Abstract
Background
Serine hydroxymethyltransferase 1 (SHMT1) is an enzyme involved in folic acid metabolism and is known to contribute to the development of hypertension. We evaluated the relationship between SHMT1 promoter methylation and essential hypertension (EH).
Methods
Quantitative methylation‐specific polymerase chain reaction was used to measure the SHMT1 promoter methylation level in 241 EH patients and 288 age‐ and gender‐matched healthy individuals. The diagnostic value of SHMT1 promoter hypermethylation was analyzed using a receiver operating characteristic (ROC) curve. The Gene Expression Omnibus (GEO) database and dual‐luciferase reporter assay were used to validate our findings.
Results
Compared with the control group, significant differences in SHMT1 promoter methylation were found in both EH and hyperhomocysteinemia groups (P < 0.001 and P = 0.029, respectively). The area under the curve of the diagnosis of SHMT1 promoter hypermethylation for EH was 0.808, with a sensitivity and specificity of 73.9% and 77.8%, respectively. The risk of SHMT1 promoter hypermethylation was significantly higher in the >65‐year group than in the ≤65‐year group (odds ratio = 3.925; 95% confidence interval = 2.141‐7.196). In addition, GEO database analysis showed that 5‐aza‐deoxycytidine increased gene expression in several carotid endothelial cell lines. A dual‐luciferase reporter assay revealed that the target sequence in the SHMT1 promoter upregulated gene expression.
Conclusion
Our findings indicate that SHMT1 promoter hypermethylation increases the risk of EH and may be a promising biomarker for EH.
Keywords: essential hypertension, methylation, promoter, SHMT1
1. INTRODUCTION
Essential hypertension (EH), the second largest risk factor accounting for 12.0% of disability‐adjusted life years and 24.6% of deaths,1 is a major public health burden.
There are more than 1.5 billion hypertensive patients worldwide, and responsible for approximately 9.4 million deaths.
However, the pathogenesis of EH is not fully understood. Currently, it is known that epigenetic modifications play an important role in the pathogenesis of EH. As one of the most common epigenetic modifications, DNA methylation affects gene expression.4 Increasing the methylation level of a promoter region will silence the transcription of the corresponding gene, whereas decreasing it may promote gene transcription.5 Aberrant gene methylation has been identified as an important contributor to the pathogenesis of EH, for example, hypomethylation of interleukin‐6 (IL‐6),6 angiotensin II type 1 receptor (AGTR1),7 angiotensin‐converting enzyme (ACE),8 Na+/K+/Cl− cotransporter protein 1 (NKCC1),9 and α‐adducin (ADD1),10 and the hypermethylation of 11β‐hydroxysteroid dehydrogenase type II (11β‐HSD‐2).11
Serine hydroxymethyltransferase 1 (SHMT1), located on chromosome 17p11.2, is a key enzyme involved in folic acid metabolism. SHMT1 reversibly catalyzes the conversion of serine and tetrahydrofolate to glycine and 5, 10‐methylenetetrahydrofolate (5, 10‐methyleneTHF).12 Besides, it also plays a key role in inducing gene methylation and DNA synthesis by providing one‐carbon units for purine, thymidylate, and methionine.12 The one‐carbon moiety of 5, 10‐methyleneTHF is in turn directed to the synthesis of purines or thymidylate or to the methionine cycle, where it remethylates homocysteine (Hcy) to synthesize methionine and S‐adenosyl methionine. Low levels of SHMT1 enzyme resulted in decreased levels of 5‐methylTHF, which is a cosubstrate for the conversion of Hcy to methionine,13 and low levels of 5‐methylTHF in turn resulted in increased levels of Hcy, which is a well‐known risk factor for hypertension.14 Based on these results taken together, we hypothesized that SHMT1 promoter hypermethylation might contribute to the development of hypertension.
Therefore, here, we aimed to investigate the association between SHMT1 promoter hypermethylation and EH in an age‐ and gender‐matched case‐control study. We also performed a data mining analysis to verify the relationship between SHMT1 promoter hypermethylation and SHMT1 gene expression.
2. MATERIALS AND METHODS
2.1. Study participants
We enrolled 241 EH patients and 288 age‐ and gender‐matched non‐EH individuals who were all Chinese, aged either 18 years or older, and lived in the Nanshan District (Shenzhen, China) community for at least 6 months. The diagnosis of EH was based on the WHO diagnostic criteria, with systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg or self‐reporting a history of antihypertensive drug use.15 All individuals provided informed consent. We excluded participants with a history of secondary hypertension; malignancy; liver and kidney failure; pregnancy; or vitamin B6, vitamin B12, or folate intake. This study was approved by the Ethics Committees of the School of Medicine at Ningbo University and Nanshan Chronic Disease Prevention Center.
2.2. Biochemical measurements
Glucose (Glu), total cholesterol (TC), triglyceride (TG), low‐density lipoprotein (LDL), uric acid (UA), and plasma homocysteine (Hcy) levels were measured. Uric enzyme method was performed for quantitative determination of uric acid levels. TC, TG, LDL, and Glu levels were determined by the enzymatic method, and Hcy level was determined by the circulating enzyme method. All these biological indicators were measured using an automatic biochemical analyzer (HITACHI 7080; Tokyo, Japan).
2.3. DNA methylation analysis
The isolation and quantification of genomic DNA were performed using a Nucleic Acid Extraction Kit (TIANLONG NP968, Xi'an, China) and a NanoDrop 2000 (Thermo Scientific, Wilmington, DE, USA). Sodium bisulfite modification was performed using the EZ DNA Methylation‐Gold™ Kit (Zymo Research, Orange, CA, USA). DNA methylation was measured by quantitative methylation‐specific polymerase chain reaction (PCR) (qMSP), using a LightCycler 480 machine (Roche Diagnostics, Mannheim, Germany), as described in previous studies.16, 17 The qMSP primer sequences for SHMT1 are shown in Table 1. The reaction conditions were as follows: initial denaturation at 95°C for 60 seconds, followed by 45 cycles of denaturation at 95°C for 20 seconds, annealing at 58°C for 45 seconds, and extension at 72°C for 20 seconds. The melting curve step was performed at 95°C for 15 seconds and 60°C for 60 seconds, with the temperature being increased at 0.11°C per second to 95°C to measure the fluorescence signal. The 2−ΔΔCt method was used to analyze the relative change in SHMT1 promoter methylation.
Table 1.
Primer sequences for quantitative methylation‐specific PCR
| Gene | Forward primer (5'‐3') | Reverse primer (5'‐3') | Product (bp) | Tm (°C) |
|---|---|---|---|---|
| SHMT1 | CGAGTTTAGGAAGGTGTATT | CCATACTTAACTACGCTCTC | 88 | 58 |
| ACTB | TGGTGATGGAGGAGGTTTAGTAAGT | AACCAATAAAACCTACTCCTCCCTTAA | 133 | 58 |
2.4. Public databases to analyze the correlation between SHMT1 gene methylation and expression
Expression values of the SHMT1 gene in the carotid artery endothelial cell lines (left carotid artery: LCA; right carotid artery: RCA) treated with 5‐aza‐deoxycytidine (5‐AZA; 0.5 μmol/L, 7 days) or left untreated were obtained from the GEO database (Accession No. GSE56143).
2.5. Luciferase reporter gene assay
The amplified promoter DNA fragment was digested with XhoI and NheI (New England Biolabs, Ipswich, MA, USA). After being purified using a Cycle‐Pure Kit (Omega, Norcross, GA, USA), the target fragment was cloned into the pGL3 basic vector (Promega, Madison city, WI, USA), using a DNA Ligation Kit (Takara, Kusatsu, Japan). Luciferase activity was determined using the dual‐luciferase reporter assay system (Dual‐Luciferase® Reporter Assay Systems, Promega).
2.6. Statistical analysis
The continuous variables with normal distribution are expressed as mean ± standard deviation (SD) and were analyzed by the t test. The frequency (percent) and chi‐square test were used to express and analyze categorical variables, respectively. The methylation level of SHMT1 promoter is expressed as the median and interquartile range, and the difference in SHMT1 methylation levels between groups was analyzed by the Mann‐Whitney U test. The chi‐square test was conducted to assess SHMT1 promoter methylation and clinical characteristics of the EH patients, including gender (male or female), age (<65 or ≥65 years), body mass index (BMI; <24 or ≥24 kg/m2), and homocysteinemia (<15 or ≥15 μmol/L). A receiver operating characteristic (ROC) curve was generated to analyze the cutoff levels of SHMT1 promoter methylation with the maximum Youden index, and the area under the ROC curve (AUC) was used to evaluate the diagnostic value of SHMT1 methylation for hypertension. The cutoff value was used to distinguish hypermethylation from hypomethylation. Bilateral P < 0.05 was considered to indicate statistically significant differences. All statistical analyses were performed using the SPSS 18.0 software (SPSS, Inc, Somers, NY, USA).
3. RESULTS
A fragment in the SHMT1 promoter island (hg19, Chr17: 18 266 824‐18 266 911) was selected for the methylation assay (Figure 1A). Sequencing of the qMSP product showed successful bisulfite conversion of the template DNA (Figure 1B). Further, capillary electrophoresis confirmed that the qMSP‐amplified fragment was 88 bp in length (Figure 1C). We observed statistically significant differences in LDL and Glu levels, BMI, SBP, DBP, and alcohol consumption between the cases and controls (Table 2), and the average Hcy level was 15.33 μmol/L in the case group, which was higher than 13.90 μmol/L in the control group (P = 0.005). There were no significant differences in age; gender; UA, TG, TC, and waist‐hip rate (WHR) levels; and smoking status between the cases and controls.
Figure 1.
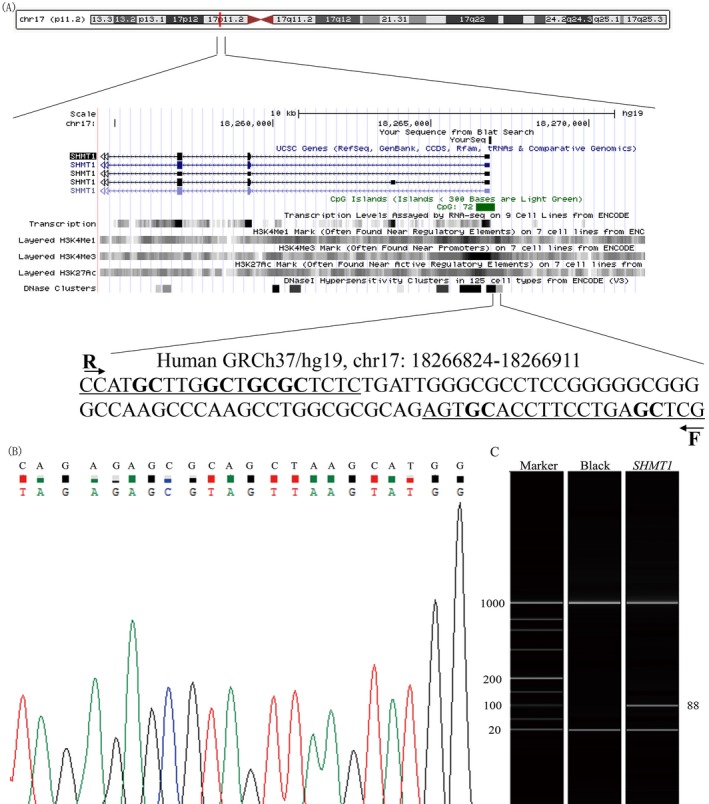
Characteristics of target sequences in SHMT1 gene. A, The location of SHMT1 on the chromosome and the sequences of primer for target gene. F stands for forward primer, and R stands for reverse primer. B, Sequencing validation of the PCR product. The top row of sequencing validation result represents the original gene sequence, and the second row shows the converted one. C, The electrophoresis experiments reveal the results of methylated sample
Table 2.
Baseline characteristics between cases and controls
| Controls | Cases | t/χ 2 | P‐value | |
|---|---|---|---|---|
| Age (y) | 65.12 ± 8.84 | 66.15 ± 8.81 | −1.34 | 0.181 |
| Gender (M/F) | 140/148 | 120/121 | 0.07 | 0.787 |
| Hcy (μmol/L) | 13.90 ± 5.37 | 15.33 ± 6.21 | −2.85 | 0.005 |
| UA (μmol/L) | 356.74 ± 89.93 | 354.02 ± 91.78 | 0.34 | 0.732 |
| TG (mmol/L) | 1.89 ± 1.56 | 1.96 ± 1.61 | −0.50 | 0.614 |
| TC (mmol/L) | 5.18 ± 1.00 | 5.20 ± 1.03 | −0.30 | 0.762 |
| LDL (mmol/L) | 3.23 ± 0.78 | 3.02 ± 0.77 | 3.06 | 0.002 |
| Glu (mmol/L) | 5.05 ± 1.33 | 5.65 ± 1.27 | 20.56 | <0.0001 |
| BMI (kg/m2) | 23.45 ± 3.17 | 24.42 ± 2.86 | −3.65 | <0.001 |
| WHR | 0.90 ± 0.10 | 0.91 ± 0.07 | −0.97 | 0.334 |
| SBP (mm Hg) | 123.81 ± 14.11 | 133.30 ± 16.78 | −7.07 | <0.001 |
| DBP (mm Hg) | 77.34 ± 8.66 | 82.78 ± 11.34 | −6.25 | <0.001 |
| Drinking (No/Yes) | 194/94 | 184/57 | 5.20 | 0.023 |
| Smoking (No/Yes) | 247/41 | 215/26 | 1.41 | 0.235 |
BMI, body mass index; DBP, diastolic blood pressure; Glu, blood glucose; Hcy, plasma homocysteine; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WHR, Waist‐hip ratio.
As shown in Figure 2, SHMT1 promoter methylation levels were significantly higher in the 241 EH patients than in the 288 age‐ and gender‐matched controls (median [interquartile range]: 26.39 [14.81, 41.34] vs 6.57 [2.41, 14.94], P < 0.001). SHMT1 promoter methylation was also significantly higher in patients with hyperhomocysteinemia than in patients with low homocysteinemia (19.42 [6.30, 31.99] vs 12.81 [3.92, 26.39], P = 0.029). As shown in Table 3, the risk of SHMT1 promoter hypermethylation was significantly higher in EH cases than in healthy controls (odds ratio = 10.104; 95% CI = 6.769‐15.086). Besides, older EH patients (odds ratio [OR] = 3.925, 95% confidence interval [CI] = 2.141‐7.196) and EH patients with hyperhomocysteinemia (OR = 2.250, 95% CI = 1.237‐4.091) had higher levels of SHMT1 promoter methylation than their counterparts. In addition, SHMT1 promoter methylation was not associated with gender, BMI, and LDL and Glu levels (Table 3). The diagnostic value of SHMT1 promoter methylation was shown in Figure 3, with AUC and 95% CI being 0.808 (0.771‐0.846), and sensitivity and specificity being 73.9% and 77.8%, respectively.
Figure 2.
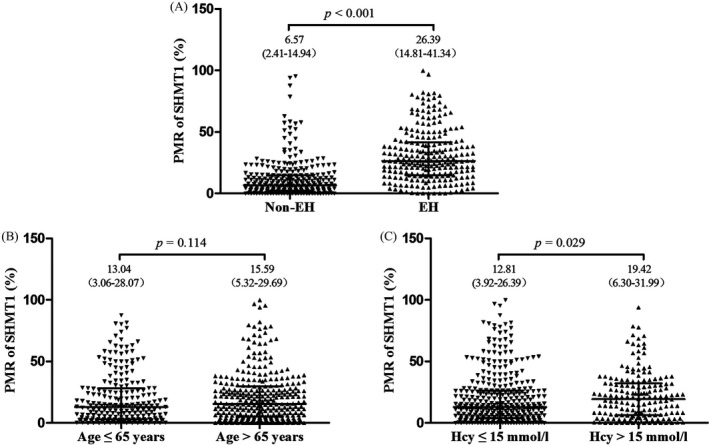
Comparison of methylation level of SHMT1 between different subgroups. The methylation levels of SHMT1 are represented as median and interquartile range
Table 3.
Association between promoter methylation of SHMT1 and baseline characteristics in EH cases
| N | SHMT1 hypomethylation | SHMT1 hypermethylation | OR (95% CI) | P‐value | |
|---|---|---|---|---|---|
| Case | 241 | 62 | 179 | 10.104 (6.769‐15.086) | <0.001 |
| Control | 288 | 224 | 64 | ||
| Gender | |||||
| Male | 144 | 39 | 105 | 1.195 (0.659‐2.167) | 0.557 |
| Female | 97 | 23 | 74 | 1 | |
| Age (y) | |||||
| >65 | 93 | 39 | 54 | 3.925 (2.141‐7.196) | <0.001 |
| ≤65 | 148 | 23 | 125 | 1 | |
| BMI (kg/m2) | |||||
| >24 | 110 | 30 | 80 | 1.160 (0.650‐2.069) | 0.615 |
| ≤24 | 131 | 32 | 99 | 1 | |
| Hcy (mmol/L) | |||||
| >15 | 120 | 40 | 80 | 2.250 (1.237‐4.091) | 0.007 |
| ≤15 | 121 | 22 | 99 | 1 | |
| LDL (mmol/L) | |||||
| >3.12 | 110 | 26 | 84 | 1.224 (0.683‐2.195) | 0.496 |
| ≤3.12 | 131 | 36 | 95 | 1 | |
| Glu (mmol/L) | |||||
| >6.18 | 47 | 15 | 32 | 0.682 (0.340‐1.368) | 0.352 |
| ≤6.18 | 194 | 47 | 147 | 1 | |
The methylation level of SHMT1 ≥15.67% was considered as hypermethylation and <15.67% was considered as hypomethylation.
BMI, body mass index; EH, essential hypertension; Glu, blood glucose; Hcy, plasma homocysteine; LDL, low‐density lipoprotein.
Figure 3.
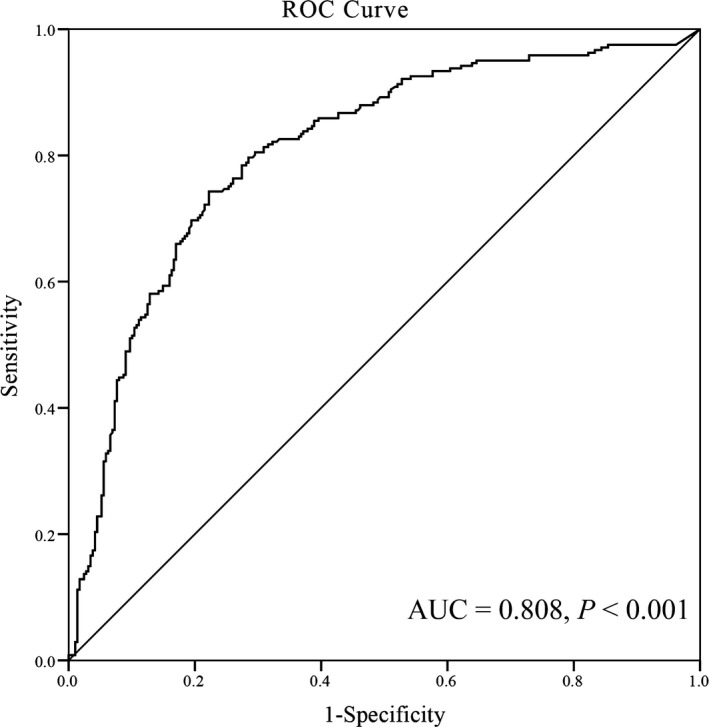
Receiver operating characteristic (ROC) curve for the diagnostic value of SHMT1 methylation. The area under the curve (AUC) of SHMT1 methylation in patients with hypertension was 0.808 (0.771, 0.846), with sensitivity and specificity being 73.9% and 77.8%, respectively
We further analyzed SHMT1 expression in the carotid endothelium (LCA and RCA) with and without 5‐AZA treatment (Accession No. GSE56143). The expression level of SHMT1 in the RCA was significantly increased after demethylation by 5‐AZA (P = 0.028, Figure 4A). The data indicated that higher levels of SHMT1 promoter methylation reduced SHMT1 expression. Subsequently, we also performed dual‐luciferase reporter assays to check whether the SHMT1 CpG island region could regulate gene expression. Our results showed that the transcriptional activity of the recombinant pGL3‐SHMT1 plasmid was higher than that of the empty vector pGL3 basic (mean: 1.39 vs 0.02, fold change = 70, P = 0.003, Figure 4B).
Figure 4.
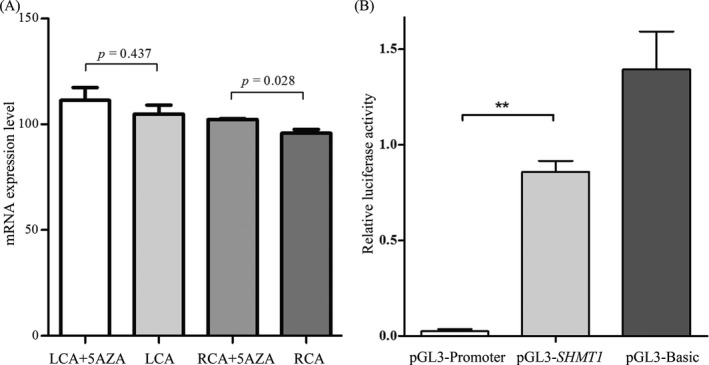
A, SHMT1 expression level with and without 5‐aza‐deoxycytidine treatment in carotid artery endothelium lines (LCA and RCA) derived from Gene Expression Omnibus database (GSE56143). 5AZA, 5‐aza‐deoxycytidine treatment; LCA, left carotid artery; RCA, right carotid artery. B, Dual‐luciferase reporter assay in HEK293T cell line. The pGL3 basic and promoter vectors were used as negative control and positive control, respectively. Relative luciferase activity was performed in triplicates. Bars represent the means ± standard deviation of three independent experiments. ** P < 0.01, pGL3‐SHMT1 promoter vector (51‐350 bp) vs pGL3 basic vector.
4. DISCUSSION
In this age‐ and gender‐matched case‐control study, SHMT1 promoter methylation levels were higher in EH patients than in healthy controls. The risk of SHMT1 promoter hypermethylation was significantly higher in both hyperhomocysteinemia and older groups. The AUC was 0.808, with a sensitivity and specificity of 73.9% and 77.8%, respectively, suggesting that SHMT1 promoter hypermethylation is a potential biomarker for EH. In addition, data from our GEO analysis showed an inverse regulatory association between SHMT1 promoter methylation and SHMT1 expression. Dual‐luciferase reporter assays indicated that the SHMT1 promoter plays an important regulatory role in its gene expression.
It is known that SHMT1 plays a key role in inducing gene methylation and DNA synthesis.12 Our study demonstrated significantly increased SHMT1 promoter methylation levels in the EH patients. The higher SHMT1 promoter methylation might have led to lower expression of the SHMT1 enzyme, which resulted in the decreased glycine levels, further restricting the production of glutathione.18 As an estimator of oxidative stress, glutathione accelerates the development of hypertension.18 Besides, low expression of SHMT1 might result in high levels of Hcy, thus contributing to the development of hypertension.19
Hyperhomocysteinemia is a well‐known risk factor for EH.19 A previous meta‐analysis suggested that elevated Hcy levels increased the risk of EH by about an additional 36%.20 In a prospective cohort study, the highest tertile of Hcy levels showed a 2.31‐fold increased risk of hypertension.21 Further, significant positive association between SHMT1 promoter methylation and high Hcy level was observed; higher Hcy level showed a 1.90‐fold increased risk of higher SHMT1 promoter methylation. Therefore, SHMT1 promoter hypermethylation may exert its effects on the risk of EH via regulating the levels of Hcy.14
In our study, increased SHMT1 promoter methylation was associated with older age. Participants older than 65 years had a 1.46‐fold increased risk of higher SHMT1 promoter methylation. Consistent with our study, previous studies also indicated a positive association between increased DNA promoter methylation, such as that for ADD1 22 and LINE‐1,23 and older age. Changes in DNA methylation may be induced by aging and environmental exposures.24, 25
Notably, smoking and drinking are also important risk factors for hypertension,27, 28 and both interact with DNA methylation of genes to affect the risk of EH.7, 30, 31 However, we did not observe any significant interactions in our study, although future studies with larger sample size are needed to confirm our findings.
The following limitations of our study should be acknowledged. First, we did not measure SHMT1 expression in our study participants. Alternatively, we used data from GEO and found an inverse correlation between SHMT1 methylation and expression. Second, owing to the nature of our case‐control study, we could not determine the causality between SHMT1 promoter hypermethylation and EH. Third, the underlying mechanism of SHMT1 promoter hypermethylation in EH remains unclear. In addition, we cannot completely exclude the influence of other genes on folic acid metabolism. Finally, we also did not measure the folic acid level; the relationship between SHMT1 promoter methylation and folic acid was therefore not explored in our study. To the best of our knowledge, this is the first age‐ and gender‐matched case‐control study to investigate the relationship between SHMT1 promoter methylation and EH.
In summary, SHMT1 promoter methylation is significantly correlated with homocysteinemia and age, and its hypermethylation increases the risk of EH SHMT1 promoter hypermethylation may therefore be a promising biomarker for diagnosing EH.
AUTHORS’ CONTRIBUTIONS
LL conceived and designed the experiments. GX, XY, FK, HJ, and CW performed the experiments. GX, CW, LZ, XZ, JZ, SD, and LH analyzed the data and wrote this manuscript.
ACKNOWLEDGMENTS
We thank GEO database for their open access. This study is supported by Natural Science Foundation of Zhejiang Province (LY17H260002), K.C. Wong Magna Fund in Ningbo University, China Postdoctoral Science Foundation funded project (156458), Jiangsu Postdoctoral Science Foundation funded project (1601121B), Natural Science Foundation of Ningbo (2017A610219, 2016A610169), Ningbo Scientific Innovation Team for Environmental Hazardous Factor Control and Prevention (2016C51001), Public welfare technology and policy science (soft science) application research of Zhejiang Province (2017C35006), Project of Science and Technology Innovation for College Students in Zhejiang Province (2018R405092), and National Natural Science Foundation of China (81803316).
Xu G, Wang C, Ying X, et al. Serine hydroxymethyltransferase 1 promoter hypermethylation increases the risk of essential hypertension. J Clin Lab Anal. 2019;33:e22712 10.1002/jcla.22712
REFERENCES
- 1. Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the global burden of disease study 2010. Lancet. 2013;381:1987‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gacd Hypertension Research Programme Writing Group , Peiris D, Thompson SR, et al. Behaviour change strategies for reducing blood pressure‐related disease burden: findings from a global implementation research programme. Implement Sci. 2015;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong J, Agha G, Baccarelli AA. The role of DNA methylation in cardiovascular risk and disease: methodological aspects, study design, and data analysis for epidemiological studies. Circ Res. 2016;118:119‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao SQ, Sun JH, Gu TL, Zhu FB, Yin FY, Zhang LN. Hypomethylation of interleukin‐6 (IL‐6) gene increases the risk of essential hypertension: a matched case‐control study. J Hum Hypertens. 2017;31:530‐536. [DOI] [PubMed] [Google Scholar]
- 7. Lin J, Lin S, Wu Y, et al. Hypomethylation of the angiotensin II type I receptor (AGTR1) gene along with environmental factors increases the risk for essential hypertension. Cardiology. 2017;137:126‐135. [DOI] [PubMed] [Google Scholar]
- 8. Zill P, Baghai TC, Schule C, et al. DNA methylation analysis of the angiotensin converting enzyme (ACE) gene in major depression. PLoS ONE. 2012;7:e40479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee HA, Baek I, Seok YM, et al. Promoter hypomethylation upregulates Na+‐K+‐2Cl‐ cotransporter 1 in spontaneously hypertensive rats. Biochem Biophys Res Commun. 2010;396:252‐257. [DOI] [PubMed] [Google Scholar]
- 10. Zhang LN, Liu PP, Wang L, et al. Lower ADD1 gene promoter DNA methylation increases the risk of essential hypertension. PLoS ONE. 2013;8:e63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alikhani‐Koopaei R, Fouladkou F, Frey FJ, et al. Epigenetic regulation of 11 beta‐hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang YM, Guo W, Zhang XF, et al. Correlations between serine hydroxymethyltransferase1 C1420T polymorphisms and susceptibilities to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma. Ai Zheng. 2006;25:281‐286. [PubMed] [Google Scholar]
- 13. Chittiboyina S, Chen Z, Chiorean EG, et al. The role of the folate pathway in pancreatic cancer risk. PLoS ONE. 2018;13:e0193298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: a population‐based, prospective cohort study. Stroke. 2015;46:1777‐1786. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462‐1536. [DOI] [PubMed] [Google Scholar]
- 16. Li B, Chen X, Jiang Y, et al. CCL2 promoter hypomethylation is associated with gout risk in Chinese Han male population. Immunol Lett. 2017;190:15‐19. [DOI] [PubMed] [Google Scholar]
- 17. Pan R, Yu H, Dai J, et al. Significant association of PRMT6 hypomethylation with colorectal cancer. J Clin Lab Anal. 2018;e22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fantinelli JC, Gonzalez Arbelaez LF, Perez Nunez IA, et al. Protective effects of N‐(2‐mercaptopropionyl)‐glycine against ischemia‐reperfusion injury in hypertrophied hearts. Exp Mol Pathol. 2013;94:277‐284. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Chen S, Yao T, et al. Homocysteine as a risk factor for hypertension: a 2‐year follow‐up study. PLoS ONE. 2014;9:e108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhong F, Zhuang L, Wang Y, et al. Homocysteine levels and risk of essential hypertension: A meta‐analysis of published epidemiological studies. Clin Exp Hypertens. 2017;39:160‐167. [DOI] [PubMed] [Google Scholar]
- 21. Kim J, Lee SK, Yoon DW, Shin C. Concurrent presence of obstructive sleep apnea and elevated homocysteine levels exacerbate the development of hypertension: a KoGES six‐year follow‐up study. Sci Rep. 2018;8:2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bayoumy N, El‐Shabrawi MM, Leheta OF, et al. alpha‐Adducin gene promoter DNA methylation and the risk of essential hypertension. Clin Exp Hypertens. 2017;39:764‐768. [DOI] [PubMed] [Google Scholar]
- 23. Wei L, Liu S, Su Z, Cheng R, Bai X, Li X. LINE‐1 hypomethylation is associated with the risk of coronary heart disease in Chinese population. Arq Bras Cardiol. 2014;102:481‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christensen BC, Houseman EA, Marsit CJ, et al. Aging and environmental exposures alter tissue‐specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604‐10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bollati V, Schwartz J, Wright R, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Das S, Purkayastha S, Roy H, et al. Polymorphisms in DNA repair genes increase the risk for type 2 diabetes mellitus and hypertension. Biomol Concepts. 2018;9:80‐93. [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Su F, Ye Q, Wang Z, Shu H, Bai F. The dose‐dependent effects of vascular risk factors on dynamic compensatory neural processes in mild cognitive impairment. Front Aging Neurosci. 2018;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krishnamoorthy P, Kalla A, Figueredo VM. Cardiovascular events in alcoholic syndrome with alcohol withdrawal history: results from the national inpatient sample. Am J Med Sci. 2018;355:425‐427. [DOI] [PubMed] [Google Scholar]
- 30. Gu T, Mao S, Fan R, et al. Interactions between CYP11B2 promoter methylation and smoking increase risk of essential hypertension. Biomed Res Int. 2016;2016:1454186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han L, Liu P, Wang C, et al. The interactions between alcohol consumption and DNA methylation of the ADD1 gene promoter modulate essential hypertension susceptibility in a population‐based, case‐control study. Hypertens Res. 2015;38:284‐290. [DOI] [PubMed] [Google Scholar]


