Skeletal muscle is a complex tissue, important for locomotion, bone health, neuromuscular function, metabolism, as well as a reservoir in catabolic conditions e.g. surgery, infection, cancer, and trauma.1, 2 Despite the aetiological differences between sarcopenia and cachexia, low muscle mass is an independent risk factor for frailty, falls, fractures, and mortality in old age as well as a wide range of acute and chronic diseases affecting millions of people.1, 2
The last two decades, the field of sarcopenia research has evolved dramatically and likewise the number of definitions and discussions about what it should include. Initially, the term sarcopenia was solely referring to low lean mass defined as 2SD below the normal mean of a young reference population.3 Combining measures of lean mass with cut‐off points for muscle strength (hand‐grip strength, knee‐extensor muscle strength) and physical function (gait speed, sit‐to‐stand test) has not simplified the number of ways the definition is used or interpreted. Consequently, the prevalence of sarcopenia is known to vary noticeably, depending on the studied population, measurements, and cut‐off points used.4
An ongoing discussion within the interest field of sarcopenia is what assessments should be included, and the lack of agreement is probably due to the diverse backgrounds of the researchers involved, ranging from epidemiology to muscle physiology and cell biology.1, 2 From a more pragmatic point of view, it makes sense to include the most sensitive and simple measurements that predict sarcopenia.5 However, we overlook many patients with some degree of muscle dysfunction if we simplify the assessments to a questionnaire and/or a measurement of hand‐grip strength.
Alternatively, we could accept the complexity of skeletal muscle health being a combination of sufficient muscle tissue and neuromuscular function translating into muscle strength, muscle power, and physical performance and use the term Muscle Failure as an umbrella term for low muscle mass (sarcopenia), low muscle strength (dynapenia), and compromised physical function.
At this point in time, we believe that a distinction between different domains of muscle dysfunction would have a huge impact on the way we treat patients of all ages with muscle failure (Figure 1), enabling targeted individualized treatments to increase muscle mass, muscle strength, or physical function or a combination of it.
Figure 1.
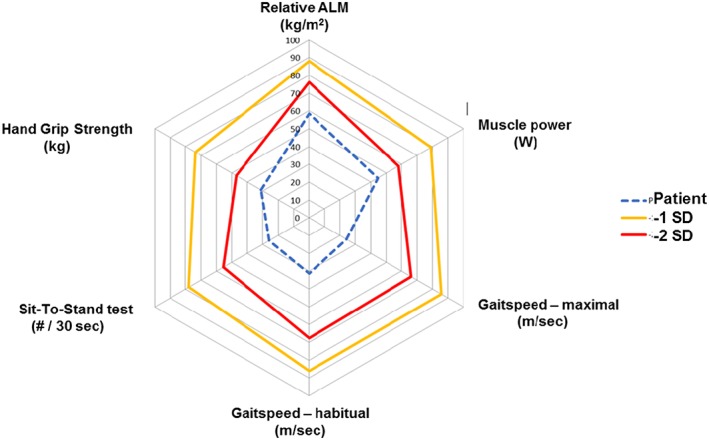
Muscle Failure web for clinical use to diagnose and monitor the different domains of muscle failure. Relative values (per cent of normal) as well as −1SD and −2SD below young reference. Blue dotted lines is an example of values from a 81 yr old woman from an outpatient clinic.
In line with heart failure, a more sophisticated approach diagnosing the type, cause, and stage of muscle failure could then guide individualized non‐pharmacological and pharmacological interventions.
Acknowledgement
The authors declare that they have no conflict of interests and certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.6
Suetta C., and Maier A. B. (2019) Is muscle failure a better term than sarcopenia?, Journal of Cachexia, Sarcopenia and Muscle, 10: 1146–1147. 10.1002/jcsm.12447.
References
- 1. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology and consequences. JAMA 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci 2010. Nov;1211:25–36, Review. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


