Abstract
Background: Transcranial photobiomodulation (t-PBM) is a noninvasive modality that may improve cognitive function in both healthy and diseased subjects.
Objective: This systematic review and meta-analysis addresses the question of whether t-PBM improves cognitive function in healthy adults.
Methods: We searched MEDLINE using PubMed, EMBASE, SCOPUS, Web of Science, and Cochrane Library up to March 2019. We also searched ProQuest and Google Scholar databases for unpublished material. The search was limited to articles on the procognitive effects of t-PBM in healthy adults. The initial search resulted in 871 studies, of which nine publications met our criteria for inclusion and exclusion. Seven studies were performed on young, healthy subjects (17–35 years), and two studies were conducted on older (≥49 years), normal subjects. A meta-analysis was performed on six full-text publications whose subjects were young adults.
Results: t-PBM administration improved cognition-related outcomes by an 0.833 standardized mean difference (SMD; 95% confidence interval (CI): 0.458–1.209, 14 comparisons) in young, healthy participants. Funnel plotting revealed asymmetry, which was validated using Egger's (p = 0.030) and Begg's regression (p = 0.006) tests. However after reanalysis, this asymmetry disappeared in the attention subgroup, but not in the memory subgroup. The trim-and-fill analysis indicated two studies were lacking required data. Thus, the effect size was adjusted from an SMD of 0.761 (95% CI: 0.573–0.949) to 0.949 (0.779–1.120). The overall quality score of the studies was modest.
Conclusions: We demonstrated a significant, beneficial effect of t-PBM on cognitive performance of young, healthy individuals; however, the heterogeneity of the data was high. This could be due to the modest quality or to the low number of included studies, or to the differences between the various subdomains assessed. These shortcomings should be meticulously addressed before concluding that t-PBM is a cognitive-enhancing intervention in healthy individuals.
Keywords: transcranial photobiomodulation, cognition, memory, learning, attention, healthy adults
Introduction
The significant impact of various cognitive enhancement strategies has been demonstrated in the scientific literature.1 Mounting evidence suggests that these interventions may help maintain cognitive performance, and preserve the quality of life and independence of both young and older adults. With the introduction of novel technologies, namely computers and videogames, these interventions or strategies could be readily applied to both the young and older populations.2,3 Among these novel approaches, the potential of photobiomodulation (PBM) has been underestimated.
PBM, also known as low-level light/laser therapy (LLLT), has been proposed to be a noninvasive modality that stimulates many different biological processes.4 Lately, transcranial PBM (t-PBM) has gained increased attention as a novel approach to stimulate the physiological functions of the brain.5,6 Mechanistically, at the appropriate light irradiance and fluence, photons at red (600–670 nm) and near-infrared (NIR; 800–1100 nm) wavelengths are absorbed by the cytochrome c oxidase (CCO) in neuronal mitochondria, leading to an increased synthesis of adenosine triphosphate (ATP), along with photodissociation of nitric oxide (NO), yet levels of reactive oxygen species remain comparably low.7 After passing through the scalp and the skull, the red/NIR photons can also affect cerebral blood vessels and subsequently increase cerebral blood flow (CBF), due to the vasodilation triggered by the release of NO.8
Both experimental9–11 and clinical research12–14 suggests that t-PBM improves cognitive function in the diseased brain. t-PBM has also shown promising results for cognitive enhancement in healthy individuals.15,16 Human studies have demonstrated that t-PBM using low powers of light from both lasers or light-emitting diodes (LEDs) emitting specific wavelengths of red and NIR light can potentially increase the oxygen consumption and metabolic activity in the frontal cortex, which may subsequently lead to improved cognitive performance in several domains.17–20
Systematic reviews and meta-analyses are meant to be careful, scientific syntheses of current data to inform the decision making of clinicians and researchers. On one hand, systematic reviews are inherently a qualitative assessment as they typically report a series of findings and address the question of whether scientific discoveries are coherent and able to be generalized over various populations and settings. On the other hand, meta-analyses, by offering a quantitative summary of findings, can more precisely estimate the overall treatment effects and exposure risks.21 Our systematic review and meta-analysis addresses whether t-PBM improves cognitive function in young and older healthy adults.
Materials and Methods
Search strategy
Bibliographic databases (i.e., MEDLINE through PubMed, EMBASE, SCOPUS, Web of Science, and Cochrane Library) were searched electronically for studies on the effects of t-PBM on the cognitive performance of healthy individuals through the keywords “low-level light therapy,” “laser therapy,” “phototherapy,” “near-infrared laser,” “transcranial laser,” “photobiomodulation,” “transcranial,” “cognition,” “attention,” “learning,” “memory,” and “executive function.” ProQuest and Google Scholar databases were also searched for unpublished material. Two independent researchers screened the title, abstract, and (whenever needed) the full text of the articles, and judged the searched materials against the inclusion and exclusion criteria. In case of any disagreement, a third independent researcher was called upon to decide on the inclusion of a contested article. There were no restrictions based on date of publication. However, our search was limited to studies performed in human subjects and to publications written in English. Therefore, animal, ex vivo, or in vitro studies were not included.
Inclusion and exclusion criteria
All clinical trials, whether presented in full or in conference articles assessing the effects of t-PBM on the cognitive performance of healthy young or older individuals were included. Studies performed on animals and ex vivo or in vitro (primary cultures or cell line) were excluded. Also, non-English language publications and studies conducted on unhealthy or diseased individuals were excluded from our review and meta-analysis. Studies that had used other diagnostic or therapeutic procedures, such as transcranial magnetic stimulation (TMS), which might alter brain excitability were also excluded. The primary outcomes of our study were cognition-related behavioral outcomes, including measures of attention, memory, learning, and executive function.
Data extraction
The author, publication year and type, number of participants, age, cognitive domain, light source/wavelength, operation mode, output power (W), irradiance (mW/cm2), irradiation time (min), fluence (J/cm2), energy (J), beam spot area (cm2), total area exposed (cm2), irradiation site, number of treatment sessions, neuropsychological tasks, and outcome(s) were extracted. Quality measures, and measures to assess and control study biases were also extracted from the included studies. Further, the number of participants in each group, the standard deviation, or the standard error of the mean were extracted from the studies. However, the time of outcome evaluation was not extracted from the studies. In case more than one experiment was conducted within the same study, each experiment was regarded as a separate study and included in the meta-analysis. However, when neurobehavioral measures were repeated in the same group of participants, only the last evaluation data were extracted from the study. Because most of the presented data in studies were geographically reported, we used Universal Desktop Ruler software, version 2.9 to extract the mandatory data.
Assessment of methodological quality of studies
The methodological attributes of the selected studies, including the measures of internal validity (e.g., performance, selection, detection, and attrition biases) and other measures of study quality, such as reporting quality and power, were evaluated using the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials. This tool identifies the risk of bias in human subject research at different steps, such as: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.22
Statistical analyses
All analyses were performed through Comprehensive Meta-Analysis Software (CMA) version 3. Due to the heterogeneity of the data, the random effects model was used to pool the data statistically. However, because of the homogeneity of the data in the attention subgroup, the fixed effects model was used in this domain. The outcome of this study was the overall effects of t-PBM on cognition-related behavioral outcomes. Funnel plotting,23 Egger's regression,24 Begg's regression, and Duval and Tweedie's nonparametric trim-and-fill approach25 were used for the assessment of publication bias.26 The chi-square test and the Higgins I2 test were applied to evaluate heterogeneity.27
Results
General study characteristics
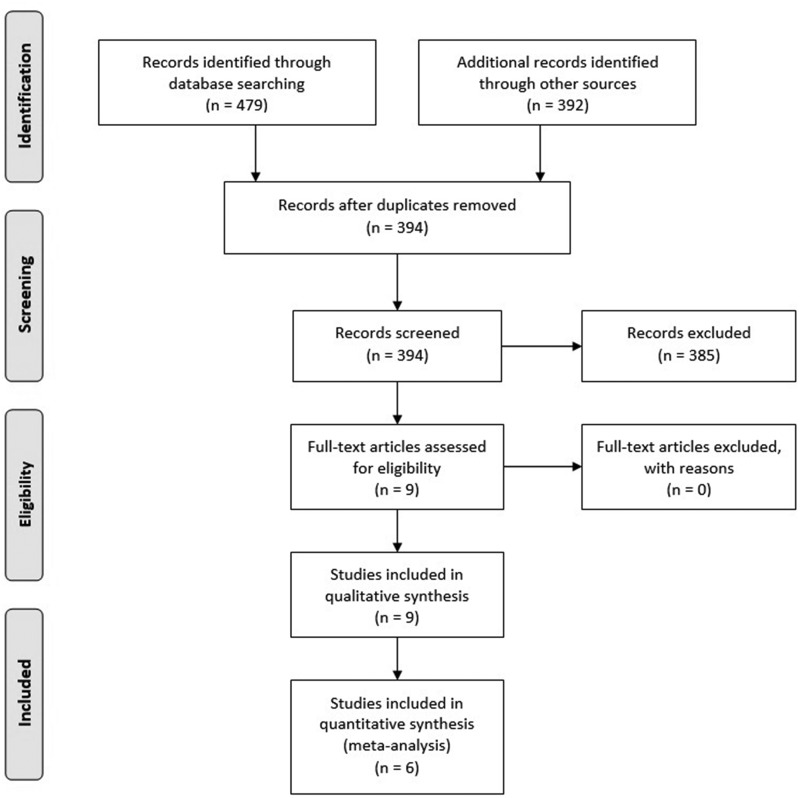
A total of 871 studies were identified during the initial systematic search, of which nine publications met our criteria for inclusion and exclusion. Seven studies were conducted in young healthy subjects, and two in older, normal subjects. Eight publications were full-text articles, and one publication was a conference article. Meta-analysis was performed on six full-text articles whose study subjects were young. Due to the very few studies in older subjects, the meta-analysis could not performed for this age group (Fig. 1).
FIG. 1.
Summary of included and excluded articles.
Seven studies assessed the effects of t-PBM on attention. Also, four studies evaluated the impacts of t-PBM on memory. Two further studies measured the effects of t-PBM on executive function. Only one study assessed the impacts of t-PBM on the learning domain of cognitive performance.
In six studies, a 1064 nm laser light source was used for t-PBM. On the other hand, two studies used an 850 nm LED light source and only one study applied a combined 633/870 nm LED cluster. The operation mode in all studies was continuous wave (CW). Other physical and treatment characteristics, such as output power, irradiance, irradiation time, fluence, beam spot area, and total area exposed are extensively summarized in Table 1. In all studies, the target of irradiation was the frontal cortex, especially the prefrontal cortex (PFC), but in one study, one of the three LED clusters was aimed at the parietal cortex (placement on Pz point on the electroencephalography [EEG] map).
Table 1.
Summary of Studies on the Procognitive Effect of Transcranial Photobiomodulation in Healthy Individuals
| Study (year) | Participants (n) | Age | Cognition domain | Light source/wavelength | Operation mode | Output power (W) | Irradiance (mW/cm2) | Irradiation time (min) | Fluence (J/cm2) | Energy (J) | Beam spot area (cm2) | Total area exposed (cm2) | Irradiation site | Number of treatment sessions | Neuropsychological tasks | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barrett and Gonzalez-Lima (2013)16 | Healthy young volunteers (40) | 18–35 years old | Attention and memory | Laser/1064 nm | CW | 3.4 | 250 | 8 (4/site) | 60/site | 816/site | 12.56 | 25.12 (12.56 × 2 sites) | 2 sites; Medial and lateral to the Fp2 point |
1 | PVT and DMS | Significant improvement of both attention and memory |
| Blanco et al. (2015)43 | Healthy young volunteers (30) | Mean age = 20.4 years | Executive function | Laser/1064 nm | CW | 3.4 | 250 | 8 (4/site) | 60/site | 816/site | 12.56 | 25.12 (12.56 × 2 sites) | 2 sites; Lower and upper portion of right lateral forehead at Fp2 and F4 |
1 | The Wisconsin Card Sorting Task | Improvement of executive function |
| Hwang et al. (2016)44 | Healthy young volunteers (60) | 18–30 years old | Attention and memory | Laser/1064 nm | CW | 3.4 | 250 | 8 (4/site) | 60/site | 816/site | 13.60 | 25.12 (12.56 × 2 sites) | 2 sites; Medial and lateral to the Fp2 point |
1 | PVT and DMS | Significant improvement of both attention and memory |
| Moghadam et al. (2017)41 | Healthy young volunteers (34) | 18–24 years old | Attention | LEDs/850 nm | CW | 0.4 | 285 | 2.5 | 60 | 60/site | 1.4 | 1.4 | 1 site; Right frontal pole at Fp2 |
1 | Level-1 of parametric Go/No-Go task | Promotion of attentional performance |
| Blanco et al. (2017)15 | Healthy young volunteers (118) | 17–35 years old | Learning | Laser/1064 nm | CW | 3.4 | 250 | 8 (4/site) | 60/site | 816/site | 13.60 | 25.12 (12.56 × 2 sites) | 2 sites; Right frontopolar at Fp2 point and right frontal at F4 and F8 |
1 | Category learning task | Enhancement of learning-related cognitive function |
| Fink et al. (2018)20 | Healthy young volunteers (40) | Mean age = 19.0 years | Verbal cognition | Laser/1064 nm | CW | 3.4 | 250 | 8 | 120/site | 1632/site | NR | NR | 1 site; Left prefrontal cortex |
1 | Interference task of verbal cognition | No effect |
| Jahan et al. (2019)42 | Healthy young volunteers (30) | Mean age = 21.2 years | Attention | LEDs/850 nm | CW | 0.4 | 285 | 2.5 | 60 | 60/site | 1.4 | 1.4 | 1 site; Right frontal pole at Fp2 |
1 | Go/No-Go task | Beneficial effects on attentional performance |
| Vargas et al. (2017)17 | Healthy old volunteers (12) | 49–90 years old | Attention and memory | Laser/1064 nm | CW | 3.4 | 250 | 8 (4/site) | 60/site | 816/site | 13.60 | 25.12 (12.56 × 2 sites) | 2 sites; Medial and lateral to the Fp2 point |
5 | PVT and DMS | Significant improvement of both attention and memory |
| Chan et al. (2019)39 | Healthy old volunteers (30) | Mean age = 66.2 years | Frontal-related cognitive function | LEDs/633 + 870 nm | CW | 2.994 (3 × 0.998) | 44.4 | 22.5 (7.5/site) | 20/site | 449/site | 67.44 (3 × 22.48) | 67.44 (3 × 22.48) | 3 sites; Left frontopolar at Fp1 and right frontopolar at Fp2 and Pz |
1 | Eriksen flanker and category fluency tests | Significant improvements in their action selection, inhibition ability, and mental flexibility |
CW, continuous wave; DMS, delayed match-to-sample; LEDs, light-emitting diodes; PVT, psychomotor vigilance task.
Two studies applied the psychomotor vigilance task, and two studies used the Go/No-Go task to assess attention in the participants. Delayed match to sample was used in three studies to evaluate memory. Also, the Wisconsin Card Sorting Task was used in one study to assess executive function. Additionally, the category learning task was applied in one study to assess learning ability in the subjects. The interference task of verbal cognition was used to assess verbal cognition in one study. Finally, Eriksen flanker and category fluency tests were used to assess frontal-related cognitive function in one study. Eight studies used a one-session treatment schedule. However, one study performed in older adults, used a five-session schedule.
All studies showed positive effects of t-PBM on various domains of cognition. Nevertheless, one study (conference article) did not show any effects of laser therapy on cognitive performance.
Global estimates of improvement in the neurobehavioral scores
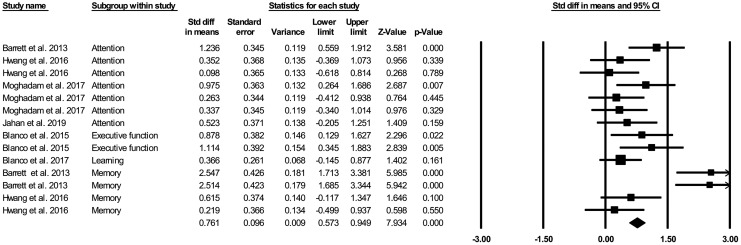
Overall, t-PBM administration in young healthy participants improved cognition-related outcomes by 0.833 standardized mean difference (SMD; 95% confidence interval (CI): 0.458–1.209, 14 comparisons; Fig. 2).
FIG. 2.
Forest plot of SMD of the included comparisons in the meta-analysis. Black diamond represents the pooled SMD. Squares indicate the SMD in each study, where square sizes are inversely proportional to the standard error of the SMD. Horizontal lines signify 95% CI. CI, confidence interval; SMD, standardized mean difference.
Our analysis showed significant heterogeneity between the included studies (χ2 = 51.094, I2 = 74.557, degree of freedom [df] = 13, p = 0.000). However, the heterogeneity was apparently due to the heterogeneous nature of included domains and their related tasks. Accordingly, the analyses were reperformed for both attention and memory subgroups. Reanalysis revealed no heterogeneity between attention data studies (χ2 = 8.217, I2 = 26.985, df = 6, p = 0.223); but, for the memory subgroup heterogeneity between studies persisted (χ2 = 28.872, I2 = 89.609, df = 3, p = 0.000).
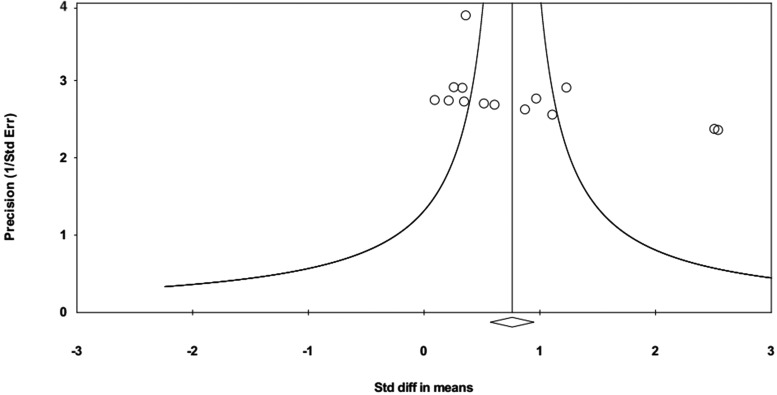
Overall, the funnel plot revealed asymmetry (Fig. 3), which was validated using Egger's (p = 0.030) and Begg's regression (p = 0.006) tests. The trim-and-fill analysis indicated two theoretically missing studies. Thus, the effect size was adjusted from an SMD of 0.761 (95% CI: 0.573–0.949) to 0.949 (0.779–1.120).
FIG. 3.
Evidence of publication (reporting) bias. Funnel plot of precision by SMD; in case of no bias, the points should look like a symmetrical inverted funnel. SMD, standardized mean difference.
On the other hand, this asymmetry disappeared in the attention subgroup, but not in the memory subgroup, after reanalysis. This was validated through Egger's (p = 0.674 for attention, and p = 0.000 for memory) and Begg's regression (p = 1.000 for attention, and p = 0.089 for memory) tests.
Study quality
On the one hand, all of the included studies had apparently complete outcome assessment and were not subject to selective reporting bias. On the other hand, only two and three studies had participants and personnel blinded to the nature of experiments and allocation concealment, respectively. Randomization of the participants was performed in six studies. Conference articles were not assessed by this checklist. Accordingly, the overall quality score of the studies was modest (Table 2).
Table 2.
Methodological Quality Assessment of the Included Publications in the Data Synthesis Based on Cochrane Study Quality Checklist
| Study (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Complete outcome data | Nonselective reporting | Other bias |
|---|---|---|---|---|---|---|
| Barrett and Gonzalez-Lima (2013)16 | + | − | − | + | + | ? |
| Blanco et al. (2015)43 | − | − | + | + | + | ? |
| Hwang et al. (2016)44 | + | + | − | + | + | ? |
| Moghadam et al. (2017)41 | + | + | − | + | + | ? |
| Blanco et al. (2017)15 | + | − | − | + | + | ? |
| Vargas et al. (2017)17 | − | − | − | + | + | ? |
| Chan et al. (2019)39 | + | − | + | + | + | ? |
| Jahan et al. (2019)42 | + | + | − | + | + | ? |
| Total | 6 | 3 | 2 | 8 | 8 | − |
+, observed; −, negated; ?, unknown.
Discussion
Our results showed a significant improvement of cognitive outcomes using t-PBM in young healthy individuals; however, the overall heterogeneity of data was high. The study design and the chosen cognitive subdomain were found to affect the cognition-related outcome scores; in fact t-PBM significantly improved the attention-related outcomes without marked heterogeneity. The heterogeneity of data in the memory subdomain remained high. This could be due to the low number of published studies or to the medium quality of the included data.
Overall, these results support the potential for a single session of t-PBM (CW mode) with red/NIR wavelengths to improve cognition-related neurobehavioral scores. However, these findings should be treated with some caution due to the limitations of the included studies.
Mechanistic insights into the observed effects
All studies conducted a single session of t-PBM and performed neuropsychological tasks accordingly, except Vargas et al.17 who administered t-PBM chronically once a week for 5 weeks. The underlying mechanisms of the procognitive effects of an acute session of t-PBM have not been completely elucidated yet; however, some possible mechanisms have been proposed.
On the one hand, medial and left inferior frontal cortices are believed to control higher-order cognitive functions.28 On the other hand, both regional CBF and frontal lobe metabolic activity are considered necessary for normal cognitive function.29 Also, NO is a well-known vasodilator, which mediates the regulation of CBF, most likely through improving the vessel extensibility and resistance.30 During t-PBM, NO is released from its binding sites in the neuronal CCO through a photodissociation process7 and consequently improves CBF in the respective areas.8 Also, t-PBM using a 1064 nm laser increases oxidized CCO concentrations and cerebral oxygenation in the frontal cortex.31 It is noteworthy that the observed beneficial impacts of t-PBM on the cerebral hemodynamics and metabolism are not due to any laser-induced heating effects on the scalp.32 Further, it has been proposed that red LEDs at 627 nm act on cerebral superficial blood vessels and alters the elasticity and resistance of the cerebrovascular endothelium, at least partly through the NO pathway.19
In addition, it has been revealed that the electrical activity of the brain changes throughout the cerebral cortex during t-PBM.33 It was shown that t-PBM (1064 nm laser, output power of 2.2 W, a beam spot area of 13.6 cm2, irradiance of 160 mW/cm2, fluence of 105.6 J/cm2, for 11 min) applied to the right forehead increased the power density of the alpha band and a small portion of the beta band in healthy subjects.33 Laser-induced increase in alpha band power was also dose dependent so that the most robust activation was observed between 8 and 10 min (77–96 J/cm2 on the scalp) after initiation of t-PBM session. This study suggested that desynchronization of alpha and beta waves and subsequent neuromodulation of an ipsilateral, fronto-parieto-occipital network and also a contralateral parieto-occipital network at the alpha frequency may in part explain the improved cognitive functions following t-PBM.33,34
Improved mitochondrial function and ATP production, reduced oxidative stress, reduced apoptosis, and neuroinflammation are other mechanisms that might be responsible for the long-lasting procognitive effects of red/NIR t-PBM.9,35
t-PBM characteristics
Cassano et al.36 used simulation modeling and reported that t-PBM with 810 nm LEDs (irradiance of 300 mW/cm2, a total exposed area of 28.4 cm2, for 5 min) on the F3 or F4 EEG points, delivered a peak fluence of 1.72 J/cm2 to the dorsolateral PFC (dlPFC). Moreover, a peak fluence of 0.82 J/cm2 was achieved at ventromedial PFC (vmPFC) using the same treatment strategy for transcranial irradiation of the Fp1-Fpz-Fp2 points.36 A cortical fluence range of 0.3–3 J/cm2 was reported to be a sufficient photoneuromodulatory dose,37 which effectively improves frontal lobe oxygenation18 and CCO levels in healthy individuals.31 Similarly, the physical and treatment parameters applied in almost all of the included studies were nearly the same as those considered by Cassano et al.36 In the included studies, the stimulation sites were the left and right vmPFC, corresponding to Fp1 and Fp2. The F4 and F8 sites that were also the target of t-PBM refer to the right dlPFC and right lateral frontal area, respectively.
Evidence suggested that only 2% of 1064 nm laser light could pass through the supraorbital frontal skull at Fp2 point.16 Light transmission percentages of 0.5% and 2.1% for the frontal skull were also reported for LED light at 633 and 830 nm wavelengths, respectively.38 In all of the enrolled studies, except one,39 the scalp fluence of 60 J/cm2 per site was applied for 1064 nm laser and 850 nm LED light. Based on the aforementioned light penetration data, it could be assumed that an ∼1.2 J/cm2 light dose was received by cortical neurons in those studies. However, in one study,39 the authors used a scalp fluence of 20 J/cm2 per site for combined 633/870 nm LED light. Thus, it is expected that a fluence of 0.1–0.4 J/cm2 reached the cortical surface which is close to the neurostimulatory range.
Whether noncoherent LED light is as effective as coherent laser light for PBM purposes is a matter of debate.40 In three studies,39,41,42 which used LED clusters, the beneficial effects on the various cognitive domains were qualitatively similar to those of laser t-PBM studies.15–17,43,44 Therefore, it might be suggested that light coherence is not necessarily needed for achieving effective procognitive outcomes. Of note, LED arrays are less expensive than lasers; moreover, flexible LED devices can be integrated into helmets for t-PBM application. Besides, wearable LED arrays with a large active area could provide a cost-effective device for ambulatory frontal lobe photostimulation.
Study quality
We applied the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials to assess the internal and external validity of the included articles. Our previous studies have revealed that articles that possess low methodological quality were predisposed to overestimating effect sizes.26 Accordingly, the quality of the articles assessed in this meta-analysis was only modest, and importantly strategies to reduce publication bias and increase quality, such as blinding of participants/personnel to the nature of experiments and allocation concealment were rarely reported or performed. Significant heterogeneity between studies was also present, which could be attributed to the difference between the assessed subdomains and to the low number of studies. However, this should be considered with caution, as the heterogeneity may indicate the existence of other factors (e.g., t-PBM parameters) that influence the impacts observed. The identification of these putative, hidden factors will further help researchers to design higher-quality studies, necessary to investigate t-PBM as a procognitive strategy.
Potential limitations
Our meta-analysis had some inevitable shortcomings; thus, the findings of this study should be considered with caution. First, this study was an observational study, which was performed on existing datasets, so our findings should only be regarded as a hypothesis. Second, as the methodological quality of the included articles was not high, our results may have overestimated and overstated the effect sizes, or vice versa. Third, our meta-analysis may also be subject to publication bias.
Conclusions
Cognitive enrichment strategies exert a protective effect against subsequent development of dementia later in life. t-PBM, as an emergent cognitive enhancing modality, has shown promising results in the improvement of cognitive function both in young and older healthy adults. Our study confirmed these effects in young healthy individuals; however, due to the limited number of studies on older subjects, we could not adequately investigate the older adults. Also, significant heterogeneity was found between studies, which could stem from either modest quality of included studies or from differences between the assessed cognitive subdomains. The low number of included studies may also affect the reliability of our findings. These shortcomings should be meticulously addressed before concluding that t-PBM is an effective cognitive-boosting intervention in healthy individuals.
Acknowledgments
The authors would like to express their gratitude to Dr. Saeed Sadigh-Eteghad for assistance with data analyses. M.R.H. was funded by US NIH Grants R01AI050875 and R21AI121700.
Author Disclosure Statement
F.S. is on the Scientific Advisory Board and consultant of Niraxx Light Therapeutics, Inc., and a consultant of ProNeuroLIGHT LLC. Dr. Cassano's salary was supported by the Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award), by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and by the Photothera, Inc. unrestricted grant. Drug donation from TEVA. Travel reimbursement from Pharmacia-Upjohn. Dr. Cassano has received consultation fees from Janssen Research and Development. Dr. Cassano has filed a provisional patent related to the use of near-infrared light in psychiatry. PhotoMedex, Inc. supplied four devices for a clinical study. Dr. Cassano is/has 1. Received unrestricted funding from Litecure, Inc. to conduct a study on transcranial photobiomodulation for the treatment of major depressive disorder; 2. Received unrestricted funding from Cerebral Sciences to conduct a study on transcranial photobiomodulation for the treatment of generalized anxiety disorder; 3. Cofounded, member of the board of directors and consultant of Niraxx Light Therapeutics, Inc., a company focused on the development of new modalities of treatment based on near-infrared light. M.R.H. is on the following Scientific Advisory Boards: Transdermal Cap, Inc., Cleveland, OH; BeWell Global, Inc., Wan Chai, Hong Kong; Hologenix, Inc. Santa Monica, CA; LumiThera, Inc., Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon, Inc., Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV, Inc., Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV, Inc., Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc., Boston, MA; Dr Hamblin has been a consultant for Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V.; Johnson & Johnson, Inc., Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; Dr Hamblin is a stockholder in Global Photon, Inc., Bee Cave, TX; Mitonix, Newark, DE. The other authors have no conflicts of interest to disclose.
References
- 1. Milgram NW, Siwak-Tapp CT, Araujo J, Head E. Neuroprotective effects of cognitive enrichment. Ageing Res Rev 2006;5:354–369 [DOI] [PubMed] [Google Scholar]
- 2. Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One 2012;7:e40588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Bastian CC, Langer N, Jäncke L, Oberauer K. Effects of working memory training in young and old adults. Mem Cognit 2013;41:611–624 [DOI] [PubMed] [Google Scholar]
- 4. Hamblin MR, Ferraresi C, Huang Y-Y, Freitas LF, Carroll JD. Low-Level Light Therapy: Photobiomodulation. SPIE Press Bellingham, WA, 2018 [Google Scholar]
- 5. Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain photobiomodulation therapy: a narrative review. Mol Neurobiol 2018;55:6601–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamblin MR. Shining light on the head: photobiomodulation for brain disorders. BBA Clin 2016;6:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 2016;22:348–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med 2010;42:566–576 [DOI] [PubMed] [Google Scholar]
- 9. Salehpour F, Ahmadian N, Rasta SH, Farhoudi M, Karimi P, Sadigh-Eteghad S. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose—induced aging mice. Neurobiol Aging 2017;58:140–150 [DOI] [PubMed] [Google Scholar]
- 10. Lu Y, Wang R, Dong Y, et al. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging 2017;49:165–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Taboada L, Yu J, El-Amouri S, et al. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis 2011;23:521–535 [DOI] [PubMed] [Google Scholar]
- 12. Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg 2017;35:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimul 2016;9:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma 2014;31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco NJ, Saucedo CL, Gonzalez-Lima F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 2017;139:69–75 [DOI] [PubMed] [Google Scholar]
- 16. Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013;230:13–23 [DOI] [PubMed] [Google Scholar]
- 17. Vargas E, Barrett DW, Saucedo CL, et al. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci 2017;32:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med 2016;48:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salgado AS, Zângaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med Sci 2015;30:339–346 [DOI] [PubMed] [Google Scholar]
- 20. Fink L, Barrett D, Poondla R, Gonzalez-Lima F. F77. Effect of transcranial infrared laser stimulation to left prefrontal cortex on verbal cognition. Biol Psychiatry 2018;83:S267 [Google Scholar]
- 21. Mulrow CD. Systematic reviews: rationale for systematic reviews. BMJ 1994;309:597–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Worp HB, Macleod MR, Bath PM, et al. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke 2014;9:642–645 [DOI] [PubMed] [Google Scholar]
- 24. Vesterinen HM, Currie GL, Carter S, et al. Systematic review and stratified meta-analysis of the efficacy of RhoA and Rho kinase inhibitors in animal models of ischaemic stroke. Syst Rev 2013;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duval S, Tweedie R. Trim and fill: a simple funnel-plot—based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463 [DOI] [PubMed] [Google Scholar]
- 26. Sadigh-Eteghad S, Majdi A, McCann SK, Mahmoudi J, Vafaee MS, Macleod MR. D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS One 2017;12:e0184122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer's disease. Neurosciences (Riyadh) 2012;17:321–326 [PubMed] [Google Scholar]
- 28. Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 1991;11:684–689 [DOI] [PubMed] [Google Scholar]
- 29. Aanerud J, Borghammer P, Chakravarty MM, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 2012;32:1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide in neurological disorders. Can J Physiol Pharmacol 2009;87:581–594 [DOI] [PubMed] [Google Scholar]
- 31. Wang X, Tian F, Reddy DD, et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: a broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab 2017;37:3789–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Reddy DD, Nalawade SS, Pal S, Gonzalez-Lima F, Liu H. Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics 2017;5:011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Dmochowski J, Husain M, Gonzalez-Lima F, Liu H. Transcranial infrared brain stimulation modulates EEG alpha power. Brain Stimul 2017;10:67–69 [Google Scholar]
- 34. Palva S, Palva JM. New vistas for α-frequency band oscillations. Trends Neurosci 2007;30:150–158 [DOI] [PubMed] [Google Scholar]
- 35. Salehpour F, Farajdokht F, Mahmoudi J, et al. Photobiomodulation and coenzyme Q10 treatments attenuate cognitive impairment associated with model of transient global brain ischemia in artificially aged mice. Front Cell Neurosci 2019;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cassano P, Tran AP, Katnani H, et al. Selective photobiomodulation for emotion regulation: model-based dosimetry study. Neurophotonics 2019;6:015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma SK, Kharkwal GB, Sajo M, et al. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med 2011;43:851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One 2012;7:e47460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan AS, Lee TL, Yeung MK, Hamblin MR. Photobiomodulation improves the frontal cognitive function of older adults. Int J Geriatr Psychiatry 2019;34:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heiskanen V, Hamblin MR. Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci 2018;17:1003–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moghadam HS, Nazari MA, Jahan A, Mahmoudi J, Salimi MM. Beneficial effects of transcranial light emitting diode (LED) therapy on attentional performance: an experimental design. Iran Red Crescent Med J 2017;19:e44513 [Google Scholar]
- 42. Jahan A, Nazari MA, Mahmoudi J, Salehpour F, Moghadam Salimi M. Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers Med Sci 2019. [Epub ahead of print]; DOI: 10.1007/s10103-018-02710-3 [DOI] [PubMed] [Google Scholar]
- 43. Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2015;11:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hwang J, Castelli DM, Gonzalez-Lima F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 2016;31:1151–1160 [DOI] [PubMed] [Google Scholar]