Abstract
The term sarcopenia was introduced in 1988. The original definition was a “muscle loss” of the appendicular muscle mass in the older people as measured by dual energy x‐ray absorptiometry (DXA). In 2010, the definition was altered to be low muscle mass together with low muscle function and this was agreed upon as reported in a number of consensus papers. The Society of Sarcopenia, Cachexia and Wasting Disorders supports the recommendations of more recent consensus conferences, i.e. that rapid screening, such as with the SARC‐F questionnaire, should be utilized with a formal diagnosis being made by measuring grip strength or chair stand together with DXA estimation of appendicular muscle mass (indexed for height2). Assessments of the utility of ultrasound and creatine dilution techniques are ongoing. Use of ultrasound may not be easily reproducible. Primary sarcopenia is aging associated (mediated) loss of muscle mass. Secondary sarcopenia (or disease‐related sarcopenia) has predominantly focused on loss of muscle mass without the emphasis on muscle function. Diseases that can cause muscle wasting (i.e. secondary sarcopenia) include malignant cancer, COPD, heart failure, and renal failure and others. Management of sarcopenia should consist of resistance exercise in combination with a protein intake of 1 to 1.5 g/kg/day. There is insufficient evidence that vitamin D and anabolic steroids are beneficial. These recommendations apply to both primary (age‐related) sarcopenia and secondary (disease related) sarcopenia. Secondary sarcopenia also needs appropriate treatment of the underlying disease. It is important that primary care health professionals become aware of and make the diagnosis of age‐related and disease‐related sarcopenia. It is important to address the risk factors for sarcopenia, particularly low physical activity and sedentary behavior in the general population, using a life‐long approach. There is a need for more clinical research into the appropriate measurement for muscle mass and the management of sarcopenia. Accordingly, this position statement provides recommendations on the management of sarcopenia and how to progress the knowledge and recognition of sarcopenia.
Keywords: Sarcopenia, Cachexia, Geriatric assessment, Muscle, Skeletal, Muscle strength
The term, sarcopenia, was coined in 1988 by Irwin Rosenberg at a meeting in Albuquerque, New Mexico, to refer to muscle wasting of the older people1. Its etymological origins are two Greek words: sarx for flesh and penia for reduced or deficiency. Baumgartner et al.2 proposed an operational definition of sarcopenia in 1998. Utilizing dual energy X‐ray absorptiometry (DXA) to measure lean soft tissue, the authors defined sarcopenia as being <2 SDs of appendicular muscle mass (ASM, kg) per height squared (m2) below the mean of a young reference group. Using this criterion, Baumgartner et al. showed that the prevalence and the severity of sarcopenia significantly increased with age and that it was associated with physical disability. In 2002, Janssen et al.,3 using bioelectrical impedance analysis (BIA), showed that in the Third National Health and Nutrition Examination Survey (NHANES III), functional impairment was three times as likely in persons with an estimated lean mass below 2 SDs of the mean. Baumgartner et al.4 found that in older persons with obesity, those who had lost muscle mass had worse outcomes than those who had maintained their muscle mass. They coined the term ‘sarcopenic obesity’ for this condition. By the early 2000s, it was recognized that there are numerous causes of age‐related sarcopenia, including loss of motor units innervating muscle, systemic inflammation, oxidative stress, decline in anabolic hormones, and the ‘anorexia of aging’ coupled with a decrease in physical activity5, 6 (Figure 1). At this stage, it was recognized that there were both primary sarcopenia (age related) and secondary sarcopenia (disease related, as with diabetes mellitus, cancer, chronic obstructive pulmonary disease, or heart failure).
Figure 1.
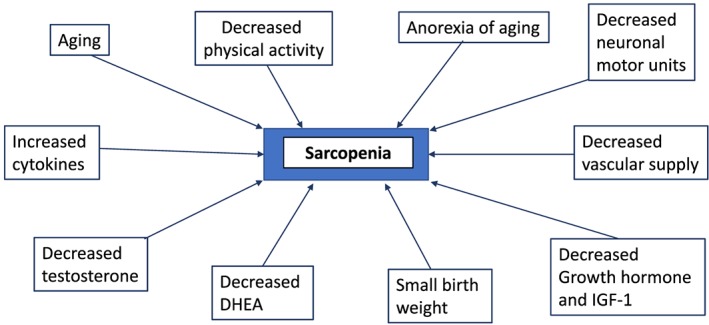
The factors involved in the pathogenesis of primary (age related) sarcopenia.
Evolving definition of sarcopenia
Primary sarcopenia (sarcopenia of aging)
In 2010, the European Working Group on Sarcopenia for Older Persons7 recommended a new operational definition of sarcopenia of aging, i.e. the presence of low muscle mass together with low muscle function (strength or performance). Over the last decade, numerous other consensus groups have agreed to this revision to the meaning of sarcopenia of aging.8, 9, 10, 11, 12 However, these groups all used different cut‐offs to define sarcopenia of aging, highlighting the fact that different cut‐offs are necessary for different ethnic groups.12
Towards the end of last year, two consensus articles on sarcopenia of aging were published. One was an update by the European Working Group on Sarcopenia (EWGSOP2),13 and the other was on the management of sarcopenia of aging by the International Clinical Practice Guidelines for Sarcopenia (ICFSR).14 The EWGSOP2 requires low muscle strength as a key characteristic of low muscle quality and the presence of low muscle quantity to confirm the diagnosis. If a person also has functional impairment, confirmed with a physical performance measure,15 this is characterized as severe sarcopenia. The authors recommended measuring muscle strength with either grip strength or the chair stand test. Muscle mass can be measured by DXA, magnetic resonance imaging, or computed tomography. Either gait speed, the short physical performance battery (SPPB), the Timed Up and Go test, or the 400‐m‐walk can be used for the assessment of physical performance.
Recognizing the limited time available during a typical visit to a health care professional, the EWGSOP2 also suggested that case finding should be used to identify older persons at risk for sarcopenia. They recommended the use of clinical symptoms usually associated with sarcopenia or the SARC‐F (Figure 2), a questionnaire with five questions, which has high specificity, albeit low sensitivity, to identify persons with sarcopenia.16, 17, 18, 19, 20 The SARC‐F has been translated into multiple languages. The SARC‐F is also recommended by the ICFSR for screening.14 The specificity of the SARC‐F can be improved by measuring calf circumference as well.21 The Ishii screening test (age, grip strength, and calf circumference) is recommended as an alternative screening test.22, 23 However, this already includes grip strength, which is a core measure of sarcopenia.
Figure 2.
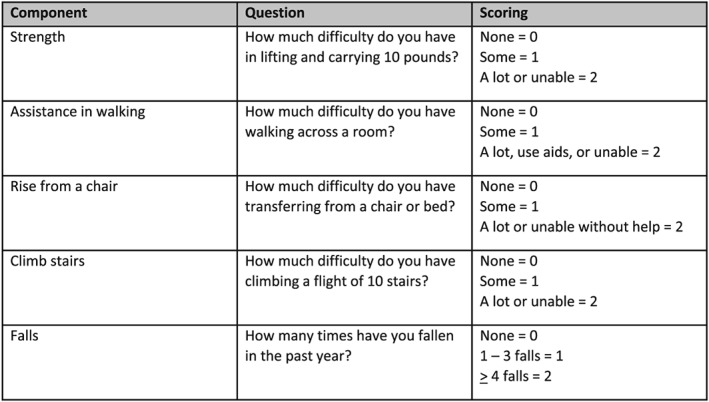
SARC‐F questionnaire (includes scoring).
While BIA was not strongly supported by the EWGSOP2, to measure muscle mass, they recognize that its portability, affordability, and availability make BIA a feasible tool to estimate muscle mass in many care settings. Ultrasound of muscle such as the quadriceps is emerging as a potential tool to measure muscle quantity and, because it excludes intermuscular adipose tissue from the measurement, also muscle quality24, 25; a protocol for using ultrasounds in sarcopenia has recently been proposed by the European Geriatric Medicine Society.26
There is increasing evidence that creatine dilution, implemented by ingesting a dose of the deuterium labelled isotope, may also offer an accurate approach for measuring muscle mass.27, 28 Studies so far suggest creatine dilution estimates of muscle mass may have good correlations with functional outcomes.27, 28 Nonetheless, its relevance and practicality in clinical settings remain to be determined.
The ICFSR consensus made similar conditional recommendations utilizing the SARC‐F for screening and applying either the original EWGSOP or Foundation for NIH (FNIH) diagnostic criteria.14
Secondary sarcopenia
Malignant disease has been the most studied secondary sarcopenia, and international consensus definitions specific to cancer sarcopenia29 are predicated on disease specific outcomes: mortality, complications of cancer surgery, and chemotherapy toxicity. Whether this secondary sarcopenia should be considered early cachexia or sarcopenia remains controversial,30 but it is becoming clearer that sarcopenia is only one of the different features of muscle changes during cancer cachexia. Owing to the prevalent use of computed tomography imaging in cancer diagnosis and follow‐up, secondary analysis of oncologic imaging for skeletal muscle cross‐sectional areas or volumes is the current standard for the quantification of muscle mass in this domain.
There are several points of relevance regarding age‐related and disease‐related loss of muscle mass. Loss of muscle mass with age occurs in a continuous fashion after reaching peak muscle mass in young adulthood (at about 30 years of age). A variety of longitudinal observational studies provide information on the rate of muscle loss per decade. The percentage loss of ASM per 10 years is of the order of ~5% in men and is usually reported to be somewhat lower in women. Chronic illness‐related muscle loss is also progressive; however, these are non‐linear and of a considerably greater magnitude than the values seen in aging. For example, cancers of advanced stage induce muscle loss over time that take an exponential course31, 32 with increasing intensity according to the disease progression, varying from 2% per 100 days to 15% per 100 days. Total cumulative loss in 12 months in colon cancer patients was 15.6%, equivalent to circa 30 years of aging31; this is partially disease‐related but also in part a consequence of cancer surgery or systemic antineoplastic therapies which induce punctate short‐term losses. Acute illness requiring hospitalization is associated with even higher intensity of muscle loss than in cancer. In elective hip replacement surgery during an average of 5.6 ± 0.3 days of hospitalization, associated with significant decline in quadriceps (−3.4 ± 1.0%) and thigh muscle cross‐sectional area (CSA) (−4.2 ± 1.1%) in the non‐operated leg (P < 0.05) aging.33, 34 This could be in part due to bed rest as 5 days of one‐legged knee immobilization using a full leg cast resulted in decline of quadriceps muscle CSA from baseline of 3.5 ± 0.5% (P < 0.0001). Acute sarcopenia secondary to hospitalization or chronic disease exacerbations may be partially recoverable or may lead to heightened risk of developing sarcopenia at a young age.35
Management of sarcopenia
For the management of sarcopenia, there is a strong recommendation that individuals with sarcopenia should be enrolled in a resistance exercise programme. There is a reasonable amount of evidence that resistance exercise will increase both muscle mass and strength.36, 37, 38 The use of a protein rich diet (1 to 1.5 g/day) or protein supplementation received a conditional recommendation based on a small amount of evidence and a previous consensus conference.39, 40, 41 Higher doses of protein (up to 2 g/day) may be appropriate in persons with severe illness or injury or when there is evidence of a pro‐inflammatory/catabolic state.39, 40 β‐hydroxy β‐methylbutyrate (HMB) has been shown to improve muscle mass and to preserve muscle strength and function in older people with sarcopenia or frailty.42 Vitamin D supplementation specifically for sarcopenia was found to have insufficient evidence, though there is evidence that persons with low vitamin D levels may improve their strength with vitamin D supplementation.43 Similarly, while testosterone can increase muscle mass and strength in older individuals and a meta‐analysis has confirmed its safety,44, 45, 46, 47 the lack of evidence in persons with sarcopenia did not lead to its integration into these recommendations. Preliminary data with anamorelin, a growth hormone secretagogue receptor type 1 (ghrelin receptor) agonist that increases muscle mass but not strength,48, 49 and anti‐myostatin antibodies49, 50, 51, 52, 53 were considered insufficient to make recommendations in favour of their use.
Recommendations
Recently, an ICD‐10‐CM code for sarcopenia as a disease has become available allowing physicians to formally include sarcopenia in the list of diagnosis that can be used and funded.54, 55, 56 Based on the available evidence and the two recent consensus recommendations, the task force of the Society for Sarcopenia, Cachexia and Wasting Disorders recommends for clinical application the following:
Health care professionals are encouraged to screen for sarcopenia using simple screening tools such as the SARC‐F.
Where available and feasible, physicians should make a formal diagnosis of sarcopenia utilizing grip strength or chair stand and—if available—a measurement of fat‐free mass. At present, we recommend ASM per height squared estimated by DXA, but recognize CT, BIA, ultrasound, or creatine dilution techniques may be as good or more accurate approaches for estimating muscle mass in the future.
Resistance exercise should be prescribed for any older person suspected of having sarcopenia both for secondary prevention and/or treatment.
A protein intake of 1 to 1.5 g/kg/day in conjunction with physical exercise seems reasonable for a person with sarcopenia.
At present, there is insufficient evidence that vitamin D, anabolic steroids, or newer pharmacological agents should be used to treat sarcopenia.
These recommendations apply to both primary (age related) sarcopenia and secondary (disease related) sarcopenia. In the second, treating the disease(s) related to sarcopenia is also essential.
Consensus documents on sarcopenia in older persons (EWGSOP and ICSFR) as well as those for sarcopenia in chronic disease29 have something in common: they intend to put sarcopenia in the clinical frontline. It is time that health professionals are educated in the diagnosis and management of sarcopenia and are encouraged to screen for sarcopenia and educate their patients on primary and secondary prevention of sarcopenia.
To progress the knowledge and recognition of sarcopenia, the task force recommends the following actions:
promotion of sarcopenia measurement in epidemiological studies of older adults, including in studies of chronic disease, and also in other disease or organ oriented specialties;
collaboration with health care policy makers and health care providers regarding medical claims using the ICD diagnosis code for sarcopenia;
development and assessment of new treatments for sarcopenia in individuals with either primary or secondary sarcopenia;
advocating common standards in the quality of clinical trials for sarcopenia, including consistent outcome measurements and recognition of such measures by regulatory agencies;
address the risk factors for sarcopenia, particularly low physical activity and sedentary behaviour in the general population, using a life‐long approach;
explore the impact of targeted nutritional approaches to countermeasure muscle loss alone and in a multimodal approach to maximize anabolic potential (e.g. exercise and anabolic therapy); and
identify the pathophysiological pathways leading to sarcopenia.
Conflict of interest
A.J.S.C. has received speaker fees and/or honoraria from Astra Zeneca, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, CVRx, Enopace, Faraday, Gore, Respicardia, Stealth Peptides, and V‐Wave. A.J.C.‐J. has received speaker fees from Abbott Nutrition, Fresenius, Nestlé, Nutricia, and Sanofi‐Aventis; is a member of advisory boards for Abbott Nutrition, Nestlé, and Pfizer; and has worked on research projects with Abbott Nutrition and Nutricia. J.C. discloses the following: consultant/independent contractor: Amgen, AstraZeneca, Coherus, Enzychem, Merck, and Pfizer; grant/research support: AstraZeneca, Genentech, and Helsinn; Chair/DSMB member: Beyond Spring. S.H. serves in Tanita and Medifast Medical Advisory Boards. A.L. has received honoraria for independent lectures at educational events organized by nutrition industry. He is a member of the scientific advisory board of Smartfish.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for editorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.57
Bauer J., Morley J. E., Schols A. M. W. J., Ferrucci L., Cruz‐Jentoft A. J., Dent E., Baracos V. E., Crawford J. A., Doehner W., Heymsfield S. B., Jatoi A., Kalantar‐Zadeh K., Lainscak M., Landi F., Laviano A., Mancuso M., Muscaritoli M., Prado C. M., Strasser F., Coats A. J. S., and Anker S. D. (2019) Sarcopenia: A Time for Action. An SCWD Position Paper, Journal of Cachexia, Sarcopenia and Muscle, 10: 956–961. 10.1002/jcsm.12483.
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127( Suppl:990S–991S. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 3. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004. [DOI] [PubMed] [Google Scholar]
- 5. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137:231–243. [DOI] [PubMed] [Google Scholar]
- 6. Clark BC, Manini TM. Sarcopeia ≠ dynapenia. J Gerontol A Biol Sci Med Sci 2008;63:829–834. [DOI] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 11. Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence‐based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 13. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dent E, Morley JE, Cruz‐Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging 2018;22:1148–1161. [DOI] [PubMed] [Google Scholar]
- 15. Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom 2015;18:467–471. [DOI] [PubMed] [Google Scholar]
- 16. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252. [DOI] [PubMed] [Google Scholar]
- 18. Bahat G, Yilmaz O, Kiliç C, Oren MM, Karan MA. Performance of SARC‐F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging 2018;22:898–903. [DOI] [PubMed] [Google Scholar]
- 19. Ida S, Kaneko R, Murata K. SARC‐F for screening among older adults: a meta‐analysis of screening test accuracy. J Am Med Dir Assoc 2018;19:685–689. [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Kim M, Won CW. Validation of the Korean version of the SARC‐F questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 2018;19:40–45.e1. [DOI] [PubMed] [Google Scholar]
- 21. Barbosa‐Silva TG, Menezes AM, Bielemann RM, Malmstrom TK, Gonzalez MC. Grupo de Estudos em Composicao corporal e Nutricao (COCONUT)Enhancing SARC‐F: Improving sarcopenia screening in the clinical practice. J Am Dir Assoc 2016;17:1136–1141. [DOI] [PubMed] [Google Scholar]
- 22. Locquet M, Beaudart C, Reginster JY, Petermans J, Bruyere O. Comparison of the performance of five screening methods for sarcopenia. Clin Epidemiol 2017;10:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int 2014;14:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle ultrasound and sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc 2017;18:290–300. [DOI] [PubMed] [Google Scholar]
- 25. Nijholt W, Scafoglieri A, Jager‐Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkisas S, Baudry S, Bauer J, Beckwee D, De Cock A‐M, Hobbelen H, et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med 2018;9:739–757. [DOI] [PubMed] [Google Scholar]
- 27. Shankaran M, Czerwieniec G, Fessler C, Wong PA, Killion S, Turner SM, et al. Dilution of oral D3‐creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 2018;9:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong relation between muscle mass determined by D3‐creatine dilation. Physical performance and incidence of falls and mobility limitations I a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 2018;Jun 12; 10.1093/Gerona/gly129 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 30. Anker SD, Coats AJ, Morley JE, Rosano G, Bernabei R, von Haehling S, et al. Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle 2014;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muscaritoli M, Lucia S, Molfino A, Cederholm T, Rossi Fanelli F. Muscle atrophy in aging and chronic diseases: is it sarcopenia or cachexia? Intern Emerg Med 2013;8:553–560. [DOI] [PubMed] [Google Scholar]
- 32. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole‐body energy demands. Am J Clin Nutr 2009;89:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 34. Kouw IWK, Groen BBL, Smeets JSJ, Kramer IF, van Kranenburg JMX, Nilwik R, et al. One week of hospitalization following elective hip surgery induces substantial muscle atrophy in older patients. J Am Med Dir Assoc 2019;20:35–42. [DOI] [PubMed] [Google Scholar]
- 35. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014;210:600–611. [DOI] [PubMed] [Google Scholar]
- 36. Vlietstra L, Hendrickx W, Waters DL. Exercise interventions in healthy older adults with sarcopenia: a systematic review and meta‐analysis. Australas J Ageing 2018;37:169–183. [DOI] [PubMed] [Google Scholar]
- 37. Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: a meta‐analysis. Scand J Med Sci Sports 2016;26:995–1006. [DOI] [PubMed] [Google Scholar]
- 38. Roth SM, Ferrell RF, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging 2000;4:143–155. [PubMed] [Google Scholar]
- 39. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz‐Jentoft AJ, Morley JE, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE study group. J Am Med Dir Assoc 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 40. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine‐enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double‐blind, placebo‐controlled trial. J Am Med Dir Assoc 2015;16:740–747. [DOI] [PubMed] [Google Scholar]
- 41. Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta‐analysis. Am J Clin Nutr 2017;106:1078–1091. [DOI] [PubMed] [Google Scholar]
- 42. Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SDR, Hart N, et al. β‐Hydroxy‐β‐methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta‐analysis. Am J Nutr 2019;109:119–1132. [DOI] [PubMed] [Google Scholar]
- 43. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta‐analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99:4336–4345. [DOI] [PubMed] [Google Scholar]
- 44. Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, et al. Muscular responses to testosterone replacement vary by administration route: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2018;9:465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha aA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta‐analysis. J Am Geriatr Soc 2006;54:1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf) 2016;85:436–443. [DOI] [PubMed] [Google Scholar]
- 47. de Spiegeleer A, Beckwe D, Bautmans I, Petrovic M. Sarcopenia Guidelines Development Group for the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta‐analyses. Drugs Aging 2018;35:719–734. [DOI] [PubMed] [Google Scholar]
- 48. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 49. Anker SD, Coats AJ, Morley JE. Evidence for partial pharmaceutical reversal of the cancer anorexia‐cachexia syndrome: the case of anamorelin. J Cachexia Sarcopenia Muscle 2015;6:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle 2014;5:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Polkey MI, Praestgaard J, Berwick A, Franssen FME, Singh D, Steiner MD, et al. Activin type II receptor blockade for treatment of muscle depletion in COPD: a randomized trial. Am J Respir Crit Care Med 2018; Aug 10; 10.1164/rccm.201802-0286OC [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int 2016;98:319–333. [DOI] [PubMed] [Google Scholar]
- 53. Vellas B, Fielding R, Bhasin S, Cerreta F, Goodpaster B, Guralnik JM, et al. Sarcopenia trials in specific diseases: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging 2016;5:194–200. [DOI] [PubMed] [Google Scholar]
- 54. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD‐10‐CM) code. J Am Med Dir Assoc 2016;17:675–677. [DOI] [PubMed] [Google Scholar]
- 56. Vellas B, Fielding RA, Bens C, Bernabei R, Cawthon PM, Cederholm T, et al. Implications of ICD‐10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging 2018;7:2–9. [DOI] [PubMed] [Google Scholar]
- 57. Von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle; Update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


