Abstract
Linear growth failure results from a broad spectrum of systemic and local disorders that can generate chronic musculoskeletal disability. Current bone lengthening protocols involve invasive surgeries or drug regimens, which are only partially effective. Exposure to warm ambient temperature during growth increases limb length, suggesting that targeted heat could noninvasively enhance bone elongation. We tested the hypothesis that daily heat exposure on one side of the body unilaterally increases femoral and tibial lengths. Mice (N = 20) were treated with 40 °C unilateral heat for 40 min/day for 14 days post-weaning. Non-treated mice (N = 6) served as controls. Unilateral increases in ear (8.8%), hindfoot (3.5%), femoral (1.3%), and tibial (1.5%) lengths were obtained. Tibial elongation rate was > 12% greater (15 μm/day) on the heat-treated side. Extremity lengthening correlated with temperature during treatment. Body mass and humeral length were unaffected. To test whether differences persisted in adults, mice were examined 7-weeks post-treatment. Ear area, hindfoot, femoral, and tibial lengths were still significantly increased ~6%, 3.5%, 1%, and 1%, respectively, on the heat-treated side. Left-right differences were absent in non-treated controls, ruling out inherent side asymmetry. This model is important for designing noninvasive heat-based therapies to potentially combat a range of debilitating growth impediments in children.
Keywords: growth plate, endochondral ossification, temperature, phenotypic plasticity, noninvasive therapeutic bone lengthening
Bone elongation disorders have multiple underlying causes, ranging from injury and illness to genetic bone disease. Advancing insight into linear growth regulation at the molecular level1 has outpaced development of strategies to offset short stature and/or leg length discrepancy caused by childhood growth failure. Limb length inequality can lead to disabling health conditions in adulthood, such as scoliosis, chronic back pain, and osteoarthritis.2 Alternatives are needed because existing limb-lengthening procedures involve invasive surgery and/or drug regimens, which are only partially effective.3 A major obstacle to successful bone lengthening by noninvasive means is difficulty in targeting therapeutics to cartilaginous growth plates, which do not have a direct blood supply. Experimental drug delivery approaches include surgically implanted catheters and localized injections into specific growth plates.4,5
Data from our lab and others demonstrate that exposure to warm ambient temperature during growth increases bone blood supply and length in young mice.6,7 While continuous whole body heating does not effectively translate to the clinic, intermittent-targeted heating could be accomplished with a heating pad or temperature cuff. Localized heat could be an alternative to surgery and a supplement to systemic bone-lengthening drugs to noninvasively achieve limb length equalization.
The objective of this project is to test a unilateral heating model to increase length of specific bones without surgical or drug intervention. We hypothesize that daily heat exposure on one side of the body will unilaterally increase femoral and tibial lengths on the heat-treated side. Our goal is to develop a low-cost, noninvasive method for lengthening bones that can translate into practical therapy to offset linear growth impediments in children.
MATERIALS AND METHODS
Animals and Experimental Design
Procedures were approved by the Institutional Animal Care and Use Committee of Marshall University (Protocol 558). A total of N = 26 male and female C57BL/6 mice were obtained from a commercial vendor or on-site breeding colony at 3 weeks weaning age.
Animals were singly caged at 21 °C in order to minimize external temperature fluctuations due to huddling.8,9 Group housing can be a major source of variation due to increased cage activity.11 Singly housed mice have reduced variance in bone and body composition,10 which is important for experimental consistency.
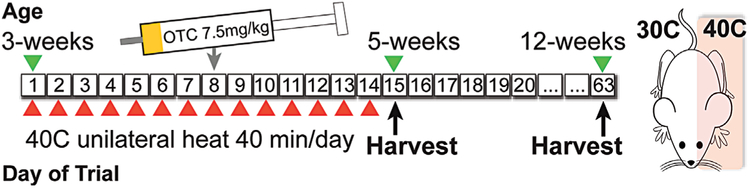
Mice (N = 20) were treated once daily to a unilateral heating regimen for 14 days post-weaning. Mice were anesthetized with 1.5% isoflurane and placed in lateral recumbency on a 40 °C heating pad for 40 min each day (Fig. 1). Temperature of the procedure room was 19 °C. Heating was deliberately scheduled at the same time each day at the light cycle start when growth plate height and growth rate are maximal.12,13 The 3–5 week age interval is a time of rapid, temperature-sensitive growth in mice.14 By comparison, this period could be considered roughly similar to human development between toddler age and entry to middle school.
Figure 1.
Unilateral heating schematic. Mice were treated with 40 °C unilateral heat for 40 min per day for 14 consecutive days. Oxytetracycline (OTC) was given at the study midpoint to quantify tibial elongation rate.
Left and right sides were heat-treated in separate trials to rule out potential side variation. Limbs on the heat-treated side were wrapped in custom fitting thermal booties to ensure uniform heat distribution (Fig. 3C inset). Foam separators prevented heat transfer to the non-treated side. Ear and hindfoot temperatures were taken three times during each daily treatment using infrared thermometry.7 Core temperature and respiration were recorded at least twice per treatment. Mice were weighed daily. A separate group of singly housed mice (N = 6) that were not subject to anesthesia or heat served as treatment controls.
Figure 3.
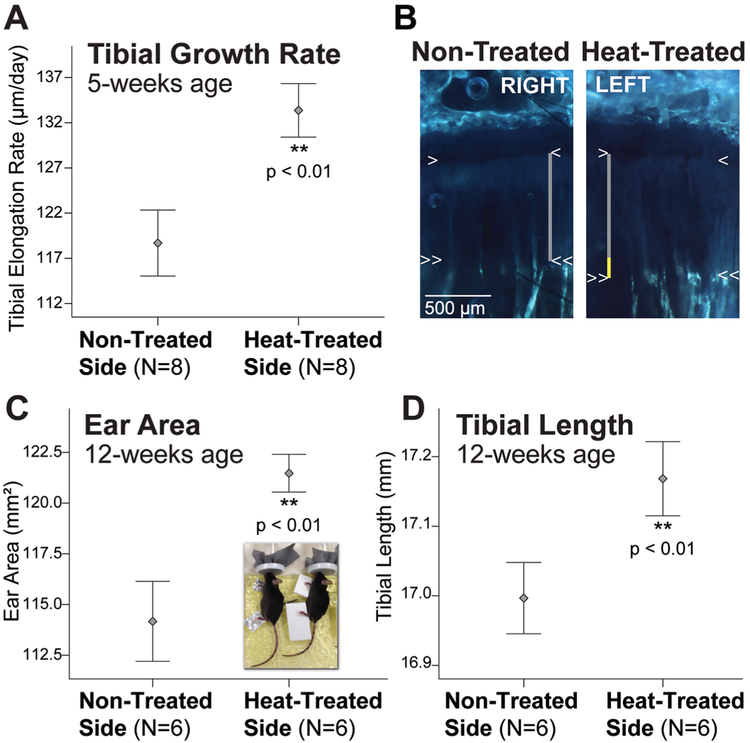
Extremities are lengthened on the heat-treated side. (A) Error bar plots show > 12% increase in tibial elongation rate on the heat-treated side. (B) Right-left tibial slab sections from the same mouse labeled with OTC. The metaphyseal chondro-osseous junction (COJ) is indicated by the single arrowheads. Double arrowheads show OTC band in metaphyseal bone. Growth rate was calculated by measuring the vertical distance between the arrowheads (gray lines). Yellow segment of the vertical line on the heat-treated side shows the total difference in length measured over 7-days. (C) Ear area and (D) tibial length remain significantly increased on the heat-treated side in skeletally mature adults after only 14 days of juvenile heat exposure (see Fig. 1). Mean ± 1 standard error plotted.
Mice were given a single intraperitoneal injection of oxytetracycline (OTC) (7.5 mg/kg, Norbrook 200 mg/ml) at the treatment midpoint to measure tibial elongation rate. OTC is a calcium chelator that becomes permanently incorporated into mineralizing tissue and leaves a band of fluorescence that has long been used as a standard for quantifying bone elongation rate in young growing animals.15-17
Tissue Collection and Elongation Rate Analyses
Experimental 5-week-old mice (N = 14 females) were euthanized for tissue harvest 1 day after the last heat-treatment. Control mice (N = 6 females) were euthanized at the same endpoint. Experimental 12-week-old mice (N = 6, mixed sex) were euthanized 49 days post-heating to evaluate persistent limb length differences at skeletal maturity (Fig. 1). In addition to limb length, cartilaginous ears were measured to document a treatment effect because ear size increases with ambient temperature.7,18
Tibial elongation rate was measured for all 5-week-old control mice (N = 6) and a subset of the 5-week-old experimental mice (N = 8). Tibiae from the remaining 5-week experimental mice (N = 6) and all 12-week-old mice (N = 6) were kept intact for lengths. Femora and humeri from all mice (N = 26 total) were reserved for length.
The proximal tibial growth plate was selected to measure elongation rate because its relatively flat contour yields a uniform growth rate across the epiphysis.17 The adjacent distal femoral growth plate was not used due to its undulating shape (with varied growth rate) and irregular geometry that changes with age,19 which introduces sampling error and measurement inconsistency. The proximal femoral and distal tibial growth plates were also not used because they contribute least to total limb lengthening,20 with correspondingly reduced growth activity.21
The OTC label was visualized in unfixed slab sections of bisected tibiae. One half of each bone was placed in a specialized holder on a glass slide, and cover-slipped with glycerol in PBS. The other half was reserved for a separate histological study. Fluorescence was visualized using a UV filter on a fluorescence stereomicroscope. Brightfield (to delineate the chondro-osseous junction) and fluorescence images were captured in tandem.
Images were calibrated manually in ImageJ software (version 1.44, National Institutes of Health, USA) from a 2mm stage micrometer, and then two lines were drawn on each image. The first line was drawn across the metaphyseal chondro-osseous junction (COJ), marked by invading vasculature at the lower edge of the growth plate (single arrowheads in Fig. 3B). The second line was drawn across the leading (proximal) edge of the OTC band in metaphyseal bone (double arrowheads in Fig. 3B). The vertical distance between the lines was measured at 5 equidistant points across the growth plate using ImageJ. Measurements were averaged and divided by the 7-day labeling period to estimate daily elongation rate (μm/day).
Long Bone, Ear, and Hindfoot Measurements
Long bones (femora, tibiae, and humeri) were dissected, cleaned, dried overnight, and scanned on a flatbed scanner. Calibrations were obtained from an included metric ruler. Measurements were acquired from the scanned and calibrated images by drawing a line between proximal and distal landmarks on the articular ends: Femoral length was measured parallel to the shaft from the proximal-most point on the greater trochanter to the distal-most point on the medial condyle; tibial length was measured between the proximal articular surface and the distal-most point on the medial malleolus; and humeral length was the distance between the most proximal and distal articular surfaces. This scan-based method of bone measurement has been previously described as a technique for reliably obtaining limb length data from mice.22,23
Left and right ears were removed by cutting along the concave (inner) base of the pinnae. Ears were placed between glass slides, scanned, and calibrated as above. Areas were acquired by manually tracing the scanned ears in ImageJ.
Hindfoot measurements were collected from digital photographs calibrated with a metric ruler. Limb positions were standardized. To account for the natural bend in the digits, hindfoot length was measured as the sum of two connecting lines drawn between the proximal-most end of the skin overlying the calcaneus (heel) to the point of metatarsophalangeal flexion (line one) and the point of metatarsophalangeal flexion to the tip of the third digit (line two).
Data Processing and Statistical Analysis
Measurements were performed separately by at least two different individuals. To minimize potential bias since observers were not blinded, at least one of the observers was trained in the methodology but was not involved in conducting experiments. No significant differences were found between observers, and so observer means were used.
Statistical analyses were performed using SPSS 21.0 software (IBM Corporation, Armonk, NY) with α = 0.05 as accepted significance. Body mass comparisons were made between non-treated control mice and heat-treated experimental mice using two-tailed independent samples t-tests. Left-right side comparisons of the heat-treated mice were done using one-tailed paired t-tests. One-tailed tests were performed because of the a priori hypothesis that the heat-treated side would be larger. Two-tailed comparisons were done in control mice to assess natural left-right variation since there was no a priori expectation of right-left asymmetry in non-treated animals. Temperature-size relationships were assessed using Pearson’s correlation.
Data are reported as mean ± standard deviation (SD) in tabular format and as mean ± standard error (SE) in graphical format to facilitate viewing. Sample sizes (minimum of N = 6 mice per variable, unless otherwise stated) were determined a priori by estimating the effect size and data variability to yield a statistical power of 80% at α = 0.05. Missing data (N = 2 femora and N = 1 tibiae pair) are the result of sample loss due to dissection damage and were excluded from statistical testing on an analysis-by-analysis basis (see Table 1).
Table 1.
Comparison of Non-Treated and Heat-Treated Sides of 5-Week-Old Experimental Mice
| Parameter | Non-Treated (30 °C) | Heat-Treated (40 °C) | Percent Increase | N |
|---|---|---|---|---|
| Ear Area (mm2) | 101.7 (6.9) | 110.6 (7.2)*** | 8.8 | 14 |
| Humeral Length (mm) | 10.06 (0.24) | 10.06 (0.25)ns | 0 | 14 |
| Femoral Length (mm) | 12.33 (0.43) | 12.49 (0.45)*** | 1.3 | 12 |
| Tibial Length (mm) | 14.96 (0.29) | 15.18 (0.27)* | 1.5 | 5 |
| Hindfoot Length (mm) | 17.54 (0.63) | 18.15 (0.42)*** | 3.5 | 14 |
| Tibial Elongation Rate (μm/day) | 118.7 (10.3) | 133.4 (8.4)** | 12.4 | 8 |
Values are mean (standard deviation). Sample size (N) is number of left-right pairs. Significantly larger on heat-treated side by one-tailed paired t-test: * p < 0.05; ** p < 0.01; *** p < 0.001; ns, non-significant.
RESULTS
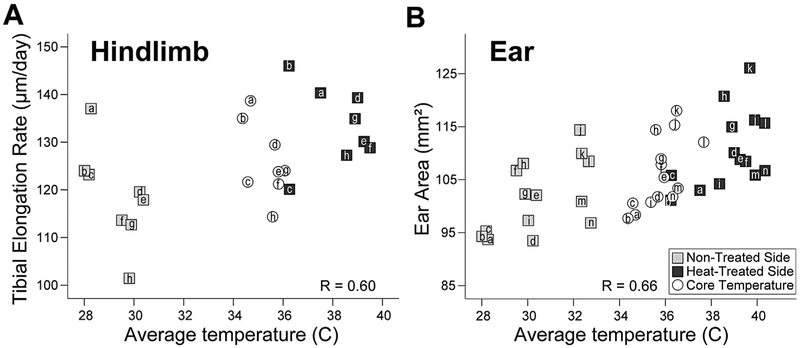
Skin temperatures of heat-treated hindfeet and ears averaged 40 °C during treatments. Non-treated side temperatures averaged 30 °C with no major fluctuations (Fig. 2). Core temperature and respiration were 36 °C and 60 breaths/min, respectively, under anesthesia (Fig. 2). When non-treated and heat-treated sides were analyzed in aggregate, there were significant positive correlations between hindlimb temperature and tibial elongation rate (Pearson’s r = 0.60, p = 0.007) (Fig. 2A), as well as between ear temperature and ear area (Pearson’s r = 0.66, p < 0.001) (Fig. 2B). Core temperature, plotted in Figure 2 against left-right side averages for reference purposes only, was not included in the analyses. Correlations were not significant in the hindlimb when each side was examined separately; however, within-side relationships were significant in the ear for both non-treated (Pearson’s r = 0.56, p = 0.0019) and heat-treated sides (Pearson’s r = 0.42, p = 0.040)(Fig. 2B).
Figure 2.
Extremity temperature correlates with extremity growth. Scatter plots of average temperatures of the hindlimb (A) and ear (B) taken during treatments show a significant positive relationship with growth of non-treated (gray squares) and heat-treated (black squares) sides. Individual 5-week-old mice are denoted by unique letters, which correspond to the same mouse in both graphs. Legend is in (B). For reference, core temperature during treatment (open circles) is plotted against the average value of heat-treated and non-treated sides for each variable. Core was not included in correlation statistics. See text for details.
Tibial elongation rate was over 12% greater on the heat-treated side (Figs. 2A, 3A-B; Table 1). The average growth acceleration was nearly 15 μm/day (paired t = 4.12, p = 0.002). Ear area (paired t = 7.19, p < 0.001) and hindfoot length (paired t = 5.49, p < 0.001) increased 8.8% and 3.5%, respectively, compared to the non-treated contralateral side at 5-weeks (Fig. 2B, Table 1). Femoral (paired t = 6.70, p < 0.001) and tibial (paired t = 3.44, p = 0.013) lengths were increased 1.3% and 1.5%, respectively (Table 1). Humeral length did not differ (paired t = 0.35, p = 0.365) (Table 1).
To test whether left-right differences were evident at skeletal maturity, mice were examined 49 days after the last treatment at 12-weeks age. Importantly, ear area (paired t = 3.98, p = 0.006), hindfoot (paired t = 5.20, p = 0.002), femoral (paired t = 2.20, p = 0.040), and tibial (paired t = 4.02, p = 0.005) lengths were still significantly increased on the heat-treated side of adults (Figs. 3C-D). Heat-treated ears were over 6% larger; hindfeet were 3.5% longer; tibiae were 1% longer; and femora were nearly 1% longer when compared to the non-treated contralateral side. Humeral length did not differ (paired t = 0.18, p = 0.431).
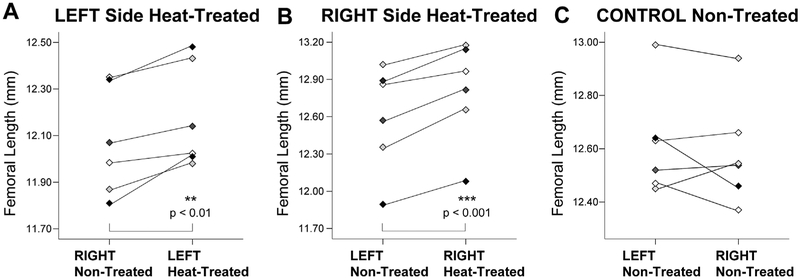
To assess potential side variation, mice were heat-treated on left and right sides in separate trials. Figure 4 shows paired comparisons of non-treated and heat-treated sides of individual 5-week-old mice that were treated on the left (Fig. 4A) and right (Fig. 4B). The average increases in femoral length on the heat-treated side were 1% and 1.6%, respectively, in the left and right side groups. Although the heat response appears to be slightly less pronounced in the left side cohort (paired t = 4.56, p = 0.003) relative to the right side group (paired t = 7.08, p < 0.001), the difference was not significant. An important technical note is that the left cohort was among the first groups examined when methods were not as well established.
Figure 4.
Comparisons of individual mice treated on left and right sides versus non-treated control mice rule out potential side asymmetry. Paired comparisons of non-treated and heat-treated sides of mice that were treated on left (A) and right (B) show an average increase in femoral length on the heat-treated side of 1% and 1.6%, respectively. In contrast, left-right differences were absent in control mice (C).
When experimental mice were compared with non-treated controls, there were no differences in 5-week ending body mass (independent samples t = 0.29, p = 0.777) or average gain in mass (t = 0.17, p = 0.870). Control mice also showed no significant left-right differences in femoral length (Fig. 4C) (paired t = 0.82, p = 0.45), ear area (paired t = 0.02, p = 0.98), humeral length (paired t = 0.94, p = 0.39), hindfoot length (paired t = 0.11, p = 0.91), or tibial elongation rate (paired t = 0.88, p = 0.42), ruling out inherent side asymmetry (Table 2). Control and experimental mice were harvested in separate trials, so differences in total extremity size are the result of variation between cohorts.
Table 2.
Comparison of Left and Right Sides of Non-Treated 5-Week-Old Control Mice
| Parameter | Non-Treated Left | Non-Treated Right | Percent Difference | N |
|---|---|---|---|---|
| Ear Area (mm2) | 98.7 (4.4) | 98.7 (4.7) | 0 | 6 |
| Humeral Length (mm) | 10.07 (0.20) | 10.08 (0.19) | 0 | 6 |
| Femoral Length (mm) | 12.62 (0.20) | 12.59 (0.20) | 0.2 | 6 |
| Hindfoot Length (mm) | 17.90 (0.22) | 17.91 (0.25) | 0 | 6 |
| Tibial Elongation Rate (μm/day) | 120.8 (6.6) | 122.7 (7.6) | 1.5 | 6 |
Values are mean (standard deviation). Sample size (N) is number of left-right pairs. There were no significant left-right differences by two-tailed paired t-tests (all p > 0.40).
DISCUSSION
The goal of this study was to establish a model system using targeted intermittent heat exposure to permanently increase extremity length in mice. Our data support the hypothesis that daily unilateral limb heating increases femoral and tibial lengths on the heat-treated side. Extremity lengthening was correlated with temperature during treatment, particularly in temperature-sensitive cartilaginous ears (Fig. 2B). The length effect persisted at skeletal maturity after only 14 days of post-weaning treatment. A significant right-left difference was measured without impacting overall body mass, and left-right differences were absent in normal non-treated control mice. Core temperature and respiration were in a physiological range under anesthesia. These results suggest that daily unilateral heating is an effective way to model temperature-enhanced hindlimb elongation in young, rapidly growing mice.
The rationale for the model is to develop methods for increasing bone length with minimally invasive methods that can apply to many different growth-limiting conditions. With these baseline data, our model will allow us to move forward and test mechanisms of heat-enhanced bone lengthening to better tailor future clinical therapies. For example, using in vivo multiphoton imaging, we have shown that short-term (30-min) hindlimb heating increases molecular uptake in mouse tibial growth plates.24 Heat could potentially be applied on a scheduled regimen with systemic bone lengthening drugs to target their delivery to specific skeletal growth plates. Routine heat exposure developed in the model here could thus provide a method for augmenting drug-induced limb elongation.
Interestingly, the humerus did not respond to heat-treatment in any of four independent trials conducted for this study (Table 1). Although it is unclear why humeral length did not differ, one potential explanation is the warmer starting temperature of the forelimb when compared to the hindlimb. The knee joint capsule is normally at least 3–4 °C lower than body core.25,26 Our treatments elevated hindlimb temperature by 10 °C on the heat-treated side (Fig. 2). However, skin temperatures in the humeral region more closely resembled body core, consistent with thermal maps for humans showing that 37 °C core temperature extends into the shoulder region, while extremity temperatures progressively decrease in a proximal-distal gradient.27
Our working hypothesis is that heat-treatments do not impact temperature of the humerus due to the proximity of this joint to the body core, and the disproportionately large volume of warm blood delivered to the shoulder region through the large subscapular artery.28 Since most elongation of the humerus occurs at its proximal growth plate (shoulder), versus in the distal (knee) growth plate of the femur,20,29-31 it is possible that the left-right symmetry in humeral length reflects its relatively constant temperature. This could be tested by decreasing shoulder temperature with cold, which stunts limb elongation in a dose-dependent manner.32
Although heat effects on extremity lengthening have been documented for over a century,6 temperature is still under-recognized for its ability to modulate bone elongation in growth plates. Brookes and May33 demonstrated up to a 20% increase in bone growth rate for every 1 °C increase in incubation temperature in growing chicks. Doyle and Smart34 used daily application of short-wave diathermy (heat generating treatment) near the growth plate in rats and showed an increase in femoral and tibial lengths. Granberry and Janes35 were not able to replicate these findings using microwave diathermy in dogs; however, their 100 watt treatment produced bone damage that may have prevented heat-related growth acceleration.
Here we found that unilateral exposure of mild, non-damaging 40 °C heat for 40-min per day for only 14 days permanently increased ear area and hindlimb length on heat-treated sides of young mice. No treatment-related damage was observed morphologically or histologically. These results suggest that heat could be a promising strategy for enhancing elongation potential of specific growth plates without affecting the entire skeleton.
One caveat is that the width of the mouse growth plate is only a fraction of that of a human growth plate. It will be important to replicate these results in a larger animal model to ensure that heat can fully penetrate a larger growth plate, so as to avoid potential angular growth deformities. This should not be problematic, however, since whole body heat-effects on bone length have already been demonstrated in experiments using large animals.18,32 Treatments could be optimized for a more robust effect by using a temperature cuff to target the most active growth plates. The distal femoral and proximal tibial growth plates contribute most to lower limb lengthening in humans.31 Use of a localized heating device around the knee could be a clinically useful tool to augment existing drug therapies (i.e., growth hormone injections) and to potentially avoid invasive surgical limb length correction. A practical strategy in children could be to wrap a noninvasive heating device around the knee during the sleep period when growth occurs13 and growth hormone naturally peaks.36
In conclusion, we believe that such heat-based therapies resulting from the model developed here will have advantages over traditional methods that are potentially painful and invasive. Our next step is to establish the effects in a larger animal model, and test them alongside pharmaceutical interventions. This approach could ultimately lead to development of alternative treatment modalities with better outcomes by reducing costs and side effects of surgery and high-dose systemic pharmaceuticals.
ACKNOWLEDGMENTS
The authors thank Marcos Serrat, D. Neff, and A. Williams for technical assistance and research aid. G. Ion, J. Kerby, and M. Gray helped collect data. Drs. B. Howard, M. Valentovic, and the Animal Resource Facility staff helped with animal protocols and husbandry. J. Denvir advised on statistical analyses. M. Crutchfield and T. Pickens, MUSOM Graphic Designers, helped create artwork. Drs. C. Farnum, R. Williams, E. Hardman, and the US Bone and Joint Initiative provided valuable advice on the study design. This work was aided by the use of facilities at the Huntington, West Virginia VA Medical Center. Dr. J. Leidy, M. Adkins, and T. Boster were critical for enabling access to these facilities, as well as for ensuring consistent temperatures in the building. The editor and two anonymous reviewers provided helpful comments that greatly improved the manuscript.
This research was made possible by generous funding and financial support from: Marshall University Joan C. Edwards School of Medicine (MUSOM) Anatomy and Pathology, Marshall University ADVANCE (NSF 0929997), NASA WV Space Grant Consortium, University of Kentucky CCTS Pilot Program (NIH UL1TR000117), National Science Foundation (MRI-R2 0959012), and ASBMR Grants in Aid Program. MUSOM provided research stipends to JG, LMS, and HLT. The content is solely the responsibility of the authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Grant sponsor: Marshall University, Joan C. Edwards School of Medicine (MUSOM) Anatomy and Pathology; Grant sponsor: Marshall University ADVANCE; Grant number: NSF 0929997; Grant sponsor: NASA WV Space Grant Consortium; Grant sponsor: University of Kentucky CCTS Pilot Program; Grant number: NIH UL1TR000117; Grant sponsor: National Science Foundation; Grant number:MRI-R2 0959012; Grant sponsor: ASBMR Grants in Aid Program; Grant sponsor: NIH/NIAMS; Grant number: 1R15AR067451-01.
Footnotes
Disclosures: T.J.S., M.L.E., J.G., L.M.S., and H.L.T. state that they have no conflicts of interest. F.D.S. is a paid consultant for Lilly for osteoporosis management strategies, which is unrelated to this study. M.A.S. has filed a provisional patent application for work described in this paper. She also has funding from NIH/NIAMS (1R15AR067451-01) for subsequent work related to this study.
REFERENCES
- 1.Karimian E,Chagin AS, Sävendahl L. 2012. Genetic regulation of the growth plate. Front Endocrinol 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurney B. 2002. Leg length discrepancy. Gait Posture 15:195–206. [DOI] [PubMed] [Google Scholar]
- 3.Hasler C, Krieg A. 2012. Current concepts of leg lengthening. J Child Orthop 6:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbaspour A, Takata S, Matsui Y, et al. 2008. Continuous infusion of insulin-like growth factor-I into the epiphysis of the tibia. Int Orthop 32:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Jiang C, Yokota H. 2012. Lengthening of mouse hind limbs with local administration of insulin-like growth factor 2. J Chin Med Assoc 75:494–500. [DOI] [PubMed] [Google Scholar]
- 6.Sumner FB. 1909. Some effects of external conditions upon the white mouse. J Exp Zool 7:97–155. [Google Scholar]
- 7.Serrat MA, King D, Lovejoy CO. 2008. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc Natl Acad Sci USA 105: 19347–19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hilli F, Wright EA. 1983. The effects of changes in the environmental temperature on the growth of the tail bones in the mouse. Br J Exp Pathol 64:34–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Bronson FH. 1984. The adaptability of the house mouse. Sci Am 250:116–125. [DOI] [PubMed] [Google Scholar]
- 10.Nagy TR, Krzywanski D, Li J, et al. 2002. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes Res 10:412–415. [DOI] [PubMed] [Google Scholar]
- 11.Meakin LB, Sugiyama T, Galea GL, et al. 2013. Male mice housed in groups engage in frequent fighting and show a lower response to additional bone loading than females or individually housed males that do not fight. Bone 54: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson S, Hunziker EB, Herrmann W, et al. 1990. Is longitudinal bone growth influenced by diurnal variation in the mitotic activity of chondrocytes of the growth plate. J Orthop Res 8:132–135. [DOI] [PubMed] [Google Scholar]
- 13.Noonan KJ, Farnum CE, Lieferman EM, et al. 2004. Growing pains: are they due to increased growth during recumbency as documented in a lamb model? J Pediatr Orthop 24:726–731. [DOI] [PubMed] [Google Scholar]
- 14.Serrat MA. 2013. Allen’s rule revisited: temperature influences bone elongation during a critical period of postnatal development. Anat Rec 296:1534–1545. [DOI] [PubMed] [Google Scholar]
- 15.Wilsman NJ, Bernardini ES, Leiferman EM, et al. 2008. Age and pattern of the onset of differential growth among growth plates in rats. J Orthop Res 26:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson LI, Menander-Sellman K, Stenström A, et al. 1972. Rate of normal longitudinal bone growth in the rat. Calcif Tissue Res 10:238–251. [DOI] [PubMed] [Google Scholar]
- 17.Farnum CE, Tinsley M, Hermanson JW. 2008. Forelimb versus hindlimb skeletal development in the big brown bat, Eptesicus fuscus: functional divergence is reflected in chondrocytic performance in autopodial growth plates. Cells Tissues Organs 187:35–47. [DOI] [PubMed] [Google Scholar]
- 18.Weaver ME, Ingram DL. 1969. Morphological changes in swine associated with environmental temperature. Ecology 50:710–713. [Google Scholar]
- 19.Chan EF, Harjanto R, Asahara H, et al. 2012. Structural and functional maturation of distal femoral cartilage and bone during postnatal development and growth in humans and mice. Orthop Clin North Am 43:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payton CG. 1932. The growth in length of the long bones in the madder-fed pig. J Anat 66:414–425. [PMC free article] [PubMed] [Google Scholar]
- 21.Serrat MA, Lovejoy CO, King D. 2007. Age- and site-specific decline in insulin-like growth factor-I receptor expression is correlated with differential growth plate activity in the mouse hindlimb. Anat Rec 290:375–381. [DOI] [PubMed] [Google Scholar]
- 22.Young NM, Hallgrímsson B. 2002. Serial homology and the evolution of mammalian limb covariation structure. Evolution 59:2691–2704. [PubMed] [Google Scholar]
- 23.Rolian C. 2008. Developmental basis of limb length in rodents: evidence for multiple divisions of labor in mechanisms of endochondral bone growth. Evol Dev 10:15–28. [DOI] [PubMed] [Google Scholar]
- 24.Serrat MA, Efaw ML, Williams RM. 2014. Hindlimb heating increases vascular access of large molecules to murine tibial growth plates measured by in vivo multiphoton imaging. J Appl Physiol 116:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris ED, McCroskery PA. 1974. The influence of temperature and fibril stability on degradation of cartilage collagen by rheumatoid synovial collagenase. N Engl J Med 290:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Moeini M, Lee KB, Quinn TM. 2012. Temperature affects transport of polysaccharides and proteins in articular cartilage explants. J Biomech 45:1916–1923. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen K. 1997. Animal physiology: adaptation and environment. New York: Cambridge University Press. [Google Scholar]
- 28.Evans HE, de Lahunta A. 2013. Miller’s anatomy of the dog. St. Louis, MO: Elsevier. [Google Scholar]
- 29.Digby KH. 1916. The measurement of diaphyseal growth in proximal and distal directions. J Anat Physiol 50:187–188. [PMC free article] [PubMed] [Google Scholar]
- 30.Bisgard JD, Bisgard ME. 1935. Longitudinal growth of long bones. Arch Surg 31:568–578. [Google Scholar]
- 31.Pritchett JW. 1991. Growth plate activity in the upper extremity. Clin Orthop Relat Res 268:235–242. [PubMed] [Google Scholar]
- 32.Serrat MA. 2014. Environmental temperature impact on bone and cartilage growth. Comprehensive Physiology 4:621–655. PMID: 24715562. [DOI] [PubMed] [Google Scholar]
- 33.Brookes M, May KU. 1972. The influence of temperature on bone growth in the chick. J Anat 111:351–363. [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle JR, Smart BW. 1963. Stimulation of bone growth by short-wave diathermy. Journal of Bone and Joint Surgery 45-A:15–24. [Google Scholar]
- 35.Granberry WM, Janes JM. 1963. The lack of effect of microwave diathermy on rate of growth of bone of the growing dog. J Bone Joint Surg 45A:773–777. [Google Scholar]
- 36.Reiter EO, Rosenfeld RG, Larsen PR, et al. 2003. Normal and aberrant growth Williams Textbook of Endocrinology, 10th ed. Philadelphia, PA: Saunders; pp 1003–1114. [Google Scholar]