Abstract
Objective: Our aim was to test the anxiolytic effect of transcranial photobiomodulation (t-PBM) with near-infrared light (NIR) in subjects suffering from generalized anxiety disorder (GAD).
Background: t-PBM with NIR is an experimental, noninvasive treatment for mood and anxiety disorders. Preliminary evidence indicates a potential anxiolytic effect of transcranial NIR.
Methods: Fifteen subjects suffering from GAD were recruited in an open-label 8-week study. Each participant self-administered t-PBM daily, for 20 min (continuous wave; 830 nm peak wavelength; average irradiance 30 mW/cm2; average fluence 36 J/cm2; total energy delivered per session 2.9 kJ: total output power 2.4 W) broadly on the forehead (total area 80 cm2) with an LED-cluster headband (Cerebral Sciences). Outcome measures were the reduction in total scores of the Hamilton Anxiety Scale (SIGH-A), the Clinical Global Impressions-Severity (CGI-S) subscale and the Pittsburgh Sleep Quality Index (PSQI) subscales from baseline to last observation carried forward.
Results: Of the 15 recruited subjects (mean age 30 ± 14 years; 67% women), 12 (80%) completed the open trial. Results show a significant reduction in the total scores of SIGH-A (from 17.27 ± 4.89 to 8.47 ± 4.87; p < 0.001; Cohen's d effect size = 1.47), in the CGI-S subscale (from 4.53 ± 0.52 to 2.87 ± 0.83; p < 0.001; Cohen's d effect size = 2.04), as well as significant improvements in sleep at the PSQI. t-PBM was well tolerated with no serious adverse events.
Conclusions: Based on our pilot study, t-PBM with NIR is a promising alternative treatment for GAD. Larger, randomized, double-blind, sham-controlled studies are needed.
Keywords: photobiomodulation, generalized anxiety disorder, near-infrared, low-level light therapy, LLLT, NIR, transcranial, neuromodulation
Introduction
Generalized anxiety disorder (GAD) is a chronic psychiatric condition characterized by a pervasive and out-of-proportion preoccupation with adverse life circumstances and with the anticipated occurrence of catastrophic, dramatic events. Oftentimes, everyday life challenges—whether potential or real—are dreaded as they are expected to escalate and to lead to irreparable consequences. Examples of everyday challenges, which are commonly experienced with excessive anxiety, are financial, health-related, professional, and relationship issues. These overproportionate worries generate an overall disturbance of mind/body wellbeing, often manifested by fatigue, tension, irritability, and problems with sleep and concentration. GAD is associated with significant distress and with poor social and professional functioning. In the United States, GAD affects a population of almost 7 million people (3.1% of the population) with a lifetime prevalence of 5.1%.1
The two cornerstones of GAD treatment are psychotherapy and FDA-approved medications. Cognitive behavioral therapy is considered a first-line psychotherapy approach, useful to patients suffering from GAD,2 however, its reach is limited by the availability of trained therapists. First-line pharmacological treatments are selective serotonin reuptake inhibitors antidepressants, such as escitalopram, and serotonin-norepinephrine reuptake inhibitors, including venlafaxine and duloxetine. Regrettably, as many as 50% of patients suffering from GAD do not respond to currently available treatments,3,4 and novel treatment approaches are warranted.
In the present study, we tested a novel treatment approach for anxiety, based on the use of transcranial photobiomodulation (t-PBM) with near-infrared light (NIR). This new technique involves noninvasive exposure of the forehead to NIR. NIR is a nonionizing electromagnetic radiation, it is invisible, it penetrates through the skin and skull into brain tissue,5 it is only minimally dissipated as thermal energy, and it is mainly absorbed by specific cellular chromophores.6 The benefits of t-PBM are wavelength specific: a mitochondrial enzyme, cytochrome c oxidase, is the primary chromophore for the t-PBM with peak absorption close to 830 nm.7 The energy absorbed by the cytochrome c oxidase leads to increased adenosine triphosphate (ATP) production, through enhanced activity of the respiratory chain. Ultimately, the increased ATP leads to increased energy metabolism in the cells, and it is hypothesized that a signaling cascade is also activated promoting cellular plasticity and neuroprotection.7 An alternative explanation of the metabolic, cellular effects of PBM has recently been proposed. It is hypothesized that the upregulation of ATP synthesis, seen after PBM, is caused by a decrease in the viscosity and density of a nanoscopic layer of H2O molecules surrounding the cells. These physical changes, affecting cellular mechanics, would allow an increased metabolic uptake by cells, as well as higher activity of the ATP synthase.8
Pilot studies in individuals with major depressive disorder (MDD) have shown that t-PBM is safe, effective, and well tolerated.9–12 In addition to depressive symptoms, anxiety symptoms appeared to respond to t-PBM. Schiffer et al.9 used t-PBM to treat nine depressed subjects with comorbid anxiety. The study showed a significant improvement in anxiety symptom severity after one session of t-PBM.9 t-PBM has also shown preliminary benefits in reducing posttraumatic stress symptoms.13 Further, one case report suggested long-term benefits of t-PBM for the treatment of anxiety.14
The present study was conducted to test the efficacy, safety, and tolerability of repeated t-PBM sessions in a group of anxious subjects with primary GAD. The study also tested the feasibility of using a take-home t-PBM device, in contrast to the more time-consuming and expensive in-office treatment delivery.
Materials and Methods
This single-site study: Transcranial Continuous Near-Infrared Light in Generalized Anxiety Disorder—Lighten GAD was approved by the Massachusetts General Hospital (MGH) Institutional Review Board (IRB). Recruitment began in September 2018 and was completed in April 2019. The main source of recruitment were people contacting the general research line of the MGH Center for Anxiety and Traumatic Stress Disorders. Cerebral Sciences LLC (located in Boca Raton, FL) created the specific study device, and sponsored this investigator-initiated pilot.
Inclusion and exclusion criteria
Adult subjects 18–70 years of age with a primary diagnosis of GAD with symptoms of at least moderate severity (Clinical Global Impression—Severity Scale, CGI-S ≥ 4), were allowed in the study after providing written informed consent. Subjects could not have used benzodiazepines or antidepressant medications within 6 weeks before the screening visit; structured psychotherapy was permitted only if started at least 8 weeks before the screening visit. Other exclusionary criteria included active suicidal or homicidal ideation, current bipolar affective disorder, active substance dependence or abuse (prior 3 months), lifetime psychotic episodes, cognitive impairment [Montreal Cognitive Assessment (MOCA) Score <21], unstable medical illness, and recent stroke (prior 3 months). Women of child-bearing potential were required to use a birth-control method if sexually active; pregnancy and lactation were exclusionary. To allow maximum light penetration and to minimize potential risks of local tissue damage from the use of NIR, the following conditions were also exclusionary: having a forehead skin condition likely to impede light penetration, such as tattoo or birth mark; taking a light-activated medication (prior 14 days); having a head implant (to prevent possible dislodgement associated with vasodilation, e.g., with endovascular stents). All subjects met all inclusion criteria and none of the exclusion criteria, except one subject with mild alcohol use disorder, whom the principal investigator (P.C.) and the IRB deemed likely to benefit from study participation and unlikely to confound study findings.
Study design and treatment
Eligible subjects participated in an 8-week pilot, open-label, treatment trial. Each subject self-administered t-PBM once daily at home, for 8 weeks. The Cerebral Science headband device emitted NIR with an output at 830 ± 15 nm, broadly across the forehead (EEG sites: FP1, FP2, F7, F8, Fpz), covering a total surface of 80 cm2, with an average irradiance of 30 mW/cm2 (total output power 2.4 W) and an average fluence of 27 or 36 J/cm2 over 15 or 20 min, respectively. Each session resulted in a total energy delivered up to 2.9 kJ, which was within the range tested in a previous study on t-PBM for depression.11 Each study subject received the first t-PBM session in office, after the baseline visit, for training purposes and to assess tolerability. t-PBM sessions were kept at 15 min during the first week and increased to 20 min thereafter. The study clinician had the option to keep the duration of light exposure at 15 min or to decrease it to 10 min during the trial, based on tolerability. Table 1 contains information on the device parameters.
Table 1.
Treatment Parameters for Transcranial Photobiomodulation (Continuous Wave)
| Peak wavelength | 830 nm |
| Irradiance | 30 mW/cm2 |
| Fluence | 36 J/cm2 |
| Duration of t-PBM session | 20 min |
| Treatment window | 80 cm2 |
| Total output | 2.4 W |
| Cumulative dose | 2.9 kJ |
t-PBM, transcranial photobiomodulation.
Assessments
After baseline assessment, subjects came back to the clinic for in-person midpoint and endpoint assessments (weeks 5 and 9, respectively), and they completed weekly check-ins over the phone with study clinicians during all other study weeks. During all check-ins, the study clinician logged adverse events and administered the CGI-S (see Primary and Secondary Clinical Outcome Measures section). During all check-ins, except weeks 5 and 7, the study clinician administered the Structured Interview Guide for the Hamilton Anxiety Scale (SIGH-A). During baseline, week 4, and week 8, the research assistant recorded the patient's vital signs; and during the same visits the clinician administered additional questionnaires exploring secondary outcomes, such as comorbid depression and suicidality, while the participant was asked to complete self-rated assessments on anxiety and other related psychological dimension, including sleep.
Primary and secondary clinical outcome measures
The primary outcome measure was the total score of the SIGH-A15 for anxiety symptoms. This clinician-rated scale uses a structured interview, specific instructions, and defined anchor points. Answers to the questions are rated on a scale of 0–4, with higher scores indicating more severe pathology. Scores fall into the following categories: mild severity (<17), mild-to-moderate severity (18–24), and moderate to severe (25–30). The Clinical Global Impressions-Severity (CGI-S) and -Improvement (CGI-I) subscales16 were also used as a secondary measure. Tolerability was assessed with an Adverse Event Form, which allowed the recording of an adverse event's description, start and end dates, intensity (severity) and seriousness, relation to the treatment, as well as any action taken and outcome. All characteristics for each adverse event (including severity) were rated by experienced, licensed, trialist physicians based on subject's report. Severity ratings were based on subject's distress, on compromised functioning, and need for action or remedies to stop the adverse event (i.e., took medication for headache or stopped using the t-PBM device). The trialist physicians also evaluated the seriousness of each adverse event based on conventional indicators of risk, defined by the IRB (i.e., hospitalization). Sleep was assessed with a self-rated measure, the Pittsburgh Sleep Quality Assessment (PSQI).17
Statistical methods
We conducted paired samples t-tests to compare baseline and endpoint scores (completers) for the SIGH-A, CGI-S, and for each of the available components of the PSQI. We repeated these analyses to include participants who dropped out of the study before the end of their course of t-PBM sessions, with a last observation carried forward (LOCF) approach. We used the Cohen's d formula for paired data to estimate effect sizes. We reported rates of responders according to the SIGH-A scores (at least 50% decrease of SIGH-A scores from baseline to endpoint or last observation) and according to the CGI-I (CGI-I equal to 1 “very much improved” or 2 “much improved” at endpoint or last observation). No corrections for multiple comparisons were performed since we identified a primary outcome measure (SIGH-A) and in line with the exploratory nature of this pilot study.
Results
Baseline, demographic information, treatment, and follow-up
We screened 17 participants (12 female) for this study. All 17 were determined eligible (of note, 1 subject met criteria for mild alcohol use disorder and the IRB approved a protocol exception), 2 dropped out of the study before receiving their t-PBM device. One of these two dropped because of time commitment and the other was lost to follow-up. Of the remaining participants, three (two female) did not complete their course of t-PBM sessions. One of these subjects dropped because of side effects, another because of time commitment, and the third did not specify the reason. Table 2 contains demographic information and baseline and endpoint scores for all participants who began treatment, as well as their t-PBM exposure and follow-up.
Table 2.
Demographic Information, Concomitant Treatment, Baseline Scores, and Endpoint Scores
| Subjects | Age | Gender | Race/ethnicity | Current diagnosis | Concomitant anxiety treatment | Weeks of treatment | Minutes per session | Average no of sessions per week | Baseline SIGH-A | Last visit SIGH-A | Baseline CGI-S | Last visit CGI-S | Last visit CGI-I | Adverse events (day of onset) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 31 | M | White/Hispanic | GAD, SAD | N/A | 8 | Switched to 20 min after 2 weeks | 6.3 | 10 | 5 | 4 | 2 | 3 | Day 26–27: headaches |
| #2 | 27 | F | White/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 2 weeks | 6.9 | 18 | 4 | 4 | 2 | 2 | N/A |
| #3 | 26 | F | Asian/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 1 week | 6 | 12 | 6 | 4 | 2 | 3 | N/A |
| #4 | 19 | F | White/non-Hispanic | GAD, AGO | Psychotherapy | 8 | Switched to 20 min after 5 weeks | 6.1 | 14 | 7 | 5 | 3 | 3 | Day 11–40: bad dreams |
| Day 12–24: memory and concentration issues | ||||||||||||||
| #5 | 64 | F | White/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 4 weeks | 7 | 28 | 7 | 5 | 2 | 1 | Day 2–4, 63–66: Insomnia |
| Day 2–4: Vivid dreams | ||||||||||||||
| Day 10: visual illusions | ||||||||||||||
| Day 41–54: increased appetite | ||||||||||||||
| #6 | 39 | F | Asian/non-Hispanic | GAD, SAD | N/A | 4 | Switched to 20 min after 1 week | 6.7 | 21 | 12 | 5 | 4 | 2 | Day 19–a: vivid dreams |
| #7 | 22 | F | White/non-Hispanic | GAD | Psychotherapy | 8 | Switched to 20 min after 1 week | 6 | 15 | 5 | 4 | 3 | 3 | N/A |
| #8 | 24 | M | Asian/non-Hispanic | GAD, AUDb | N/A | 2 | 15 min | 6 | 22 | 17 | 5 | 4 | 3 | Day 5–11, 16–21: headaches |
| #9 | 28 | M | White/non-Hispanic | GAD, PD | N/A | 8 | Switched to 20 min after 1 week | 7 | 21 | 14 | 5 | 3 | 3 | N/A |
| #10 | 25 | F | Asian/non-Hispanic | GAD | Psychotherapy | 1 | 15 min | 6 | 15 | 16 | 4 | 4 | 4 | N/A |
| #11 | 22 | F | Asian/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 1 week | 7 | 20 | 2 | 5 | 2 | 2 | N/A |
| #12 | 58 | M | White/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 1 week | 6.6 | 11 | 7 | 5 | 3 | 3 | Day 9–17: detailed dreams |
| Day 36–43: headaches | ||||||||||||||
| #13 | 18 | F | White/Hispanic | GAD | N/A | 8 | Switched to 20 min after 1 week | 6.4 | 21 | 8 | 4 | 3 | 2 | Day 21–36: worsening of insomnia |
| #14 | 27 | M | White/non-Hispanic | GAD | N/A | 8 | Switched to 20 min after 1 week | 7 | 15 | 3 | 4 | 3 | 3 | Day 1–3: hypersomnia |
| Day 1–9: loose stools | ||||||||||||||
| Day 37: headaches | ||||||||||||||
| #15 | 21 | F | White/non-Hispanic | GAD | Psychotherapy | 8 | Switched to 20 min after 1 week | 6.8 | 16 | 14 | 5 | 4 | 4 | Day 17–29: vivid dreams |
Indicates that participant did not report end date to side effects.
This subject was included after consultation with our Institutional Review Board, which provided a protocol exception based on mild symptomatology despite the diagnosis of alcohol use disorder.
AGO, agoraphobia; AUD, alcohol use disorder; CGI, Clinical Global Impressions Severity or Improvement; F, female; GAD, Generalized Anxiety Disorder; M, male; PD, panic disorder; SAD, Social Anxiety Disorder; SIGH-A, Structured Interview Guide for the Hamilton Anxiety Scale.
The final sample consisted of n = 15 participants (10 female) who received t-PBM. The mean age of the sample was M = 30 years (standard deviation [SD] = 13.61). Most of the participants (53.33%) identified as white and non-Hispanic, whereas 33.33% of the sample identified as Asian, and 13.33% identified as white and Hispanic. The mean age of onset was M = 21.58 years (SD = 13.34). The mean baseline SIGH-A score for the sample was M = 17.27, and the mean baseline CGI-S score was M = 4.53.
Effect on anxiety
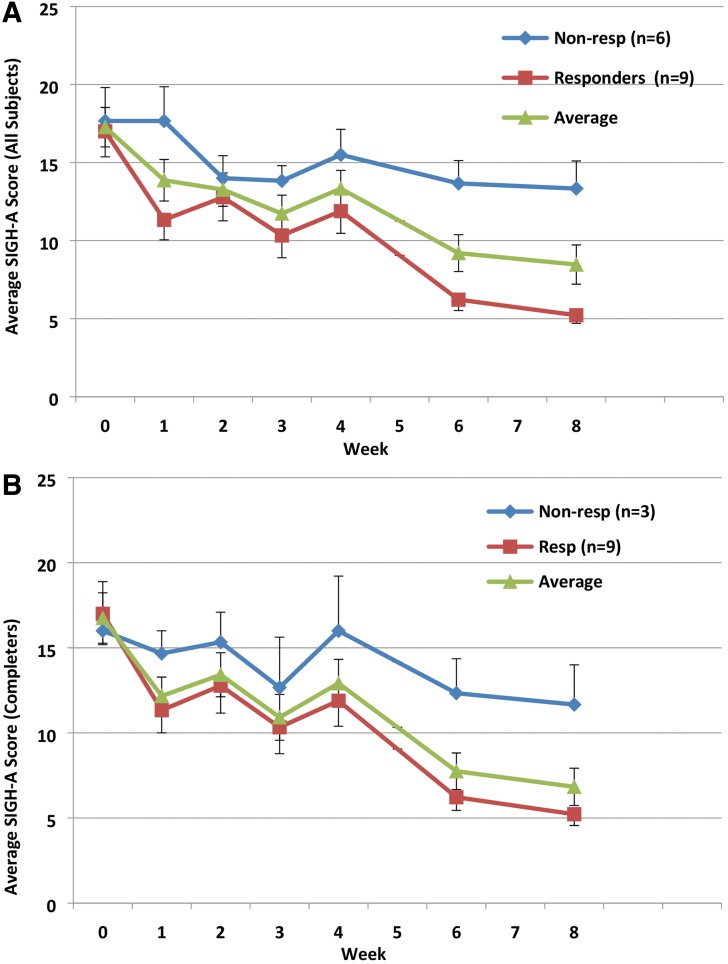
In completers (n = 12), there was a significant difference in SIGH-A scores at baseline (M = 16.75, SD = 5.14) and endpoint (M = 6.83, SD = 3.79); t(11) = 5.90, p < 0.001, Cohen's d = 1.68. There was also a significant difference in the CGI-S scores at baseline (M = 4.5, SD = 0.52) and endpoint (M = 2.58, SD = 0.67); t(11) = 9.91, p < 0.001, Cohen's d effect size = 3.51. These results suggest that participants had lower SIGH-A and CGI-S scores, and thus lower levels of anxiety, after completing their course of t-PBM sessions. Among completers, 75% and 33% were responders as per the SIGH-A and as per the CGI-I, respectively. Figure 1 displays the average, weekly SIGH-A scores, for all completers as well as for responders and nonresponders according to the SIGH-A.
FIG. 1.
Average, weekly SIGH-A scores, for all samples (A) and for completers (B), as well as for responders and nonresponders, according to the SIGH-A. SIGH-A, Structured Interview Guide for the Hamilton Anxiety Scale.
We repeated these analyses to also include participants who did not complete the study (LOCF, n = 15). Their scores at their last assessment were used as their endpoint scores. SIGH-A scores still significantly differed between baseline (M = 17.27, SD = 4.89) and endpoint (M = 8.47, SD = 4.87); t(14) = 5.71, p < 0.001, Cohen's d effect size = 1.47. CGI-S scores also significantly differed between baseline (M = 4.53, SD = 0.52) and endpoint (M = 2.87, SD = 0.83); t(14) = 7.91, p < 0.001, Cohen's d effect size = 2.04. In the evaluation carried-forward sample, 60% and 33% were responders as per the SIGH-A and as per the CGI-I, respectively. Figure 1 displays the average, weekly SIGH-A scores, for the evaluation carried forward—as well as for responders and nonresponders according to the SIGH-A.
Effect on sleep
One participant did not complete the final PSQI assessment despite completing the clinician-rated forms, so there was one less completer for the sleep analysis. In completers (n = 11), their sleep latency at the PSQI decreased significantly from baseline (M = 1.47, SD = 0.99) to endpoint (M = 0.82, SD = 0.75); t(10) = 2.76, p < 0.02, suggesting a shorter lag to fall asleep after 8 weeks of t-PBM. Since self-report forms were administered infrequently, the whole sample (n = 15) did not yield additional data points for the PSQI.
Side effects
Overall, the treatment was well tolerated. The side effects incurred by each participant—excluding the ones deemed unrelated to t-PBM—are presented in Table 2. The most common side effects were headaches, experienced by four subjects, and vivid, detailed, or bad dreams, experienced by five subjects. Other participants experienced memory and concentration issues (n = 1), insomnia (n = 2), visual illusions (n = 1), hypersomnia (n = 1), loose stools (n = 1), and increased appetite (n = 1). All adverse events were rated as mild to moderate by the trialist physicians—except for one case of severe headache, which prevented dose increase and led to t-PBM discontinuation—; none of the adverse events was deemed serious based on risk assessment.
Discussion
In our pilot trial, t-PBM NIR for the treatment of GAD appeared effective and well tolerated, with no serious adverse events. Most noticeably, participants who completed the 8-week trial of t-PBM showed the most pronounced reduction in anxiety symptoms.
These results are consistent with the previous studies that found an anxiolytic effect combined with the antidepressant effect of t-PBM. Schiffer et al.9 treated nine MDD subjects with comorbid anxiety and traumatic stress disorders (mean [SD] HAM-A total score 23.0 [12.2]) with a single session of t-PBM. Their study showed a significant improvement in anxiety at weeks 2 and 4 (delta HAM-A total score were 14.9 and 9, respectively). We demonstrated that similar anxiolytic effects could be attained, and maintained up to week 8, with repeated t-PBM sessions in a well-characterized sample of primary GAD patients. Since, in our sample, no subject experienced concurrent MDD, the effects on anxiety could not be ascribed to an underlying antidepressant effect. While the exact mechanisms for the anxiolytic effects of t-PBM in humans are still under investigation, a preclinical study in mice showed that t-PBM increased serotonin levels and decreased nitric oxide in both the prefrontal cortex and the hippocampus.18 The same authors showed that t-PBM also decreased cortisol levels in the serum.
Further, our results indicate that t-PBM might improve sleep in GAD patients, in terms of decreased latency. Our findings on sleep are in line with previous findings of improved sleep in patients treated with t-PBM for dementia19 and traumatic brain injury.13,20
Last but not least, our study suggests that t-PBM, delivered by an LED-cluster device, is feasible as in-home self-administration. In fact, a single in-office session was sufficient to demonstrate the method of use for the device and none of our study participants reported any difficulties with self-administration.
Our study has several limitations: (1) The lack of a placebo control group prevents an accurate estimate of the effect size of t-PBM for the reduction of anxiety symptoms. (2) The generalizability of our findings is likely to be affected by our small sample size. Yet, by selecting as an entry diagnosis a single anxiety disorder, the specificity of our findings is greatly improved as compared with prior studies reporting similar effects of t-PBM in multiple anxiety disorders, often with MDD as a comorbidity. (3) The lack of a long follow-up prevents any inference being made concerning both long-term efficacy and tolerability; however, a case report suggests that long-term use of t-PBM for anxiety might be a viable option.14 (4) Biomarkers of anxiety or fear were not included, thus preventing any correlation of clinical findings with underlying biological processes.
Conclusions and Summary
Overall, this pilot study suggests that t-PBM, has the potential to be an effective, novel treatment for GAD. Our subjects experienced a significant decrease in levels of anxiety, with relatively few, mostly mild, and transient side effects and no serious adverse events. Our encouraging findings deserve further testing in well-powered, double blind, sham-controlled trials. Safety, tolerability, and acceptability of t-PBM cannot be ascertained in the absence of rigorous trials and long-term follow-up studies.
If more rigorous studies confirmed our findings, t-PBM could be an attractive alternative for patients who decline medications and who cannot commit to weekly psychotherapy sessions. t-PBM could also be an alternative anxiolytic treatment for patients who did not respond to medications or to psychotherapy.
Acknowledgments
M.R.H. was supported by US NIH Grants R01AI050875 and R21AI121700.
Author Disclosure Statement
M.M., O.L., E.B., V.S., and L.M. have no competing financial interests. E.B. receives royalties from Springer for a textbook. M.R.H. is on the following Scientific Advisory Boards: Transdermal Cap, Inc., Cleveland, OH; BeWell Global, Inc., Wan Chai, Hong Kong; Hologenix, Inc., Santa Monica, CA; LumiThera, Inc., Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon, Inc., Bee Cave, TX; Medical Coherence, Boston, MA; NeuroThera, Newark, DE; JOOVV, Inc., Minneapolis-St. Paul, MN; AIRx Medical, Pleasanton, CA; FIR Industries, Inc., Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV, Inc., Lansing, MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp., Incline Village, NV; and Niraxx Light Therapeutics, Inc., Boston, MA. Dr Hamblin has been a consultant for Lexington Int, Boca Raton, FL; USHIO Corp., Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland; B.V. Johnson & Johnson, Inc., Philadelphia, PA; and Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Dr Hamblin is a stockholder in Global Photon, Inc., Bee Cave, TX; Mitonix, Newark, DE. Dr. Cassano has received consultation fees from Janssen Research and Development and from Niraxx Light Therapeutics, Inc. Dr. Cassano has received unrestricted funding from Photothera, Inc. and then from Litecure, Inc. to conduct studies on t-PBM for the treatment of major depressive disorder and a study on healthy subjects. He has also received funding from Cerebral Sciences to conduct a study on t-PBM for GAD. Dr. Cassano cofounded a company (Niraxx Light Therapeutics, Inc.) focused on the development of new modalities of treatment based on NIR. Dr. Cassano has filed several patents related to the use of NIR in psychiatry.
References
- 1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51:8–19 [DOI] [PubMed] [Google Scholar]
- 2. Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. J Clin Psychiatry 2001;62 Suppl 11:37–42; discussion 43–35 [PubMed] [Google Scholar]
- 3. Yonkers KA, Warshaw MG, Massion AO, Keller MB. Phenomenology and course of generalised anxiety disorder. Br J Psychiatry 1996;168:308–313 [DOI] [PubMed] [Google Scholar]
- 4. Hunot V, Churchill R, Silva de Lima M, Teixeira V. Psychological therapies for generalised anxiety disorder. Cochrane Database Syst Rev 2007:CD001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Q, Ma H, Nioka S, Chance B. Study of near infrared technology for intracranial hematoma detection. J Biomed Opt 2000;5:206–213 [DOI] [PubMed] [Google Scholar]
- 6. Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 2002;323:207–210 [DOI] [PubMed] [Google Scholar]
- 7. Eells JT, Henry MM, Summerfelt P, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A 2003;100:3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sommer AP. Revisiting the photon/cell interaction mechanism in low-level light therapy. Photobiomod Photomed Laser Surg 2019; 37:336–341 [DOI] [PubMed] [Google Scholar]
- 9. Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cassano P, Cusin C, Mischoulon D, et al. Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatry J 2015;2015:352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassano P, Petrie SR, Mischoulon D, et al. Transcranial photobiomodulation for the treatment of major depressive disorder. The ELATED-2 Pilot Trial. Photomed Laser Surg 2018. DOI: 10.1089/pho.2018.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caldieraro MA, Cassano P. Transcranial and systemic photobiomodulation for major depressive disorder: a systematic review of efficacy, tolerability and biological mechanisms. J Affect Disord 2019;243:262–273 [DOI] [PubMed] [Google Scholar]
- 13. Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma 2014;31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caldieraro MA, Sani G, Bui E, Cassano P. Long-term near-infrared photobiomodulation for anxious depression complicated by Takotsubo Cardiomyopathy. J Clin Psychopharmacol 2018;38:268–270 [DOI] [PubMed] [Google Scholar]
- 15. Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety 2001;13:166–178 [PubMed] [Google Scholar]
- 16. Guy W, National Institute of Mental Health, Psychopharmacology Research Branch, Early Clinical Drug Evaluation Program. ECDEU assessment manual for psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976 [Google Scholar]
- 17. Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 18. Eshaghi E, Sadigh-Eteghad S, Mohaddes G, Rasta SH. Transcranial photobiomodulation prevents anxiety and depression via changing serotonin and nitric oxide levels in brain of depression model mice: a study of three different doses of 810 nm laser. Lasers Surg Med 2019. DOI: 10.1002/lsm.23082 [DOI] [PubMed] [Google Scholar]
- 19. Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg 2017;35:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat 2015;11:2159–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]