Abstract
Background
To investigate the correlation between hyperandrogenism (HA) and insulin resistance (IR) in women with polycystic ovary syndrome (PCOS) by measuring serum total testosterone (TT) using a liquid chromatography and tandem mass spectrometry assay (LC‐MS/MS).
Methods
This cohort study included 332 patients with PCOS, 63 patients with IR and 276 with controls. TT levels were measured by LC‐MS/MS and chemiluminescent immunoassay (CLIA); glucose and insulin levels were determined by an oral glucose tolerance test (OGTT).
Results
Compared with CLIA, LC‐MS/MS differentiated more cases with high TT levels among the non‐PCOS subjects with IR In patients with PCOS, LC‐MS/MS‐based TT levels or a combination with the mFG score detected a significantly higher incidence of HA in subjects with IR identified by hyperinsulinemia (HIN), HOMA‐IR or impaired fasting glucose (IFG) than in those without IR Conversely, the IR rates demonstrated by HIN, HOMA‐IR, or IFG were remarkably higher in the LC‐MS/MS‐defined high TT subgroup than in the normal TT subgroup. However, the CLIA platform could not discern a difference in HA incidence between IR and non‐IR subgroups or in IR rate between high and normal TT populations. ROC curves also proved that HIN, HOMA‐IR, and IFG were positive contributors to HA as measured by LC‐MS/MS
Conclusions
The correlation between HA and IR has always been underestimated, partly owing to the less accurate methods previously used to measure TT. HIN, HOMA‐IR, and IFG are likely to contribute to the development of HA from a clinical perspective.
Keywords: chemiluminescent immunoassay, hyperandrogenism, insulin resistance, liquid chromatography with tandem mass spectrometry, polycystic ovary syndrome
1. INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among young women, affecting 5%‐10% of women of childbearing age.1, 2 Hyperandrogenism (HA) and insulin resistance (IR) are regarded as two essential physiopathological characteristics of PCOS.3 A previous study reported a significant positive correlation between insulin and androgen levels in PCOS4 and the intense debate on the causal relationship between HA and IR Lately, animal experiments suggest that IR may play a more important role in the pathogenesis and development of PCOS, and more recent studies implicate IR as one of the causes responsible for the occurrence and progress of HA.5, 6 However, to the best of our knowledge, the strong correlation between IR and HA observed in animal experiments is not supported by the clinical evidence, and this observation is largely attributed to the inaccurate measurement of androgens7 as demonstrated in the existing reports.
Recent recommendations by professional societies and experts have explicitly stated the importance and high accuracy of using liquid chromatography and tandem mass spectrometry (LC‐MS/MS) to measure total testosterone (TT) levels in women.8, 9 In a previous study, we found that the TT level measured by LC/MS‐MS was more closely related to the modified Ferriman Gallwey (mFG) score (r = 0.642, P < 0.001) than the TT level measured by chemiluminescent immunoassay (CLIA) was. Therefore, we wondered whether a more accurate measurement of TT would lead to an elevated incidence of IR in PCOS women with HA and whether the correlation between TT and IR was previously underestimated in clinical practice owing to the less accurate method of TT measurement. In the present study, we re‐evaluated the potential correlation between HA and IR by comparing a non‐PCOS IR cohort and a control group, as well as by comparing the subgroups among PCOS patients, based on LC‐MS/MS measurements of serum TT levels.
2. MATERIALS AND METHODS
2.1. Study populations
2.1.1. PCOS patients
A total of 332 PCOS women aged 18‐44 years were recruited at Sun Yat‐sen Memorial Hospital from September 21, 2014, to October 10, 2015. Patients with complaints of oligomenorrhea/amenorrhea, dramatic weight gain, excess facial or body hair, acne, recurrent pregnancy loss, and infertility were systematically evaluated, and PCOS was diagnosed when two of the following three criteria were met according to the Rotterdam 2003 criteria1: (a) oligomenorrhea and/or anovulation; (b) clinical and/or biochemical HA; and (c) polycystic ovaries (12 or more follicles measuring 2‐9 mm in diameter in each ovary and/or an increased ovarian volume of more than 10 mL). Subjects with mimicking manifestations or other androgen excess disorders were excluded, such as androgen‐secreting neoplasms, adrenal hyperplasia, iatrogenic androgen excess, thyroid dysfunction, or hyperprolactinemia. In this study, biochemical HA was defined as TT ≥2.39 nmol/L, free testosterone (FT) ≥26.00 pmol/L or dehydroepiandrosterone sulfate (DHEAS) ≥4.92 μmol/L or free androgen index (FAI) ≥6.1, and clinical HA (hirsutism) was defined as an mFG score of 5 or higher, as described in our previous reports.10, 11
2.1.2. IR cohort
Between September 21, 2014, and October 10, 2015, a total of 63 patients who were 18‐44 years old and did not meet the criteria for a diagnosis of PCOS were enrolled in the IR group at Sun Yat‐sen Memorial Hospital according to the criteria for impaired fasting glucose (IFG; FG ≥5.6 mmol/L), impaired glucose tolerance (IGT; glucose level at 2 hours after glucose administration ≥7.8 mmol/L), HOMA‐IR ≥2.14, diabetes mellitus, or acanthosis nigricans.12 Patients with PCO or any other endocrine disorder were excluded.
2.1.3. Control group
The control group consisted of women aged 18‐44 years, who consulted at the outpatient department of Sun Yat‐sen Memorial Hospital from September 21, 2014, to October 10, 2015, due to infertility caused by male factors or tubal blockage. After screening out pregnant women and those with hirsutism, IR, PCO, or other endocrine disorders, 276 subjects with regular menstruation were included in the control group.
2.2. Collection of clinical, biochemical, and metabolic data
Information concerning menstrual regularity, hirsutism, acne, androgenic alopecia, gynecologic and obstetric history, medications, and family history of related disorders were collected for all patients. Physical examination was also conducted to obtain parameters such as blood pressure, body mass index (BMI, calculated as weight in kilograms divided by height in square meters), and waist‐to‐hip ratio. The mFG system was used to evaluate terminal hair growth. Levels of pituitary hormone, ovarian and adrenal steroids, and thyroid‐stimulating hormone were measured during the first five days of spontaneous menstrual cycles or at progestin‐withdrawal bleeding. An OGTT using 75 g of glucose was performed after an overnight fast of at least 10 hours, with blood samples taken at baseline, 1 and 2 hours after the glucose load for glucose and insulin measurement. Systematic transvaginal ultrasound for the evaluation of polycystic ovary morphology was carried out in the early follicular phase if menses were regular or randomly if menses were irregular. Oligomenorrhea/amenorrhea and polycystic ovaries were defined according to the revised Rotterdam criteria.1
2.3. Total testosterone (TT) measurement
Chemiluminescent immunoassay was conducted using a Beckman DXI 800 automatic chemiluminescence analyzer (Beckman Coulter, Inc, Brea, CA, USA) to obtain the measurements of TT and other hormones, with a sensitivity of 10 ng/dL (0.35 nmol/L), as well as intra and interassay CVs of 1.67%‐3.93% and 4.22%‐7.08%, respectively. The cut‐off value for CLIA‐based TT was determined to be 2.39 nmol/L by k‐means cluster analysis according to the data from 450 well‐characterized healthy controls with the same genetic background.13
Total testosterone levels were measured in serum previously collected from our subjects and stored at −80°C. TT measurement was performed by LC‐MS/MS at the Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, using an API 4000 tandem mass spectrometer with electrospray ionization (ESI) and Analyst® 1.4.1 software (Applied Biosystem Corporation, Foster City, CA, USA). Testosterone standard stock solution (20 ng/mL) was diluted to prepare calibration solutions at 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 ng/mL, and 200 μL of each concentration of calibration solution was spiked with 50 μL of 250 ng/mL norethisterone prepared from 1.0 mg/mL norethisterone standard stock solution and mixed well. After 1 mL of methyl tertiary‐butyl ether was added, the mixture was placed on a rotary mixer (100 rpm) for 15 minutes, followed by a 10‐minutes centrifugation (9 455 g). With the upper phases separated and dried under nitrogen, the residues were dissolved in 100 μL of 0.1 mol/L hydroxylamine hydrochloride, incubated at 60°C for 70 minutes, and then re‐suspended in 50 μL of acetonitrile for subsequent LC‐MS/MS analysis. The assay was conducted for all the samples, with a limit of quantitation of 0.47 ng/dL (0.016 nmol/L) and intra‐ and inter‐assay CVs of 5.52% and 7.41%, respectively, at a low TT level of 20.54 ng/mL (0.713 nmol/L).
2.4. Statistical analyses
Relevant IR parameters, including IFG, IGT, hyperinsulinemia (HIN, fasting plasma insulin ≥11.87 mIU/L),12 increased HOMA‐IR, INS peak shift and acanthosis nigricans (AN), were used to differentiate the IR subgroup from the non‐IR subgroup among PCOS patients. TT level was adopted as an indicator to distinguish the biochemically high TT (HT) and normal TT (NT) subgroups, with the cut‐off value set at 1.85 nmol/L for LC‐MS/MS (determined by k‐means cluster analysis from 198 healthy reproductive‐age women with the same genetic background) and 2.39 nmol/L for CLIA measurements (determined by k‐means cluster analysis from 450 well‐characterized healthy controls with the same genetic background). Clinical HA was considered equivalent to hirsutism and was defined by an mFG score ≥5 based on our previous study.10
In the present study, data are presented as the means ± standard deviation (SD). The Kolmogorov‐Smirnov test was used to examine the normal distribution. A t test or one‐way analysis of variance (ANOVA) was used for intergroup comparisons, and a chi‐squared test was used for enumerative data comparisons. Receiver operating characteristics (ROC) curves were plotted to assess the predictability of IR‐related parameters for HA. Statistical analyses were performed using the IBM SPSS 19.0 (IBM, Armonk, NY) statistical package, with P < 0.05 considered to indicate statistical significance.
2.5. Ethical approvals
The study was approved by the Institutional Review Board of the Sun Yat‐sen Memorial Hospital, Sun Yat‐sen University, under the Chinese Clinical Trial Registry (https://www.chictr.org.cn/enIndex.aspx) number ChiCTR‐DDT‐14005186. Informed consent was obtained from all individual participants included in the study.
3. RESULTS
3.1. LC‐MS/MS detected a higher level of TT in the IR population than in the control group
LC‐MS/MS revealed a significantly higher level of TT in the IR cohort (1.65 ± 0.88 nmol/L) than in the control group (1.02 ± 0.46 nmol/L; P < 0.001; Figure 1), which was in contrast to CLIA‐based TT measurements that showed no statistically significant difference between the two populations (1.22 ± 0.56 nmol/L vs 1.12 ± 0.49 nmol/L, P = 0.239).
Figure 1.
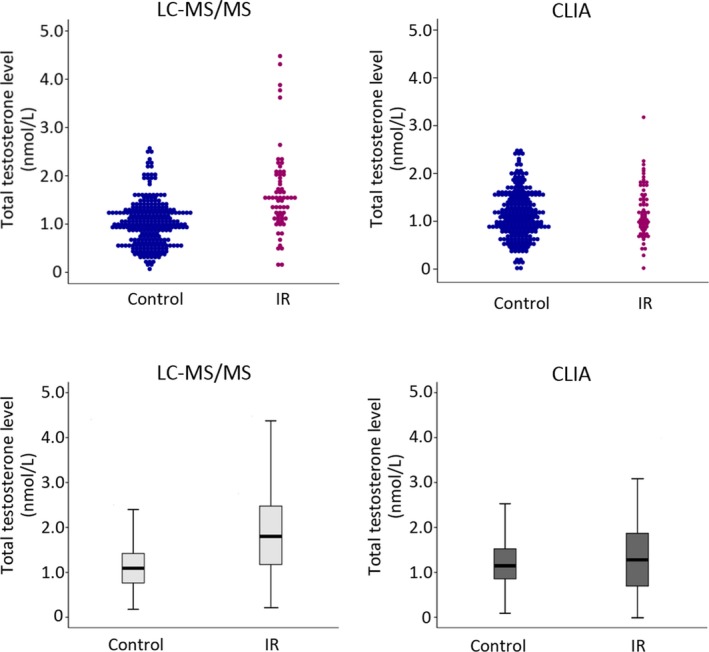
Comparison of the distribution of TT assessed by LC‐MS/MS and CLIA measurements in IR and control groups. TT measured by LC‐MS/MS: IR group vs control group (means ± SD) = 1.65 ± 0.88 nmol/L vs 1.02 ± 0.46 nmol/L (P < 0.001). TT measured by CLIA: IR group vs control group (means ± SD) = 1.22 ± 0.56 nmol/L vs 1.12 ± 0.49 nmol/L (P = 0.239). The line in the middle of the box represents the median TT level; the lower boundary of the box indicates the 25th percentile, and the upper boundary indicates the 75th percentile. CLIA, chemiluminescent immunoassay; IR, insulin resistance; LC‐MS/MS, liquid chromatography and tandem mass spectrometry assay; TT, total testosterone
3.2. Notably, there was an increased incidence of HA in the IR subgroup compared with the non‐IR subgroup among the PCOS patients
In addition to the comparison between the IR cohort and control group of non‐PCOS women, we also analyzed 332 PCOS patients by comparing IR and non‐IR subgroups based on parameters including IFG, IGT, HIN, HOMA‐IR, INS peak shift, and signs of AN. The rates of HA determined by LC‐MS/MS (TT ≥ 1.85 nmol/L) were significantly higher in the IR subgroup than in the non‐IR subgroup when the IR subgroups were defined by HIN (80.97% vs 37.89%, P < 0.001), increased HOMA‐IR (68.53% vs 38.52%, P < 0.001) and IFG (80.36% vs 51.45%, P < 0.001; Figure 2A). In contrast, CLIA‐based measurements failed to reveal a statistically significant difference in HA (TT ≥ 2.39 nmol/L) incidence between the subgroups with or without IR (Figure 2B).
Figure 2.
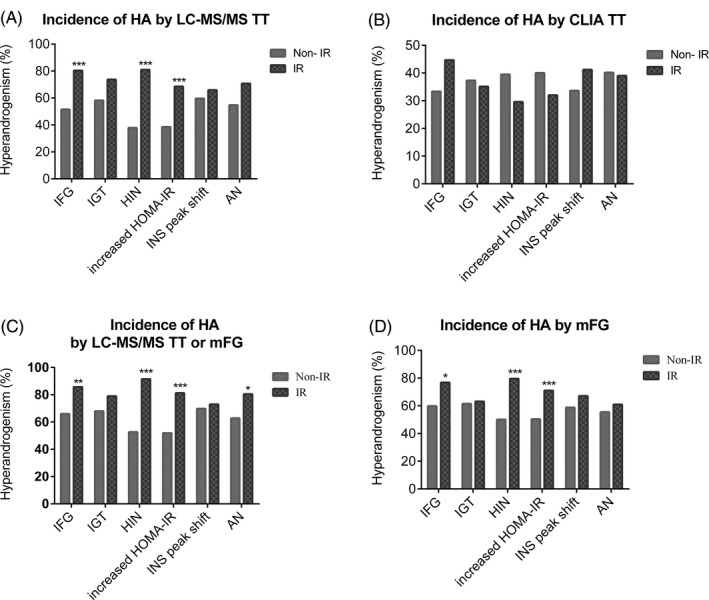
Comparison of HA incidence between PCOS IR and non‐IR subgroups identified using different IR‐relevant parameters#. The incidence of HA was assessed by (A) LCMS‐based TT (≥ 1.85 nmol/L), (B) CLIA‐based TT (≥ 2.39 nmol/L), (C) LCMS‐based TT or mFG score (TT ≥1.85 nmol/L or mFG ≥5), and (D) mFG score alone (≥ 5). AN, acanthosis nigricans; HIN, hyperinsulinemia; HOMA‐IR, fasting glucose (mmol/L) × fasting insulin (mIU/L)/22.5, homeostatic model assessment of IR; IGT, impaired glucose tolerance; IFG, impaired fasting glucose; IR, insulin resistance;. *P < 0.05; **P < 0.01; ***P < 0.001. #The numbers of subjects were 332 for IFG, HIN, and HOMA‐IR, 210 for IGT, 204 for INS peak shift and 178 for AN
In addition, when HA was identified by either a high mFG score (mFG ≥ 5) or an increased LC‐MS/MS‐based TT, the HA rate was also higher in the IR subgroup defined by HIN (91.55% vs 52.63%, P < 0.001), increased HOMA‐IR (81.22% vs 51.85%, P < 0.001), and IFG (85.71% vs 65.94%, P < 0.01) than in the non‐IR subgroup (Figure 2C). Similar results were obtained for the rate of HA determined by a higher mFG score alone when the IR subgroup was differentiated from the non‐IR subgroup by HIN and increased HOMA‐IR (P < 0.001) or by IFG (P < 0.05; Figure 2D).
3.3. LC‐MS/MS revealed a higher IR incidence in PCOS patients with HT than in those with NT
To compare the IR rate between the patients with HT identified by LC‐MS/MS and those identified by CLIA, we divided the PCOS population into HT and non‐HT subgroups using cut‐off TT values of ≥1.85 nmol/L for LC‐MS/MS (HT‐LCMS and NT‐LCMS subgroups) and ≥2.39 nmol/L for CLIA (HT‐CLIA and NT‐CLIA subgroups). The baseline characteristics of the subgroups with and without increased TT levels are demonstrated in Table S1.
The results showed that the incidence of IR was significantly higher in the HT‐LCMS subgroup than in the NT‐LCMS subgroup when IR was defined by HIN (61.50% vs 18.62%, P < 0.001), increased HOMA‐IR (72.19% vs 42.76%, P < 0.001), and IFG (24.06% vs 7.59%, P < 0.001; Figure 3A), whereas the CLIA platform could not discern differences in IR between women with HT and NT (Figure 3B).
Figure 3.
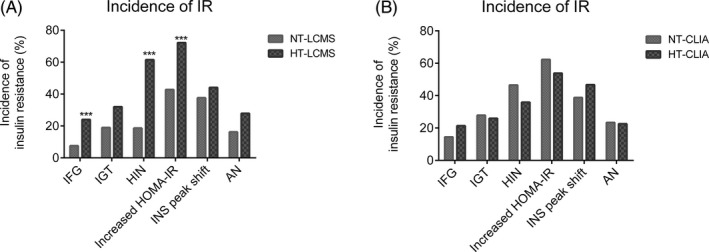
Comparison of the incidence of IR identified by different parameters among PCOS patients with or without HA assessed by LC‐MS/MS or CLIA#. AN, acanthosis nigricans; HIN, hyperinsulinemia; HOMA‐IR, fasting glucose (mmol/L) × fasting insulin (mIU/L)/22.5, homeostatic model assessment of IR; HT‐CLIA, the subgroup with TT ≥2.39 nmol/L determined by CLIA; HT‐LCMS, the subgroup with TT ≥1.85 nmol/L determined by LC‐MS/MS; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NT‐CLIA, the subgroup with normal TT (<2.39 nmol/L) determined by CLIA; NT‐LCMS, the subgroup with normal TT (<1.85 nmol/L) determined by LC‐MS/MS. ***P < 0.001. #The numbers of subjects were 332 for the analysis of IFG, HIN, and HOMA‐IR, 210 for IGT, 204 for INS peak shift and 178 for AN
3.4. OGTT dynamic glucose and insulin curves for HT and non‐HT PCOS patients
The results of the 75‐g OGTT for PCOS women revealed significantly elevated levels of relevant IR parameters (GLU‐0h, GLU‐2h, INS‐0h, INS‐1h, and INS‐2h) in the HT‐LCMS subgroup compared to those in the NT‐LCMS subgroup, as shown below: GLU‐2h (7.31 ± 2.50 nmol/L vs 6.67 ± 1.82 nmol/L, P < 0.05), INS‐0h (15.98 ± 12.87 mIU/L vs 7.42 ± 4.04 mIU/L, P < 0.001), INS‐1h (125.11 ± 105.00 mIU/L vs 89.71 ± 62.02 mIU/L, P < 0.01), and INS‐2h (112.84 ± 86.55 mIU/L vs 73.28 ± 51.17 mIU/L, P < 0.001; Figure 4A,B). Again, no statistically significant difference was found between women with CLIA‐based HT and NT according to the dynamic glucose (Figure 4C) and insulin (Figure 4D) curves.
Figure 4.
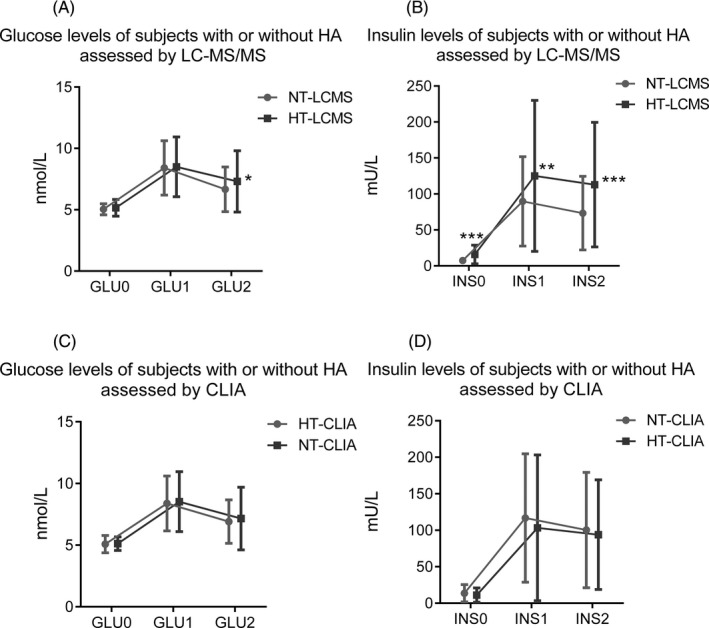
Glucose and insulin levels of PCOS women with or without HA (different phenotypes of PCOS) during a 2‐h OGTT. A, Glucose levels of the PCOS women with or without HT assessed by LC‐MS/MS (n = 210); B, Insulin levels of the PCOS women with or without HT assessed by LC‐MS/MS (n = 201); C, Glucose levels of the PCOS women with or without HT assessed by CLIA (n = 210); D, Insulin levels of the PCOS women with or without HT assessed by CLIA (n = 201). GLU0, fasting plasma glucose; GLU1, plasma glucose level at 1 h after glucose administration; GLU2, plasma glucose level at 2 h after glucose administration; INS0, fasting plasma insulin; INS1, plasma insulin level at 1 h after glucose administration; INS2, plasma insulin level at 2 h after glucose administration. *P < 0.05; ***P < 0.001
3.5. Predictive ability of IR for HA by ROC curves
ROC curves were plotted to predict HA using IR‐associated parameters, including increased IFG, IGT, HIN, INS peak shift, HOMA‐IR, and AN. The areas under the curve (AUCs) of HIN, increased HOMA‐IR and IFG were measured as 0.714 (95% CI: 0.658, 0.770, P < 0.001), 0.647 (95% CI: 0.587, 0.707, P < 0.001), and 0.582 (95% CI: 0.521, 0.643, P < 0.05), respectively, indicating a significant predictive ability for HA determined by LCMS‐TT (Table 1). In contrast, these parameters were not considered good predictors for HA identified by CLIA‐TT, as the AUCs of HIN, increased HOMA‐IR and IFG were calculated to be 0.447 (95% CI: 0.383, 0.511, P = 0.110), 0.458 (95% CI: 0.392, 0.523, P = 0.202), and 0.535 (95% CI: 0.469, 0.601, P = 0.292), respectively (Table 1).
Table 1.
Receiver operating characteristic (ROC) curves used to predict HA with IR determined by various parameters
| IR‐relevant parameters | N | HT‐LCMS | HT‐CLIA | ||||
|---|---|---|---|---|---|---|---|
| AUC | 95% CI | P value | AUC | 95% CI | P value | ||
| IFG | 332 | 0.582 | 0.521‐0.643 | 0.010 | 0.535 | 0.469‐0.601 | 0.296 |
| IGT | 210 | 0.565 | 0.487‐0.644 | 0.113 | 0.491 | 0.41‐0.572 | 0.824 |
| HIN | 332 | 0.714 | 0.658‐0.770 | 0.000 | 0.447 | 0.383‐0.511 | 0.110 |
| Increased HOMA‐IR | 332 | 0.647 | 0.587‐0.707 | 0.000 | 0.458 | 0.392‐0.523 | 0.202 |
| INS peak shift | 204 | 0.532 | 0.451‐0.614 | 0.442 | 0.540 | 0.457‐0.622 | 0.347 |
| AN | 178 | 0.558 | 0.474‐0.643 | 0.185 | 0.496 | 0.409‐0.583 | 0.925 |
Bold values be of statistically significance.
AN, acanthosis nigricans; AUC, area under the curve; CI, confidence interval; HA, hyperandrogenism; HIN, hyperinsulinemia; HOMA‐IR, fasting glucose (mmol/L) × fasting insulin (mIU/L)/22.5, homeostatic model assessment of IR; HT‐CLIA, HA group with TT ≥2.39 nmol/L determined by CLIA; HT‐LCMS, HA group with TT ≥1.85 nmol/L determined by LC‐MS/MS; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IR, insulin resistance.
4. DISCUSSION
As LC‐MS/MS enables TT measurement with high accuracy, we compared LC‐MS/MS and CLIA measurements of TT levels of the subjects in the present study, aiming to reassess the potential correlation between HA and IR Based on the LC‐MS/MS measurements, an HA incidence of up to 80.99% was observed in patients with IR in the PCOS population, which was significantly higher than that in the women without IR, and vice versa, a higher IR rate (61.50%) was found in those women with increased TT levels compared with that in women with normal TT levels. Therefore, we speculate that IR is not considered one of the diagnostic criteria for PCOS in the Rotterdam consensus because of the less accurate methods previously used to measure androgens and that these methods might have been unable to detect a correlation between IR and HA/PCOS.
In the present study, we first compared TT levels measured by LC‐MS/MS and CLIA between the non‐PCOS IR cohort and the control group. As expected, the mean TT level measured by LC‐MS/MS was significantly higher in the IR cohort than in the control group (Figure 1). Among the patients with PCOS, the incidence of HA was also observed to be much higher in the IR subgroup than in the non‐IR subgroup when increased fasting insulin (80.97% vs 37.89%, P < 0.001), increased HOMA‐IR (68.53% vs 38.52%, P < 0.001), and IFG (80.36% vs 51.45%, P < 0.001) were used to define IR In contrast, such a notable difference in the incidence of HA was not seen between the CLIA‐based IR and non‐IR subgroups.
On the other hand, a higher IR rate was also found in the HT subgroup in comparison to that of the NT subgroup when subgroups were classified on the basis of TT measured by LC‐MS/MS Furthermore, the intersubgroup difference in the PCOS population was particularly significant when IR was identified by HIN (61.50% vs 18.62%, P < 0.001), increased HOMA‐IR (72.19% vs 42.76%, P < 0.001), and IFG (24.06% vs 7.59%, P < 0.001). Not surprisingly, CLIA measurement of TT levels failed to discriminate a difference in IR status between women with HT and NT. Moreover, the OGTT dynamic glucose tolerance curves reinforced these findings (Figure 4). Although serum glucose was slightly increased, which is in part owing to younger age, the dynamic insulin responses in the HT‐LCMS subgroup showed dramatic changes compared with those in the NT‐LCMS subgroup. In addition, the PCOS patients with IR defined by HIN, HOMA‐IR, and IFG also showed a high incidence of hirsutism (Figure 2), the clinical HA diagnosed on the basis of a specific cut‐off value of mFG.10, 14
In vitro studies have shown that insulin can directly stimulate ovarian steroidogenesis and/or augment luteinizing hormone (LH)‐stimulated androgen secretion or indirectly enhance the amplitude of gonadotropin‐releasing hormone (GnRH)‐stimulated LH pulses. According to the literature, lowering insulin levels with insulin‐sensitizing drugs, such as metformin and thiazolidinediones, can reduce circulating androgen levels or the bioactivity of testosterone and increase sex hormone binding globulin (SHBG) levels.4, 15 Our study supports the idea that IR or compensatory HIN is associated with the occurrence or progression of HA among patients with PCOS if, of course, the TT levels can be appropriately measured. This finding was further confirmed by ROC curve analysis and logistic regression analysis, which both indicate a close correlation between IR and a higher risk of HA among PCOS women.
In conclusion, our study revealed a close relationship between HA and IR by LC‐MS/MS measurement of TT, which may have been underestimated for a long time due to the lack of an accurate method for quantifying TT. HIN, HOMA‐IR, and IFG are likely to contribute to the development of HA from a clinical perspective. In addition to biochemical HA, which is defined by TT levels, IR could also be related to the development of clinical HA, which is identified by mFG score.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81471425 and 81370680), the Natural Science Foundation of Guangdong Province (2016A030313266), the Science Technology Research Project of Guangdong Province (2014A020213006 and 2015A030313091), the Medical Scientific Research Foundation of Guangdong Province (A2016345) and the Key Project of Fundamental Research Funds for the Central Universities (16ykzd15).
Yang Y, Ding M, Di N, Azziz R, Yang D, Zhao X. Close correlation between hyperandrogenism and insulin resistance in women with polycystic ovary syndrome—Based on liquid chromatography with tandem mass spectrometry measurements. J Clin Lab Anal. 2019;33:e22699 10.1002/jcla.22699
Clinical Trial Registration Number: ChiCTR‐DDT‐14005186.
REFERENCES
- 1. The Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41‐47. [DOI] [PubMed] [Google Scholar]
- 2. Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237‐4245. [DOI] [PubMed] [Google Scholar]
- 3. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic‐hyperinsulaemic clamp. Hum Reprod. 2013;28:777‐784. [DOI] [PubMed] [Google Scholar]
- 4. Diamanti‐Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legro RS, Kunselman AR, Dodson WC, et al. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165‐169. [DOI] [PubMed] [Google Scholar]
- 6. Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome ‐ Part 2. Endocr Pract. 2015;21:1415‐1426. [DOI] [PubMed] [Google Scholar]
- 7. Rosner W, Auchus RJ, Azziz R, et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405‐413. [DOI] [PubMed] [Google Scholar]
- 8. Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713‐1719. [DOI] [PubMed] [Google Scholar]
- 9. Salameh WA, Redor‐Goldman MM, Clarke NJ, et al. Specificity and predictive value of circulating testosterone assessed by tandem mass spectrometry for the diagnosis of polycystic ovary syndrome by the National Institutes of Health 1990 criteria. Fertil Steril. 2014;101:1135‐1141 e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X, Ni R, Li L, et al. Defining hirsutism in Chinese women: a cross‐sectional study. Fertil Steril. 2011;96:792‐796. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Z, Ni R, Hong Y, et al. Defining hyperandrogenaemia according to the free androgen index in Chinese women: a cross‐sectional study. Clin Endocrinol (Oxf). 2012;77:446‐452. [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55‐S60. [DOI] [PubMed] [Google Scholar]
- 13. Zhao X, He Z, Mo Y, et al. Determining the normal cut‐off levels for hyperandrogenemia in Chinese women of reproductive age. Eur J Obstet Gynecol Reprod Biol. 2011;154:187‐191. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Han Y, Wang W, et al. Assessing new terminal body and facial hair growth during pregnancy: toward developing a simplified visual scoring system for hirsutism. Fertil Steril. 2016;105:494‐500. [DOI] [PubMed] [Google Scholar]
- 15. Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nat Rev Endocrinol. 2014;10:624‐636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


