Abstract
Background
Human papillomavirus (HPV) infection can lead to the development of cervical cancer. This study assessed the genotype distribution of HPV of high‐grade squamous intraepithelial lesion (HSIL) and invasive cervical cancer (ICC) in Ganzhou population.
Methods
A total of 935 females who got HPV testing from January 2016 to July 2018 in the maternal and child health hospital of Ganzhou were enrolled in the study, including 806 HSIL and 129 ICC. HPV detection and genotyping were tested by HPV Geno‐Array test kit.
Results
The overall HPV‐positive rate was 74.0% in Ganzhou. Among the HSIL and ICC patients, the positive rates of HPV detection were 75.6% and 64.3%. Among the HSIL individuals, the most prevalent hr‐HPV genotype was HPV 16. And the 4 common subtypes in decreasing order were HPV 52, 58, 33, and 18. Of the ICC patients, the most frequently hr‐HPV subtype was HPV 16 followed by 18, 52, 58, and 59. Among the squamous cell carcinoma (SCC) patients, for hr‐HPV genotypes, HPV 16, 18, 52, 58, and 59 were five most common subtypes. In patients with adenocarcinoma (ADC), the most common hr‐HPV genotype was HPV 18, followed by HPV 16, 52, 56, 68, 73. And, we found U‐shaped and S‐shaped curves in the HPV distribution of different age groups.
Conclusion
The prevalence and distribution of HPV genotypes in Ganzhou differed from other regions of China and Western countries. These results can serve as valuable reference for HPV vaccination programs for Ganzhou women.
Keywords: Genotype, high‐grade squamous intraepithelial lesion, Human papillomavirus, infection, invasive cervical cancer
1. INTRODUCTION
Cervical carcinoma (CC) is the fourth most frequent cancer among female around the world.1 Most of new cases of CC occur in less‐developed regions.2 It is estimated that the number of new cases of CC in China was one hundred thousand per year.3 Persistent infection with carcinogenic human papillomavirus (HPV) is usually subclinical, and persistent infection can lead to tissue damage, malignancy, and, ultimately, invasive cervical cancer (ICC).4, 5 More than 70% of women will be infected at least once in their lifetime, and 10%‐15% of them will turn into persistent infection.6 Persistent infection of high‐risk HPV (hr‐HPV) is critical for the development of almost all cervical cancers and precancerous lesion.4 The detection of HR‐HPV in cervical cancer can provide a reliable and sensitive clinical reference in screening cervical cancer.7 The International Agency for Research on Cancer suggested that hr‐HPV was divided into 25 genotypes, mainly including HPV 16, 18, 31, 33, 35, 45, 52, 58.8 Previous study indicated that HPV 16 and HPV 18 were the most common genotypes around the worldwide.9 However, in different countries and regions, the distribution of HPV genotypes in cervical cancer and precancerous lesion varies was different.10, 11, 12, 13 Many studies proved that the genotype distribution of HPV differed in the various provinces of China.14, 15, 16, 17 The most frequent hr‐HPV genotypes were HPV 16 and HPV 58, HPV 52 and HPV 16, HPV 16 and HPV 18, and HPV 16 and HPV 52 in Shanxi, Sichuan, Jiangxi, and Fujian province, respectively.
China has a high prevalence of HPV; the disease burden on cervical cancer is very heavy. China has a large population in the world, and its CC burden has a significant impact on estimates of disease burdens worldwide.18 The HPV vaccine has been proven to be effective in the prevention of cervical cancer.19 Nowadays, there are two types of vaccines in China, the bivalent vaccine (targeted at HPV 16, 18) and the tetravalent vaccine (targeted at HPV 6, 11, 16, 18). The implementation of these vaccines in China may not achieve the expected preventive effect. However, research of HPV infection among HSIL and ICC females was rare in China. Therefore, we conducted a retrospective study and evaluated HPV test histories of women with high‐grade squamous intraepithelial lesion (HSIL) and ICC diagnoses.
2. MATERIALS AND METHODS
2.1. Study population
The research was conducted from January 2016 to July 2018 in maternal and child health hospital of Ganzhou City, Jiangxi Province. A total of 935 women getting HPV test were enrolled in this study, including 806 HSIL (average age 39.3 years, range 17‐77 years) and 129 ICC (average age 48.8 years, range 29‐81 years). And 129 ICC patients were classified into 108 squamous cell carcinoma (SCC) and 21 adenocarcinoma (ADC). Inclusion criteria were as follows: (a) no sexual intercourse within 48 hours; (b) without vaginal medications history within 48 hours; (c) without history of total uterus or cervix resection; and (d) no currently pregnant.
2.2. Ethical statement
The present study was approved by the ethics committee of maternal and child health hospital of Ganzhou according to the World Medical Association’s Declaration of Helsinki of 1975, revised in 2008. All participants signed the informed consent before sample collection.
2.3. Histopathological diagnosis
In this study, tissue samples were collected from biopsies of colposcopy or operations, including cervical conization and/or loop electrosurgical excisional procedure and/or hysterectomy or radical hysterectomy. HSIL (CIN2/3) and ICC (SCC or ADC) were diagnosed according to the standard criteria.20 At least 2 experienced pathologists evaluated the patient specimens.
2.4. HPV genotyping and PCR
HPV genotyping was tested using HPV Geno‐Array test kit (Genetel Pharmaceuticals CO., Ltd. Shenzhen, China) with American ABI 7500 Real‐Time PCR System. The HPV detection kit could identify 28 HPV subtypes, including 18 hr‐HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 10 low‐risk HPV (lr‐HPV) genotypes (6, 11, 40, 42, 43, 44, 54, 61, 81, 83). PCR was performed in a 25 μL reaction mixture containing 5 μL extracted DNA, 0.75μL DNA Taq polymerase, and 19.25 μL PCR‐mix solution with GP5+/6+ primer system. PCR methods: The samples denatured at 95℃ for 15 minutes, followed by 40 cycles of denaturation, annealing, and extension at 94℃, 55℃, and 72℃ for 40 seconds, respectively. The final extension was performed at 72℃ for 7 minutes. β‐Globin gene and pure water were as positive and negative controls to confirm the accuracy of the PCR result. All experiments were carried out in accordance with the manufacturer’s instructions.
2.5. Statistical analysis
Data were analyzed with the SPSS 16.0 software (SPSS, Chicago, IL, USA) and Prism 5 software (GraphPad, San Diego, CA, USA). The chi‐square test was used in our study, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. The overall HPV prevalence
A total of 935 women including 806 HSIL and 129 ICC individuals were involved in this research. The overall HPV‐positive rate was 74.0% (692/935) in Ganzhou. Of the 806 HSIL women, the overall HPV infection rate accounted for 75.6% (609/806), which included 600 (74.4%, 600/806) hr‐HPV infections. Among the 129 ICC patients, the overall HPV infection rate was 64.3% (83/129). And the high‐risk HPV infection rate was 64.3% (83/129).
3.2. Distribution of single and multiple hr‐HPV infections
Among the HSIL infections, the single hr‐HPV infection rate was 52.2% (421/806), accounting for 76.1% (421/553) of hr‐HPV‐positive samples. The multiple hr‐HPV infection rate was 22.6% (182/806) of all women, accounting for 22.2% of hr‐HPV‐positive cases. Among the multiple hr‐HPV infections, the prevalence of two genotypes was the highest with the rate of 13.5%. The maximum number of HPV infection genotype was 5 genotypes of individuals (Table 1).
Table 1.
The prevalence of HPV infection among the HSIL and ICC patients
| HPV infection | HSIL | ICC | ||
|---|---|---|---|---|
| Positive case (n) | Percentage (%) | Positive case (n) | Percentage (%) | |
| 1hr‐HPV only | 421 | 52.2 | 66 | 51.2 |
| 2hr‐HPV only | 109 | 13.5 | 14 | 10.9 |
| ≥3hr‐HPV only | 23 | 2.9 | 0 | 0 |
| lr‐HPV only | 9 | 1.1 | 0 | 0 |
| Combination of hr and lr‐HPV | 47 | 5.8 | 3 | 2.3 |
| Negative | 197 | 24.4 | 46 | 35.7 |
| total | 806 | 100 | 129 | 100 |
HPV, human papillomavirus; hr‐HPV, high‐risk human papillomavirus; lr‐HPV, low‐risk human papillomavirus; HSIL, high‐grade squamous intraepithelial lesion; ICC, invasive cervical cancer
Of the ICC women, the prevalence of single and multiple hr‐HPV infections was 51.2% (66/129) and 10.9% (14/129) of ICC patients, accounting for 79.5% (66/83) and 16.9% of hr‐HPV‐positive cases, respectively. There was no infection with three or more hr‐HPV and only lr‐HPV genotypes (Table 1).
3.3. hr‐HPV genotype distribution
Among the HSIL individuals, the most prevalent hr‐HPV genotype was HPV 16 (29.9%). The 4 common subtypes in decreasing order were HPV 52 (17.5%), 58 (17.2%), 33 (10.7%), and 18 (3.6%). The lowest percentage of hr‐HPV genotype was HPV 45 (0.1%). In lr‐HPV population, the most common genotypes were HPV 42 (1.7%) and 44 (1.7%), and the 4 common subtypes in decreasing order were 40 (1.6%), 6 (0.9%), 81 (0.7%), and 11 (0.6%). No cases of HPV 83 infection were found (Table 2, Figure 1).
Table 2.
The HPV genotype distribution in HSIL and ICC patients
| HPV type | HSIL | ICC | ||
|---|---|---|---|---|
| Case (n) | Percentage(%) | Case (n) | Percentage(%) | |
| hr‐HPV | ||||
| 16 | 241 | 29.9 | 58 | 45.0 |
| 18 | 29 | 3.6 | 12 | 9.3 |
| 26 | 5 | 0.6 | 2 | 1.6 |
| 31 | 23 | 2.9 | 1 | 0.8 |
| 33 | 86 | 10.7 | 0 | 0 |
| 35 | 11 | 1.4 | 1 | 0.8 |
| 39 | 13 | 1.6 | 0 | 0 |
| 45 | 1 | 0.1 | 0 | 0 |
| 51 | 25 | 3.1 | 1 | 0.8 |
| 52 | 141 | 17.5 | 10 | 7.8 |
| 53 | 15 | 1.9 | 0 | 0 |
| 56 | 10 | 1.2 | 1 | 0.8 |
| 58 | 139 | 17.2 | 6 | 4.7 |
| 59 | 10 | 1.2 | 3 | 2.3 |
| 66 | 17 | 2.1 | 0 | 0 |
| 68 | 9 | 1.1 | 2 | 1.6 |
| 73 | 2 | 0.2 | 1 | 0.8 |
| 82 | 10 | 1.2 | 0 | 0 |
| lr‐HPV | ||||
| 6 | 7 | 0.9 | 1 | 0.8 |
| 11 | 5 | 0.6 | 0 | 0 |
| 40 | 13 | 1.6 | 2 | 1.6 |
| 42 | 14 | 1.7 | 0 | 0 |
| 43 | 4 | 0.5 | 0 | 0 |
| 44 | 14 | 1.7 | 0 | 0 |
| 54 | 1 | 0.1 | 0 | 0 |
| 61 | 2 | 0.2 | 0 | 0 |
| 81 | 6 | 0.7 | 0 | 0 |
| 83 | 0 | 0 | 0 | 0 |
HPV, human papillomavirus; hr‐HPV, high‐risk human papillomavirus; lr‐HPV, low‐risk human papillomavirus; HSIL, high‐grade squamous intraepithelial lesion; ICC, invasive cervical cancer
Figure 1.
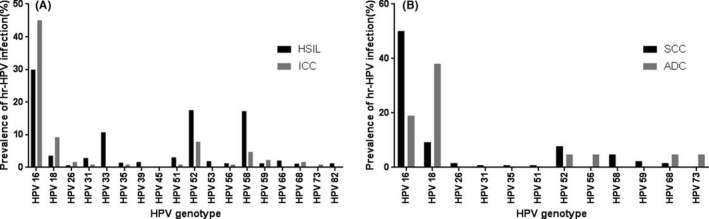
The prevalence of hr‐HPV genotype infection. A, HISL and SCC women. B, SCC and ADC women
Of the ICC patients, the most frequently hr‐HPV subtype was HPV 16 (45.0%) followed by 18 (9.3%), 52 (7.8%), 58 (4.7%), and 59 (2.3%). And ICC patients were classified into SCC and ADC individuals. Among the SCC patients, for hr‐HPV genotypes, HPV 16 (50.0%), 18 (9.3%), 52 (7.8%), 58 (4.7%), and 59 (2.3%) were five most common subtypes. In patients with ADC, the most common hr‐HPV genotype was HPV 18 (38.1%), followed by HPV 16 (19.0%), 52 (4.8%), 56 (4.8%), 68 (4.8%), 73 (4.8%) (Tables 2, 3, Figure 1).
Table 3.
The HPV genotype distribution in SCC and ADC patients
| HPV type | SCC | ADC | ||
|---|---|---|---|---|
| Case (n) | Percentage (%) | Case (n) | Percentage (%) | |
| hr‐HPV | ||||
| 16 | 54 | 50.0 | 4 | 19.0 |
| 18 | 4 | 9.3 | 8 | 38.1 |
| 26 | 2 | 1.6 | 0 | 0 |
| 31 | 1 | 0.8 | 0 | 0 |
| 35 | 1 | 0.8 | 0 | 0 |
| 51 | 1 | 0.8 | 0 | 0 |
| 52 | 9 | 7.8 | 1 | 4.8 |
| 56 | 0 | 0 | 1 | 4.8 |
| 58 | 6 | 4.7 | 0 | 0 |
| 59 | 3 | 2.3 | 0 | 0 |
| 68 | 1 | 1.6 | 1 | 4.8 |
| 73 | 0 | 0 | 1 | 4.8 |
| lr‐HPV | ||||
| 6 | 1 | 0.8 | 0 | 0 |
| 40 | 2 | 1.6 | 0 | 0 |
ADC, adenocarcinoma ADC; HPV human papillomavirus; hr‐HPV, high‐risk human papillomavirus; lr‐HPV, low‐risk human papillomavirus; SCC, squamous cell carcinoma.
3.4. HPV infection among women of different ages
Among the 806 cases of HSIL, the prevalence rates of HPV infection were 76.2%, 75.3%, 75.7%, 72.5%, and 88.0% for women aged under 25, 25‐34, 35‐44, 45‐54, and over 55 years old, respectively. Among the different age groups, the prevalence rates were not significantly different (P = 0.259). The overall prevalence of hr‐HPV was 73.8%, 74.5%, 74.6%, 71.5%, and 86.0% for women aged under 25, 25‐34, 35‐44, 45‐54, and over 55 years old, respectively, but there was no statistical difference in the different age groups (P = 0.163) (Table 4, Figure 2).
Table 4.
The infection rate of HPV and hr‐HPV in different age groups of HSIL (n, %)
| Age | HPV | P value | hr‐HPV | P value | ||
|---|---|---|---|---|---|---|
| + | − | + | − | |||
| <25 | 32 (76.2) | 10 (23.8) | 0.259 | 31 (73.8) | 10 (26.2) | 0.163 |
| 25‐34 | 177 (75.3) | 58 (24.7) | 175 (74.5) | 58 (25.5) | ||
| 35‐44 | 206 (75.7) | 66 (24.3) | 203 (74.6) | 66 (25.4) | ||
| 45‐54 | 150 (72.5) | 57 (27.5) | 148 (71.5) | 57 (28.5) | ||
| ≥55 | 44 (88.0) | 6 (12.0) | 43 (86.0) | 7 (14.0) | ||
Figure 2.

The prevalence of total HPV and hr‐HPV. A, The prevalence of total HPV and hr‐HPV among HISL women with different age groups. B, The prevalence of hr‐HPV in different age groups of ICC
None of the ICC patients was infected by lr‐HPV. Among the 129 cases of ICC, the infection rates of both HPV and hr‐HPV were 66.7%, 62.2%, 58.6%, and 78.6% for women aged 25‐34, 35‐44, 45‐54, and over 55 years old, respectively. There was no statistical difference among the different age groups (P = 0.335) (Table 5, Figure 2).
Table 5.
The prevalence of HPV and hr‐HPV in different age groups of ICC (n, %)
| Age | HPV | P value | hr‐HPV | P value | ||
|---|---|---|---|---|---|---|
| + | − | + | − | |||
| 25‐34 | 4 (66.7) | 2 (33.3) | 0.335 | 4 (66.7) | 2 (33.3) | 0.335 |
| 35‐44 | 23 (62.2) | 14 (37.8) | 23 (62.2) | 14 (37.8) | ||
| 45‐54 | 34 (58.6) | 24 (41.4) | 34 (58.6) | 24 (41.4) | ||
| ≥55 | 22 (78.6) | 6 (21.4) | 22 (78.6) | 6 (21.4) | ||
HPV, human papillomavirus; hr‐HPV, high‐risk human papillomavirus; HSIL, high‐grade squamous intraepithelial lesion; ICC, invasive cervical cancer
4. DISCUSSION
Previous study has confirmed that HPV infection, especially hr‐HPV, is the main cause of cervical cancer.21 In addition, persistent HPV infection is related to the development of cervical cancer.22 However, there is a contrast between different countries and areas on the genotype distribution of HPV infection.23 Therefore, in this research, the prevalence and distribution of HPV genotypes were discussed in Ganzhou, China.
The previous studies from different areas in China proved that the overall HPV infection rates were 22.4%,16 38.3%,17 17.6%,24 and 24.1%,15 respectively. However, our research suggested that the overall prevalence of HPV infection was 74.0% among the HSIL and ICC women. The prevalence of our result was higher than that of previous researches because of the different populations. Our participants were HSIL and ICC females, while those researches were general population. In addition, for hr‐HPV genotypes, the 5 common subtypes in decreasing order were HPV 16, 52, 58, 33, and 18. The results of this study are similar to other literature.14, 15, 16 While other researches suggested that the most frequent HPV genotypes of HSIL were HPV 16, 58, and 18; HPV 16 and 52; and HPV 53, 58, and 52 in Sichuan, Shanxi, and Korea, respectively.12, 15, 25 Our results found that there was only one HSIL infected with HPV 45 (0.1%), while other study suggested that the HPV 45 infection rate was 0.45%.16 The sample size of the two studies was different, but all results suggested that the most infrequency hr‐HPV genotype was HPV 45. And it was different in patient population. The most two common HPV genotypes were HPV 16 and 18 of ICC; this result was consistent with previous study.26 However, HPV 18 and 16 were the most two most common subtypes among the ADC women. Our study also reflected that there still exist a part of HSIL and cervical carcinoma women who were HPV test negative, and we recommend HPV test and cytology screening as the first‐line screening methods.
In China, the bimodal curve of age‐specific HPV distribution is still controversial.27, 28 In our report, for women aged 55 years or older, the hr‐HPV prevalence was 88.0% and 78.6% among HSIL and ICC patients, respectively, but there was no statistical difference in the overall age‐specific HPV/hr‐HPV distribution. Among the HSIL and ICC patients, our results found an approximate U‐shaped curve in the HPV distribution of different age groups (Figure 2). A similar trend was also observed in Guangdong province,28 but we also found an S‐shaped curve of hr‐HPV distribution (Figure 2). The higher prevalence in older women was more likely caused by viral persistence or reactivation of latent HPV or immunological disorders associated with hormone fluctuations during the menopausal transition.29
At present, two different prophylactic HPV vaccines have been licensed and are now available in most countries including Mainland China, but HPV infection type was different in various regions of China, and the bivalent vaccine and the quadrivalent vaccine may not achieve the desired preventive efficacy. 9‐Valent HPV vaccine 6/ 11/ 16/ 18/ 31/ 33/ 45/ 52/ 58 might provide more effective prevention outcomes on cervical HSIL and ICC in China, but nonvaccine types 26/ 35/ 39/ 40/ 42/ 44/ 51/ 53/ 56/ 59/ 66/ 68/ 82 may be still responsible for some HSIL and ICC. In addition, obtaining data from this area could develop the optimal and effective screening and vaccination programs. Our data provided a baseline for HPV infection, and we will monitor changes in HPV infection rates caused by future HPV vaccines in China.
Our research has some limitations. Firstly, all patients came from one hospital only. Secondly, the study was retrospective with a limited sample size, especially invasive cervical cancer cases. And we will conduct a multicenter cooperation research in the future.
5. CONCLUSION
The prevalence and distribution of HPV genotypes in Ganzhou differed from other regions of China and Western countries. These results can serve as valuable reference for HPV vaccination programs for Ganzhou women.
CONFLICT OF INTEREST
The authors declare no conflict of interests in this research.
AUTHORS’ CONTRIBUTIONS
LH carried out studies, participated in the study, and drafted the manuscript. CH participated in the design of the study and performed the statistical analysis. ZF collected the questionnaire and helped in drafting manuscript and performing the experiment. ZW and DY conducted and checked the studies. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank all medical workers and patients in Department of Pathology, Maternal and Child Health Hospital of Ganzhou, for help in this research. And we would like to thank my supervisors Yanqing Ding and Weijie Zhou for guidance.
Liao L, Cheng H, Zeng F, Zhou W, Ding Y. Prevalence and distribution of human papillomavirus genotypes among women with high‐grade squamous intraepithelial lesion and invasive cervical cancer in Ganzhou, China. J Clin Lab Anal. 2019;33:e22708 10.1002/jcla.22708
Linhong Liao and Hui Cheng contributed equally to this work.
Contributor Information
Weijie Zhou, Email: weijiezhouum@163.com.
Yanqing Ding, Email: dingyqgz@sina.com.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 3. Women’s health in rural China . Lancet. 2009;374:358. [DOI] [PubMed] [Google Scholar]
- 4. Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. [DOI] [PubMed] [Google Scholar]
- 5. Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steben M, Duarte‐Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107:S2–S5. [DOI] [PubMed] [Google Scholar]
- 7. Ruan G, Song Y, Dong B, et al. Cervical cancer screening using the Cervista high‐risk human papillomavirus test: opportunistic screening of a hospital‐based population in Fujian province, China. Cancer Manag Res. 2018;10:3227–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IARC . Working group on the evaluation of carcinogenic risks to humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 9. de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta‐analysis. Lancet Infect Dis. 2007;7:453–459. [DOI] [PubMed] [Google Scholar]
- 10. Moore RA, Ogilvie G, Fornika D, et al. Prevalence and type distribution of human papillomavirus in 5,000 British Columbia women‐implications for vaccination. Cancer Causes Control. 2009;20:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson LA, O'Rorke MA, Wilson R, et al. HPV prevalence and type‐distribution in cervical cancer and premalignant lesions of the cervix: a population‐based study from Northern Ireland. J Med Virol. 2016;88:1262–1270. [DOI] [PubMed] [Google Scholar]
- 12. Ouh YT, Min KJ, Cho HW, et al. Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in the Republic of Korea, 2014–2016. J Gynecol Oncol. 2018;29:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hooi DJ, Lissenberg‐Witte BI, Kenter G, et al. Human papillomavirus (HPV) prevalence and associated risk factors in women from Curacao. PLoS ONE. 2018;13:e0199624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Li Z, Li J, et al. Prevalence and Distribution of HPV Genotypes in 1387 Women with Cervical Intraepithelial Neoplasia 2/3 in Shanxi Province, China. J Cancer. 2018;9:2802–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li B, Wang H, Yang D, et al. Prevalence and distribution of cervical human papillomavirus (HPV) genotypes in women with cytological results from Sichuan Province, China. J Med Virol. 2018. [DOI] [PubMed] [Google Scholar]
- 16. Zhong TY, Zhou JC, Hu R, et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J Infect Public Health. 2017;10:783–788. [DOI] [PubMed] [Google Scholar]
- 17. Wu C, Zhu X, Kang Y, et al. Epidemiology of Humanpapilloma virus infection among women in Fujian, China. Bmc Public Health. 2018;18:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population‐based cervical cancer screening in China. Asian Pac J Cancer Prev. 2015;16:7401–7407. [DOI] [PubMed] [Google Scholar]
- 19. Giuliano AR, Kreimer AR, de Sanjose S. The beginning of the end: vaccine prevention of HPV‐driven cancers. J Natl Cancer Inst. 2015;107:djv128. [DOI] [PubMed] [Google Scholar]
- 20. Kurman RJ, Ellenson LH, Ronnett BM. Blaustein’s Pathology of the Female Genital Tract. Progresos De Obstetricia Y Ginecología. 2003;35:457–457. [Google Scholar]
- 21. Pierce Campbell CM, Menezes LJ, Paskett ED, et al. Prevention of invasive cervical cancer in the United States: past, present, and future. Cancer Epidemiol Biomark Prev. 2012;21:1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boumba LM, Qmichou Z, Mouallif M, et al. Human papillomavirus genotypes distribution by cervical cytologic status among women attending the General Hospital of Loandjili, Pointe‐ Noire, Southwest Congo (Brazzaville). J Med Virol. 2015;87:1769–1776. [DOI] [PubMed] [Google Scholar]
- 23. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens‐ part B: biological agents. Lancet Oncol. 2009;10:321–322. [DOI] [PubMed] [Google Scholar]
- 24. Xiang F, Guan Q, Liu X, et al. Distribution characteristics of different human papillomavirus genotypes in women in Wuhan, China. J Clin Lab Anal. 2018;3:e22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao XL, Hu SY, Zhang Q, et al. High‐risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017;28:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hopenhayn C, Christian A, Christian WJ, et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 US cancer registries before vaccine introduction. J Low Genit Tract Dis. 2014;18:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu JF, Shen GR, Li Q, et al. Genotype distribution characteristics of multiple human papillomavirus in women from the Taihu River Basin, on the coast of eastern China. BMC Infect Dis. 2017;17:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin M, Yang LY, Li LJ, et al. Genital human papillomavirus screening by gene chip in Chinese women of Guangdong province. Aust N Z J Obstet Gynaecol. 2008;48:189–194. [DOI] [PubMed] [Google Scholar]
- 29. Ye J, Cheng X, Chen X, et al. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population‐based study. Virol J. 2010;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]


