Abstract
Background
Loss of appetite and body weight are potentially devastating, highly prevalent cancer complications. The ghrelin receptor is a mediator of appetite and metabolism, and anamorelin is a novel, orally administered ghrelin receptor agonist. Effects on appetite and food intake may influence body‐weight gain but can be difficult to measure in multi‐site studies. Here, we summarize two single‐centre trials.
Methods
Both trials were phase I, randomized, double‐blind, placebo‐controlled, partly/wholly crossover studies of healthy young adults. Study 102 tested single anamorelin doses of 1–20 mg. Assessments included post‐dose self‐ratings on 100 mm visual analogue scales for hunger, anticipated eating pleasure, and anticipated quantity of food consumption. Study 101 tested single 10, 25, and 50 mg doses. Assessments included the same scales plus caloric intake beginning 4 h post‐dose.
Results
Study 102 treated 16 male subjects (mean age, 26.3 years). Mean hunger scores generally increased after all treatments, with significant differences from placebo (P < 0.05) in the 5 mg anamorelin group at 0.5 and 1 h post‐dose (+8.2 and +13.2 mm). Results for other scales were similar. Study 101 treated nine male subjects (mean age, 26.3 years). Pooled findings for anamorelin 25 and 50 mg vs. placebo showed significant mean increases in hunger scores at all but 1 pre‐prandial time point, including the first assessment, 0.5 h post‐dose (+10.9 vs. +0.7 mm, P = 0.0077), and the last assessment, 4 h post‐dose (+32.7 vs. +7.0 mm, P = 0.0170), with a significant mean 18.4% increase vs. placebo in caloric intake (P = 0.0148).
Conclusions
In single‐centre studies of healthy adults, single anamorelin doses of 1–20 mg elicited modest increases in hunger, and single doses of 25 and 50 mg achieved significant increases in hunger and caloric intake. The findings are consistent with dose‐related weight gain reported in a prior phase I study as well as multi‐centre findings in cachectic cancer patients and expand the evidence supporting anamorelin as a potential intervention.
Keywords: Anamorelin, Cancer cachexia, Appetite, Food intake, Phase I studies
Introduction
Loss of appetite and involuntary loss of body weight, particularly wasting of muscle mass, are potentially devastating, highly prevalent complications of cancer, especially in the late stages of many malignancies.1, 2, 3 Increasing evidence suggests that these two complications are important markers of cachexia, which is a multifactorial metabolic syndrome resulting largely from cancer‐related up‐regulation of proinflammatory cytokines and dysregulation of orexigenic signals to the hypothalamus.2, 4, 5, 6, 7 Despite their severe adverse impact, including a strong association between cachexia and mortality in cancer patients,1, 8, 9 no interventions addressing anorexia and weight loss in cancer have been approved in the USA. Progestins, such as megestrol acetate, have been employed in acquired immune deficiency syndrome and have been approved in Europe for use in cancer as well as acquired immune deficiency syndrome, but they promote weight gain mostly as adipose tissue and fluids without consistent improvements in lean body mass/muscle mass or quality of life.7, 10, 11 Other drug classes, such as cannabinoids, have offered strong rationales but equivocal clinical data.7, 12
In 1999, the identification of ghrelin as the endogenous ligand of the growth hormone (GH) secretagogue receptor, now called the ghrelin receptor, opened a new approach for potential pharmacological intervention. Ghrelin is a 28‐amino‐acid peptide hormone secreted chiefly by the stomach.13 Via its stimulation of the ghrelin receptor, which is expressed in the brain at sites including the hypothalamic arcuate nucleus,14 ghrelin acts as a mediator of appetite, feeding, and metabolism.15 It does so partly by triggering increases in circulating levels of GH,16, 17 which in turn has been found to reduce circulating levels of proinflammatory cytokines and increase insulin‐like growth factor‐1, which is involved in anabolic pathways in skeletal muscle.18, 19 Endogenous plasma ghrelin concentrations are increased by fasting and decreased by meals,20, 21 implying a role as a signal of hunger.22 Indeed, in a study of ghrelin administered by continuous intravenous infusion to healthy volunteers, the hormone was associated with acute increases in appetite and in food consumption compared with saline infusion.23 In animal models of cancer cachexia, ghrelin promoted feeding, weight gain, and retention of lean body mass.24, 25 As a peptide, however, ghrelin must be administered parenterally. Moreover, the acylated, biologically active form of the peptide has a plasma elimination half‐life of only about 10 min.26 To avoid one or both of these drawbacks, a variety of ghrelin receptor ligands have been tested for potential clinical benefit.27 Among them, a non‐peptide agent, capromorelin, has been approved in the USA for veterinary use as an appetite stimulant.28
Anamorelin (RC‐1291; ONO‐7643) is a novel, orally administered, small‐molecule drug currently under investigation that acts as a highly selective agonist of the ghrelin receptor.29 Because of this mechanism of action, anamorelin is hypothesized to have beneficial effects on cancer anorexia and cachexia by increasing appetite and by activating anabolic hormonal pathways that promote development of muscle mass. Its actual effects on appetite and body weight are therefore crucial measures of its potential efficacy. In a phase I anamorelin study in healthy volunteers, which tested multiple doses of once or twice daily anamorelin, significant dose‐related increases in total body weight were seen after 6 days of treatment and began on Day 2.30 In two phase II anamorelin trials in cachectic patients with advanced or incurable cancer31 and two phase III trials in cachectic patients with unresectable non‐small cell lung cancer,32 significant gains were recorded in lean body mass and in total body weight over 12 weeks. In the phase II studies, significant improvement in overall quality of life was identified by the Anderson Symptom Assessment Scale (ASAS),31 and in the phase III studies, significant improvement in anorexia/cachexia symptom burden was identified by a specific, validated questionnaire, the Functional Assessment of Anorexia–Cachexia Therapy (FAACT) anorexia–cachexia scale (A/CS).32, 33, 34
However, there are some limitations when evaluating the phase II and phase III studies for detailed mechanism‐based information on weight and appetite changes. For example, accurate changes in body weight can be hard to obtain at multiple diverse clinical research sites without proper training and detailed procedures to ensure consistency. Furthermore, while both the ASAS and FAACT A/CS questionnaires contain many questions around key symptoms, appetite loss is only directly addressed as a single item, and food diaries/caloric intake measures to support appetite changes were not determined to be feasible in international, multi‐centre trial settings.
Therefore, data obtained during single‐centre anamorelin trials may help provide additional details. In addition to the phase I study showing dose‐related weight gain,30 two other such studies are available. Here, we summarize the results of two randomized, double‐blind, placebo‐controlled, phase I studies of healthy volunteers. In Study 102 (initial investigation of anamorelin in humans), the evaluations of single‐dose treatment effects included subjects' self‐ratings of appetite on scales for hunger, anticipated eating pleasure, and anticipated quantity of food consumption. In Study 101,35, 36 the evaluations of single‐dose effects included the same appetite self‐ratings, as well as assessments of post‐dose food consumption.
Methods
Study subjects
Participants in Study 102 (conducted from July to October 2003) were required to be healthy men aged 21–35 years old, weighing 50–100 kg, and with a body mass index of 18–29 kg/m2. For Study 101 (January–February 2004), surgically sterile women were also permitted, and the permitted age range was 18–40 years. In both studies, subjects were excluded for any condition that would prevent proper study drug evaluation; any significant medical, laboratory, or cardiac abnormality; and any participation in a clinical trial within the 30 days preceding study drug treatment. Throughout each study, and starting 48 h beforehand, subjects were forbidden to use any non‐study drug medications.
Study 102 design
During each of four treatment periods, eight subjects in total received a single dose of study treatment: anamorelin (six subjects) or matching placebo (two subjects). By a computer‐generated randomization schedule, each subject's overall dosing consisted of anamorelin three times and placebo once. In Period 1, the active drug was a 1 mg solution; in Period 2, it was a 2.5 mg solution; in Period 3, it was a 5 mg solution; and in Period 4, it was a 5 mg capsule. Based on preclinical evidence, all of these doses were expected to be subtherapeutic. (In dogs, the threshold dose for producing a modest increase in circulating GH had been 0.1 mg/kg.) After an interim data analysis confirming study drug safety and tolerability, the protocol was amended to include an additional eight subjects who underwent an extended sequence of treatments, consisting of the first four periods plus two additional periods, in which anamorelin was dosed at 10 mg (two 5 mg capsules) in Period 5 and 20 mg (four 5 mg capsules) in Period 6. Throughout the study, each treatment was administered with 200 mL of water at 07:00–11:00 a.m., after a fast beginning at midnight the previous night. Until 4 h post‐dose, subjects were permitted to ingest only water. A standardized meal was provided 4 h post‐dose. Each treatment was separated from the next by at least a 7 day washout. For each period, subjects reported to the clinic the day before dosing and were released approximately 24 h after dosing.
The study's tools for assessing treatment effects included a set of three appetite self‐ratings on 100 mm visual analogue scales (VASs) adapted from scales utilized to investigate the effects of ghrelin in healthy humans.23 A hunger scale (‘How hungry do you feel right now?’) was anchored by ‘Not at all’ at the left end (0 mm) and ‘Extremely’ at the right end (100 mm). An anticipated pleasure scale (‘How pleasant would it be to eat right now?’) was anchored the same way. An anticipated quantity scale (‘How much do you think you could eat right now?’) was anchored by ‘Nothing’ at the left end and ‘A large amount’ at the right end. All scores were obtained by measuring a subject's vertical mark as distance in millimetres from the left end of a scale, and scores were obtained within 15 min pre‐dose and at 0.5, 1, 2, and 4 h post‐dose.
Study 101 design
During each of three treatment periods, nine subjects in total received a single dose of study drug: six subjects received anamorelin and three subjects received matching placebo. By a computer‐generated randomization schedule, each subject's overall dosing consisted of anamorelin twice and placebo once. In Period 1, the anamorelin dose was 10 mg (two 5 mg capsules); in Period 2, it was 25 mg (one 25 mg capsule); and in Period 3, it was 50 mg (two 25 mg capsules). Each treatment was administered with 200 mL of water as a morning dose, after an overnight fast beginning at midnight the previous night. Until 4 h post‐dose (roughly noon on each treatment day), subjects were permitted to ingest only water. At 4 h, subjects were offered a buffet lunch consisting of items pre‐selected by each subject and were allowed to consume as much as they wished for approximately 30 min. Each treatment was separated from the next by a 7 day washout. For each period, subjects reported to the clinic the day before dosing and were released approximately 24 h after dosing.
The study's tools for assessing treatment effects included the same appetite VASs utilized in the preceding study. For each dosing of each subject, scores were obtained within 15 min pre‐dose and at 0.5, 1, 2, 4, and 6 h post‐dose. The amount of food each subject ingested 4 h post‐dose at the buffet lunch was measured by caloric content.
Statistical methods
Changes from baseline in appetite VAS scores were assessed by paired t‐test. In Study 101, per cent difference between post‐anamorelin and post‐placebo caloric intake was also assessed by paired t‐test. Because of the pilot nature of these small phase 1 studies, multiple comparisons were made without formally adjusting for multiplicity. In all studies, differences from placebo were considered to be statistically significant at P < 0.05.
Sample size calculation
In Study 102, no formal sample size calculation was performed. In Study 101, the sample size was based on a published study of ghrelin effects on appetite and food consumption.23
Results
Study 102
The planned 16 subjects were enrolled, randomized, and treated. One subject participating in the expanded treatment sequence was withdrawn after Period 5, when results of his admission urine toxicology screen were reported as positive. All other subjects completed the study. Subjects' demographic characteristics are summarized in Table 1 (middle column), and baseline characteristics were well balanced across the treatment sequences (data not shown).
Table 1.
Baseline demographic characteristics in Studies 102 and 101
| Characteristic | Study 102 | Study 101 |
|---|---|---|
| n | 16 | 9 |
| Age (year) | ||
| Mean (SD) | 26.3 (4.6) | 26.3 (7.2) |
| Range | 21–34 | 20–36 |
| Sex, n (%) | ||
| Male | 16 (100) | 9 (100) |
| Female | 0 | 0 |
| Race, n (%) | ||
| White | 14 (88) | 8 (89) |
| Black | 1 (6) | 1 (11) |
| Other | 1 (6) | 0 |
| Weight (kg) | ||
| Mean (SD) | 73.1 (5.6) | 74.9 (13.4) |
| Range | 58.3–80.5 | 57.9–91.9 |
| BMI (kg/m2) | ||
| Mean (SD) | 23.6 (2.3) | 23.5 (2.9) |
| Range | 18.2–27.3 | 18.8–28.1 |
BMI, body mass index; SD, standard deviation.
In the anamorelin groups, the mean pre‐dose hunger VAS score (Supporting Information, Table S1, middle column) differed significantly from that for placebo only among subjects assigned to receive 1 mg. Post‐dose changes in hunger score are presented in Figure 1A. In general, the mean score increased after all treatments, especially at later time points, 2 and 4 h post‐dose. However, anamorelin differed significantly from placebo only for the 5 mg dose administered as a capsule and only at early time points, 0.5 and 1 h post‐dose. Results for the other appetite scales (anticipated pleasure and anticipated quantity) were consistent with those for the hunger scale (Figures 2A and 3A).
Figure 1.
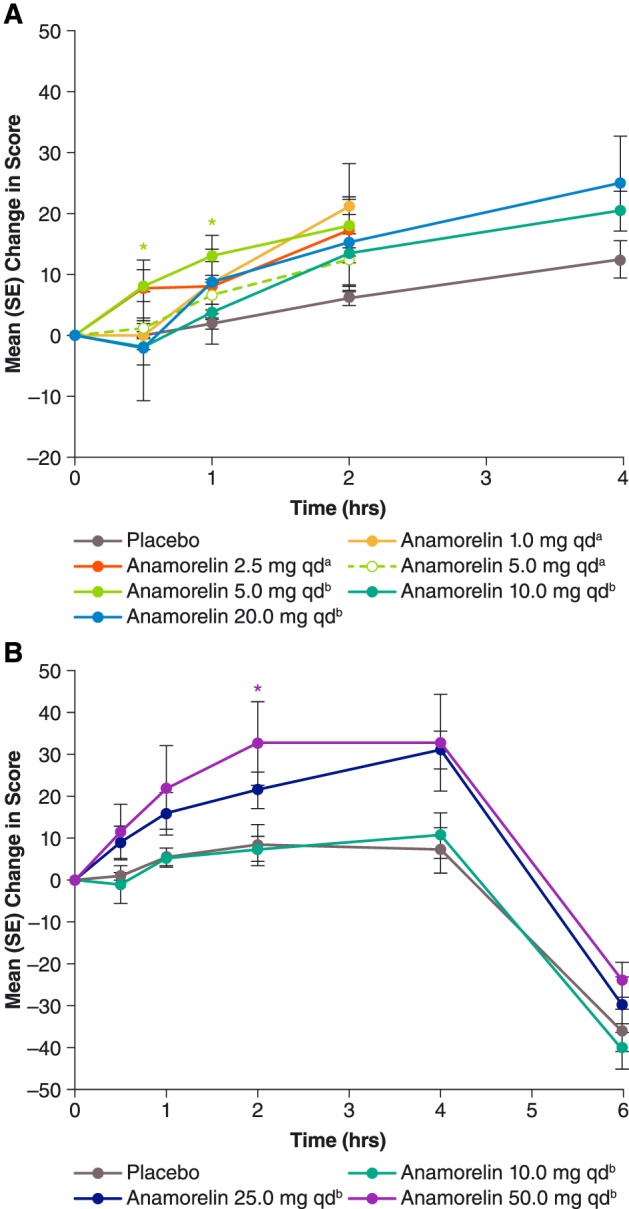
Mean [standard error (SE)] post‐dose changes in hunger visual analogue scale scores during Study 102 (A, top chart) and Study 101 (B, bottom chart). For each curve, n = 6, except for anamorelin 20 mg once daily (qd) in Study 102 (n = 5), placebo in Study 102 (n = 12 except at 4 h, where n = 4), and placebo in Study 101 (n = 9). aAdministered as a solution. bAdministered in capsules. *P < 0.05 vs. placebo, paired t‐test.
Figure 2.
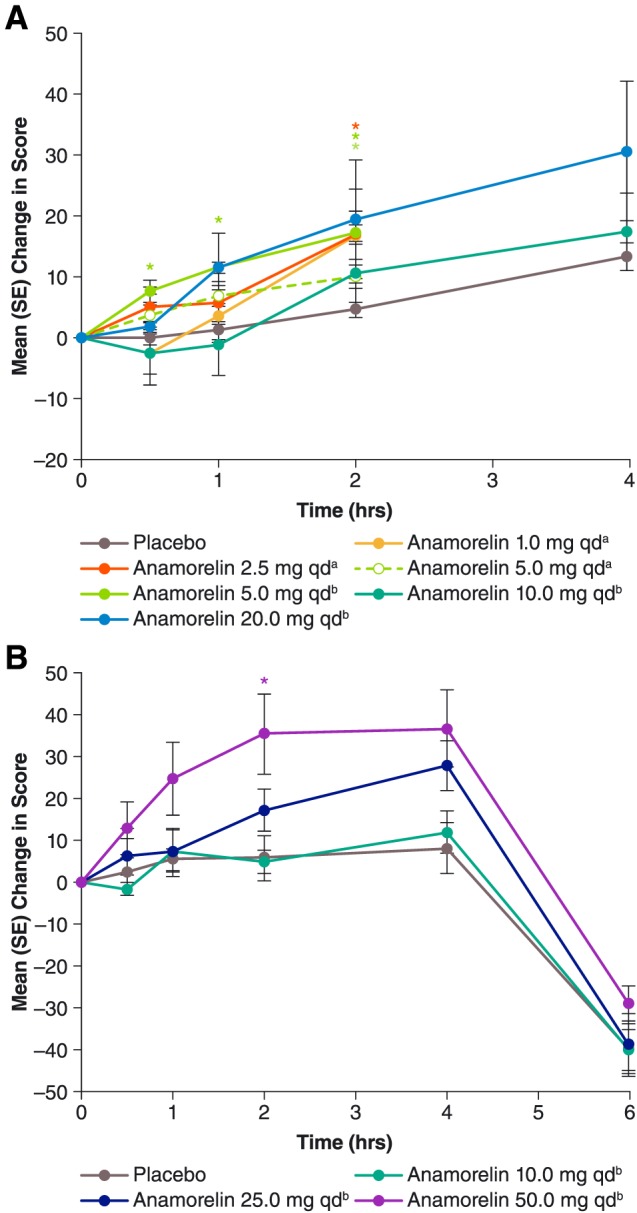
Mean [standard error (SE)] post‐dose changes in visual analogue scale scores for anticipated eating pleasure during Study 102 (A, top chart) and Study 101 (B, bottom chart). For each curve, n = 6, except for anamorelin 20 mg once daily (qd) in Study 102 (n = 5), placebo in Study 102 (n = 12 except at 4 h, where n = 4), and placebo in Study 101 (n = 9). aAdministered as a solution. bAdministered in capsules. *P < 0.05 vs. placebo, paired t‐test.
Figure 3.
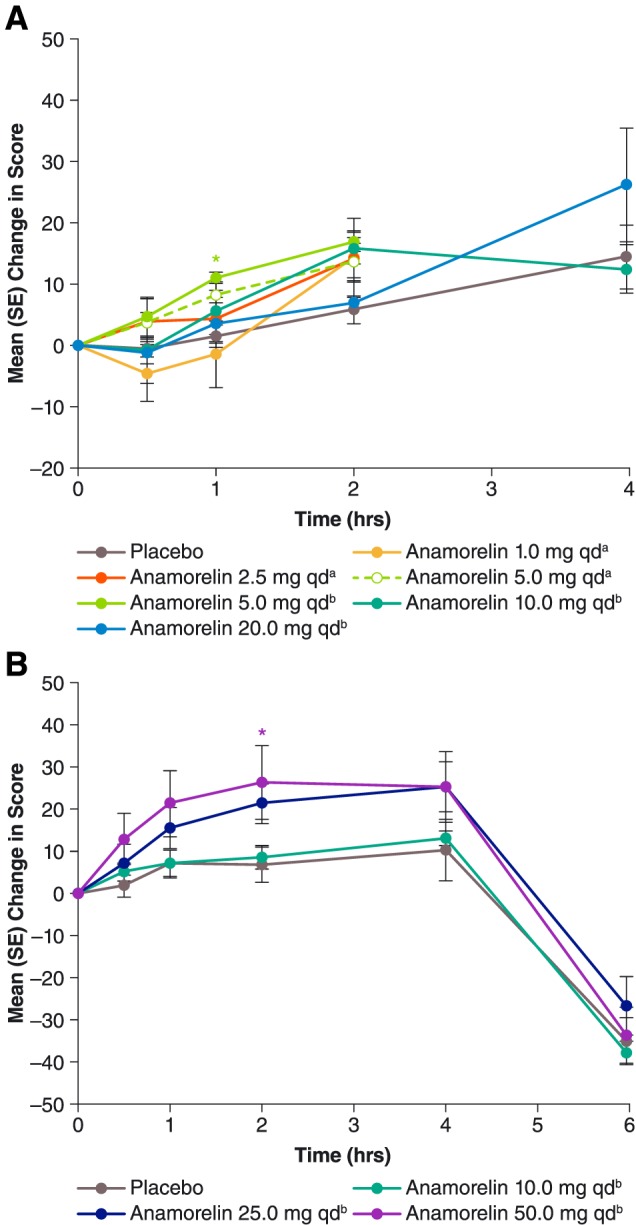
Mean [standard error (SE)] post‐dose changes in visual analogue scale scores for anticipated quantity of food consumption during Study 102 (A, top chart) and Study 101 (B, bottom chart). For each curve, n = 6, except for anamorelin 20 mg once daily (qd) in Study 102 (n = 5), placebo in Study 102 (n = 12 except at 4 h, where n = 4), and placebo in Study 101 (n = 9). aAdministered as a solution. bAdministered in capsules. *P < 0.05 vs. placebo, paired t‐test.
All anamorelin doses were well tolerated, with no clinically consequential safety findings.
Study 101
The planned nine subjects were enrolled, randomized, treated, and all completed the study. Subjects' demographic characteristics are summarized in Table 1 (right column). Across the study's four treatments, baseline characteristics were well balanced (data not shown).
There was no significant difference in hunger VAS scores between anamorelin groups and the placebo group at baseline (Supporting Information, Table S1, right column). Post‐dose changes in hunger score are presented in Figure 1B. In general, anamorelin dosing led to dose‐related increases in hunger. The 10 mg dose was not statistically significantly different from placebo at all assessment time points and was considered a ‘no effect’ dose. Pooled findings for the other dose levels, 25 and 50 mg, showed statistically significant mean increases in hunger score at all but 1 pre‐prandial time point, including the first assessment, 30 min post‐dose (+10.9 vs. +0.7, P = 0.0077), and the last pre‐prandial assessment, 4 h post‐dose (+32.7 vs. +7.0, P = 0.0170).
Caloric intake at the subjects' buffet meal, beginning 4 h post‐dose, is summarized in Table 2. Anamorelin stimulated food intake at a threshold dose of 25 mg; again, the 10 mg dose was found to be ineffective. Pooled findings for 25 and 50 mg showed a statistically significant 18.4% increase compared with placebo (P = 0.0148). Numerically, the mean intake was 4.8% greater among the six subjects receiving 25 mg than among the six subjects receiving 50 mg (2156.8 vs. 2057.8 calories). Post‐prandial hunger VAS scores obtained 6 h post‐dose (i.e. 2 h after the buffet meal) showed appetite abatement in all treatment groups, as expected. At this time point, pooled findings for 25 and 50 mg resembled those for placebo (–25.7 vs. –36.0, P = 0.1507), indicating no exaggeration of appetite decrease among active‐drug recipients, despite their greater intake of food. Results for the other appetite scales were consistent with those for the hunger VAS (Figures 2B and 3B).
Table 2.
Post‐dose caloric intake in Study 101a
| Treatment group | |||||
|---|---|---|---|---|---|
| 10 mgb (n = 6) | 25 mgb (n = 6) | 50 mgb (n = 6) | Pooled 25 and 50 mgb (n = 9) | Placebo (n = 9) | |
| Intake (calories), mean (SD) | 1542.8 (485.2) | 2156.8 (1394.4) | 2057.8 (1155.0) | 1983.1 (1033.7) | 1765.9 (1075.4) |
| Difference from placebo (%), mean (SD) | –0.8 (13.7) | 27.9 (14.7)** | 12.6 (19.2) | 18.4 (17.8)* | — |
SD, standard deviation.
As ingested 4 h post‐dose, when each subject was permitted ad libitum consumption at a buffet lunch.
Administered in capsules.
P < 0.05 vs. placebo, paired t‐test.
P < 0.01 vs. placebo, paired t‐test.
Adverse events reported during the study were mild and transient, with no pattern suggesting a relation to study drug. Four anamorelin‐treated subjects had headache (one of whom also had upper abdominal pain), while one placebo subject reported dizziness. Physical examination and clinical laboratory findings showed no clinically relevant changes.
Discussion
This report of two phase I studies demonstrates the orexigenic activity of anamorelin, which complements the results from the earlier phase I study and larger phase II and III studies that have already been completed.
In the first study reported here (Study 102), anamorelin administered as single oral doses of 1 to 20 mg to healthy young adults elicited modest increases in self‐ratings of hunger. In the second study (Study 101), designed to extend these findings, single doses of 25 and 50 mg achieved more substantial orexigenic effects, manifested not only by subjective increases in hunger but also by objective increases in caloric intake, with statistical significance vs. placebo for the pooled 25 and 50 mg dose levels and for the 25 mg dose. Lack of statistical significance for the 50 mg dose despite a mean caloric intake only 5% lower than for the 25 mg dose may reflect the contribution of data by only six subjects per dose level. Among the hunger ratings obtained in these studies, dose dependence and numerical differences from placebo began to emerge at the earliest assessment time points, 0.5 and 1 h post‐dose (Figure 1). In cancer cachexia, a rapid treatment effect would be highly desirable.
As anamorelin is a ghrelin receptor agonist, weight gain among its recipients might reflect not only increased appetite and caloric intake (as observed in Studies 101 and 102) but also activation of anabolic pathways such as the GH/insulin‐like growth factor‐1 path (as observed in other anamorelin research37, 38), which may promote nutrient incorporation into lean body mass. A prior phase I study investigated the effect of anamorelin on weight gain.30, 37 The study was conducted in three sequential panels of healthy volunteers. In Panel A, eight subjects received anamorelin 25 mg or placebo once daily for 5 days. In Panel B, 15 new subjects participated in a crossover evaluation of anamorelin 50 mg taken once daily and 25 mg taken twice daily, each for a planned 5 days. In another treatment arm, subjects received placebo for all 11 days. In Panel C, nine subjects received anamorelin 75 mg or placebo once daily for 6 days. In all panels, body weight was recorded each morning before breakfast.
Significant weight gain was identified throughout anamorelin treatment, beginning on Day 2 in the 50 mg/day (as once daily 50 mg and twice daily 25 mg doses) and 75 mg/day (once daily) treatment groups. At Day 6, weight gain (standard deviation) was 0.75 (0.043) kg in the twice daily 25 mg group, 1.25 (0.65) kg in the once daily 50 mg group, and 1.16 (0.65) kg in the 75 mg/day group (P ≤ 0.0375), while mean weight change of the once daily 25 mg dose was not significant.30 Crossover subjects took anamorelin totalling 50 mg/day for 11 days. At Day 12, their mean (standard deviation) increase in body weight was 1.54 (0.80) kg compared with 0.32 (0.58) kg for placebo (P = 0.0312, by t‐test of within‐group change). Among these subjects, a divided‐dose regimen conferred no advantage in weight gain. Indeed, mean weight gain was numerically greater during once daily dosing of 50 mg than during twice daily dosing of 25 mg, at 1.34 (0.64) vs. 1.02 (0.93) kg on the last day of treatment, Day 5 or 6 (P = 0.3399), suggesting that for promoting weight gain, peak anamorelin concentration may be more consequential than trough concentration.30, 37
Phase II and III studies of anamorelin administered to cachectic cancer patients have yielded additional documentation of weight gain. In a phase II, randomized crossover trial of anamorelin 50 mg and placebo, each for 3 days of once daily treatment, mean change in total body weight favoured anamorelin, at +0.77 vs. –0.33 kg (P = 0.016).38 On Day 1 of each crossover treatment, mean post‐dose appetite score (on a VAS) and mean caloric intake (at an ad libitum breakfast) were numerically higher for anamorelin than for placebo.38 On Day 3, mean appetite score (on the ASAS questionnaire appetite item) was significantly higher for anamorelin than for placebo (mean increase of 2.67 vs. 0.50, P = 0.011).38 In a pooled analysis of two phase II trials of anamorelin 50 mg vs. placebo taken once daily for 12 weeks, the adjusted mean change in total body weight was +0.48 vs. –1.80 kg (P = 0.0057).31
In a pair of phase III trials of anamorelin 100 mg vs. placebo in cachectic patients with non‐small cell lung cancer, change in total body weight during 12 weeks of treatment also favoured anamorelin, at a mean (averaged for Weeks 6 and 12) of +2.20 vs. +0.14 kg in one trial (P < 0.0001) and +0.95 vs. –0.57 kg in the other (P < 0.0001).32 In both of the phase III trials, mean change in total body weight was statistically significant at 3 weeks (the first observation time point) and was sustained throughout the next 9 weeks. Additionally, a significant and sustained improvement was noted in the FAACT A/CS domain score, a 12‐item anorexia–cachexia symptom burden scale33, 34 that included an appetite item (the patient's agreement, on a 5‐point scale, that ‘I have a good appetite’), along with other anorexia‐related symptom items and concern items pertaining to weight loss and eating. Mean FAACT A/CS domain scores (averaged over the 12 weeks) for anamorelin compared with placebo were 4.12 vs. 1.92 in one trial (P = 0.0004) and 3.48 vs. 1.34 in the other (P = 0.0016).32 Lean body mass (a co‐primary endpoint) also increased during both trials, by a median of +0.99 vs. –0.47 kg in one trial (P < 0.0001) and +0.65 vs. –0.98 kg in the other (P < 0.0001). Handgrip strength (the other co‐primary endpoint) showed no difference between treatment arms. Together, the phase III findings of significant improvement in body weight, body composition, and anorexia/cachexia burden in cachectic cancer patients are suggestive of an increased food intake, which was not feasible to assess accurately across multiple international sites and therefore was not monitored in the phase III trials. These data also imply that nutritional intervention might further enhance anamorelin's benefits,39 although this is still an untested area of potential future research.
The findings obtained in the phase I anamorelin studies reported here are limited by a shorter duration of treatment and lower doses than those administered to cachectic cancer patients in the more recently completed phase III programme. In particular, hunger and caloric intake were measured only after single doses of 1 to 50 mg, while weight gain was measured after multiple dosing of 25 to 75 mg/day but during a treatment interval lasting no longer than 11 days. By contrast, the phase III programme tested a 100 mg/day dose level administered for 12 weeks.32 In these phase I studies, moreover, weight gain was measured as an increase in total body weight, without also assessing increases in lean body mass, which is a key goal in treating the muscle wasting of cancer cachexia.40 Throughout their treatment, however, the subjects in the phase I studies did not show any signs or symptoms of fluid retention, implying that gain in body weight was due to increase in lean and/or fat body mass rather than water.
The phase I anamorelin findings are further limited by the subjects' medical status as healthy young adults. Nevertheless, the findings are consistent with phase II and III findings in cachectic cancer patients and with anamorelin's action as a ghrelin receptor agonist. Although the phase III studies did not identify rescue of muscle function in advanced cancer, improvements in body weight, lean body mass, and anorexia/cachexia symptom burden were demonstrated.32 The present phase I results supplement this by providing additional data concerning treatment effects on appetite and caloric intake, albeit in a young, healthy population. Thus, they expand the overall characterization of the effects of anamorelin in humans and support its development as a potential option for making cancer‐related anorexia and weight loss less devastating for patients.
Conflict of interest
R.A.B. is the Executive Director of Buffalo Clinical Research Center, LLC (Buffalo, NY, USA), which provided clinical research support in the original studies by Rejuvenon (now Helsinn Therapeutics). S.M. is the Medical Director and employee of Quotient Clinical Ltd (Nottingham, UK), which provided clinical research support in the original studies by Rejuvenon (now Helsinn Therapeutics). E.M.D. was an employee of Helsinn Therapeutics (US), Inc.
Ethical conduct
All authors comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.41 Each study was conducted in accordance with the Declaration of Helsinki and its amendments, guidelines of the International Conference on Harmonisation, and the principles of Good Clinical Practice. Each study's protocol, any amendments, and the informed consent form were approved by the relevant independent ethics committee before subject enrolment. All subjects provided written informed consent before undergoing any study procedures.
Supporting information
Table S1. Baseline hunger VAS scores in Studies 102 and 101
Acknowledgements
Support for developing this manuscript was provided by Helsinn Healthcare SA, and copyediting, editorial assistance, and production assistance were provided by The Curry Rockefeller Group, LLC (Tarrytown, NY, USA).
Blum R. A., Mair S., and Duus E. M. (2019) Appetite and food intake results from phase I studies of anamorelin, Journal of Cachexia, Sarcopenia and Muscle, 10: 1027–1035. 10.1002/jcsm.12439.
References
- 1. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 2. Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ 1997;315:1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 4. Sonti G, Ilyin SE, Plata‐Salamán CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol 1996;270:R1394–R1402. [DOI] [PubMed] [Google Scholar]
- 5. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–871. [DOI] [PubMed] [Google Scholar]
- 6. Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer 2003;97:1211–1216. [DOI] [PubMed] [Google Scholar]
- 7. Mantovani G, Madeddu C. Cancer cachexia: medical management. Support Care Cancer 2010;18:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med 1988;148:1586–1591. [PubMed] [Google Scholar]
- 9. LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, et al. Correlation between the international consensus definition of the Cancer Anorexia–Cachexia Syndrome (CACS) and patient‐centered outcomes in advanced non‐small cell lung cancer. J Pain Symptom Manage 2015;49:680–689. [DOI] [PubMed] [Google Scholar]
- 10. Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body‐composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol 1993;11:152–154. [DOI] [PubMed] [Google Scholar]
- 11. Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer‐associated anorexia and weight loss. J Clin Oncol 2005;23:8500–8511. [DOI] [PubMed] [Google Scholar]
- 12. Davis MP. Cannabinoids for symptom management and cancer therapy: the evidence. J Natl Compr Canc Netw 2016;14:915–922. [DOI] [PubMed] [Google Scholar]
- 13. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 14. Bailey AR, von Engelhardt N, Leng G, Smith RG, Dickson SL. Growth hormone secretagogue activation of the arcuate nucleus and brainstem occurs via a non‐noradrenergic pathway. J Neuroendocrinol 2000;12:191–197. [DOI] [PubMed] [Google Scholar]
- 15. Guillory B, Splenser A, Garcia J. The role of ghrelin in anorexia–cachexia syndromes. Vitam Horm 2013;92:61–106. [DOI] [PubMed] [Google Scholar]
- 16. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000;141:4325–4328. [DOI] [PubMed] [Google Scholar]
- 17. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A 2004;101:4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med 2000;133:111 – 122. [DOI] [PubMed] [Google Scholar]
- 19. Adamopoulos S, Parissis JT, Georgiadis M, Karatzas D, Paraskevaidis J, Kroupis C, et al. Growth hormone administration reduces circulating proinflammatory cytokines and soluble Fas/soluble Fas ligand system in patients with chronic heart failure secondary to idiopathic dilated cardiomyopathy. Am Heart J 2002;144:359–364. [DOI] [PubMed] [Google Scholar]
- 20. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714–1719. [DOI] [PubMed] [Google Scholar]
- 21. Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post‐prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001;24:RC19–RC21. [DOI] [PubMed] [Google Scholar]
- 22. Patterson M, Bloom SR, Gardiner JV. Ghrelin and appetite control in humans—potential application in the treatment of obesity. Peptides 2011;32:2290–2294. [DOI] [PubMed] [Google Scholar]
- 23. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992. [DOI] [PubMed] [Google Scholar]
- 24. Hanada T, Toshinai K, Kajimura N, Nara‐Ashizawa N, Tsukada T, Hayashi Y, et al. Anti‐cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun 2003;301:275–279. [DOI] [PubMed] [Google Scholar]
- 25. DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 2007;148:3004–3012. [DOI] [PubMed] [Google Scholar]
- 26. Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol 2004;150:447–455. [DOI] [PubMed] [Google Scholar]
- 27. Ali S, Chen J‐A, Garcia JM. Clinical development of ghrelin axis derived molecules for cancer cachexia treatment. Curr Opin Support Palliat Care 2013;7:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rhodes L, Zollers B, Wofford JA, Heinen E. Capromorelin: a ghrelin receptor agonist and novel therapy for stimulation of appetite in dogs. Vet Med Sci 2017;4:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietra C, Takeda Y, Tazawa‐Ogata N, Minami M, Yuanfeng X, Duus EM, et al. Anamorelin HCl (ONO‐7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia–cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle 2014;5:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia JM, Polvino WJ. Effect on body weight and safety of RC‐1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo‐controlled, multiple‐dose study in healthy volunteers. Oncologist 2007;12:594–600. [DOI] [PubMed] [Google Scholar]
- 31. Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo‐controlled, double‐blind trials. Lancet Oncol 2015;16:108–116. [DOI] [PubMed] [Google Scholar]
- 32. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 33. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 34. LeBlanc TW, Samsa GP, Wolf SP, Locke SC, Cella DF, Abernethy AP. Validation and real‐world assessment of the Functional Assessment of Anorexia–Cachexia Therapy (FAACT) scale in patients with advanced non‐small cell lung cancer and the cancer anorexia–cachexia syndrome (CACS). Support Care Cancer 2015;23:2341–2347. [DOI] [PubMed] [Google Scholar]
- 35. Kumor K, Polvino W. Biological activity of RC‐1291, a novel ghrelin mimetic for cancer anorexia/cachexia: results from a phase I randomized, double‐blind, placebo‐controlled trial in healthy volunteers [abstract]. Support Care Cancer 2006;14: abstr‐03‐009. [Google Scholar]
- 36. Leese PT, Trang JM, Blum RA, de Groot E. An open‐label clinical trial of the effects of age and gender on the pharmacodynamics, pharmacokinetics and safety of the ghrelin receptor agonist anamorelin. Clin Pharmacol Drug Dev 2015;4:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res 2009;19:267–273. [DOI] [PubMed] [Google Scholar]
- 38. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer‐related cachexia: a multicenter, randomized, double‐blind, crossover, pilot study. Support Care Cancer 2013;21:129–137. [DOI] [PubMed] [Google Scholar]
- 39. Muscaritoli M. Targeting cancer cachexia: we're on the way [editorial]. Lancet Oncol 2016;17:414–415. [DOI] [PubMed] [Google Scholar]
- 40. Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pract 2012;27:593–598. [DOI] [PubMed] [Google Scholar]
- 41. van Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline hunger VAS scores in Studies 102 and 101


