Abstract
Background:
Prostate-specific membrane antigen (PSMA) is a rational target for noninvasive detection of recurrent prostate cancer (PCa) and for therapy of metastatic castration-resistant prostate cancer (mCRPC) with PSMA-targeted agents. Here we conducted serial measurements of PSMA expression on circulating tumor cells (CTCs) to evaluate patterns of longitudinal PSMA dynamics over the course of multiple sequential therapies.
Methods:
A retrospective investigation of men with mCRPC undergoing evaluation at medical oncology clinics at our institution assessed the dynamics of PSMA expression in the context of different systemic treatments administered sequentially. Eligibility included patients who began systemic therapies with androgen receptor (AR)-directed agents or taxane agents for whom peripheral blood samples were tested for CTC mRNA of AR splice variant-7 (AR-V7), prostate-specific antigen (PSA), and PSMA (with >2 CTC + results) in a CLIA-accredited laboratory.
Results:
From August 2015 to November 2017, we identified 96 eligible men. Fifteen had greater than or equal to 2 sequential therapies and evaluable CTC samples, greater than or equal to 1 expressing PSMA (PSMA+). Among the 15 patients included in this analysis, a total of 54 PSMA status evaluations were performed in the context of 48 therapies during a median follow-up of 18 months. At baseline, PSMA signal was detected (“positive”) in 11 of 15 (73.3%) patients, while for 4 of 15 (26.7%) patients PSMA signal was undetectable (“negative”). In all but two patients, the baseline collection corresponded with a change in treatment. On the second assessment, PSMA increases were detected in all 4/4 (100%) PSMA-negative patients and 8 of 11 (72.7%) PSMA-positive patients. PSMA significantly decreased in a patient treated with 177Lu-PSMA-617. Serum PSA declines were seen in 7 of 8 (88%) of the treatment periods where PSMA decreased.
Conclusions:
PSMA expression in CTCs is a dynamic marker. PSMA transcript declines appear to be associated with concurrent decreases in serum PSA. Sequential CTC sampling could provide a noninvasive response assessment to systemic treatment for mCRPC.
Keywords: circulating tumor cells, metastatic prostate cancer, prostate-specific membrane antigen, PSA, PSMA
1 |. INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer death in men in the United States resulting in an estimated 31 620 deaths in the United States in 2019.1 Over the past decade, the treatment landscape for patients with metastatic castration-resistant PCa (mCRPC) has expanded with a range of effective new systemic agents approved by the Food and Drug Administration (FDA).2 A major clinical priority is finding and validating biomarkers that identify men most likely to benefit from a given therapy, and biomarkers of early response or progression to optimize outcomes.
Prostate-specific membrane antigen (PSMA), a transmembrane glycoprotein, is a promising target for noninvasive detection of recurrent PCa3 and for therapy of mCRPC using PSMA-targeted agents such as radioligands,4 and is gaining an increasingly important role in the clinical management of PCa patients. PSMA has a limited expression in benign prostatic tissues but is overexpressed 100- to 1000-fold in PCa, and further overexpressed in metastatic and castration-resistant PCa.5 PSMA-targeted radioligand therapies, including 177Lu-PSMA-617, successfully deliver β-radiation directly to PCa cells.6 PSMA positron emission tomography (PET) imaging has demonstrated superiority to traditional imaging in the initial staging of intermediate- and high-risk disease7 and in the detection of biochemical relapse in patients with low PSA values.8,9 PSMA imaging using the radiotracer,18F-DCFPyL, also demonstrated improved sensitivity and specificity over conventional imaging modalities in the metastatic castration-resistant space.10
Most metastatic prostate cancers spread through the blood as circulating tumor cells (CTCs), and enumeration of CTCs correlates with baseline and postbaseline prognosis in the mCRPC population.11 PSMA expression on circulating tumor cells is emerging as a potential method of measuring treatment response.12 PSMA and PSA biomarkers are being used to identify circulating PCa cells in blood,13,14 and high concordance with results of FDA-cleared CellSearch assays have been demonstrated in studies using real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) methods for messenger RNA (mRNA) detection and quantification.15 Our group has demonstrated the use of real-time RT-PCR for determining the presence of CTCs using multiplex PCR assays based on the examination of PSMA, PSA, EGFR, and Actin transcript expression.13,14 In the current study, we performed serial blood-based PSMA sampling across the treatment landscape for mCRPC to describe temporal changes in CTC dynamics with a focus on PSMA dynamics. Our objective was to determine if PSMA expression in CTCs changes at the time of therapeutic decisions and if it correlates with PSA and/or radiographic progression, or response, in patients undergoing sequential therapies using AR-targeting agents, taxane chemotherapies, and PSMA-directed therapies.
2 |. METHODS
2.1 |. Patients study population and laboratory analyses
The patient population was comprised of men with mCRPC undergoing evaluation in the outpatient clinical practice setting at Johns Hopkins University from August 2015 to November 2017. To aid in treatment decisions, peripheral blood samples were obtained and tested at a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory for mRNA expression in CTCs of full-length androgen receptor (AR-FL), androgen receptor splice variant-7 (AR-V7), PSA, and PSMA, using previously published methods.14 Samples were obtained either at the time of clinical/radiographic progression, often coinciding with the initiation of a new therapeutic regimen, or during therapy. No restriction on prior anticancer therapies was applied.
2.2 |. Study design and assessments
A retrospective investigation was conducted to assess the dynamics of PSMA expression in the context of different treatments as sequentially administered across the standard-of-care continuum. Only patients with greater than or equal to 2 CTC evaluations and greater than or equal to 1 expressing PSMA (PSMA+) were included. For simplicity, PSMA dynamics are described as “PSMA increase” and “PSMA decrease” in relation to the first CTC assessment that is considered the “baseline” assessment. qPCR data were analyzed using the 2−ΔΔCt method relative to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PSMA dynamics were evaluated either as a signal increase or as a signal decrease from baseline. Since patients received multiple sequential therapies, every subsequent draw provided a new threshold for further PSMA status assessment.
2.3 |. CTC enrichment and real-time PCR
CTC enrichment and real-time PCR were performed as previously described.14 Briefly, 2 × 10 mL of peripheral blood were collected in acid citrate dextrose tubes (yellow top, BD, Franklin Lakes, NJ), and processed within 4 hours. CTCs were enriched using the AdnaTest Prostate Cancer Select kit (Qiagen, Hilden, Germany), mRNA isolated using the AdnaTest Prostate Cancer Detect kit using oligo-dT coated beads (Qiagen), and reverse-transcribed using SensiScript reverse transcriptase (Qiagen). Complementary DNA (cDNA) was stored at −70°C and analyzed in batches. Real-time PCR for housekeeping genes (GAPDH) and prostate-specific markers (PMSA and PSA) were assayed using predefined ABI TaqMan™ gene expression assays (ThermoFisher, Waltham, MA). For AR-full length (FL) we used 5′-CCTGCTCAA GACGCTTCTAC-3′ (forward), 5′-GAACTGATGCAGCTCTCTCG-3′ (reverse) and 5′-CTCCTGGACTCCGTGCAGCCTA-3′ (probe) and for AR-V7, 5′-TGAAGCAGGGATGACTCTGG-3′ (F), 5′-TCAGCCTTTCTT-CAGGGTCTG-3′ (R) and 5′-CCGGGTTGGCAATTGCAAGCATCT-3′ (probe). All probes were labeled with fluorescein amidite and a minor groove binding nonfluorescent quencher, and all reactions were run in duplicate in a single plate on an ABI7900 HT qPCR instrument (ThermoFisher) using a two-step protocol of 95°C × 15 seconds, 60°C × 1 minute, × 50 cycles.
3 |. RESULTS
3.1 |. Patient characteristics
From August 2015 to November 2017, we enrolled 96 men with metastatic PCa beginning therapy with AR-directed agents or taxane agents and who were followed at medical oncology clinics at our institution. We identified 15 men who had consented to CTC collections over the course of greater than 2 consecutive systemic treatments and who had undergone CLIA-grade AR-V7 testing with at least two CTC+ results. As shown in Figure 1, 52 patients did not have CTC+ results and were excluded (since CTC– patients were unevaluable for mRNA analyses). Of the remaining 44 patients, 10 were excluded because they had only one CTC+ result, and 19 were excluded because they had no detectable PSMA transcripts. The 15 remaining patients had a least one PSMA value and are included in this study.
FIGURE 1.
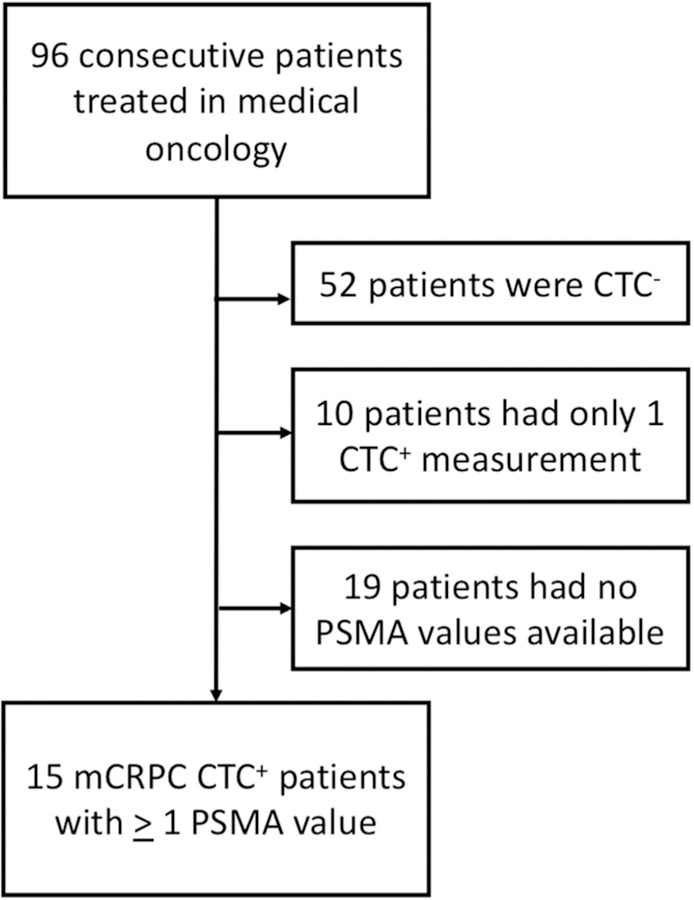
CONSORT diagram for patient exclusion. CTC, circulating tumor cell; CTC−, CTC negative; CTC+, CTC positive; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen
3.2 |. PSMA dynamics
The present study examined PSMA dynamics of the selected 15 mCRPC patients (CTC+, ≥1 PSMA+), whose baseline characteristics along with disease burden and previous treatment(s), are summarized in Table 1.
TABLE 1.
Patient characteristics at baseline
| Pt. | Age | Years since Dx | Gleason score | Metastatic sites | Previous treatments | PSA, ng/mL |
|---|---|---|---|---|---|---|
| 1† | 81 | 13 | 10 | B, LN | L, B, D, C, E, A | 9.6 |
| 2 | 68 | 17 | 7 | B | L, E, D, O, Le | 4.2 |
| 3 | 81 | 14 | 8 | B, LN | L, A, E, B | 3.7 |
| 4 | 76 | 7 | 8 | LN | L, B, A, E, D, C, D, O, P | 29.9 |
| 5† | 66 | 13 | 9 | B, LN | L, A, E, C | 2.9 |
| 6† | 94 | 25 | 6 | B, LN | L, A, E | 169.3 |
| 7 | 72 | 21 | 8 | B | L, B, Ap, A, D, C | 9.8 |
| 8† | 76 | 4 | 8 | B, L | L, E, D, C, A | 18.9 |
| 9 | 65 | 5 | 9 | B | L, D, C, B | 57.1 |
| 10† | 73 | 0 | Unknown | B | L, A, E, D, B | 38.2 |
| 11 | 71 | 1 | Unknown | B | L, D | 21.3 |
| 12 | 79 | 6 | 8 | B | L, A, D | 3.2 |
| 13† | 61 | 2 | 10 | L, LN | L, D, BET, E, A | 3.3 |
| 14† | 68 | 1 | 10 | B | L, A, D | 2.4 |
| 15† | 67 | 10 | 8 | B, LN | L, B, A, E, F, N | 6.4 |
Abbreviations: A, abiraterone; Ap, apalutamide; B, bicalutamide; BET, BET inhibitor; C, cabazitaxel; D, docetaxel; E, enzalutamide; F, flutamide; L, LHRH agonist/antagonist; Le, lenalidomide; N, nilutamide; O, orteronel; P, pembrolizumab.
deceased patient.
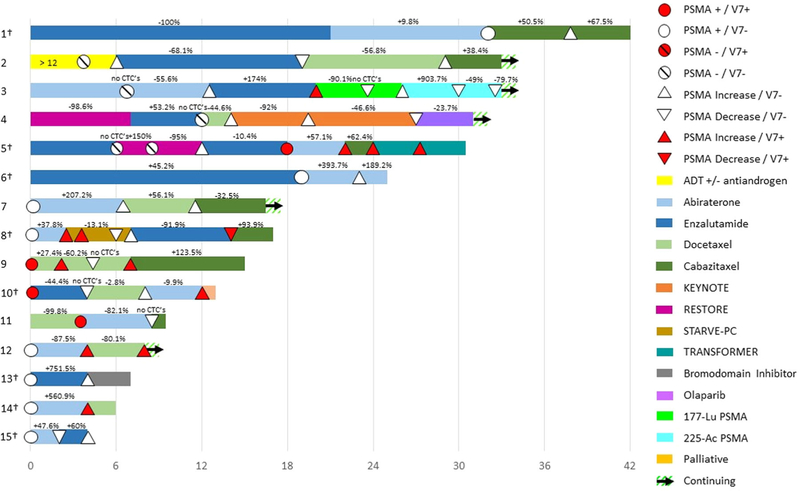
Predominant sites of metastatic disease included bones and lymph nodes. All 15 of 15 (100%) patients received standard-of-care androgen deprivation therapy [ADT: luteinizing hormone-releasing agonist/antagonist (LHRH) ± first generation antiandrogen] and second-line novel AR-targeting agents (abiraterone/enzalutamide), aside from patient #9, who received taxane chemotherapy. In 11 of 15 (73.3%) patients, PSMA signal was detected (ie, “PSMA positive”) upon the first CTC collection. A total of 54 PSMA status evaluations (mean, 3.4 [range, 2-7]) were performed in the context of 48 therapies (mean, 4.1 [range, 2-7]) during a median follow-up of 10 months (range, 4-28). Type and duration of each treatment, and corresponding PSA response status (±%), are summarized in Figure 2.
FIGURE 2.
Swimmer plot indicating treatments that patients received, along with timing (and PSMA status) of CTC sampling, and whether or not PSA responses occurred during each therapy. Patients had greater than 2 CTC+ evaluations; the first was the baseline. Percentage values indicate the best PSA response. Daggers indicate deceased patient [Color figure can be viewed at wileyonlinelibrary.com]
Based on the selection criteria, the CTC assay for all 15 patients showed PSMA signal in at least one out of two or more evaluations. Notably, the sequential CTC samples were collected in the context of disease progression to inform the subsequent therapeutic approach.
3.3 |. PSMA-based treatment
One patient (#3) underwent treatment with PSMA-targeted radioligand therapies, 177Lu-PSMA-617 followed by 225Ac-PSMA-617 (Figure 2). That patient, who showed evidence of both AR-V7 and rising PSMA levels in CTCs, demonstrated a decline in CTC-derived PSMA expression while receiving PSMA-directed treatment with 177Lu-PSMA-617.
3.4 |. Correlation between PSMA and PSA
PSA increased during the 14 treatments and decreased in 18 treatments before PSMA assessment. As shown in Figure 3, PSA declines were observed in 7 of 8 (87.5%) of the treatment switches where PSMA decreased, while only 1 of 8 (12.5%) treatment switches showed a PSA increase while PSMA was decreasing.
FIGURE 3.
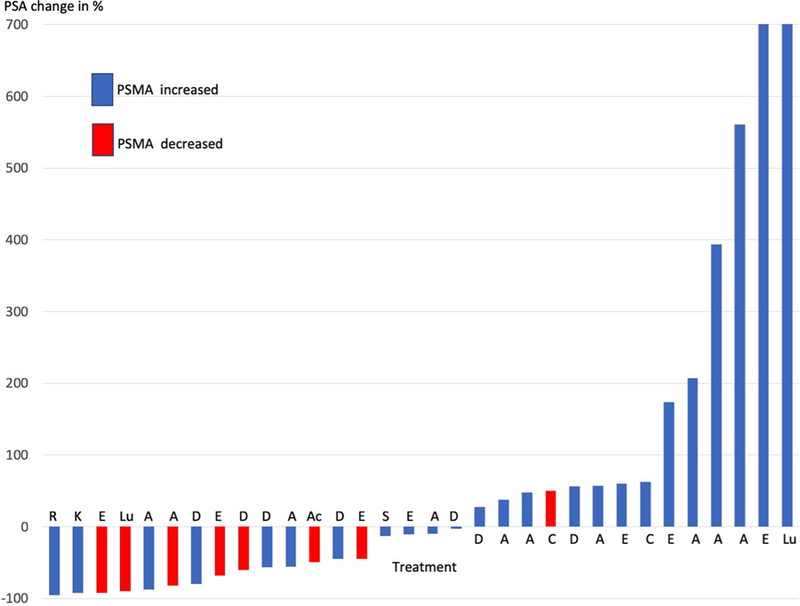
Association between PSA change and PSMA change by treatment type. A, abiraterone; Ac,225-Ac-PSMA-617; Ap, apalutamide; C, cabazitaxel; D, docetaxel; E, enzalutamide; K, KEYNOTE (pembrolizumab + enzalutamide); Lu,177-Lu-PSMA-617; R, RESTORE (bipolar androgen therapy: testosterone); S, STARVE-PC (Ipilimumab + nivolumab) [Color figure can be viewed at wileyonlinelibrary.com]
3.5 |. PSMA expression reversals
In 7 of 15 (46.7%) patients (#2, #3, #8-11, #15) PSMA levels decreased, and in 6 of 7 (85.7%) of those cases increased at a later point along their treatment trajectory. In 4 of 7 (57.1%) of the cases of PSMA decreases (#3, #9-11), PSA decreases were also observed, and no CTCs were observed.
PSMA levels increased during 28 of 37 (75.7%) of treatment switches, with 19 of 24 (79.2%) increasing for patients who were PSMA-positive when treatment began and 9 of 13 (69.2%) increasing for patients who were PSMA-negative when treatment began (Table 2).
TABLE 2.
Numbers of patients whose PSMA increased and decreased by treatment and baseline PSMA status
| Treatment | PSMA-positive at baseline |
PSMA-negative at baseline |
||
|---|---|---|---|---|
| PSMA increase | PSMA decrease | PSMA increase | PSMA decrease | |
| AR-directed | ||||
| ADT | 1 | |||
| Abiraterone | 7 | 2 | 1 | |
| Enzalutamide | 2 | 2 | 2 | 1a |
| All AR-directed | 9 | 4 | 4 | 1 |
| Taxane | ||||
| Docetaxel | 5 | 1 | 2 | |
| Cabazitaxel | 2 | |||
| All taxanes | 7 | 1 | 2 | |
| PSMA-directed | ||||
| 177Lu PSMA-617 | 1 | 1b | ||
| 225Ac PSMA-617 | 1c | |||
| All PSMA-directed | 1 | 2 | ||
| Trials | ||||
| RESTORE | 1 | |||
| TRANSFORMER | 1 | |||
| KEYNOTE-199 | 1 | |||
| STARVE-PC | 2 | 1 | ||
| All treatments | 19 | 6 | 9 | 3 |
Abbreviations: ADT, androgen deprivation therapy; PSMA, prostate-specific membrane antigen.
PSMA decrease followed a PSMA increase on previous treatment with ADT (patient #2).
PSMA decrease followed sequential PSMA increases on previous treatment with abiraterone and enzalutamide (patient #3, who also showed ARV7 expression in his CTCs).
PSMA decrease on 225Ac PSMA-617 followed a PSMA increases on previous treatment with 177Lu-PSMA-617 (patient #4).
Patients experienced PSMA decreases during five of eighteen (27.8%) periods of AR-directed treatments, but in only 1 of 10 (10%) periods of taxane treatments. Overall, no differences were seen in the percentage of patients experiencing PSMA decreases between those who had positive or negative baseline PSMA.
4 |. DISCUSSION
This is a descriptive study examining temporal changes in PSMA transcript levels in men undergoing sequential AR-directed therapies, taxane chemotherapies, and other treatments on clinical trials including bipolar androgen therapy and immunotherapy for metastatic PCa. Given the interest in PSMA as a diagnostic and therapeutic tool, we reasoned that PSMA dynamics may provide insight into disease status in patients with PCa. We, therefore, selected patients who had multiple CTC evaluations as well as PSMA expression during their sequentially administered treatments. CTCs were generally obtained at the time of clinical/radiographic progression, often coinciding with the initiation of a new therapeutic regimen. We focused specifically on PSMA analysis because of PSMA’s relative abundance in advanced disease and its growing role in both diagnosis and staging of PCa, as well as its emerging use in targeting radioligand therapy and chemotherapy in the treatment of advanced PCa.6,7,10,12,16
The current analysis represents the first study on dynamic changes of CTC-derived PSMA expression over the course of sequential therapies, and our data suggest that PSMA on CTCs is a dynamic marker. In this study, we observed five patients in whom sequential PSMA assessments showed increases followed by decreases followed by more increases, providing hypothesis-generating data on potentially differential effects of the therapies on detection of PSMA on CTCs. PSMA declines were due mainly to the eradication of PSMA-positive CTCs. Of note in this era of increasing interest in PSMA-based therapeutics for metastatic PCa, one patient (#3) with evidence of both AR-V7 and rising PSMA expression in CTCs demonstrated a decline in PSMA in CTCs while receiving PSMA-targeted treatment with 177Lu-PSMA-617.
The emergence of PSMA is likely to be important in the setting of PSMA-directed diagnostics that have previously demonstrated efficacy as predictive biomarkers of response to PSMA-targeted therapies. Because PSMA activity in PCa cell lines has been shown to increase as cells became more androgen independent,17 PSMA might not be expected to correlate with PSA levels if both are evaluated by gene expression, but we report here that a preponderance of PSMA decreases occurred when patients were undergoing systemic treatments during which serum PSA declined as well, and in four cases were associated with no observed CTCs. Concurrent declines in PSA and PSMA are to be expected when the tumor is treated and is no longer producing either PSA or PSMA.18 Thus, these CTC-negative samples corresponded to samples taken during clinical responses to therapy and were associated with declines in serum PSA. Our results are consistent with a recent report that shorter PSA doubling time, indicating faster tumor growth, may predict PSMA-targeted PET positivity in patients with biochemically recurrent PCa.19
This study established the feasibility of serial noninvasive CTC sampling for PSMA analysis in routine clinical practice. The clinical significance of these changes in PSMA are still unknown, but as diagnostic applications of PSMA-PET imaging continue to evolve, concurrent CTC assessments may establish CTC-based PSMA measurement as a companion biomarker to other measures of disease progression and response to therapy. A preliminary analysis of sequential 68Ga-PSMA-11 PET testing in 23 metastatic PCa patients before and after multiple cycles of docetaxel showed that such testing may outperform conventional CT imaging for treatment response, although the differences were not statistically significant.20 PSMA imaging routinely outperforms conventional bone scans and CT, and thus CTC-based PSMA testing may decrease the possibility of false negatives on scans as benign lesions have rarely been associated with PSMA radiotracer uptake.21
The present analysis is limited by the inclusion of a heterogeneous patient population undergoing a broad array of different treatments rather than a specific homogeneous population. A further limitation is that the included patients may not reflect the general population of CRPC patients. By including only mCRPC patients with ≥ 2 CTC+ samples, with greater than or equal to 1 PSMA+ expression across greater than or equal to 2 therapies, we selected patients with more advanced disease, as evidenced by higher Gleason scores and higher PSA levels at baseline. In addition, the criteria for the current analysis enriched for patients regularly monitored at our institution who agreed to longitudinal follow-up and periodic blood donations. Although PSMA testing value might be greatest in high-risk mCRPC patient populations, the identification of other subpopulations that may benefit from serial PSMA expression analysis will require additional studies.
5 |. CONCLUSION
This descriptive analysis suggests that PSMA transcripts can be reliably detected from peripheral blood CTCs, providing a noninvasive means of serially probing PSMA expression in mCRPC patients. We show that PSMA is a dynamic marker and that CTC-based PSMA declines are associated with concurrent decreases in serum PSA. Sequential CTC sampling could provide insights into disease evolution and might yield important clues into optimal sequencing strategies. Follow-up studies will shed further light on the clinical significance of CTC-based measures of PSMA dynamics.
ACKNOWLEDGMENTS
We thank the study participants and their families. This study was supported by the Prostate Cancer Foundation (PCF), the Department of Defense (DOD) Prostate Cancer Research Program grant (W81XWH-12-1-0605), the Patrick C. Walsh Fund, the Johns Hopkins Prostate SPORE grant (P50 CA058236), NIH (P30 CA006973, CA134675, CA184228, EB024495, and CA183031) and NCI (K23 CA197526).
Funding information
Patrick C. Walsh Fund; U.S. Department of Defense, Grant/Award Number: W81XWH-12-1-0605; Prostate Cancer Foundation; National Cancer Institute, Grant/Award Numbers: K23 CA197526, P50 CA058236, P30 CA006973, CA183031, CA134675, CA184228
CJP has served as a paid advisor for Omnitura and has received research funding from Lilly. ESA has served as a paid consultant/advisor for Janssen, Astellas, Bayer, AstraZeneca, Clovis, Merck, Bristol Myers-Squibb, Sanofi, Dendreon, Essa, and Medivation; he has received research funding from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, AstraZeneca, Clovis, Merck, Bayer, Bristol Myers-Squibb and Tokai; he is a coinventor of a technology that has been licensed to Tokai. JL has served as a paid consultant/advisor for Sun Pharma, Janssen, and Astellas, has been a speaker for Sanofi and Gilead, has received research funding from Sanofi, Mirati, Orion, Astellas, and Constellation, and is a coinventor of a technology that has been licensed to Tokai, A&G, and Qiagen. MGP is a coinventor on a US patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. SPR is a consultant for Progenics Pharmaceuticals, Inc., the licensee of 18F-DCFPyL. MGP and SPR receive research funding from Progenics Pharmaceuticals, Inc. Relevant disclosures have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
CONFLICT OF INTERESTS
Remaining authors declare that there are no conflict of interests.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe SP, Gorin MA, Pomper MG. Imaging of prostate-specific membrane antigen with small-molecule pet radiotracers: from the bench to advanced clinical applications. Annu Rev Med. 2019;70: 461–477. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni HR, Singh A, Langbein T, et al. Theranostics of prostate cancer: from molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. Br J Radiol. 2018;91(1091):20180308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–640. [DOI] [PubMed] [Google Scholar]
- 6.Hofman MS, Violet J, Hicks RJ, et al. [(177)Lu]-PSMA-617 radio-nuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–833. [DOI] [PubMed] [Google Scholar]
- 7.Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–1443. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [(18)F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18(3):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller G, McCormack R, Kheoh T, et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol. 2018;36(6):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autio KA, Dreicer R, Anderson J, et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4(10):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lokhandwala PM, Riel SL, Haley L, et al. Analytical validation of androgen receptor splice variant 7 detection in a clinical laboratory improvement amendments (CLIA) laboratory setting. J Molecular Diagn. 2017;19(1):115–125. [DOI] [PubMed] [Google Scholar]
- 15.Helo P, Cronin AM, Danila DC, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55(4):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sathekge M, Bruchertseifer F, Knoesen O, et al. 225)Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46(1):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denmeade SR, Sokoll LJ, Dalrymple S, et al. Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. Prostate. 2003;54(4): 249–257. [DOI] [PubMed] [Google Scholar]
- 18.Holland JP, Cumming P, Vasdev N. PET of signal transduction pathways in cancer. J Nucl Med. 2012;53(9):1333–1336. [DOI] [PubMed] [Google Scholar]
- 19.Pereira Mestre R, Treglia G, Ferrari M, et al. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: a meta-analysis. Eur J Clin Invest. 2018;49:e13063. [DOI] [PubMed] [Google Scholar]
- 20.Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45(4):602–612. [DOI] [PubMed] [Google Scholar]
- 21.Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017; 44(12):2117–2136. [DOI] [PubMed] [Google Scholar]