Abstract
Background
Cancer‐related inflammation promotes gallbladder tumorigenesis and metastasis of gallbladder cancer (mGBC). The levels of circulating inflammatory‐related cell and protein as well as the ratios of them may imply the severity of chronic inflammation in GBC patients, and all of them are candidate prognostic biomarkers for mGBC.
Materials and methods
In our study, pre‐treatment circulating immune cell, fibrinogen (Fib), albumin (Alb), and pre‐albumin (pAlb) were detected in 220 mGBC patients, and we calculated neutrophil‐to‐lymphocyte ratio (NLR), derived neutrophil‐to‐lymphocyte ratio (dNLR), lymphocyte‐to‐monocyte ratio (LMR), platelet‐to‐lymphocyte ratio (PLR), Alb‐to‐Fib ratio (AFR), and Fib‐to‐pAlb ratio (FPR) replying on the detection. Three years’ follow‐up was carried out in those patients, and we investigated the possible associations between those biomarkers and three years’ overall survival (OS) of these patients using X‐tile software, Kaplan‐Meier curve, Cox regression, and time‐dependent receiver operating characteristics (ROC).
Results
Our results showed that OS of the patients with high pAlb and LMR was significantly superior to the cases with the low biomarkers, respectively. However, survival of the cases with high CEA, dNLR, and FPR was significantly inferior to the patients with low levels of those biomarkers. Area under the curve (AUC) of time‐dependent ROC of CEA and dNLR was higher than pAlb, LMR, and FPR, respectively. Additionally, higher CEA‐dNLR score (adjusted HR = 3.09, 95% CI = 1.01‐4.51 for the score one; adjusted HR = 4.99, 95% CI = 2.32‐7.21 for the score two) was significantly associated with reduced survival of the patients, and AUC of the score for predicting clinical outcome of mGBC patients was 0.756, and it was significantly higher than the single CEA and dNLR, respectively.
Conclusion
Our findings implied that pretreatment CEA‐dNLR score was superior to the other biomarkers to predict OS of mGBC patients, and it was an independent prognostic factor for the disease.
Keywords: dNLR, metastatic gallbladder cancer, prognostic biomarker
1. INTRODUCTION
Gallbladder cancer (GBC) is the sixth common gastrointestinal cancer and the sixth cause of cancer‐related death in China.1 Although improvement in diagnosis and treatment of GBC, the majority of the patients are usually confirmed as metastatic GBC (mGBC).2, 3 Due to the lymph node or distant metastasis of the disease, its five years’ overall survival rate was merely 5%.3, 4 Thus, it is urgent for us to clarify the mechanisms of GBC occurrence and progression and to explore the effective clinical biomarker to predict the prognosis of the individuals.
Emerging evidences showed that up to 20% of the malignancies were caused by chronic inflammation,5 and cancer‐related inflammation played a decisive role in tumorigenesis, malignant conversion, and distant metastasis of GBC.6 Systemic inflammation triggered by chronic infection, smoking, drinking, and obesity contributed to oncogene activation and inhibition of tumor suppressor gene, leading to occurrence of the disease. Moreover, inflammatory microenvironment protected GBC cell to escape from immune surveillance and promoted the specific organic metastasis through the formation of pre‐metastatic niche.7 Thus, we speculate that circulating inflammatory biomarker may reflect progression and predict clinical outcome of the disease.
Recently, there were lots of studies reported association of circulating inflammatory immune cell ratio and albumin‐to‐fibrinogen ratio (AFR) and fibrinogen‐to‐pre‐albumin ratio (FPR) and prognosis of gastric cancer, non‐small‐cell lung cancer, colorectal cancer, and GBC.8, 9, 10, 11, 12 Zhang et al reported that FPR was a feasible prognostic biomarker in surgical stage II and III gastric cancer and it could precisely distinguish stage III patients who appeared to obviously benefit from adjuvant chemotherapy.8 Pretreatment AFR was reported to be a clinical biomarker to predict clinical efficacy of surgical resection and adjuvant chemo‐radiotherapy and to be a prognostic biomarker for non–small‐cell lung cancer individuals.9 According to GBC, circulating neutrophil‐to‐lymphocyte ratio (NLR) was closely related to poor prognosis of surgical GBC patient.10 However, there is no study reported the prognostic roles of derived neutrophil‐to‐lymphocyte ratio (dNLR), lymphocyte‐to‐monocyte ratio (LMR), AFR, and FPR in mGBC, and the prognostic significance of them remain to be determined.
Therefore, we detected and calculated pretreatment circulating immune cell, Fib, Alb, pAlb, NLR, dNLR, platelet‐to‐lymphocyte ratio (PLR), LMR, AFR, and FPR and obtained the overall survival (OS) of them by means of three years’ follow‐up in 220 mGBC cases to investigate the prognostic values of them in predicting clinical outcome of the disease.
2. MATERIALS AND METHODS
In our study, we collected eligible mGBC patients in accordance with the following inclusion and exclusion criteria. First of all, the selected patients were clinically confirmed as mGBC without acute infection, autoimmune and hematological as well as live diseases; secondly, clinical feature and outcome could be obtained from the included patients; thirdly, all of the included cases were from the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine and Longyan People Hospital between October 2009 and May 2015. All of the informed consents were signed, and this study was approved by Medical Ethics Committee of the two hospitals.
The baseline characteristics such as name, gender, age, status of drinking and smoking, hypertension, diabetes, and metastatic information were retrieved and collected from medical record of each individual. Two milliliter EDTA anticoagulation peripheral blood, serum, and plasma samples were collected between 7:00 am and 9:00 am from the each individual, respectively. The peripheral blood counting was detected by semiconductor laser flow cytometry and nucleic acid staining method using SYSMEX XS‐500i (SYSMEX CORPORATION, Chuo‐ku, Kobe, Hyogo, Japan) automatic blood analyzer, and the internal and extraval coefficient of variations (CVs) of the method were less than 5%. Bromocresol green method, immunity transmission turbidity, and coagulation method were selected to detect serum Alb and pAlb, and plasma Fib, respectively. The ratios of NLR, dNLR (total white blood cell counting‐lymphocyte/lymphocyte), PLR, LMR, FPR, and AFR were calculated according to the detection results. We performed the three years’ OS by retrieving medical record, telephone, and correspondent letter, OS was the first endpoint of the study, and the deadline of follow‐up was May 2018. The time from the diagnosis date to death or alive until the deadline was presented as OS.
In order to further investigate the prognostic values of CEA and dNLR, we established a novel CEA‐dNLR score replying on the two biomarkers. Both CEA and dNLR less or higher than the cutoff values were defined as CEA‐dNLR zero and two score, respectively. Either CEA or dNLR higher than the cutoff values was defined as the score one.
In present study, the optimal cutoff values of PLR, NLR, dNLR, AFR, and FPR were obtained using X‐tile software. Kaplan‐Meier curve, log‐rank test, univariable and multivariable Cox proportion regression models were used to investigate the associations between the ratios and clinical outcome of the patients. Hazard ratio (HR) and 95% confidential interval (CI) were selected to measure the strength between them. Time‐dependent receiver operating characteristics (ROC) were used to compare the efficacies of the ratios for mGBC survival prediction. All statistics were conducted using R 3.5.0 and IBM SPSS statistics 22 software (IBM Corporation, Armonk, NY, USA), and P < 0.05 implied significance between the groups.
3. RESULTS
A total of 220 mGBC patients were included in the study, and the baseline characteristics were presented in Table 1. As shown from the Table 1, only 60.45% of the patients received adjuvant chemotherapy, the numbers of the patient harbored CEA‐dNLR score zero, one, and two were 100 (45.45%), 85 (38.64%), and 35 (15.91%), respectively. In the follow‐up period, 139 patients were dead from the disease, and the others were alive, and the median overall survival (OS) was 9.00 (3.00‐30.00) months.
Table 1.
The baseline characteristics of 220 included metastatic gallbladder cancer patients
| Variables | Categories | No. of patients (%) |
|---|---|---|
| Gender | Male | 122 (55.45) |
| Female | 98 (44.54) | |
| Age | ≤60 years | 125 (56.81) |
| >60 years | 95 (43.18) | |
| Tobacco | Yes | 23 (10.45) |
| No | 197 (89.55) | |
| Alcohol | Yes | 56 (25.50) |
| No | 164 (74.50) | |
| Hypertension | Yes | 61 (27.73) |
| No | 159 (72.27) | |
| Diabetes | Yes | 43 (19.55) |
| No | 177 (80.45) | |
| Chemotherapy | Yes | 133 (60.45) |
| No | 87 (39.55) | |
| Fib | mg/L | 3.71 (3.14‐4.53) |
| Alb | g/L | 37.00 (34.37‐40.13) |
| pAlb | mg/L | 134.80 (94.90‐185.50) |
| CEA | ng/ml | 2.68 (1.42‐10.77) |
| CA199 | U/ml | 95.45 (25.98‐700.00) |
| NLR | 3.36 (2.07‐5.62) | |
| dNLR | 3.94 (2.53‐6.07) | |
| PLR | 154.95 (114.06‐216.84) | |
| LMR | 2.92 (1.84‐4.39) | |
| AFR | 9.57 (7.85‐12.34) | |
| FPR | 31.84 (19.08‐43.82) | |
| CEA‐dNLR score | Score 0 | 100 (45.45) |
| Score 1 | 85 (38.64) | |
| Score 2 | 35 (15.91) | |
| Survival status | Alive | 81 (36.82) |
| Death | 139 (63.18) | |
| Median OS | 9.00 (3.00‐30.00) |
Fib, fibrinogen; Alb, albumin; pre‐Alb, pre‐albumin; AFR, albumin‐to‐fibrinogen ratio; FPR, fibrinogen‐to‐pre‐albumin ratio; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19‐9; NLR, neutrophil‐to‐lymphocyte ratio; dNLR, derived neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; LMR, lymphocyte‐to‐monocyte ratio; OS, overall survival; score 0 means both CEA and dNLR were less than the cutoff values; score 1 means either CEA or dNLR was higher than the cutoff values; score 2 means both CEA and dNLR were higher than the cutoff values.
In present study, X‐tile software, Kaplan‐Meier curve with log‐rank test and univariable and multivariable Cox proportion regression models were selected to calculate the optimal cutoff values of the inflammatory biomarkers based on OS of the patients and to investigate the prognostic values of clinical baseline characteristics and each biomarker in mGBC patients. The optimal cutoff values based on OS were 4.35 g/L for Fib, 35.70 g/L for Alb, 90.00 for pAlb, 15.45 for AFR, 5.00 for dNLR, 178 for PLR, 2.92 for LMR, and 5.1 for NLR as well as 31.84 for FPR (Figure 1). There was no significant survival difference in the patients stratified by sex, age, status of smoking and drinking, hypertension, and diabetes (all P‐values >0.05). Whereas we observed that OS of the patients with treatment of chemotherapy (crude HR = 0.57, 95% CI = 0.14‐0.85; adjusted HR = 0.66, 95% CI = 0.37‐0.82), high pAlb (crude HR = 0.43, 95% CI = 0.28‐0.65; adjusted HR = 0.58, 95% CI = 0.38‐0.90), and LMR (crude HR = 0.52, 95% CI = 0.37‐0.72; adjusted HR = 0.69, 95% CI = 0.35‐0.91) was significantly superior to the cases without chemotherapy, low pAlb and LMR, respectively, the survival of the cases with high CEA (crude HR = 2.18, 95% CI = 1.49‐3.22; adjusted HR = 1.91, 95% CI = 1.26‐2.89), dNLR (crude HR = 2.64, 95% CI = 2.09‐4.14; adjusted HR = 2.66, 95% CI = 2.10‐4.68) and FPR (crude HR = 1.93, 95% CI = 1.26‐2.97; adjusted HR = 1.57, 95% CI = 1.00‐2.46) was significantly inferior to the patients with the low levels of those biomarkers (Figure 2 and Table 2).
Figure 1.
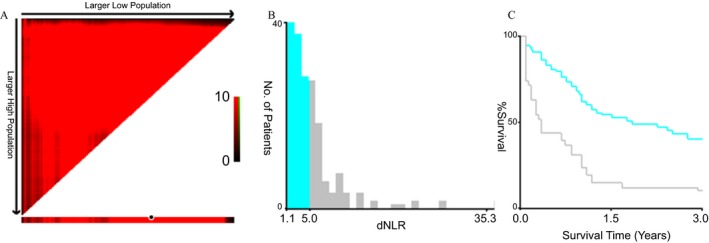
Optimal cutoff value of dNLR. A, 3Pop X‐tile plot of dNLR using X‐tile software; B, histogram of dNLR using X‐title software; C, Kaplan‐Meier curve of dNLR using X‐tile software; dNLR, derived neutrophil‐to‐lymphocyte ratio
Figure 2.
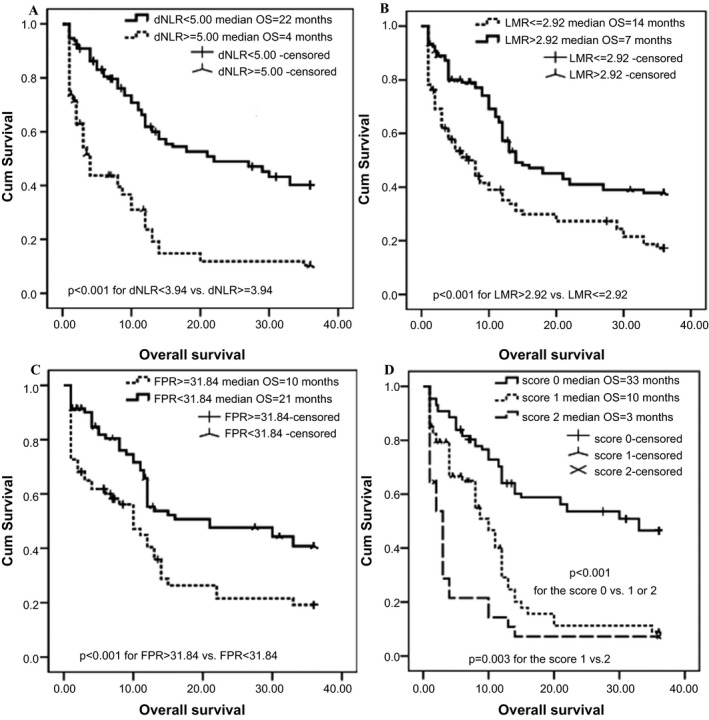
Kaplan‐Meier curves of dNLR, LMR, FPR, and CEA‐dNLR score in 220 mGBC patients. A, dNLR; B, LMR; C, FPR; D, CEA‐dNLR score; dNLR, derived neutrophil‐to‐lymphocyte ratio; LMR, lymphocyte‐to‐monocyte ratio; FPR, fibrinogen‐to‐pre‐albumin ratio; mGBC, metastatic gallbladder cancer
Table 2.
Cox regression model analyses of prognostic factors for 3 years’ OS within 220 metastatic gallbladder cancer patients by Cox regression model
| Variables | Three years’ overall survival | |||
| Univariate analysis | Multivariate analysis | |||
| P‐value | Crude HR (95%CI) | P‐value | Adjusted HR (95%CI)* | |
| Sex (male) | 0.58 | 1.10 (0.79‐1.54) | 0.64 | 1.57 (0.94‐2.22) |
| Age (>60 years) | 0.04 | 2.05 (1.46‐2.86) | 0.08 | 1.32 (0.91‐1.91) |
| Smoking (yes) | 0.43 | 0.74 (0.42‐1.31) | 0.41 | 0.69 (0.37‐1.29) |
| Drinking (yes) | 0.56 | 0.93 (0.50‐1.73) | 0.64 | 1.28 (0.54‐2.04) |
| Hypertension (yes) | 0.48 | 0.89 (0.25‐1.36) | 0.47 | 0.78 (0.44‐1.46) |
| Diabetes (yes) | 0.73 | 1.54 (0.93‐2.31) | 0.72 | 1.48 (0.44‐2.32) |
| Chemotherapy (yes) | 0.03 | 0.57 (0.14‐0.85) | <0.01 | 0.66 (0.37‐0.82) |
| Fib | 0.41 | 0.79 (0.54‐1.14) | 0.58 | 0.92 (0.63‐1.35) |
| Alb | 0.52 | 1.08 (0.87‐2.03) | 0.64 | 1.41 (0.99‐2.01) |
| pAlb | <0.01 | 0.43 (0.28‐0.65) | <0.01 | 0.58 (0.38‐0.90) |
| CA199 | 0.76 | 1.60 (0.81‐2.38) | 0.51 | 1.32 (0.88‐1.98) |
| CEA | <0.01 | 2.18 (1.49‐3.22) | <0.01 | 1.91 (1.26‐2.89) |
| NLR | 0.70 | 1.35 (0.85‐2.49) | 0.62 | 1.38 (0.41‐1.84) |
| dNLR | <0.01 | 2.64 (2.09‐4.14) | <0.01 | 2.66 (2.10‐4.68) |
| PLR | 0.40 | 0.63 (0.37‐1.23) | 0.44 | 0.75 (0.53‐1.06) |
| LMR | <0.01 | 0.52 (0.37‐0.722) | 0.03 | 0.69 (0.35‐0.91) |
| AFR | 0.82 | 1.97 (0.96‐3.67) | 0.51 | 0.89 (0.46‐1.73) |
| FPR | <0.01 | 1.93 (1.26‐2.97) | 0.02 | 1.57 (1.00‐2.46) |
Fib, fibrinogen; Alb, albumin; pre‐Alb, pre‐albumin; AFR, albumin/fibrinogen ratio; FPR, fibrinogen/pre‐albumin ratio; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19‐9; NLR, neutrophil/lymphocyte ratio; dNLR, derived neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; HR, hazard ratio; CI, confidence interval; HR (95% CI)* was adjusted by sex, age, alcohol, tobacco, hypertension, diabetes.
Significance results are shown in bold.
In our study, time‐dependent ROC was used to compare the prognostic efficacy of each biomarker. The highest area under the curve (AUC) within the inflammatory biomarkers was dNLR (0.673), and AUC of CEA (0.652) was higher than pAlb (0.649), LMR (0.624), and FPR (0.622), respectively (Figures 3 and 4). To further investigate the prognostic values of CEA and dNLR, we established and assessed the prognostic value of a novel CEA‐dNLR score replying on the two biomarkers in these patients. The higher CEA‐dNLR score (crude HR = 2.85, 95% CI = 1.86‐4.36 and adjusted HR = 3.09, 95% CI = 1.01‐4.51 for the score one; crude HR = 4.80, 95% CI = 2.89‐7.99 and adjusted HR = 4.99, 95% CI = 2.32‐7.21 for the score two) was significantly associated with reduced survival of the patients (Table 3), and AUC of CEA‐dNLR score for predicting clinical outcome of mGBC patients was 0.756, and it was significantly higher than both the single CEA and the dNLR, respectively (Figures 3 and 4).
Figure 3.
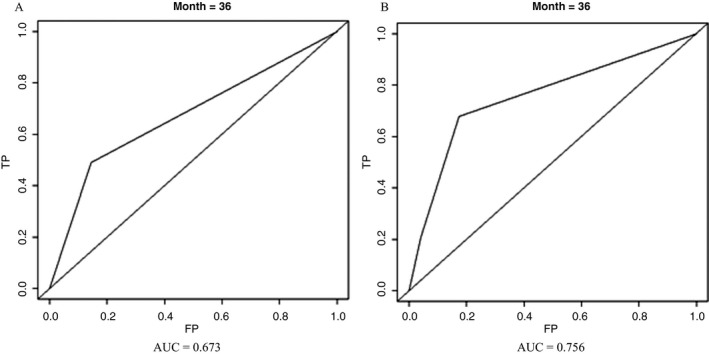
Time‐dependent receiver operating characteristics of dNLR and CEA‐dNLR score in 220 mGBC patients. A, dNLR; B, CEA‐dNLR score; dNLR, derived neutrophil‐to‐lymphocyte ratio; mGBC, metastatic gallbladder cancer; AUC, area under the curve
Figure 4.
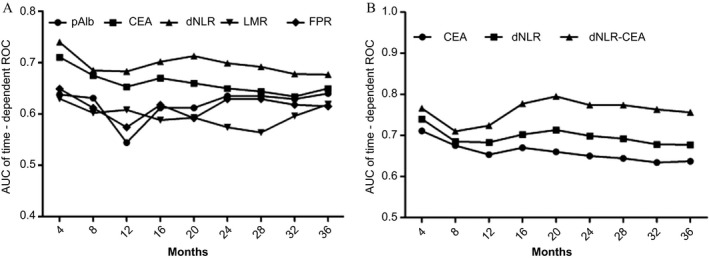
Comparison of area under curves of the time‐dependent receiver operative characteristics within pAlb, LMR, dNLR, CEA, FPR, and CEA‐dNLR score. A, comparison between pAlb, LMR, dNLR, CEA, and FPR; B, comparison between CEA, dNLR, and CEA‐dNLR score; pAlb, pre‐albumin; LMR, lymphocyte‐to‐monocyte ratio; dNLR, derived neutrophil‐to‐lymphocyte ratio; FPR, fibrinogen‐to‐pre‐albumin ratio
Table 3.
Univariate and multivariate analyses of CEA‐dNLR score for 3 years’ OS in metastatic gallbladder cancer patients by Cox regression model
| Ratio | Score | Three years’ overall survival | |||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | ||
| CEA‐dNLR | 0 | 1 | 1 | ||
| 1 | 2.85 (1.86‐4.36) | <0.001 | 3.09 (1.01‐4.51) | 0.001 | |
| 2 | 4.80 (2.89‐7.99) | <0.001 | 4.99 (2.32‐7.21) | <0.001 | |
dNLR, derived neutrophil‐to‐lymphocyte ratio; HR, hazard ratio; CI, confidential interval.
4. DISCUSSION
Gallbladder cancer was recognized as a complex disease causing by interaction of personal genetic predisposition and environmental factor such as substantial intake of alcohol and tobacco as well as chronic inflammation.11 Persistent chronic inflammatory response triggered by these environmental exposures in the patients, contributed to constitutive activation of pro‐inflammatory signaling pathways, promoted mutation of KRAS, BRAF, PIK3CA, and IDH1/2,12, 13, 14, 15 consequently leading to onset and metastasis of GBC.
In our study, we comprehensively investigated circulating inflammatory‐related cell, protein, and ratios of them to determine the simple, economical, practical clinical biomarker to effectively predict the survival of mGBC patients. We found that pretreatment pAlb, LMR, CEA, dNLR, and FPR were significantly associated with clinical outcome of mGBC patients, indicating that all of them were independent prognostic factors for the disease. Moreover, we found that the predicted efficacy of dNLR was the highest between them, implying that dNLR was superior to the other inflammatory biomarkers to predict the survival of the patients. Additionally, survival of the patients harbored high CEA‐dNLR score was significantly inferior to the cases with the low score, and cancer‐death risk of the patient with the score two and one was approximately twofold comparing to the score one and zero patients, respectively, the survival predicted AUC of the score was significantly higher than CEA and dNLR. These results suggested that a novel CEA‐dNLR score was an independent prognostic factor for mGBC patients, and it was superior to the other inflammatory biomarkers to predict 3 years’ OS of the patient.
Till now, several studies reported the association of inflammatory‐related biomarker with clinical outcome of GBC.16, 17, 18 However, the controversial results were observed between circulating ratio of peripheral blood cell and survival of the disease.16, 17 In our study, we confirmed that the pAlb, dNLR, PLR, LMR, and FPR were independent prognostic factors for mGBC, and we found that dNLR was superior to PLR, LMR, and FPR to precisely predict prognosis of the disease. Neutrophil and monocyte are the main components of circulating leukocyte, and tumor‐associated macrophage derived from monocytic precursors is recruited at the tumor site by CCL2/MCP‐1 and EGF.19, 20 Tumor‐associated neutrophil and macrophage are important infiltrated components within mGBC patients, there are all key regulators of the tumor‐related inflammation.21, 22, 23, 24 Secondary, lymphocyte, especially CD3+ and CD8+ T cell, plays anti‐tumor role in various malignancies including GBC.25 Study of Fluxa et al have showed that the presence of CD8+ and the absence of Foxp3+ T‐cell populations in mGBC tissue were correlated with improved survival of the patient.25 Furthermore, circulating Alb and pAlb are indicators of both chronic inflammation and status of nutrition. However, GBC is a chronic consumption disease, and most of the patients are emaciated with different degree of systematic inflammation.6 IL‐6 secreted by the tumor or surrounding cell stimulated high level of Fib,26 and Fib interacted with several growth factors to promote invasion and metastasis of GBC cell, leading to a poor survival of the patients.27
This study is the first time for us to construct CEA‐dNLR score and to investigate the predictive and prognostic roles of AFR, FPR, and the score in mGBC patients. Moreover, pAlb, LMR, dNLR, and FPR were reliable, economical, and practical biomarkers to predict the prognosis of mGBC, and CEA‐dNLR score was superior to the single inflammatory biomarker to predict the survival of the disease, avoiding false‐positive or false‐negative result. However, several limitations of our study should be addressed. Firstly, only 220 eligible patients were included in our study, since small sample size might lead to unstable result. Secondary, this study was a retrospective design, all of the included patients were included from the two hospitals, and the conclusion was not validated by other centers. Thirdly, due to the incomplete survival data of the patient, we did not obtain sufficient progression‐free survival data, and it remained unknown the association between the score and progression of mGBC patient. For this, further large sample size and multi‐center studies are warrant to confirm our result.
In conclusion, this study suggested that pretreatment pAlb, LMR, dNLR, and FPR were independent prognostic factors of mGBC patient and CEA‐dNLR score was superior to these biomarkers to predict 3 years’ clinical outcome of the patients.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
Du J‐H, Lu J. Circulating CEA‐dNLR score predicts clinical outcome of metastatic gallbladder cancer patient. J Clin Lab Anal. 2019;33:e22684 10.1002/jcla.22684
REFERENCES
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 2. Shi JS, Liu G, Yu YL, et al. Early diagnosis of primary gallbladder carcinoma. China J Hepatobiliary Surg. 2000;6(6):436‐438. [PubMed] [Google Scholar]
- 3. Hueman MT, Vollmer CM Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16(8):2101‐2115. [DOI] [PubMed] [Google Scholar]
- 4. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ulich TR, del Castillo J, Keys M, et al. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor‐alpha‐induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987;139(10):3406‐3415. [PubMed] [Google Scholar]
- 6. Li Y, Zhang J, Ma H. Chronic inflammation and gallbladder cancer. Cancer Lett. 2014;345(2):242‐248. [DOI] [PubMed] [Google Scholar]
- 7. Nakagawa H, Suzuki N, Hirata Y, et al. Biliary epithelial injury‐induced regenerative response by IL‐33 promotes cholangiocarcinogenesis from peribiliary glands. Proc Natl Acad Sci U S A. 2017;114(19):E3806–E3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Li SQ, Liao ZH, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195‐75205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li SQ, Jiang YH, Lin J, et al. Albumin‐to‐fibrinogen ratio as a promising biomarker to predict clinical outcome of non‐small cell lung cancer individuals. Cancer Med. 2018;7(4):1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saqib R, Pathak S, Smart N, et al. Prognostic significance of pre‐operative inflammatory markers in resected gallbladder cancer: a systematic review. ANZ J Surg. 2018;88(6):554‐559. [DOI] [PubMed] [Google Scholar]
- 11. Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol. 2017;23(22):3978‐3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu M, Sasaki M, Igarashi S, et al. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119(9):1669‐1674. [DOI] [PubMed] [Google Scholar]
- 13. Sicklick JK, Fanta PT, Shimabukuro K, et al. Genomics of gallbladder cancer: the case for biomarker‐driven clinical trial design. Cancer Metastasis Rev. 2016;35(2):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande V, Nduaguba A, Zimmerman SM, et al. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad‐based tumor genotyping. Oncologist. 2012;17(1):72‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Jiang C, Li J, et al. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol. 2015;17(10):810‐818. [DOI] [PubMed] [Google Scholar]
- 17. Tao Z, Li SX, Cui X, et al. The prognostic value of preoperative inflammatory indexes in gallbladder carcinoma with hepatic involvement. Cancer Biomark. 2018;22(3):551‐557. [DOI] [PubMed] [Google Scholar]
- 18. Wu XS, Shi LB, Li ML, et al. Evaluation of two inflammation‐based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Onco. 2014;21(2):449‐457. [DOI] [PubMed] [Google Scholar]
- 19. McClellan JL, Davis JM, Steiner JL, et al. Linking tumor‐associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP‐1. Am J Physiol Gastrointest Liver Physiol. 2012;303(10):G1087–G1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guven Maiorov E, Keskin O, Gursoy A, et al. The structural network of inflammation and cancer: merits and challenges. Semin Cancer Biol. 2013;23(4):243‐251. [DOI] [PubMed] [Google Scholar]
- 21. Subbannayya T, Leal‐Rojas P, Barbhuiya MA, et al. Macrophage migration inhibitory factor ‐ a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortez‐Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor‐associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109(7):2491‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elpek KG, Cremasco V, Shen H, et al. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol Res. 2014;2(7):655‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung KY, Cho SW, Kim YA, et al. Cancers with higher density of tumor‐associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49(4):318‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fluxá P, Rojas‐Sepúlveda D, Gleisner MA, et al. High CD8+ and absence of Foxp3+ T lymphocytes infiltration in gallbladder tumors correlate with prolonged patients survival. BMC Cancer. 2018;18(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamaguchi T, Yamamoto Y, Yokota S, et al. Involvement of interleukin‐6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28(12):740‐744. [DOI] [PubMed] [Google Scholar]
- 27. Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. [DOI] [PMC free article] [PubMed] [Google Scholar]


