEUD was excluded from the multivariable model, D2cc was selected as the most predictive (highest AUC) dose metric for grade ≥3 esophagitis, whereas D2cc and MD had the next highest AUC value for grade ≥2 esophagitis. For an estimated risk of grade ≥3 esophagitis of 5%, the threshold values for gEUD and D2cc were 59.3 Gy and 68 Gy, respectively (Figs 1 and 2).
Table 3.
Univariate and ROC analysis for grade ≥2 esophagitis
| Variable | OR | AUC | P value |
|---|---|---|---|
| Patient characteristics | |||
| Body mass index | 0.99 | 0.51 | .51 |
| Current smoker | 1.27 | 0.53 | .44 |
| AJCC stage (II vs III) | 0.56 | 0.54 | .02 |
| Concurrent chemotherapy | 3.18 | 0.55 | <.01 |
| Volumetric variables (cc) | |||
| V10 | 1.02 | 0.56 | .08 |
| V20 | 1.03 | 0.59 | <.01 |
| V30 | 1.05 | 0.63 | <.01 |
| V40 | 1.05 | 0.63 | <.01 |
| V50 | 1.06 | 0.63 | <.01 |
| V60 | 1.08 | 0.61 | <.01 |
| Mean dose | 1.06 | 0.65 | <.01 |
| D2cc | 1.05 | 0.65 | <.01 |
| gEUD | 1.07 | 0.66 | <.01 |
Table 4.
Univariate and ROC analysis for grade ≥3 esophagitis
| Variable | OR | AUC | P value |
|---|---|---|---|
| Patient characteristics | |||
| Body mass index | 0.95 | 0.63 | .32 |
| Current smoker | 0.56 | 0.57 | .67 |
| AJCC Stage (II vs III) | 0.67 | 0.52 | .70 |
| Concurrent chemotherapy | 0.71 | 0.52 | .77 |
| Volumetric variables (cc) | |||
| V10 | 1.03 | 0.49 | .36 |
| V20 | 1.05 | 0.58 | .11 |
| V30 | 1.06 | 0.62 | .05 |
| V40 | 1.08 | 0.66 | .01 |
| V50 | 1.09 | 0.66 | <.01 |
| V60 | 1.16 | 0.70 | <.01 |
| Mean dose | 1.08 | 0.67 | 0.02 |
| D2cc | 1.16 | 0.76 | <.01 |
| gEUD | 1.20 | 0.76 | <.01 |
Abbreviations as in Table 2.
Figure 1.
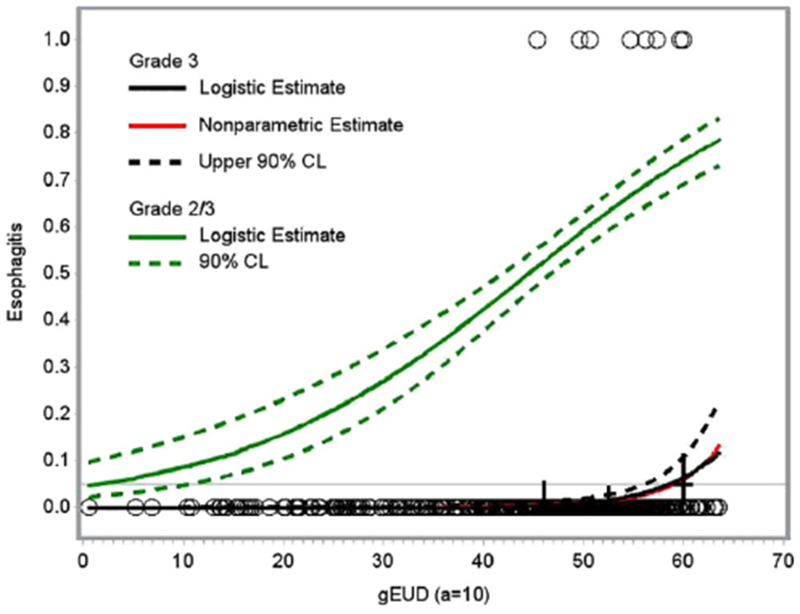
Logistic and nonparametric estimates of risk of grade ≥2 (green) and grade ≥3 (black) esophagitis as a function of generalized equivalent uniform dose (gEUD). A horizontal line is drawn at an estimated risk of 5% and intersects the grade ≥3 curve at gEUD = 59.3 Gy. CL, confidence limit.
Figure 2.
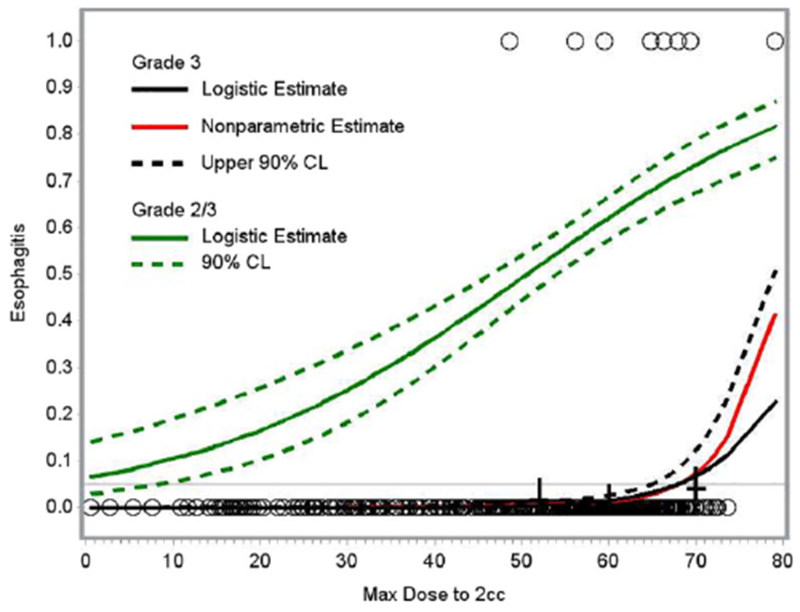
Logistic and nonparametric estimates of risk of grade ≥2 (green) and grade ≥3 (black) esophagitis as a function of maximum dose of 2cc (D2cc). A horizontal line is drawn at an estimated risk of 5% and intersects the grade ≥3 curve at D2cc = 68 Gy.
Early onset of esophagitis (weeks 1-3) was highly predictive of subsequent grade ≥3 toxicity. A classification tree analysis identified patients with either (1) early development of esophagitis (weeks 1-3) or (2) gEUD >54 Gy and onset of esophagitis within weeks 4 to 5 as being at high risk of subsequent grade 3 esophagitis. This prediction rule had an estimated sensitivity of 100% and specificity of 75% for prediction of grade ≥3 esophagitis.
Discussion
Despite improvements in treatment planning and delivery, acute esophagitis remains a significant clinical problem during combined modality therapy, and current remedies, such as viscous lidocaine and sucralfate, have limited efficacy. Previous attempts to prevent esophagitis with interventions such as amifostine and manuka honey have been largely unsuccessful, although agents such as glutamine and soy have shown promise in early clinical and preclinical studies and are the subject of prospective trials.8–11 Aside from pharmacologic prevention of esophagitis, there has also been growing interest in using the improvement in the planning and delivery of thoracic radiation, particularly with intensity modulated radiation therapy (IMRT), to mitigate toxicity.12 Critical to reducing clinically relevant esophagitis is prevention with better definition of the dosimetric variables that predict for esophagitis, an area in which comparatively less advancement has been made over the past decade.
In 1991, Emami et al published their landmark paper establishing a set of normal tissue constraints for use in modern radiation therapy practice.13 In this paper, partial volume dose constraints for each organ site were recommended using a combination of clinical experience and limited clinical outcome data. For esophagus, the clinical endpoint was defined as late toxicity occurring within 5 years of treatment. For 5% (tolerance dose 5/5), the chosen volumetric constraints based on length of esophagus radiated were 1/3 <6000 cGy, 2/3 <5800 cGy, and whole organ <5500 cGy.
Since the publication of the Emami tables, subsequent clinical work has been directed at better defining the dose metrics that reliably predict for acute esophagitis. In 2010, the Quantitative Analysis of Normal Tissue Effects in the Clinic report was published, in which a new set of comprehensive dose constraints were recommended on the basis of empirical clinical outcomes data.3 In this report, various volumetric constraints were identified as predictors of esophagitis, with MD listed as the only metric predictive for grade ≥3 toxicity, whereas V35, V50, and V70 were the recommended dosimetric criteria for grade ≥2 esophagitis.
MD has been widely adopted as one of the most commonly used dose constraints in the United States. In 2003, the report of a single institutional retrospective study demonstrated that MD to the esophagus >34 Gy, along with administration of concurrent chemotherapy and maximum dose >58 Gy, were predictive of grade ≥3 esophagitis on univariate analysis.14 On multivariate analysis, however, only concurrent chemotherapy and maximum dose remained predictive. These findings were substantiated in another retrospective series in which concurrent chemotherapy, maximum dose >60 Gy, MD >40 Gy, and presence of subcarinal lymphadenopathy were all associated with esophagitis on univariate analysis but, as with the previously mentioned study, only concurrent chemotherapy and maximum dose were predictive on multivariate analysis.15 In addition, it has been reported that the length of the esophagus in the radiation field is not predictive of esophagitis.16 In the present study, we found that, although MD was predictive of grade ≥2 and grade ≥3 esophagitis, gEUD and D2cc were the most predictive of the dose metrics on ROC analysis.
The gEUD is the mathematical expression of the expected biologic effect of a given dose gradient across a structure, as if it were instead delivered as a single uniform dose across the entire structure.17 Although this is conceptually similar to the simpler concept of MD, gEUD differs in that it accounts for maximum dose depending on the numeric “a” value, which is generally low for parallel organs and high for serial organs. In the present study, an “a” value of 10 was selected to reflect the serial nature of the esophagus and thus maximum dose was preferentially weighted in the gEUD calculation, suggesting that areas of high dose accounted for the statistical relevance of gEUD over MD in predicting esophagitis. Similarly, we found that the predictive ability of the individual dose metrics for grade ≥3 esophagitis increases with increasing dose, with D2cc being the most predictive.
The relationship between the volume of esophagus receiving high dose and esophagitis has been reported in multiple retrospective studies.14,15,18,19 In 2013, the report of a collaborative group meta-analysis to determine dosimetric predictors of pneumonitis and esophagitis was published.4 In this study, 1082 patients treated in Europe, North America, Asia, and Australia were randomly divided into training and validation sets. On multivariable analysis, V60 was the strongest predictor of grade ≥2 and ≥3 esophagitis with subsequent recursive partitioning analysis dividing patients into low (V60 <0.07%), intermediate (V60 0.07% to 16.99%), and high (V60 ≥17%) risk groups. When applied to the validation set, V60 remained significantly predictive only for grade ≥3 esophagitis, remarkably similar to our findings for V60, which was the most predictive of the volumetric variables.
The advent of IMRT has been a huge advancement in the field of radiation oncology, allowing for improved target coverage, sparing of critical normal structures, and reduction of late toxicity to a degree not possible with 3-dimensional (3D) conformal therapy in certain situations.20,21 With specific regard to esophagitis, IMRT has previously been shown to reduce dose to the esophagus while maintaining adequate target coverage, particularly in patients with mediastinal nodal involvement.22,23 The recently reported results of a prospective trial in which IMRT was used to spare the esophageal wall contralateral to the primary tumor from high dose exposure showed promising results, with no incidences of grade ≥3 esophagitis.12 There has been some hesitation to adopt IMRT for definitive lung radiation therapy due to concerns regarding increased low dose exposure to the lung and the potential for higher rates of radiation pneumonitis, specifically because of increasing lung V5.24 Recent secondary analyses of RTOG 0617, however, have shown that V5 was not predictive of pneumonitis for patients treated with IMRT and that quality of life was demonstrably improved for patients receiving IMRT compared with 3D conformal therapy.25,26 A separate analysis comparing our outcomes for patients treated with IMRT versus 3D conformal therapy is currently under way, but our finding that high dose is preferentially associated with severe esophagitis suggests that the use of IMRT in patients with mediastinal involvement may be warranted in situations in which a steep dose gradient or concave dose distribution may reduce the volume of the esophagus receiving high doses.
One of the inherent difficulties in an observational study such as this, in which patients are not treated per a specific protocol, is the potential for significant variability in target delineation, total dose, dose per fraction, and particularly in organ at risk contouring. Indeed, significant interobserver variability in esophageal contouring was reported in a secondary analysis of RTOG 0617.27 In the present study, we attempted to mitigate the effect of contouring variability by reporting dose metrics as a function of absolute volume as opposed to volumetric percentage. The reported rate of grade ≥3 esophagitis in the current study is relatively low compared with the rates in recently reported prospective trials2,28; this may be a function of our selected patient population, 22% of which did not receive concurrent chemotherapy, but may also be due in part to variability in toxicity grading among different practitioners.29 Additionally, the toxicity is physician-reported rather than patient-reported, and this may lead to potential bias; analysis of our experience with patient-reported outcomes is ongoing. Finally, although we found that gEUD was a strong predictor of esophagitis, institutions should be mindful of using this objective alone within the inverse planning cost function. As cautioned in American Association of Physicists in Medicine TG 166, gEUD objectives have the potential to allow small volume, high dose hotspots, and overall high MDs (especially for more serial organs), which may result in a nonconventional dose distribution.30 Until such metrics are more strongly validated, we would recommend that conventional optimization objectives also be applied so as to avoid high dose hotspots or MDs above levels necessary to meet the target coverage objectives. Despite these potential limitations, the finding that high dose metrics are associated with grade ≥3 esophagitis is remarkably consistent with the existing literature. In addition, the relevance of D2cc as a dose metric for prediction of severe esophagitis is unique to this study and, in our estimation, of significant clinical relevance. We believe that these findings are strengthened by the prospective nature by which the data were collected, as well as the “real-world” variability of participating sites, including both community and academic practices.
Conclusion
In this prospective, multi-institutional observational study for patients with locally advanced lung cancer treated across the state of Michigan, we find that gEUD and D2cc, rather than MD, were the most predictive dose metrics for severe esophagitis. To limit the estimated risk of grade ≥3 esophagitis to <5%, thresholds of 59.3 Gy and 68 Gy were identified for gEUD and D2cc, respectively. Patients who developed esophagitis in the first 3 weeks of therapy were at higher risk of developing severe esophagitis by the conclusion of therapy. Future work will be directed at validation of these findings in a separate prospective cohort.
Acknowledgments
Sources of support: Funding provided by Blue Cross and Blue Shield of Michigan and Blue Care Network.
References
- 1.Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chcmoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011; 103:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley JD. Paulus R, Komaki, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carbo-platin plus paclitaxcl with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma DA, Senan S, Oberjie C, et al. Predicting esophagitis after chcmoradiation therapy for non-small cell lung cancer: An individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87: 690–696. [DOI] [PubMed] [Google Scholar]
- 5.Moran JM, Feng M, Benedetti L, et al. Development of a model web-based system to support a statewide quality consortium in radiation oncology. Pract Radiat Oncol. 2017;7:e205–e213. [DOI] [PubMed] [Google Scholar]
- 6.Georg P, Lang S, Dimopoulos JC, et al. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. [DOI] [PubMed] [Google Scholar]
- 7.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 8.Algara M, Radriguez N, Vinais P, et al. Prevention of radiochemotherapy-induced esophagitis with glutamine: results of a pilot study. Int J Radiat Oncol Biol Phys. 2007;69:342–349. [DOI] [PubMed] [Google Scholar]
- 9.Fountain MD, Abernathry LM, Lonardo F, et al. Radiation-induced esophagitis is mitigated by soy isoflavones. Front Oncol. 2015;5:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence YR, Paulus R, Langer C, et al. The addition of amifostine to carboplatin and paclitaxel based chemoradiation in locally advanced non-small cell lung cancer: Long-term follow-up of Radiation Therapy Oncology Group (RTOG) randomized trial 9801. Lung Cancer. 2013;80:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fogh SE, Deshmukh S, Berk LB, et al. A randomized phase 2 trial of best supportive care: Manuka Honey Liquid and Manuka Honey Lozenges for prevention of radiation esophagitis during chemotherapy and radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2017;90:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Halabi H, Paetzold P, Sharp GC, Olsen C, Willers H. A contralateral esophagus-sparing technique to limit severe esophagitis associated with concurrent high-dose radiation and chemotherapy in patients with thoracic malignancies. Int J Radiat Oncol Biol Phys. 2015;92:803–810. [DOI] [PubMed] [Google Scholar]
- 13.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 14.Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:337–341. [DOI] [PubMed] [Google Scholar]
- 15.Qiao WB, et al. Clinical and dosimetric factors of radiation-induced esophageal injury: radiation-induced esophageal toxicity. World J Gastroenterol. 2005; 11:2626–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner-Wasik M, Pequignot E, Leeper D, Hauck W, Curran W. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiated esophagus: A multivariate analysis of patients with lung cancer treated with nonoperative therapy. Int J Radiat Oncol Biol Phys. 2000;48:689–696. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Yorke ED, Belderbos JS, et al. Using generalized equivalent uniform dose atlases to combine and analyze prospective dosimetric and radiation pneumonitis data from 2 non-small cell lung cancer dose escalation protocols. Int J Radiat Oncol Biol Phys. 2013;85: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley J, Deasy JO, Bentzen S, El-Naga I. Dosimetric correlates for acute esophagitis in patients treated with radiotherapy for lung carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:1106–1113. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Deasy JO, Bentzen S, et al. Dose-volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:995–1002. [DOI] [PubMed] [Google Scholar]
- 20.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys. 2010;76:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 22.Niedzielski J, Bluett JB, Williamson, et al. Analysis of esophageal-sparing treatment plans for patients with high-grade esophagitis. J Appl Clin Med Phys. 2013;14:4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: A comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66: 1399–1407. [DOI] [PubMed] [Google Scholar]
- 25.Movsas B, Hu C, Sloan J, et al. Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A Secondary analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016;2:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun SG, Hu C, Choy H, et al. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG Oncology/RTOG 0617. Int J Radiat Oncol Biol Phys. 2017;93:S1–S2. [Google Scholar]
- 27.Giaddui TG, et al. Interobserver variability in esophageal contours for patients with non-small cell lung cancer treated with definitive chemoradiation therapy: RTOG 0617 experience. Int J Radiat Oncol Biol Phys. 2014;90:S347. [Google Scholar]
- 28.Brade A, MacRae R, Laurie SA, et al. Phase II study of concurrent pemetrexed, cisplatin, and radiation therapy for stage IIIA/B unresectable non-small cell lung cancer. Clin Lung Cancer. 2016;17:133–141. [DOI] [PubMed] [Google Scholar]
- 29.Huynh-Le M-P, et al. Low interrater reliability in grading of rectal bleeding using National Cancer Institute Common Toxicity Criteria and Radiation Therapy Oncology Group Toxicity scales: A survey of radiation oncologists. Int J Radiat Oncol Biol Phys. 2014;90: 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen Li X, Alber M, Deasy JO, et al. The use and QA of biologically related models for treatment planning: Short report of the TG-166 of the therapy physics committee of the AAPM. Med Phys. 2012;39: 1386–1409. [DOI] [PubMed] [Google Scholar]


