Abstract
Introduction: Tick-borne encephalitis virus (TBEV) and Borrelia burgdorferi, the causative agent of Lyme disease (LD), are widespread in Western Ukraine. However, relatively little is known about Anaplasma phagocytophilum in this region. This study examined patterns of infection with A. phagocytophilum in two tick vectors compared with the better studied TBEV and B. burgdorferi.
Materials: Ticks were collected in three different ecosystems of the Western Ukraine during 2009–2014. Samples were examined for pathogen detection using real-time polymerase chain reaction (PCR), and logistic regression models were developed to assess the significance of different factors.
Results: Among the three selected ecological systems of the Western region of Ukraine, 5130 ticks belonging to Ixodes ricinus and Dermacentor reticulatus were collected between 2009 and 2014. They were grouped into 366 pools and were tested by PCR for A. phagocytophilum. A subsample (1620 ticks, 162 pools) of the ticks was concurrently tested by PCR for A. phagocytophilum, B. burgdorferi, and TBEV. Overall, there was no trend in the proportion of positive ticks across years (p > 0.05). However, the prevalence of A. phagocytophilum was higher (27.4%) in I. ricinus than in D. reticulatus (15.9%) (OR = 2.69; 95% CI, 1.52–4.94 (Lower, Upper 95% CI)). Infection was more common in forested habitats (OR = 1.89; 95% CI, 1.07–3.36) and during the later summer–early autumn (3.78; 95% CI, 1.79–8.06). B. burgdorferi was found in 29.3% and 31.9% of I. ricinus and D. reticulatus, respectively; and TBEV was found in 6.3% and 14.5% of I. ricinus and D. reticulatus. Coinfection of A. phagocytophilum and B. burgdorferi occurred more often than chance and was more frequent than any other combination of pathogens (p = 0.031).
Conclusions: Our study is the first to explore the potential relationship between the ecosystems, vectors, and the presence of Human Granulocytic Anaplasmosis (HGA) and other tick-borne infections in Western Ukraine. Anaplasma demonstrated a greater prevalence in I. ricinus in the forested area in Western Ukraine. Altogether, HGA, LD, and tick-borne encephalitis (TBE) pathogens are actively circulating in these ecosystems and have the potential to coinfect vectors that might increase the risk of transmitting multiple pathogens to humans during host feeding by individual ticks.
Keywords: human granulocytic anaplasmosis, Anaplasma phagocytophilum, Borrelia burgdorferi, tick-borne encephalitis virus, tick-borne infections, ticks, Ukraine
Introduction
Tick-borne diseases affect humans and other mammals when infectious ticks feed on them, most commonly ticks of the Ixodidae family. Clinical presentation and disease severity in humans mostly depend on the infecting pathogen (Hamel et al. 2013, Reye et al. 2013, Vynograd 2014). Although multiple tick species may act as vectors and transmit the same pathogen to vertebrate hosts, coinfections in ticks with multiple pathogens also can occur so that one tick bite can transmit one or more pathogens to the infested host (Pañczuk et al. 2016, Asman et al. 2017). The strong seasonality of tick-borne diseases depends on the tick activity, which, in turn, depends on temperature and hygrometry (i.e., climate and environment). Altogether, climate and ecosystems favorable to tick reproduction, natural host abundance for various tick life stages, human occupations and recreational activities, as well as an active circulation of various pathogens in the region, drive the zoonotic risk for both humans and animals (Alexander et al. 2012, Dantas-Torres 2015).
Previous studies in Ukraine (Movila et al. 2009) have provided insight into tick-borne encephalitis (TBE), a disease caused by a Flavivirus, and Lyme disease (LD), caused by the spirochete, Borrelia burgdorferi (Biletska et al. 2008). From 2004 to 2014, the highest rate of registered cases of LD was 4.1 per 100,000 people in Ukraine (Biletska et al. 2014). From 2002 through 2015, inclusive, 100 cases of TBE also were locally reported (and four imported cases were identified) indicating the presence of virus in areas with different landscape and climatic characteristics (Biletska et al. 2011, 2012, Lozynskyi et al. 2013, Pavlikovska et al. 2014).
Human Granulocytic Anaplasmosis (HGA) is not a subject of mandatory reporting in Ukraine so there are no official data about the disease incidence. The presence of HGA in Ukraine was first described in 2007 in a pilot study conducted in nine oblasts of Ukraine (Ben and Biletska 2008). ELISA was used to detect IgM and IgG to Anaplasma phagocytophilum in 98 suspected cases of tick-borne infections (fever after tick bite). The first 13 cases of HGA were identified in Volyn (3), Dnipropetrovsk (1), Zaporizhzhya (4), Lviv (1), Poltava (1), Rivne (1), and Cherkassy (2) Oblasts in 2007 (Ben and Biletska 2008, 2009). In addition, 328 healthy persons were tested with ELISA in Western Ukraine (Volyn, Zakarpattia, and Lviv Oblasts) in 2007–2009. The level of positive IgG to A. phagocytophilum was between 4.8–10.3% in population of Volyn Oblast; 3.3–6.4% in Lviv Oblast; and 1.4% in Zakarpattia Oblast (Biletska and Ben 2009). ELISA was also used to test 82 patients with tick bites for presence of IgG to A. phagocytophilum in Eastern Ukraine (Kharkiv Oblast) in 2007–2009; 7.7% cases were positive (Malyj et al. 2010). Despite serological evidence of HGA pathogen circulation in Western Ukraine, there appears to be limited knowledge of HGA prevalence in ticks.
Lviv and Volyn oblasts of Western region of Ukraine cover three landscape zones: forest ( = Ukrainian Polissia); forest steppe; and Ukrainian Carpathian Mountains (Ben and Biletska 2015). This area is characterized by a mild wet climate, a wide range of plants and animals, the presence of vector ticks, and reservoir hosts (Biletska et al. 2012). In addition, HGA has been reported in countries neighboring this region of Ukraine (Kiewra et al. 2014).
The goals of this study were: (1) to identify the likely vectors of A. phagocytophilum in Western Ukraine and (2) to compare the prevalence and risk factors of Anaplasma infection with other, better studied tick pathogens (B. burgdorferi and TBEV) in the region.
Materials and Methods
Study period
Sample collection and testing were performed from April to September (except July when tick activity naturally decreases) every year during 2009–2014.
Study sites
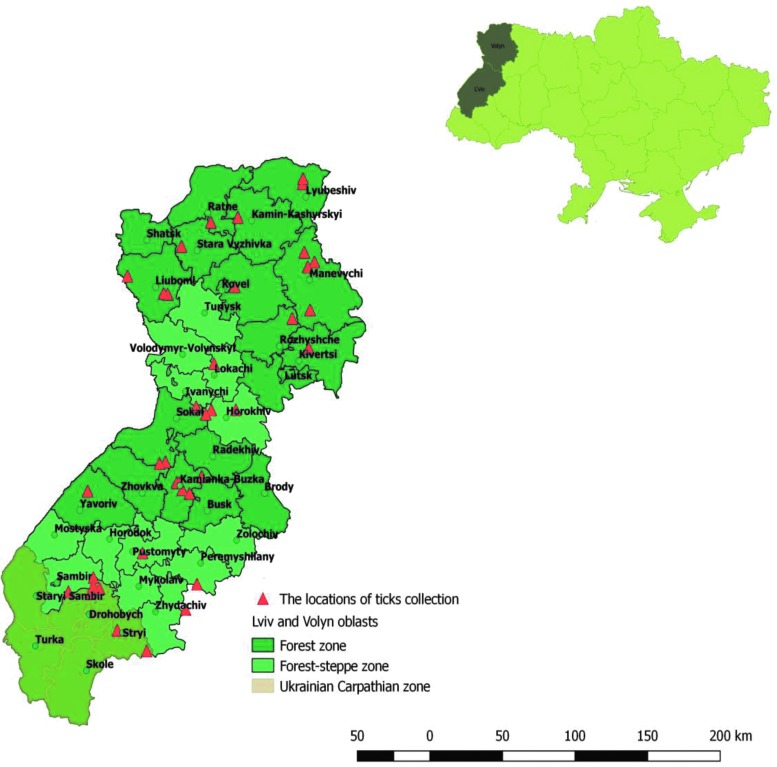
Ticks were collected in Lviv and Volyn oblasts of Western Ukraine in the forest (Ukrainian Polissia) (16 districts), forest-steppe (12 districts), and Ukrainian Carpathian zone (5 districts) range characterized by wetlands with pastures and meadows (Fig. 1).
FIG. 1.
Locations of tick collections in Lviv and Volyn oblasts, Ukraine, 2009–2014.
Collection of ticks
Sample collection was performed in locations identified as sampling sites for national routine vector surveillance. Ixodid ticks were collected by flagging (Chong et al. 2013) and identified to species using external characteristics (Balashov Yu 1998). Live ticks were pooled to represent groupings of the same species, sex, the date, and site of collection. Of the total 5130 ticks, 3170 ticks collected by our institute were pooled as adults in groups of 10 individuals. The remaining 1960 ticks were collected as a part of routine monitoring for tick-borne infections with the staff of the Volyn Oblast Laboratory Center of the Ministry of Health of Ukraine and were pooled as 40 individuals in a pool (49 pools). Pools were stored at −70°C until the end of each season of collection. All pools were tested for DNA of A. phagocytophilum; samples collected from areas where tick-borne infections had been previously reported were also tested for presence of B. burgdorferi and TBEV to assess risk of human coinfection after a tick bite.
Polymerase chain reaction
Pools of ticks were tested for the causative agents of tick-borne infections using real-time polymerase chain reaction (qPCR). DNA was extracted with established methods (Grzeszczuk 2006) using UltraClean™ Tissue® & Cell DNA Isolation Kit® (MO BIO Laboratories, Inc.) according to the manufacturer's instructions. The quality of extracted DNA was tested with a spectrophotometer to confirm extraction of quality DNA (NanoDrop ND-2000; Thermo Scientific®). PCR for Anaplasma sp. DNA detection used the commercial diagnostic test system Ampli-HGA® (Omnix, St. Petersburg, Russian Federation). Multiprime-AmpliSens® (InterLabService, Russian Federation) was used for simultaneous detection of DNA for Anaplasma and Borrelia and cDNA fragments of TBEV, B. burgdorferi, and A. phagocytophilum (Karan 2012). During amplification of the study samples, we used amplification controls: negative control (C−)–10 mL of DNA buffer and positive control (C+)–10 mL of c DNA of TBEV, B. burgdorferi s.l., and A. phagocytophilum. The total volume of reaction composition was 25 mL. Amplification and detection of A. phagocytophilum, B. burgdorferi, and TBEV used a thermocycler (Rotor-GeneTM 6000) and software Thermal Cycler System (Mysterud et al. 2013). A system for real-time detection of fluorescent signal used the following cycling conditions: initial denaturation at 95°C for 15 min; then 5 cycles of 95°C 10 s, 60°C 35 s, 72°C 15 s; and 40 cycles of 95°C 10 s, 56°C 35 s, 72°C 15 s. Detection of fluorescent signal was performed at FAM, JOE, ROX fluorophore channels for TBEV and A. phagocytophilum and FAM, JOE channels for B. burgdorferi s.l.
Statistical analyses
Logistic regression models were developed to assess the significance of tick species, seasonal habitat effects, and coinfection conditions for detecting of any of the three pathogens. Estimation of odds ratios and confidence intervals was performed using R software. To evaluate whether coinfections were more common than expected under chance, alone, the prevalence of pathogen combinations in individual tick pools was tested by chi-squared test with Yates' continuity correction. Adjusted p values were generated using Holm's (Holm 1979) adjustment to account for multiple comparisons. P value <0.05 was regarded as statistically significant.
Results
A total of 5130 ticks (366 pools) were analyzed for A. phagocytophilum. They belonged to only two species: Ixodes ricinus—3210 ticks (190 pools) and Dermacentor reticulatus—1920 ticks (176 pools) (Tables 1 and 2). A. phagocytophilum was found in both tick vectors with 21.9% of all pools testing positive by PCR (Table 1). After controlling for year, season, and eco-zone, A. phagocytophilum was more frequent in I. ricinus than in D. reticulatus (OR = 2.69; 95% CI, 1.52–4.94 Table 3).
Table 1.
The Results of Screening for DNA of Anaplasma phagocytophilum with PCR in Ticks Collected in Different Ecosystems of LVIV and Volyn Oblasts, 2010–2014
| The results of screening for A. phagocytophilum with PCR | ||||||
|---|---|---|---|---|---|---|
| Total | By species | |||||
| I. ricinus | Dermacentor reticulatus | |||||
| Landscape-geographical zones | Number of ticks tested/number of pools tested | Number of positive pools (% positive) | Ticks tested/pools tested | Number of positive pools (% positive pools) | Ticks tested/pools tested | Number of positive pools (% positive pools) |
| Forest | 3010/172 | 48 (27.9%) | 2070/94 | 35 (37.2%) | 940/78 | 13 (16.7%) |
| Forest steppe | 1640/146 | 28 (19.2%) | 990/81 | 15 (18.5%) | 650/65 | 13 (20%) |
| Carpathians | 480/48 | 4 (8.3%) | 150/15 | 2 (13.3%) | 330/33 | 2 (6.1%) |
| Total | 5130/366 | 80 (21.9%) | 3210/190 | 52 (27.4%) | 1920/176 | 28 (15.9%) |
Table 2.
Number of Ticks Positive for Anaplasma phagocytophilum, Borrelia burgdorferi, Tick-Borne Encephalitis Virus and Coinfections by Different Risk Factors
| Variable | Anaplasma phagocytophilum (n = 366) | Borrelia burgdorferi (n = 190) | Tick-borne encephalitis virus (n = 162) | ||||
|---|---|---|---|---|---|---|---|
| Type | Value | POS | NEG | POS | NEG | POS | NEG |
| Tick Species | Dermacentor reticulatus | 28 | 148 | 29 | 62 | 12 | 71 |
| Ixodes ricinus | 52 | 138 | 29 | 70 | 5 | 74 | |
| Eco-zone | Forest | 48 | 124 | 27 | 63 | 12 | 67 |
| Forest steppe | 28 | 118 | 29 | 51 | 4 | 68 | |
| Carpathians | 4 | 44 | 2 | 18 | 1 | 10 | |
| Area | Rural | 66 | 280 | 53 | 122 | 17 | 130 |
| Urban | 14 | 6 | 5 | 10 | 0 | 15 | |
| Collection Month |
Apr | 3 | 22 | 4 | 15 | 1 | 18 |
| May | 27 | 102 | 9 | 18 | 0 | 27 | |
| Jun | 28 | 112 | 25 | 76 | 15 | 66 | |
| Aug | 1 | 11 | 6 | 2 | - | - | |
| Sep | 21 | 39 | 14 | 21 | 1 | 34 | |
| Collection Year |
2009 | 1 | 7 | 3 | 3 | 0 | 6 |
| 2010 | 14 | 71 | 3 | 46 | 7 | 42 | |
| 2011 | 2 | 22 | 2 | 1 | 0 | 3 | |
| 2012 | 45 | 137 | 41 | 54 | 10 | 85 | |
| 2013 | 6 | 16 | 8 | 2 | 0 | 2 | |
| 2014 | 12 | 33 | 1 | 26 | 0 | 7 | |
| A resultsa | POS | — | — | 25 | 42 | — | — |
| NEG | — | — | 33 | 90 | — | — | |
| A or b resultsb | POS | — | — | — | — | 6 | 81 |
| NEG | — | — | — | — | 11 | 64 | |
| Subtotals | 80 | 286 | 58 | 132 | 17 | 145 | |
POS, positive test result; NEG, negative test result.
Anaplasma phagocytophilum (A) presence.
Anaplasma phagocytophilum (A) or Borrelia burgdorferi (B) presence.
Table 3.
Results from the Multiple Logistic Regressions for Three Pathogens
| Anaplasma phagocytophilum | Borrelia burgdorferi | Tick-borne encephalitis virus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter Estimates | 95% CI | 95% CI | 95% CI | |||||||
| Type | Value | OR | LL | UL | OR | LL | UL | OR | LL | UL |
| Intercept | 0.07* | 0.02 | 0.2 | 0.3* | 0.1 | 0.82 | 0.07* | 0.01 | 0.37 | |
| Tick Species | Derma-centor reticu-latus | Ref | — | — | Ref | — | — | Ref | — | — |
| Ixodes ricinus | 2.69* | 1.52 | 4.94 | 0.82 | 0.38 | 1.74 | 1.01 | 0.24 | 4.22 | |
| Eco-zone | Forest | 1.89* | 1.07 | 3.36 | 0.55 | 0.26 | 1.15 | 4.29* | 1.2 | 18.98 |
| Forest steppe | Ref | — | — | Ref | — | — | Ref | — | — | |
| Carpathians | Ref | — | — | Ref | — | — | Ref | — | — | |
| Area | Rural | Ref | — | — | Ref | — | — | — | — | — |
| Urbana | 8.26* | 2.93 | 24.82 | 1.84 | 0.4 | 8.26 | — | — | — | |
| Month | Apr-May | 0.92 | 0.48 | 1.77 | 0.81 | 0.29 | 2.17 | 0.12* | 0.01 | 0.81 |
| Jun | Ref | — | — | Ref | — | — | Ref | — | — | |
| Aug-Sep | 3.78* | 1.79 | 8.06 | 1.96 | 0.83 | 4.57 | 0.10* | 0.01 | 0.66 | |
| A resultb | NEG | — | — | — | Ref | — | — | — | — | — |
| POS | — | — | — | 1.66 | 0.8 | 3.45 | — | — | — | |
| A or B resultsc | NEG | — | — | — | — | — | — | Ref | — | — |
| POS | — | — | — | — | — | — | 0.46 | 0.12 | 1.62 | |
| Year (continuous) | 1.01 | 0.8 | 1.26 | 1.12 | 0.84 | 1.51 | 1.22 | 0.7 | 2.13 | |
Year variable has been recoded from 2009 to 2014 into 1–6.
Indicates a significant effect on 95% Confidence Level.
Area type is not included in the regression for TBEV to avoid problems with numerical estimation.
Anaplasma phagocytophilum (A) presence.
A. phagocytophilum (A) or Borrelia burgdorferi (B) presence.
OR, odds ratio; 95% CI, 95% confidence interval.
LL, lower limit of the confidence interval.
UL, upper limit of the confidence interval.
Infection with A. phagocytophilum was more commonly found in ticks collected from forest habitats (27.9%) and were less likely in forest steppe (19.2%) or the Carpathian zone (8.3%); multiple logistic regression showed significant association with forest habitats (OR = 1.89; 95% CI, 1.07–3.36). Collecting in urban areas was strongly associated with higher chances of detecting the pathogen in ticks (OR = 8.26; 95% CI, 2.93–24.82).
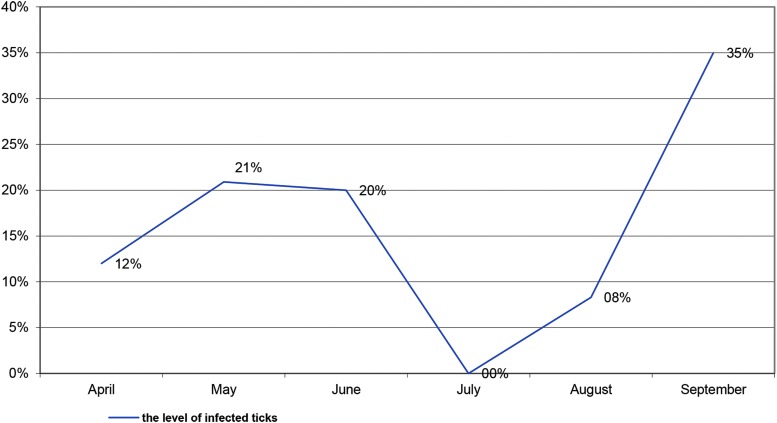
A. phagocytophilum had a bimodal seasonal pattern of infection in ticks (Fig. 2). PCR positive ticks were found between April and September (no surveys were performed in July of any year of the study period). The prevalence of infected ticks had a spring-summer peak reaching nearly 21% in May before decreasing in late summer. Prevalence peaked again in autumn (September) at more than 30%. There was a significant increase in the proportion of positive pools with A. phagocytophilum (OR = 3.78; 95% CI, 1.79–8.06) identified in the autumn compared to the early summer (Table 3). There was no evidence of an annual trend in detecting A. phagocytophilum during the study period (Table 3; variable Year, OR = 1.01; p > 0.05).
FIG. 2.
Seasonal distribution of infected with A. phagocytophylum ticks by months, % of positive pools in PCR, in 2009–2014 in Lviv and Volyn Oblast.
Among 366 tick pools tested for A. phagocytophilum, 190 pools (99 of I. ricinus and 91 of D. reticulatus) were additionally tested for presence of B. burgdorferi; 162 of them (79 of I. ricinus and 83 of D. reticulatus) were also tested for presence of B. burgdorferi and TBEV (Table 2).
The prevalence of B. burgdorferi in all pools was 30.5%; with 31.9% in D. reticulatus and 29.3% in I. ricinus. In contrast to A. phagocytophilum, B. burgdorferi positive pools were not associated with tick species, eco-zone, or area.
The prevalence of TBEV in total was found to be 10.5%, with 6.3% in I. ricinus and 14.5% in D. reticulatus. TBEV infection patterns differed from A. phagocytophilum and B. burgdorferi (Table 3). As with A. phagocytophilum, there were significant effects of eco-zones with forest habitats significantly more likely to yield positive pools than forest-steppe zones (OR = 4.29; 95% CI, 1.20–18.98). However, both I. ricinus and D. reticulatus were comparable in yielding positive pools (OR = 1.01; 95% CI, 0.24–4.22). TBEV also showed a seasonal variation in prevalence. However, in contrast to A. phagocytophilum and B. burgdorferi, the proportion of positive pools was highest in the summer (June) and lowest in the spring (OR = 0.12; 95% CI, 0.01–0.81) or the autumn (OR = 0.10; 95% CI, 0.01–0.66). As with the previous pathogens, the prevalence of infection did not differ significantly across years of the study.
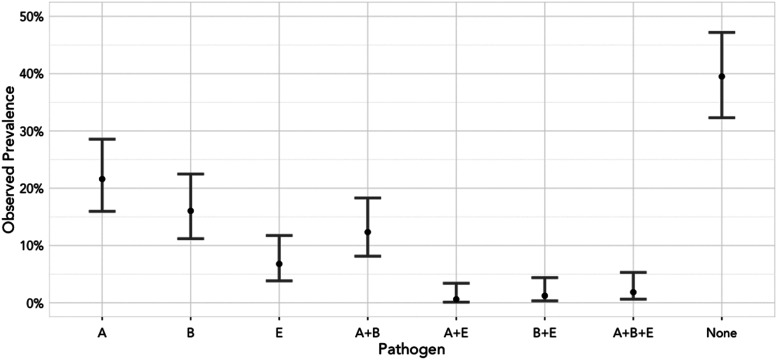
When analyzing frequencies of the three pathogens and their combination, the most frequently observed pathogen or pathogen combination was A. phagocytophilum (A), followed by B. burgdorferi (B) and the combination of these two pathogens (A+B) (Fig. 3). Thirty-five samples (21.6%) had only A. phagocytophilum (A), 26 samples (16%) were only positive for B. burgdorferi (B), and 20 samples (12.3%) had both A. phagocytophilum and B. burgdorferi (A+B). TBEV was found in 11 samples (6.8%). The smallest observed prevalence belonged to A. phagocytophilum and B. burgdorferi pathogen combinations with TBEV (A+E, B+E, or A+B+E) with about 1% each.
FIG. 3.
Observed pathogen and pathogen combination prevalence with 95% Confidence Interval for samples that were tested for all three pathogens. A, A. phagocytophilum; B, B. burgdorferi; E–TBEV, None–none of the three pathogens was detected in the sample.
After adjusting for multiple comparisons, we concluded that tick samples that were positive exclusively for TBEV were significantly less common than for A. phagocytophilum or B. burgdorferi (p value 0.001 and 0.04, respectively). In addition, the pathogen combination of A. phagocytophilum and B. burgdorferi occurred significantly more often than any other pathogen combination (p value 0.03). Despite the difference in infection prevalence, the tick pools with multiple pathogens occurred randomly. None of the combinations had odds ratios that differed significantly from 1.0 (Table 3 and Fig. 3).
Discussion
Our study is the first to explore the potential relationship between the ecosystems, vectors, and the presence of HGA and other tick-borne infections in Western Ukraine. The prevalence of Anaplasma in I. ricinus and D. reticulatus is an important indicator of epidemicity of natural foci provided by immediate evidence of the ticks' roles in development and persistence of natural foci of HGA, rather than relying on serological evidence from vertebrate species.
The presence of the HGA pathogen in ticks (21.9% of all pools) showed that the agent is common in Western Ukraine. Our results are similar to the data obtained in the active natural foci of HGA in many European countries, including those adjoining Ukraine: Poland, Belarus, and Russia, but with rates higher than in Hungary (Grzeszczuk and Stańczak 2006, Rigó et al. 2011, Nordberg 2012, Víchová et al. 2014). The absence of any annual trends in infection prevalence during the 6 years study (Tables 2 and 3) suggests that the infection is endemic and is not dramatically increasing.
Based on our data (Tables 1–3), there are at least two vector species maintaining A. phagocytophilum. Although there are eco-zone and seasonal differences in infection prevalence, Anaplasma was consistently more common in I. ricinus (OR = 2.69; 95% CI, 1.52–4.94) compared with D. reticulatus. As reported in other Eurasian countries (Belarus, Lithuania, Serbia, and Russian Asia) (Nordberg, 2012, Stuen et al. 2013), among the 10 species of Ixodid ticks identified in the Western region of Ukraine, these two species are the most abundant in the region. Consequently, during our 5-year study period, I. ricinus was the main vector of Anaplasma, while D. reticulatus served as a secondary vector (Stuen et al. 2013). I. ricinus is mainly found in forest areas and caught during April-October. D. reticulatus is more often found in forest-steppe and Carpathian areas; its activity starts in late March and may last until November. The involvement of two vector species in the transmission of Anaplasma increased the duration of the risk season (up to 8–9 months per year), as well as the geographic range of the risk area, thus, increasing the risk of human infection.
To determine the epidemiological significance of coinfection in Ixodid ticks, we simultaneously tested the presence of additional pathogens: LD and TBE. While 12.3% of pools were positive for both A. phagocytophilum and B. burgdorferi, the co-occurrence of A. phagocytophilum and/or B. burgdorferi with TBEV was much less common, at about 1% each. The data are consistent with observations reported in other countries (Nordberg 2012, Asman and Nowak 2013). Observations of coinfection in I. ricinus with the pathogens of two (HGA-LD) agents in Europe have been described since 2000 in Italy (Aureli et al. 2012) and were equal to 0.9% in Germany (Tappe et al. 2014), 1.6% in the Netherlands (Wielinga et al. 2006), 5% in Slovakia (Derdakova et al. 2003), from 0.93% (Wójcik-Fatla et al. 2009) to 8.3% (Stańczak et al. 2004) in Poland, and 9.3% in Bulgaria (Nader et al. 2018). Infection of ticks with three pathogens occurs much less frequently—0.3% of sampled I. persulcatus in Russia (Swanson et al. 2006).
The presence of two or three pathogens in the same vector allows simultaneous infection and development of mixed infection in individual humans. In future studies, patterns of coinfection should examine individual ticks rather than pools for the presence of the three causative agents (HGA, LD, and TBE). This would better clarify if individuals were carrying multiple human pathogens compared to identifying sites where multiple pathogens are circulating in close proximity of space and time (pools were grouped by site and date).
In general, it would be beneficial for future studies to standardize tick collection technique among participating institutions, namely, the approach to selection of study sites and sample number and, if using pools, the number of ticks in a pool. While we tested only ticks that were collected by flagging, examination of ticks collected from humans would provide an important insight about the level of risk for human infection. In addition, it is also important to compare infection rate in ticks and rodents in future studies to assess the role of mammal host species in maintaining endemicity.
Understanding the spatial and temporal ecology of the tick-borne pathogens, including ticks and natural hosts (wild vertebrate), and the risk and vulnerability of human population (density, proximity, behavior, etc.) with respect to environmental factors (e.g., season, chorology, altitude, and so on) appears essential for the control and prevention of tick-borne diseases. In addition, efficient survey and control of HGA in Ukraine will encompass eco-epidemiology of other tick-borne pathogens, including parasitic or viral diseases, such as Babesiosis and TBE.
Conclusions
Active foci of HGA were identified in the Western region of Ukraine. Of the two main tick species circulating in Western Ukraine, the predominantly observed vector of A. phagocytophilum in Lviv and Volyn oblasts is wood tick I. ricinus with the average prevalence of 27.4%. The secondary vector is the marsh tick D. reticulatus, which had average infection rates of 15.9%. Based on tests of these samples, HGA was the second most common infection after LD. The recognition of HGA in Western Ukraine is the key rationale for the public health system to develop protocols for differential diagnosis and targeted preventive measures against tick-borne infections. Such high prevalence of A. phagocytophilum strengthens the choice of doxycycline as agent of choice for LB treatment since active also against HGA.
Acknowledgments
The authors acknowledge the United States Department of Defense, Defense Threat Reduction Agency (DTRA), and Cooperative Biological Engagement Program (CBEP) for their assistance and financial support in publication of this article. While DTRA/CBEP did not support the research described in this publication, the Program supported the article development and publication. The contents of this publication are the responsibility of the authors and do not necessarily reflect the views of DTRA or the United States Government.
The authors express their gratitude to Dr. Gregory Glass, University of Florida, for supporting them in developing of this article, multiple review, and revision he competed.
The authors also thank Volodymyr Serhiienko, Metabiota, Inc., for assistance in statistical analysis and describing of the data.
Research Institute of Epidemiology and Hygiene Danylo Halytsky Lviv National Medical University, Lviv, Ukraine.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander KA, Lewis BL, Marathe M, Eubank S, et al. Modeling of Wildlife-Associated Zoonoses: Applications and Caveats. Vector Borne Zoonotic Dis 2012; 12:1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asman M, Nowak-Chmura M, Solarz K, Szilman E, et al. Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi sensu lato, and Toxoplasma gondii in Ixodes ricinus (Acari, Ixodida) ticks collected from Slowinski National Park (Northern Poland). J Vector Ecol 2017; 42:200–202 [DOI] [PubMed] [Google Scholar]
- Asman M, Nowak M. The risk of exposure to Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Babesia sp. and co-infections in Ixodes ricinus ticks on the territory of Niepołomice forest (southern Poland). Ann Parasitol 2013; 59:13–19 [PubMed] [Google Scholar]
- Aureli S, Foley JE, Galuppi R, Rejmanek D, et al. Anaplasma phagocytophilum in ticks from parks in the Emilia-Romagna region of northern Italy. Vet Ital 2012; 48:413–423 [PubMed] [Google Scholar]
- Balashov Yu S. Ixodid Ticks–Parasites and Vectors. St. Petersburg: Science, 1998:287 [Google Scholar]
- Ben I, Biletska H. Study of human granulocytic anaplasmosis in the Western Ukraine. Materials of the conference devoted to the Day of Science Modern problems of epidemiology, microbiology and hygiene. Lviv: LRIEH; 2008; 6:297–304 [Google Scholar]
- Ben I, Biletska H. Epidemiologic aspects of human granulocytic anaplasmosis in the Western region of Ukraine. Lik Sprava 2015; 167–171 [PubMed] [Google Scholar]
- Biletska H, Ben I. Human granulocytic anaplasmosis in Ukraine: Serologic Study National priorities of Russia. Omsk: Omsk Scientific Herald, 2009; 2:110–111 [Google Scholar]
- Biletska H, Lozynskiy I, Drul O, Semenyshyn O, et al. Natural Focal Transmissible Infections with Neurological Manifestations in Ukraine. In: Flavivirus Encephalitis. Ruzek Daniel, Rijeka, Croatia, InTech, 2011:273–292 [Google Scholar]
- Biletska H, Semenychyn O, Lozinskyi I, Ben I. The main directions of prevention of tick-borne infections in persons affected by tick bite No. 208–2014. SI Lviv research Institute epidemiology and hygiene Ministry of health of Ukraine; Kyiv; 2014:5 [Google Scholar]
- Biletska H, Podavalenko L, Semenyshyn O, Lozynskyi I, et al. Study of Lyme borreliosis in Ukraine. IJMM 2008; 4:154–160 [Google Scholar]
- Biletska H, Semenyshyn O, Ben I, Shulgan A, et al. Modern parasitic system of tick-born infections in Lviv region. Ann Mechnikov Inst 2012; 4:126–132 [Google Scholar]
- Chong ST, Kim HC, Lee IY, Kollars TM Jr, et al. Comparison of dragging and sweeping methods for collecting ticks and determining their seasonal distributions for various habitats, Gyeonggi Province, Republic of Korea. J Med Entomol 2013; 50:611–618 [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int J Parasitol Parasites Wildl 2015; 4:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdakova M, Halanova M, Stanko M, Stefancikova A, et al. Molecular evidence for Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from eastern Slovakia. Ann Agric Environ Med 2003; 10:269–271 [PubMed] [Google Scholar]
- Grzeszczuk A. Anaplasma phagocytophilum in Ixodes ricinus ticks and human granulocytic anaplasmosis seroprevalence among forestry rangers in Białystok region. Adv Med Sci 2006; 51:283–286 [PubMed] [Google Scholar]
- Grzeszczuk A, Stańczak J. Highly variable year-to-year prevalence of Anaplasma phagocytophilum in Ixodes ricinus ticks in northeastern Poland: A 4-year follow-up. Ann N Y Acad Sci 2006; 1078:309–311 [DOI] [PubMed] [Google Scholar]
- Hamel D, Silaghi C, Zapadynska S, Kudrin A, et al. Vector-borne pathogens in ticks and EDTA-blood samples collected from client-owned dogs, Kiev, Ukraine. Ticks Tick Borne Dis 2013; 4:152–155 [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70 [Google Scholar]
- Karan LS. The possibility of applying molecular methods in the diagnosis of tick-borne encephalitis: Tick-borne encephalitis, Ixodes tick-borne borreliosis. Bull Lab DNA Diagn 2012; 1:11–16 [Google Scholar]
- Kiewra D, Zaleśny G, Czułowska A. The prevalence of Anaplasma phagocytophilum in questing Ixodes ricinus ticks in SW Poland. Pol J Microbiol 2014; 63:89–93 [PubMed] [Google Scholar]
- Lozynskyi I, Biletska H, Semenyshyn O, Fedoruk V, et al. Active Natural Foci of Tick-Borne Neuroinfection in the North-West Region of Ukraine 10.5772/54912 in Encephalitis Edited by Tkachev Sergey, ISBN 978-953-51-0925-9, 292 pages, Publisher: InTech, Chapters published January 09, 2013 under CC BY 3.0 license DOI: [DOI]
- Malyj VP, Shepilova NV, Tkachenko LV. Tick-borne infections in the Kharkiv region. Int Med J 2010; 3:99–102 [Google Scholar]
- Movila A, Rolain JM, Podavalenko A, Toderas I, et al. Detection of spotted fever group rickettsiae and family Anaplasmataceae in Ixodes ricinus ticks from Republic of Moldova and Eastern Ukraine. Clin Microbiol Infect 2009; 15 Suppl 2:32–33 [DOI] [PubMed] [Google Scholar]
- Mysterud A, Easterday WR, Qviller L. Spatial and seasonal variation in the prevalence of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in questing Ixodes ricinus ticks in Norway. Parasites Vectors 2013; 6:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Król N, Pfeffer M, Ohlendorf V, et al. The diversity of tick-borne bacteria and parasites in ticks collected from the Strandja Nature Park in south-eastern Bulgaria. Parasit Vectors 2018; 11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg M. Tick-Borne Infections in Humans. Aspects of immunopathogenesis, diagnosis and co-infections with Borrelia burgdorferi and Anaplasma phagocytophilum/Linköping University Medical Dissertations No. 1315–Linköping, Sweden, 2012:139 [Google Scholar]
- Pañczuk A, Tokarska-Rodak M, Kozioł-Montewka M, Plewik D. The incidence of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microticoinfections among foresters and farmers in eastern Poland. J Vector Borne Dis 2016; 53:348–354 [PubMed] [Google Scholar]
- Pavlikovska TM, Chyzh V, Hlavachek , Lozynskyi I, et al. Tick-borne viral encephalitis (TBE) in Ukraine in 2013: information letter No 04.3/346 from 03.12.2014. DZ Ukrainian center for disease control and monitoring of the Ministry of Health of Ukraine, Lviv Scientific Research Institute of epidemiology and hygiene, Ministry of health of Ukraine; Kyiv, 2014: 6 [Google Scholar]
- Reye AL, Stegniy V, Mishaeva NP, Velhin S, et al. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One 2013; 8:e54476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigó K, Gyuranecz M, Tóth AG, Földvári G. Detection of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in small mammals and ectoparasites in Hungary. Vector Borne Zoonotic Dis 2011; 11:1499–1501 [DOI] [PubMed] [Google Scholar]
- Stańczak J, Gabre RM, Kruminis-Łozowska W, Racewicz M, et al. Ixodes ricinus as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann Agric Environ Med 2004; 11:109–114 [PubMed] [Google Scholar]
- Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum–a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 2013; 3:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ, Neitzel D, Reed K, Belongia EA. Coinfections acquired from ixodes ticks. Clin Microbiol Rev 2006; 19:708–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe J, Jordan D, Janecek E, Fingerle V, et al. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit Vectors 2014; 7:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Víchová B, Majláthová V, Nováková M, Stanko M, et al. Anaplasma infections in ticks and reservoir host in Slovakia. Infect Genet Evol 2014; 22:265–272 [DOI] [PubMed] [Google Scholar]
- Vynograd N. Natural foci diseases as a stable biological threat. Arch Immunol Ther Exp (Warsz) 2014; 62:445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielinga PR, Gaasenbeek C, Fonville M, de Boer A, et al. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl Environ Microbiol 2006; 72:7594–7601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik-Fatla A, Szymańska J, Wdowiak L, Buczek A, et al. Coincidence of three pathogens (Borrelia burgdorferi Sensu Lato, Anaplasma phagocytophilum and Babesia microti) in Ixodes ricinus ticks in the Lublin Macroregion. Ann Agric Environ Med 2009; 16:151–158 [PubMed] [Google Scholar]