Abstract
Favorable long-term functional outcomes after severe traumatic brain injury (TBI) may be underestimated. We analyzed 24-month functional outcomes from a consecutive series of severe TBI survivors. A prospective, observational database of severe TBI survivors from a single institution was analyzed. Glasgow Outcome Scale-Extended (GOS-E) scores were obtained at 3, 6, 12, and 24 months post-injury. GOS-E scores were dichotomized into unfavorable and favorable outcomes, and the proportion of survivors changing from unfavorable to favorable outcomes was calculated using Wilcoxon signed-rank tests. Surviving adults (N = 304; mean age ± standard deviation = 35.06 ± 15.11; 80.92% male; mode of initial GCS = 7) were analyzed. A statistically significant mean increase in GOS-E was noted from 3 to 6, 6 to 12, 12 to 24, and 6 to 24 months after injury (0.65 [p < 0.0001], 0.42 [p < 0.0001], 0.23 [p = 0.020], and 0.61 [p < 0.0001], respectively). Moreover, 43% of survivors from 3 to 6 months, 36% from 6 to 12 months, 38% from 12 to 24 months, and 54% from 6 to 24 months progressed from an unfavorable to a favorable outcome. Two thirds of survivors in the unfavorable category at 3 months had favorable outcomes at 2 years. Overall, 74% of surviving adults with a documented GOS-E at 2 years after injury had a favorable outcome. Severe TBI survivors demonstrated significant improvement in functional outcomes from 3 to 24 months after injury. At 2 years, three fourths of survivors had a favorable outcome. Long-term prognosis in severe TBI is better than broadly appreciated.
Keywords: Glasgow Outcome Scale-Extended, long-term functional status, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major global public health problem with at least 200 per 100,000 people per year sustaining such an injury.1 It is estimated that 5.3 million Americans are living with TBI-related disabilities.2 Because of the high variability in the mechanism of TBI, individual patient factors (e.g., age at injury), and the complexity of physical, cognitive, and emotional sequelae that can affect outcomes, it is challenging for providers to predict accurate prognoses and time course of recovery. Such uncertainty impacts decision making in the acute care setting after severe TBI.
Long-term outcomes after severe TBI are underestimated and underappreciated by many healthcare providers. Further, prognostication of TBI outcomes is notoriously inaccurate, especially when predictions are made within 24 h after injury and based on Glasgow Coma Scale (GCS) score or computed tomography (CT) scan findings.3–6 When predictions are made, neurosurgeons overestimate long-term poor outcomes by 50% and underestimate long-term good outcomes by 50%.3 As such, family discussions regarding treatment decisions are often based on insufficient information.
TBI is a heterogeneous disorder, and no two TBIs exactly alike.3,5 There is also individual variability in the degree of cognitive, motor, and psychological recovery and the timeline of recovery after severe TBI. Previous studies have frequently followed severe TBI survivors for only 6 months.2 As a consequence, significant functional improvement occurring beyond that arbitrary time point has been missed.2 As a result, the common assumption that TBI survivors plateau at 6 months has driven the use of the 6-month Glasgow Outcome Scale-Extended (GOS-E) as the primary endpoint in TBI clinical trials.7 However, as demonstrated by several studies, severe TBI survivors continue to show improvement well beyond 6 months after injury.8–13 Corral and colleagues demonstrated a significant improvement in their severe TBI population outcomes from 6 months to 1 year. In addition, a more recent study demonstrated a steady continued progression in outcome up to 5 years in their severe TBI population.8
In the current study, we analyzed a consecutive series of severe TBI survivors in a prospective, observational database to review longer-term outcomes (up to 2 years after injury) and assess the frequency with which patients convert to more favorable GOS-E outcome categories. We aim to reinforce past research conclusions that indicate that severe TBI survivors have more favorable long-term outcomes than previously appreciated. In addition, we have expanded our analyses to include a statistical comparison of demographic characteristics between TBI survivors and those who died.
Methods
Participants
Between 2003 and 2017, a consecutive series of severe TBI patients were enrolled into the Brain Trauma Research Center (BTRC) database, an institutional review board– (IRB)-approved prospective, longitudinal cohort study. Five hundred fifty-nine severe TBI patients were enrolled during the study period, and neurological outcome assessments were performed at 3, 6, 12, and 24 months post-injury on the surviving adults. To be included in the study, patients must be 16–80 years old with a post-resuscitation GCS ≤8 and not following commands (GCS motor score ≤5). Patients were excluded if they had a GCS of 3 and bilateral fixed pupils, neurological deterioration more than 24 h after admission, or imminent brain death. Because this study focuses on long-term outcome trajectory, we choose to exclude those patients who did not survive the acute hospital setting.
Measures
The database included clinical data collection and functional outcome (as measured by GOS-E) at 3, 6, 12, and 24 months. GOS-E is widely used for assessing global long-term outcome after a TBI and was administered by structured in-person or telephone interview by trained, qualified neuropsychology technicians. It consists of eight outcome categories: upper and lower good recovery, upper and lower moderate disability, upper and lower severe disability, vegetative state, and death.7,14–16 Need of assistance, work restrictions, and social limitations differentiate between upper and lower scores. Upper good recovery included survivors who resumed normal life with the capacity to work with or without minor neurological or psychological deficits.2 If these deficits are disabling, the survivor would fall within the lower good recovery category.2 Upper moderate disability described survivors who are independent at home, but dependent outside of home.2 These survivors have some disability, such as aphasia, hemiparesis, epilepsy, and/or memory or personality deficits.2 If they are not able to return to work, the rating is lower moderate disability.2 Upper severe disability included survivors who were dependent for daily support for mental and/or physical disabilities.2 If they could not be left alone at home for more than 8 h, they are rated as having lower severe disability.2
Outcome scores were dichotomized as favorable outcome (upper severe disability or better [GOS-E score of 4–8]) or unfavorable outcome (lower severe disability or worse [GOS-E score of ≤3]), based on the dichotomization utilized in the RESCUE-ICP trial and other trials.17–19 The favorable outcome category included survivors who were independent in the home, but relied on others for assistance outside the home. As with previous trials, the dichotomization was chosen with the understanding that survival with considerable disability instead of death may be acceptable to some survivors and caregivers and not acceptable to others.19
Procedures
Trained research assistants collected information regarding injury severity (initial GCS) and medical course from hospital and emergency medical service records. All initial GCS scores were recorded upon admission by a trained neurosurgeon post-resuscitation and off paralytics and/or sedative medications. A verbal subscale score of 1 was assigned to subjects who were intubated. Demographic information, such as date of birth, education, and pre-morbid functioning, was collected in interviews with the subjects or family/significant others. Subjects and their families were contacted at 3, 6, 12, and 24 months post-injury to assess the subjects' level of functioning, including completion of GOS-E. If the patient was not able to provide accurate information, data were collected from family members or care providers who were familiar with the subject.
Statistical analysis
The distributions of the data were checked for normality. Wilcoxon signed-rank tests were used to assess whether the observed changes in GOS-E scores between time points was significantly different from no change in GOS-E scores. Descriptive statistics were used to quantify the nature of the change. The proportion of survivors who exhibited an increase in GOS-E score was calculated. Moreover, the proportion of survivors changing from unfavorable to favorable outcomes between each time point was calculated. An additional descriptive calculation was used to assess the mean initial GCS between the unfavorable and favorable outcome groups. A comparison of demographic characteristics was made between survivors with favorable outcomes and unfavorable outcomes at 2 years. Demographic characteristics of those deceased at 3 months were compared to survivors. Also, patients who were deceased at 24 months were compared with those who were alive at 24 months and patients deceased at 3 months. Last, among those with GOS-E at three or more time points, a within-subjects analysis using a generalized estimating equation (GEE) model was applied to evaluate the change in GOS-E across time points.
Results
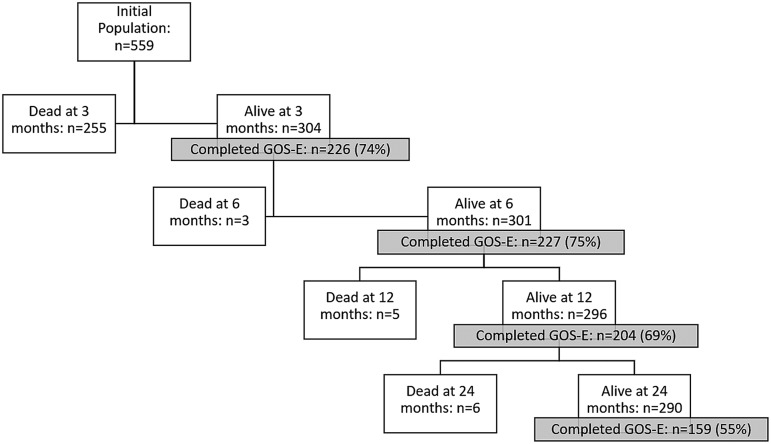
Study enrollment included 559 severe TBI patients; of these, 255 (46%) died within 90 days and 14 more died between 3 and 24 months after injury (Fig. 2). Patients who died before their 3-month follow-up were excluded from analysis.
FIG. 2.
Patient population over time. The flow diagram follows the patient population from the initial number of patients enrolled through 24 months showing how many were dead and alive at each time point. Of the surviving patients at each time point, the number and percent of patients who completed GOS-E is shown. GOS-E, Glasgow Outcome Scale-Extended.
As seen in Table 1, patients who died before 3 months (n = 255) significantly differed in many characteristics than those that survived (n = 304). Those who died early were older (mean age = 49.22 ± 17.19), had lower GCS scores on admission (42% had an initial GCS score of 3), and 44% suffered from falls compared to survivors (all p < 0.0001). TBI survivors' mean age was 35.06 ± 15.11, and 44% had an initial GCS score of 7. Patients who were deceased at 24 months (n = 14) were similar to survivors, in that 43% had an initial GCS score of 7 and most suffered from traffic accidents (43%; p < 0.0001). Demographic and clinical characteristics of study participants with GOS-E data at 24 months are listed in Table 2. On logistic regression, there were no significant differences between the unfavorable and favorable outcome groups at 2 years. Survivors at 2 years were younger and less likely to have been injured in a fall, compared to those patients who died by 3 months (p < 0.0001). Survivors demonstrated significant improvements in mean GOS-E scores between each time point. From 3 to 6 months, on average, survivors demonstrated a statistically significant 0.65-point increase in mean GOS-E score (p < 0.0001).
Table 1.
Demographics of All Enrolled Participants
| Survivors | Patients deceased at 3 months | Patients deceased at 24 months | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | |
| Agea | 35.06 ± 15.11 | 304 | 49.22 ± 17.19 | 255 | 47.71 ± 17.84 | 14 |
| % | % | % | ||||
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 91.42 | 277 | 92.55 | 236 | 93.86 | 13 |
| Non-white | 8.58 | 26 | 7.45 | 19 | 7.14 | 1 |
| Sex | ||||||
| Male | 80.92 | 246 | 76.86 | 196 | 85.71 | 12 |
| Female | 19.08 | 58 | 23.14 | 59 | 14.29 | 2 |
| GCS at admissiona | ||||||
| 3 | 12.83 | 39 | 41.96 | 107 | 14.29 | 2 |
| 4 | 6.58 | 20 | 13.33 | 34 | 14.29 | 2 |
| 5 | 6.91 | 21 | 12.55 | 32 | 7.14 | 1 |
| 6 | 20.39 | 62 | 11.37 | 29 | 21.43 | 3 |
| 7 | 43.75 | 133 | 16.47 | 42 | 42.86 | 6 |
| 8 | 9.54 | 29 | 4.31 | 11 | 0 | 0 |
| Mechanism of injury | ||||||
| Traffic accident | 62.82 | 191 | 38.04 | 97 | 42.86 | 6 |
| Fall | 14.14 | 43 | 43.53 | 111 | 21.43 | 3 |
| Off-road vehicle accident | 06.25 | 19 | 2.75 | 7 | 7.14 | 1 |
| Pedestrian struck | 06.25 | 19 | 7.06 | 18 | 14.29 | 2 |
| Other | 04.28 | 13 | 4.31 | 11 | 7.14 | 1 |
| Assault | 03.95 | 12 | 2.35 | 6 | 7.14 | 1 |
| Bicycle | 02.30 | 7 | 1.96 | 5 | 0 | 0 |
Among all survivors between 3 and 24 months, mean age was 35 years with 81% being male and 91% white. Mechanism of injury is listed showing that 63% of severe TBIs were attributed to traffic accidents. The most common initial Glasgow Coma Scale (GCS) score on admission was 7. In addition, descriptors for the patients who died before 3 months are shown. Mean age for this population was 49 years with 77% being male and 93% white. The most common mechanism of injury among these patients was falls (44%). Forty-two percent of patients in this population had an initial GCS of 3 on admission.
Survivors were, on average, younger (p < 0.0001). Patients who died in the first 3 months had a higher rate of GCS 3.
SD, standard deviation.
Table 2.
Demographic Characteristics among Those with GOS-E Data at 24 Months
| Unfavorable (GOS-E 1–3) | Favorable (GOS-E ≥4) | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age | 38.35 ± 16.94 | 33.90 ± 14.54 | ||
| % | n | % | n | |
|---|---|---|---|---|
| Race | ||||
| White | 95.56 | 43 | 93.70 | 119 |
| Non-white | 4.44 | 2 | 6.30 | 8 |
| Sex | ||||
| Male | 73.91 | 34 | 76.38 | 97 |
| Female | 26.09 | 12 | 23.62 | 30 |
| GCS at admission | ||||
| 3 | 13.04 | 6 | 9.45 | 12 |
| 4 | 19.57 | 9 | 4.72 | 6 |
| 5 | 10.87 | 5 | 8.66 | 11 |
| 6 | 26.09 | 12 | 18.90 | 24 |
| 7 | 28.26 | 13 | 44.88 | 57 |
| 8 | 2.17 | 1 | 13.39 | 17 |
| Mechanism of Injury | ||||
| Traffic accident | 69.57 | 32 | 61.42 | 78 |
| Fall | 8.70 | 4 | 17.32 | 22 |
| Off-road vehicle accident | 4.35 | 2 | 7.09 | 9 |
| Pedestrian struck | 8.70 | 4 | 4.72 | 6 |
| Other | 4.35 | 2 | 3.94 | 5 |
| Assault | 2.17 | 1 | 2.36 | 3 |
| Bicycle | 2.17 | 1 | 3.15 | 4 |
This table demonstrates the characteristics among survivors with favorable and unfavorable outcomes at 24 months. There were no significant differences between survivors in the favorable and unfavorable outcome groups at 2 years. This table is only among those survivors with GOS-E data at 24 months. N = 173.
GCS, Glasgow Coma Score; GOS-E, Glasgow Outcome Scale-Extended; SD, standard deviation.
Half of survivors demonstrated an increased GOS-E score, whereas 36.6% showed no change at all (Table 3). As seen in Table 4, 43% of survivors progressed from an unfavorable to a favorable outcome. From 6 months to 12 months, survivors demonstrated a statistically significant 0.42-increase in mean GOS-E score (p < 0.0001). During this time, 39.0% of survivors demonstrated an increased GOS-E score, whereas 47.6% survivors demonstrated no change at all (Table 3). Over one third (36%) of survivors progressed from an unfavorable outcome to a favorable outcome between 6 and 12 months after injury (Table 4).
Table 3.
Changes in GOS-E across Time Post-Injury
| From 3m to 6m | From 3m to 12m | From 3m to 24m | From 6m to 12m | From 6m to 24m | From 12m to 24m | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| GOS-E | ||||||||||||
| Decrease | 27 | 13.37 | 20 | 11.24 | 18 | 13.43 | 25 | 13.37 | 26 | 18.71 | 23 | 17.04 |
| No change | 74 | 36.63 | 47 | 26.40 | 24 | 17.91 | 89 | 47.59 | 43 | 30.94 | 64 | 47.41 |
| increase | 101 | 50.00 | 111 | 62.36 | 92 | 68.66 | 73 | 39.04 | 70 | 50.36 | 48 | 35.56 |
| Missed time point | 74 | 166 | 297 | 92 | 223 | 131 | ||||||
| Deceased | 3 | 8 | 14 | 5 | 11 | 6 | ||||||
| Subtotal n | 279 | 352 | 445 | 284 | 373 | 272 | ||||||
The percentage of survivors decreasing, increasing, or having no change in GOS-E scores over each time interval is shown. Only 13% of survivors had a decline in their functional status from 3 to 24 months, whereas 69% of severe TBI survivors demonstrated an increase in their functional status within this time interval. More important, this table demonstrates from 6 to 24 months there is an increase in GOS-E scores of 50% during a time interval thought to be when survivors plateau in their progression. Patients who died between 3 and 24 months have been excluded from this table.
GOS-E, Glasgow Outcome Scale-Extended; m, months; TBI, traumatic brain injury.
Table 4.
Conversion Rates from Unfavorable and Favorable Outcomes
| Unfavorable to favorable | Favorable to unfavorable | Remained unfavorable | Remained favorable | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 3–6 months | 45 | 42.86 | 11 | 11.34 | 60 | 57.14 | 86 | 88.66 |
| 3–12 months | 48 | 52.17 | 7 | 8.14 | 44 | 47.83 | 79 | 91.86 |
| 3–24 months | 52 | 66.67 | 3 | 5.36 | 26 | 33.33 | 53 | 94.64 |
| 6–12 months | 25 | 36.23 | 7 | 5.93 | 44 | 63.77 | 111 | 94.07 |
| 6–24 months | 32 | 54.24 | 6 | 7.50 | 27 | 45.76 | 74 | 92.50 |
| 12–24 months | 15 | 37.50 | 5 | 5.26 | 25 | 62.50 | 90 | 94.74 |
The percentage of survivors converting between favorable and unfavorable outcomes is shown.
There is a small portion of severe TBI survivors who progressed from favorable to unfavorable functional outcomes by 2 years. Most severe TBI survivors show a progressive increase in their functional status over 2 years. Over half of survivors demonstrated a conversion from the unfavorable category to the favorable category from 6 to 24 months and 3 to 24 months. The 14 patients that died between 3 and 24 months were excluded from this analysis. The percentages calculated are based on the total number of cases in each row.
TBI, traumatic brain injury.
From 12 to 24 months post-injury, survivors demonstrated a statistically significant 0.23-increase in mean GOS-E score (p = 0.02). From 12 to 24 months post-injury, 35.6% of survivors demonstrated an increase in GOS-E score, whereas 47.4% survivors demonstrated no change (Table 3). Similarly, 38% of survivors progressed from an unfavorable to a favorable outcome (Table 4). Among those with GOS-E at three or more time points, a GEE model revealed that survivors' GOS-E scores increased 0.62 points from 3 to 6 months, 1.01 from 3 to 12 months, and 1.21 from 3 to 24 months (p < 0.0001).
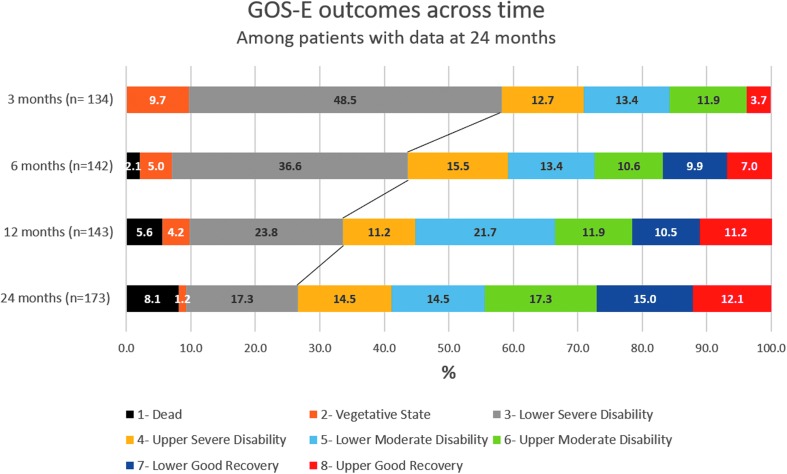
Figure 1 represents how survivors with data at 24 months moved through each GOS-E category over time. Further, it shows the delineation of survivors who shifted from unfavorable to favorable outcomes over time. The mean initial GCS was 6.25 ± 1.44 among the survivors with favorable outcomes at 2 years. Survivors with unfavorable outcomes at 2 years had a mean initial GCS of 5.4 ± 1.47.
FIG. 1.
GOS-E outcomes across time among survivors with data at 24 months. Severe TBI survivors show a significant progressive increase in favorable functional outcome from 3 to 24 months. There were 10% of survivors in a vegetative state (GOS-E of 2) at 3 months and only 1% by 2 years. Again, at 3 months, there were 49% of survivors within the lower severe disability (GOS-E of 3) category, which decreased to 17% by 2 years. We dichotomized survivors into two groups; those with unfavorable outcomes (GOS-E scores 1–3) and favorable outcomes (GOS-E scores 4–8), which the black line delineates. Over half of the survivors fell within the unfavorable group at 3 months. However, this percentage decreased to 26% by 2 years. More important, the percentage of survivors with favorable outcomes increased from 42% at 3 months to 74% at 2 years. This figure is among survivors with data at 24 months accounting for the difference in number of survivors at each time point. N = 134 at 3 months, N = 142 at 6 months, N = 143 at 12 months, and N = 173 at 24 months. GOS-E, Glasgow Outcome Scale-Extended; TBI, traumatic brain injury.
In the unfavorable category, 67% of survivors at 3 months had favorable outcomes at 2 years (Table 4). Overall, 74% of surviving adults with a documented GOS-E score at 2 years after injury had a favorable outcome (Fig. 1).
Discussion
We recognize that the mortality in severe TBI patients within 3 months was high; however, this study was planned to assess recovery trajectories for long-term severe TBI patients who survived the acute hospital setting. In this consecutive series, severe TBI survivors demonstrated significant improvement in functional outcomes from 3 to 24 months after injury. Though 2-year mortality was 46%, three fourths of severe TBI survivors achieved favorable outcomes. Significant improvement in functional outcomes in the 6–24 months after injury was independent of initial GCS. Further, two thirds of survivors in the unfavorable category at 3 months post-injury converted to the favorable outcome (independent in the home, at minimum) by 2 years after injury. Even though some may consider a GOS-E score of 4 (upper severe disability) unfavorable, there is a reduced demand on caregivers and potential for further improvement. These results buttress the perspective that long-term prognosis in severe TBI survivors is better than broadly appreciated.
There is evidence that TBIs can trigger progressive degenerative processes affecting cognitive and neurological function.20 Physical, cognitive, and psychological disturbances can dramatically affect survivors' ability to live and work independently. An improved understanding of long-term clinical outcomes after severe TBI will aide in refining prognostic tools and can guide clinicians who are counseling families on treatment decisions. The favorable outlook of survivors in this series also highlights the need for ongoing rehabilitation and community reintegration strategies that may further mitigate long-term disability. Unfortunately, in numerous regions, current insurance regulations require that a patient demonstrate signs of responsiveness to be eligible for acute inpatient rehabilitation.9,11 However, in our series and in many other studies, survivors who failed to show early improvements still experienced considerable recovery after hospital discharge and most survivors recover enough to participate in rehabilitation services.9,11,12,21 Regrettably, data from the Traumatic Brain Injury Model System centers indicate that few of these survivors are secondarily referred to appropriate rehabilitation facilities.9
Rehabilitation impacts emergence from coma, improving vocational capacity, as well as regaining independence at home, which, in turn, leads to decreased caregiver burden and societal costs, and overall quality of life of survivors as well as their families.9,10,13,21 Recent studies have not proven a causal relationship in which rehabilitation hastens recovery. However, the positive prognosis, prolonged survival, and considerable recovery potential after severe TBI intuitively suggest the importance of continuous, individualized rehabilitation.
The use of a global measure of functional outcome, such as the GOS-E, may limit our study because of the subjective nature of questions and lack of quantitation. It does not reflect subtle changes with respect to cognition or symptom manifestation, such as psychological issues or sleep changes that may occur.2,13 In addition, information regarding post-acute phase interventions and rehabilitation treatments are not available. Although most patients do receive rehabilitation, no data were compiled because patients received this treatment in other centers. Further, we focused this analysis on TBI survivors. Excluding TBI deaths biases, the overall outlook toward a more positive picture, but our goal was to understand better the long-term outcomes in patients who survive a severe TBI. Pessimism exists regarding TBI outcomes, and this analysis provides important clarity on the bimodal distribution of real-world TBI care (some patients die; survivors overall do quite well and continue to improve). Focusing on TBI survivors also underscores the public health need for stronger rehabilitation and community reintegration programs.
Future studies will attempt to incorporate more detailed measures of severity of intracranial injuries, such as CT imaging, functional measures, broader range of predictors, and rehabilitation information. Understanding the social support at the community level available to individual patients can aide in better discerning the long-term impact of injury on various functional outcomes as well as tailor rehabilitation programs to meet the long-term needs of this population.
Conclusion
In this large consecutive series of severe TBI survivors, substantial and ongoing recovery was observed from 3 to 24 months after injury. At 2 years, three fourths of survivors had a favorable outcome. Long-term prognosis in severe TBI survivors is better than broadly appreciated.
Acknowledgments
This work was supported, in part, by the National Institutes of Health (NS30318; Brain Trauma Research Center: Department of Neurosurgery, University of Pittsburgh): NIH/NINR R00 NR013176 (AMP).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Bryan-Hancock C., and Harrison J. (2010). The global burden of traumatic brain injury: preliminary results from the global burden of disease project. Inj. Prev. 16, 1720179030 [Google Scholar]
- 2. Sandsmark D. (2016). Clinical outcomes after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 16, 52. [DOI] [PubMed] [Google Scholar]
- 3. Kaufmann M., Buchmann B., Scheidegger D., Gratzl O., and Radü E. (1992). Severe head injury: should expected outcome influence resuscitation and first-day decisions? Resuscitation 23, 199–206 [DOI] [PubMed] [Google Scholar]
- 4. Bonds B., Dhanda A., Wade C., Massetti J., Diaz C., and Stein D. (2015). Prognostication of mortality and long term functional outcomes following traumatic brain injury: can we do better? J. Neurotrauma. July 31. doi: 10.1089/neu.2014.3742. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5. Forslund M., Roe C., Perrin P., Sigurdardottir S., Lu J., Berntsen S., and Andelic N. (2017). The trajectories of overall disability in the first 5 years after moderate and severe traumatic brain injury. Brain Inj. 31, 329–335 [DOI] [PubMed] [Google Scholar]
- 6. Edlow B., Giacino J., Hirschberg R., Gerrard J., Wu O., and Hochberg L. (2013). Unexpected recovery of function after severe traumatic brain injury: the limits of early neuroimaging-based outcome prediction. Neurocrit. Care 19, 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilde E., Whiteneck G., Bogner J., Bushnik T., Cifu D., Dikmen S., French L., Giacino J., Hart T., Malec J., Millis S., Novack T., Sherer M., Tulsky D., Vanderploeg R., and Steinbuechel N. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Phys. Med. Rehabil. 91, 1650–1660 [DOI] [PubMed] [Google Scholar]
- 8. Corral L., Ventura J., Herrero J., Monfort J., Juncadella M., Gabarrós A., Bartolomé C., Javierre C., and García-Huete L. (2007). Improvement in GOS and GOSE scores 6 and 12 months after severe traumatic brain injury. Brain Inj. 21, 1225–1231 [DOI] [PubMed] [Google Scholar]
- 9. Nakase-Richardson R., Whyte J., Giacino J., Pavawalla S., Barnett S., Yablon S., Sherer M., Kalmar K., Hammond F., Greenwald B., Horn L., Seel R., McCarthy M., Tran J., and Walker W. (2012). Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI model systems programs. J. Neurotrauma 29, 59–65 [DOI] [PubMed] [Google Scholar]
- 10. Whyte J., Nakase-Richardson R., Hammond F., McNamee S., Giacino J., Kalmar K., Greenwald B., Yablon S., and Horn L. (2013). Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the national institute on disability and rehabilitation research traumatic brain injury model systems. Arch. Phys. Med. Rehabil. 94, 1855–1860 [DOI] [PubMed] [Google Scholar]
- 11. Luaute´ J., Maucort-Boulch D., Tell L., Quelard F., Sarraf T., Iwaz J., Boisson D., and Fischer C. (2010). Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 75, 246–252 [DOI] [PubMed] [Google Scholar]
- 12. Walker W., Stromberg K., Marwitz J., Sima A., Agyemang A., Graham K., Harrison-Felix C., Hoffman J., Brown A., Kreutzer J., and Merchant R. (2018). Predicting long-term global outcome after traumatic brain injury: development of a practical prognostic tool using the traumatic brain injury model systems national database. J. Neurotrauma 35, 1587–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dams-O'Connor K., Pretz C., Billah T., Hammond F., and Harrison-Felix C. (2015). Global outcome trajectories after TBI among survivors and nonsurvivors: a national institute on disability and rehabilitation research traumatic brain injury model systems study J. Head Trauma Rehabil. 30, E1–E10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennett B., and Bond M. (1975). Assessment of outcome after severe brain damage: a practical scale. Lancet 305, 480–484 [DOI] [PubMed] [Google Scholar]
- 15. Wilson J., Pettigrew L., and Teasdale G. (1998). Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J, Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 16. Wright J. (2011). Glasgow Outcome Scale, in: Encyclopedia of Clinical Neuropsychology. Kreutzer J.S., DeLuca J., and Caplan B. (eds). Springer, New York [Google Scholar]
- 17. Hutchinson P., Kolias A., Timofeev I., Corteen E., Czosnyka M., Timothy J., Anderson I., Butlers D., Belli A., Eynon C., Wadley J., Mendelow A., Mitchell P., Wilson M., Critchely G., Sahuquillo J., Unterberg A., Servadei F., Teasdale G., Pickard J., Menon D., Murray G., and Kirkpatrick P. (2016). Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 375, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 18. Vahedi K., Hofmeijer J., Jüttler E., Vicaut E., George B., Algra A., Amelink G., Schmiedeck P., Schwab S., Rothwell P., Bousser M., van der Worp H., and Hacke P. (2007). Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomized controlled trials. Lancet Neurol. 6, 215–222 [DOI] [PubMed] [Google Scholar]
- 19. Jüttler E., Unterberg A., Woitzik J., Bösel J., Amiri H., Sakowitzm O., Gondan M., Schiller P., Limprecht R., Luntz S., Schneider H., Pinzer T., Hobohm C., Meixensberger J., and Hacke W. (2014). Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N. Engl. J. Med. 370, 1091–1100 [DOI] [PubMed] [Google Scholar]
- 20. Corrigan J., and Hammond F. (2013). Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 94, 1199–1201 [DOI] [PubMed] [Google Scholar]
- 21. Estraneo A., Moretta P., Loreto V., Lanzillo B., Santoro L., and Trojano L. (2010). Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 75, 239–245 [DOI] [PubMed] [Google Scholar]